Summary
Hypoxia within the tumor microenvironment (TME) is a key factor contributing to immunosuppression in tumors, co‐relating with poor treatment outcome and decreased overall survival in advanced oral cancer (OC) patients. Vδ2 is a dominant subset of gamma delta T cells (γδT cells) present in the peripheral blood which exhibits potent anti‐tumor cytotoxicity and is evolving as a key player of anti‐cancer cellular therapy. However, the fate of γδT cells in hypoxic oral tumors remains elusive. In the present study, we compared the effect of hypoxia (1% O2) and normoxia (21% O2) on the expansion, proliferation, activation status, cytokine secretion and cytotoxicity of γδT cells isolated from OC patients and healthy individuals. Hypoxia‐exposed γδT cells exhibited reduced cytotoxicity against oral tumor cells. Our data demonstrated that hypoxia reduces the calcium efflux and the expression of degranulation marker CD107a in γδT cells, which explains the decreased anti‐tumor cytotoxicity of γδT cells observed under hypoxia. Hypoxia‐exposed γδT cells differentiated to γδT17 [γδ T cells that produce interleukin (IL)‐17] cells, which corroborated our observations of increased γδT17 cells observed in the oral tumors. Co‐culture of γδT cells with CD8 T cells in the presence of hypoxia showed that programmed cell death ligand 1 (PD‐L1)high γδT cells brought about apoptosis of programmed cell death 1 (PD‐1)high CD8 T cells which could be significantly reversed upon blocking PD‐1. Thus, future immunotherapeutic treatment modality for oral cancer may use a combined approach of blocking the PD‐1/PD‐L1 signaling and targeting hypoxia‐inducible factor 1α, which may help in reversing hypoxia‐induced immunosuppression.
Keywords: γδT cells, γδT17, hypoxia‐induced immune dysfunction, tumor microenvironment
γδT cells exhibit enhanced tumor infiltration and their ability to lyse oral tumors categorise them as unique players of cancer immunotherapy against oral cancers. Our data demonstrates that under hypoxic conditions, although the activation status, cytokines secretion and proliferation of γδT cells is not affected their anti tumor cytotoxic ability is severely compromised, thereby inducing immune dysfunction in γδT cells. Hypoxia exposed γδT cells differentiate to γδT17 cells and thus contribute to tumor progression.

Introduction
Oral cancer is a major public health problem in India, where it ranks among the top three types of cancers in the country [1]. Hypoxia within the tumor microenvironment is co‐related with poor treatment outcome and decreased overall survival in advanced cancer patients [2]. Hypoxia is a common feature in a majority of advanced solid cancers, which arises due to a deficiency in the amount of oxygen reaching the tissue, and depends on the tissue, size and stage of the tumor [3, 4]. It is a crucial contributor of tumor heterogeneity through which the tumor acquires hallmark traits: most importantly, resistance to therapy [3]. Changes in immune surveillance can either be tumor hypoxia‐dependent or ‐independent, which results in the consecutive generation of an immune escape phenotype [5, 6]. Hypoxia induces resistance of tumor cells to cytotoxic T lymphocyte (CTL)‐mediated cytotoxicity indirectly affecting the cytolytic capacities [7]. The hypoxic zone in solid tumor interferes with the homing of immune effector cells into tumors [8, 9]. Hypoxia recruits suppressive cells such as regulatory T cells [10], polarizes macrophages to immune inhibitory M2‐like phenotypes [11] and promotes the maintenance of myeloid‐derived suppressor cells, which suppress T cell effector cytotoxic functions [12].
Hypoxia‐inducible factor 1α (HIF‐1α) plays an important role in the modulation of immune cell functions under hypoxic conditions. Hypoxia has a detrimental effect on T cells when combined with activation/T cell receptor (TCR) signaling [13] and alters the effector and differentiation functions of CD4+ and CD8+ T cells [14, 15, 16, 17]. Hypoxic T cells exhibit enhanced expression of the inhibitory receptors in a HIF‐1α‐dependent manner [18, 19]. Studies have shown that hypoxia impairs natural killer (NK) cell cytotoxicity [20], reduces the expression of NK group 2, member D (NKG2D) ligand–MICA on tumor cells and down‐regulates the expression of activating receptor NKG2D on NK cells [21]. Hypoxia‐induced immune dysfunction plays a pivotal role in impeding immunotherapy, resulting in cancer progression and reduction in patient survival [18]. Surprisingly, most of the studies to investigate the effect of hypoxia on T cells are reported in CD8 T, CD4 T and NK cells, with very scanty reports available on gamma delta T cells (γδT cells).
γδT cells represent 1–5% of peripheral blood lymphocytes and are categorized as unconventional T cells bridging both the innate and adaptive arms of the immune system. γδT cells exhibit distinctive antigen recognition properties in a major histocompatibility complex (MHC)‐unrestricted fashion, thus contributing uniquely to host immune competence. The Vγ9Vδ2 subset of γδT cells is evolving as a promising candidate for immunotherapy due to their relatively high abundance in the peripheral blood and the possibility to efficiently culture them using amino‐bisphosphonates (N‐BP) or synthetic phosphoantigens (pAg) [22]. A meta‐analysis of the clinical trials demonstrated that adoptive transfer of Vγ9Vδ2 T cells has shown promise in improving overall survival in advanced or metastatic cancer patients because of their broad range of antigen recognition ability [23, 24].Vγ9Vδ2 T cells recognize self and microbial phosphorylated metabolites, which are the intermediates of the eukaryotic mevalonate pathway and the microbial Rhomer pathway [25]. Vγ9Vδ2T cells can recognize intermediates of the dysregulated mevalonate pathway in cancer cells as antigens [isopentyl pyrophosphate and its synthetic analog 1‐hydroxy‐2‐methyl‐2‐buten‐4‐yl 4‐diphosphate (HDMAPP)] via butyrophilin 3A1 [26]. Vγ9Vδ2 subset of γδT cells exhibit anti‐tumor responses through recognition of the non‐peptide pAg [27] and heat shock protein 60 expressed on tumor cells [28].
In the present report, we investigated the immunophenotype of γδT cells sequestered in oral tumors versus those in circulation and co‐related the data with the functional responses. We isolated γδT cells from peripheral blood of healthy individuals and oral cancer (OC) patients, cultured and stimulated γδT cells under 1% O2 tension (hypoxia) or 21% O2 tension (normoxia) and compared their cytolytic ability and differentiation potential. We observed that the expansion, proliferation and activation status of γδT cells was unaltered under hypoxia. A marked decrease was observed in the anti‐tumor cytotoxic ability of γδT cells cultured under hypoxia due to the decreased calcium efflux and reduced expression of degranulation marker CD107a, which are responsible for regulating the cytotoxic effector functions of γδT cells. γδT cells cultured under hypoxia displayed elevated RAR‐related orphan receptor gamma t (RORγt) expression and an increase in cytokines favoring γδT17 [γδ T cells that produce interleukin (IL)‐17] differentiation. Interestingly, γδT17 cells were found to be increased in OC tumor‐infiltrating lymphocytes (TILs). To the best of our knowledge, this is the first report demonstrating the effect of hypoxia on differentiation and anti‐tumor effector functions of γδT cells in OC. Our data show that hypoxia drives the differentiation of γδT17 in oral tumors, a pro‐tumorigenic phenotype that may be promoting tumor progression.
Material and methods
Study group
Newly diagnosed stages III and IV treatment‐naive OC patients (n = 25) were recruited from Tata Memorial Hospital, Mumbai, India. The study protocol was approved by the ACTREC‐TMC Institutional Review Board for human studies. Peripheral blood of OC patients was collected prior to chemotherapy/radiotherapy or surgery after obtaining written informed consent. Tumor tissues were obtained from treatment‐naive OC patients post‐surgery. Peripheral blood was obtained from age‐matched healthy individuals (n = 25) who participated voluntarily, and written informed consent was obtained.
Cell isolation
Peripheral blood lymphocytes (PBLs) were isolated from heparinized blood of OC patients and healthy individuals (HI) using Ficoll Hypaque (Sigma‐Aldrich, St Louis, MO, USA) ultra‐density centrifugation. γδT cells were purified from PBLs of HI using immunomagnetic microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany) by positive selection, as per the manufacturer’s instructions. The purity of separated γδT cells was > 97%, as confirmed by flow cytometry [fluorescence activated cell sorter (FACS) Aria; BD Biosciences, San Jose, CA, USA] and the purified γδT cells were used for the experiments (Supporting information, Fig. S1a).
Surgically resected OC tumors were minced finely and incubated in double‐strength RPMI medium containing enzyme mixture (0·1% trypsin, 300 U/ml collagenase, 0·02% DNase and 100 U/ml hyaluronidase; Sigma‐Aldrich) at 370C for 2 h with intermittent shaking. The tissue was passed through the wire mesh and washed with saline to obtain a single‐cell suspension. HI‐PBLs, OC‐PBLs and OC‐TILs were fixed with 1% paraformaldehyde and permeabilized with 0·1% saponin and used for immunophenotyping.
Assay set‐up of γδT cells under hypoxic and normoxic conditions
PBLs were expanded from HI and OC patients by stimulating with rhIL‐2 (30 U/ml; PreProtech, London, UK) alone, rhIL‐2 (30 U/ml) + αCD3 monoclonal antibody (mAb) (1 μg/ml; BD Biosciences) or rhIL‐2 (30 U/ml) + HDMAPP (1 nM/ml; Echelon, Salt Lake City, UT, USA). The cells were cultured in RPMI‐1640 supplemented with 10% heat‐inactivated fetal bovine serum (FBS) (Thermo Fisher, Fremont, CA, USA), 2 mM glutamine (Sigma‐Aldrich) and ×1 antibiotic–anti‐mycotic (GIBCO; Thermo Fisher). The culture was maintained in a normoxic (21% O2) or hypoxic (1% O2) environment for 12 days at 37°C with intermettient addition of 30 U/ml rhIL‐2 every 3 days. Expanded γδT cells were immunomagnetically purified and further used for cytotoxicity experiments.
The γδT cells immunomagnetically purified from HI‐PBLs were stimulated with rhIL‐2 alone (100 U/ml), rhIL‐2 (100 U/ml) + αCD3 mAb (1 μg/ml) or rhIL‐2 (100 U/ml) + HDMAPP (1 nM/ml) or kept unstimulated and exposed to a normoxic (21% O2) and hypoxic (1% O2) environment for 72 h at 37°C; 1% O2 was maintained using an InvivO2 400 (Baker Ruskin, Sanford, ME, USA) hypoxic work‐station.
Immunostaining
γδT cells stimulated under normoxia or 1% hypoxia for 72 h, as described previously, were fixed with 1% paraformaldehyde (Sigma‐Aldrich) and permeabilized with 0·1% saponin (Sigma‐Aldrich). For staining the intracellular cytokines, lymphocytes/γδT cells were stimulated with phorbol myristate acetate (PMA) (10 ng/ml) and ionomycin (1 μg/ml) for 4 h in the presence of brefeldin A (5 μg/ml; all from Sigma‐Aldrich). Cells were washed with ×1 phosphate‐buffered saline (PBS) and cold‐fixed with 1% paraformaldehyde for 15 min at 40C, followed by permeabilization for 5 min with 0·1% saponin at room temperature. Cells were washed and stained with the following antibodies: CD3‐phycoerythrin‐cyanin 7 (PECy7)/AF700, Vδ2 TCR‐PE, CD25‐PECy7, CD69‐allophycocyanin (APC), CD71‐ fluorescein isothiocyanate (FITC), CD3ζ‐PE, IL‐17a‐APC, CD4‐AF700, CD8‐PB, interferon (IFN)‐γ‐PECy7, RORγt‐PE, CD132‐PE (BD Biosciences), HIF1α‐FITC, programmed cell death 1 (PD‐1)‐PE‐CF594, programmed cell death ligand 1 (PD‐L1)‐peridinin chlorophyll (PerCP) Cy5·5 (BioLegend, San Diego, CA, USA) and NKG2D‐APC, killer immunoglobulin‐like receptor (KIR)D2L2/3‐PE (Miltenyi Biotech) for 30 min at room temperature. Appropriate isotype controls were used. A minimum of 50 000 events were acquired using a FACS AriaIII flow cytometer. Cells were analyzed using FlowJo software (TreeStar, Ashland, OR, USA).
Degranulation assay
For analysing the expression of degranulation marker Lamp‐1 (CD107a), γδT cells sorted from HI‐PBLs were stimulated under normoxia or 1% hypoxia for 72 h as described previously. These cells were harvested and washed with ×1 PBS for 10 min at 100 g. Cells were then restimulated for 4 h with PMA (50 ng/ml; Sigma‐Aldrich) and ionomycin (1 μg/ml; Sigma‐Aldrich) in polypropylene tubes (BD Biosciences) in the presence of monensin (5 mg/ml; Sigma‐Aldrich) and brefeldin (5 μg/ml; Sigma‐ Aldrich). CD107a‐APC antibody (BD FastImmune™ CD107a; BD Biosciences) was added at the start of the assay. After 4 h, cells were washed, fixed and stained with anti‐human TCR Vδ2 PE (BD Biosciences). Cells were acquired on a FACS AriaIII (BD Biosciences) and analysis was performed using FlowJo software (TreeStar). The expression of CD107a was analyzed on the Vδ2 TCR+ gated populations.
Proliferation assay
γδT cells (1 × 105) sorted from HI‐PBLs were stimulated under normoxia or 1% hypoxia for 72 h, as described previously in 96‐well cell culture plates. The γδT cells were pulsed with 1 µCi [3H]‐thymidine (Board of Radiation and Isotope Technology, Mumbai, India) 18 h prior to termination of the assay. Following the incubation, cells were transferred onto glass‐wool filters using a cell harvester (Perkin Elmer, Beaconsfield, UK). The radioactivity incorporated into the DNA was measured in a liquid beta scintillation counter (Packard, Meriden, CT, USA). Data were expressed as counts per minute (cpm).
Cytokine measurement
γδT cells sorted from HI‐PBLs were stimulated under normoxia or 1% hypoxia for 72 h as described previously for 24 h. Cell‐free supernatant was collected after 24 h from the culture wells. Cytokines in the culture supernatants were measured using the T helper type 1 (Th1)/Th2/Th17 cytometric bead array kit, as per the manufacturer’s instructions (BD Biosciences). Samples were acquired on FACS AriaIII and analyzed using the BD FCAP array (BD Biosciences).
Cytotoxicity assay
Chromium release assay was performed to measure the cytotoxic potential of γδT cells exposed to 1% hypoxia against OC cell lines AW13516 and AW8507 [29], which were used as target cells. γδT cells were stimulated under normoxia or 1% hypoxia for 72 h as described previously. The killing ability of hypoxia‐ and normoxia‐exposed stimulated γδT cells was measured against aminobisphosphonate‐treated oral tumor targets.These tumor cells were exposed to a normoxic and hypoxic environment for 48 h prior to co‐culture. Cell lines (oral tumor targets) were treated for 18 h with zoledronate, an aminobisphosphonate (100 μM; Sigma‐Aldrich). Targets were labeled with 51chromium for 90 min at 370C. Labeled target cells were co‐cultured with γδT cells in 96‐well plates (Nunc, Roskilde, Denmark) at a 30 : 1 effector (Eff) : target (T) ratio at 370C in 5% CO2 for 4 h. After incubation, plates were centrifuged, 80 μl supernatant was collected and radioactive chromium released in the supernatant was measured using a 1470 Wallac automated gamma counter. Spontaneous release was determined by incubating the target cells with medium alone, and maximum release was determined by incubating target cells with 10% Triton X‐100 (Sigma Aldrich). The percentage of cytotoxicity was calculated as (experimental release – spontaneous release)/(maximum release – spontaneous release) × 100.
Estimation of calcium flux
γδT cells sorted from HI‐PBLs were stimulated under normoxia or 1% hypoxia for 72 h, as described previously. After 72 h, cells were labeled with 5 μM Fluo‐3‐AM (Sigma Aldrich) for 30 min at room temperature and washed with ×1 PBS for 10 min at 100 g. The cells were resuspended in calcium estimation buffer [137 mMNaCl, 5 mMKCl, 1 mM Na2HPO4, 5 mM glucose, 0·5 mM MgCl2, 10 mM HEPES, 1 mM CaCl2 and 1 g/l bovine serum albumin (BSA); Sigma Aldrich]. The baseline expression of Fluo‐3‐AM was assessed for the initial 30 s of acquisition and stimulated with PMA (10 ng/ml; Sigma Aldrich) and ionomycin (1 μg/ml) and acquired for 300 s. Calcium flux was acquired on a FACS ARIA III (BD Biosciences) and calcium kinetics was calculated using FlowJo software (TreeStar).
Co‐culture studies to assess the apoptosis on CD8 T cells by γδT cells
γδT cells and CD8 T cells were immunomagnetically purified from HI‐PBLs by magnetic‐activated cell sorter (MACS), as per the manufacturer’s protocol (Miltenyi Biotech). γδT cells and CD8 T cells were co‐cultured (1 : 1) and stimulated with 100 IU/ml rhIL‐2 alone, 100 IU/ml rhIL‐2 + αCD3 mAb (1 μg/ml) or kept unstimulated for 72 h at 370C in the presence of 1% hypoxia or normoxia. Additionally, anti‐PD‐1 (1 μg/ml; BioLegend) and anti‐PD‐L1 (1 μg/ml; BioLegend) blocking antibodies were added to the culture before 4 h of stimulation. After 72 h, the cells were harvested and washed with 1 × PBS at 100 g for 10 min. The viability of CD8 T cells and γδT cells was assessed by staining with annexin V APC (BD Biosciences), 7‐amino‐actinomycin D (7‐AAD; BioLegend), Vδ2 PE (BD Biosciences) and CD8 PB (BD Biosciences) and kept at room temperature for 30 min, and were acquired on a FACS AriaIII (BD Biosciences). The data were analyzed using FlowJo software (TreeStar).
Statistical analysis
Data analysis was performed by Student’s t‐test using GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA) and considered significant at P < 0·05.
Results
Increased HIF1α expression in tumor‐infiltrating γδT cells
HIF1α is the first protein to be activated in response to hypoxia. In order to assess the effect of hypoxia on γδT cells, we analyzed the expression of HIF1α in the Vδ2 subset of γδT cells (henceforth referred to as γδT cells), CD4 and CD8 T cells present in the paired samples of peripheral blood and tumor tissues of stages III and IV advanced OC patients (n = 25) and HI‐PBLs (n = 15). γδT cells were increased in the PBLs of the OC patients compared to the HI. Levels of γδT cells were found to be further elevated in the tumor compared to the peripheral compartment of the same patient (Fig. 1a,b). The expression of HIF1α in the CD4, CD8 and γδ T cells in both OC patients and HI was analysed. Compared to the PBL of OC patients, a statistically significant increase in the expression of HIF1α was observed in CD4, CD8 and γδ T cells in the tumor. A relative increase in expression of HIF1α was observed in γδ T cells compared to CD4 and CD8 T cells in the tumor compartment (Fig. 1c–e).
Fig. 1.
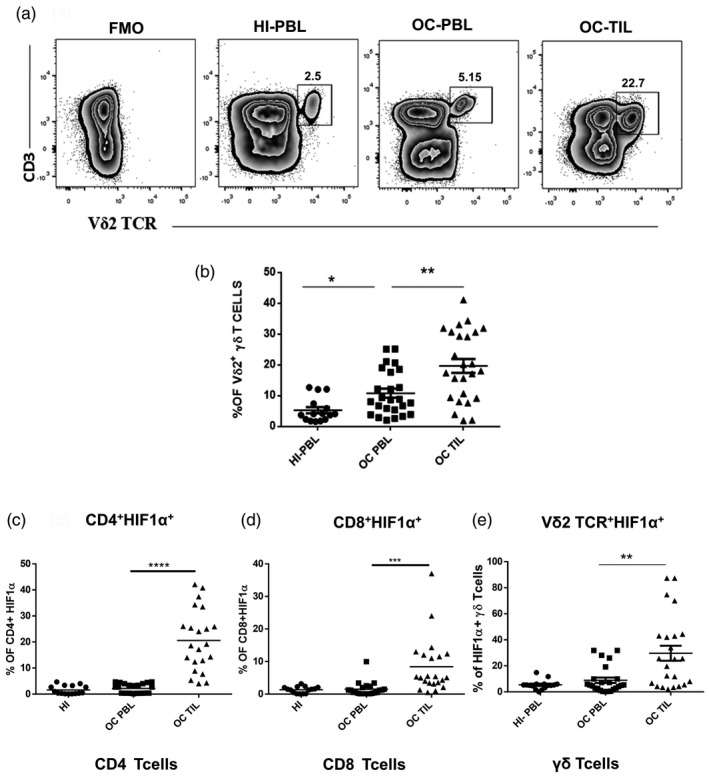
Increased hypoxia‐inducible factor 1α (HIF1α) expression in tumor‐infiltrating gamma delta T (γδT) cells. (a) Representative figure showing the expression of CD3+ Vδ2 T cell receptor (TCR)+ γδT cells in healthy individuals’ peripheral blood lymphocytes (HI‐PBL) and PBLs and tumor‐infiltrating lymphocytes (TILs) (paired samples) of the oral cancer (OC) patient with the fluorescence minus one (FMO) control. The values shown in the figure indicate the percentage‐positive population. (b) Immunophenotyping of γδT cells (Vδ2 subset) in HI‐PBL (n = 15) and OC patient paired‐samples PBLs and TILs (n = 25) by multi‐color flow cytometry. Gating was performed on lymphocytes. (c–e) Expression of HIF1α was assessed on CD4, CD8 T cells and γδT cells in HI‐PBL (n = 15) and OC patient paired‐samples PBLs and TILs (n = 22) as assessed by multi‐color flow cytometry. The expression of HIF1α was analysed on CD3+CD4+, CD3+CD8+ T cells and CD3+Vδ2+ (subset of γδT cells), respectively (*P < 0·05; **P < 0·01; ***P < 0·007; ****P < 0·001).
Expansion of γδT cells upon TCR stimulation is unaltered under hypoxia
To investigate how hypoxia influences the expansion of γδT cells in vitro, we incubated HI‐PBLs in the presence of rhIL‐2 + αCD3 or rhIL‐2 + HDMAPP or rhIL‐2 alone in independent sets under normoxia and hypoxia for 12 days and phenotyped the cultures for the presence of γδT cells (Vδ2 subset). A marked expansion of the CD3+Vδ2 TCR+ subset of γδT cells was observed after stimulation of PBLs with rhIL‐2 + αCD3 or rhIL‐2 + HDMAPP (compared to the rhIL‐2 control), which was comparable under both hypoxic and normoxic conditions (Fig. 2a,b). The data indicate that expansion of γδT cells in HI‐PBLs upon stimulation with rhIL‐2 + αCD3 or rhIL‐2 + HDMAPP was not hindered under hypoxia (Fig. 2a,b). HI‐PBLs stimulated with rhIL‐2 alone, rhIL‐2+αCD3 or rhIL‐2 + HDMAPP cultured under hypoxia showed a significant increase in the intracellular expression of HIF1α in the γδT cells compared to γδT cells in HI‐PBLs stimulated under normoxia (Fig. 2c).
Fig. 2.
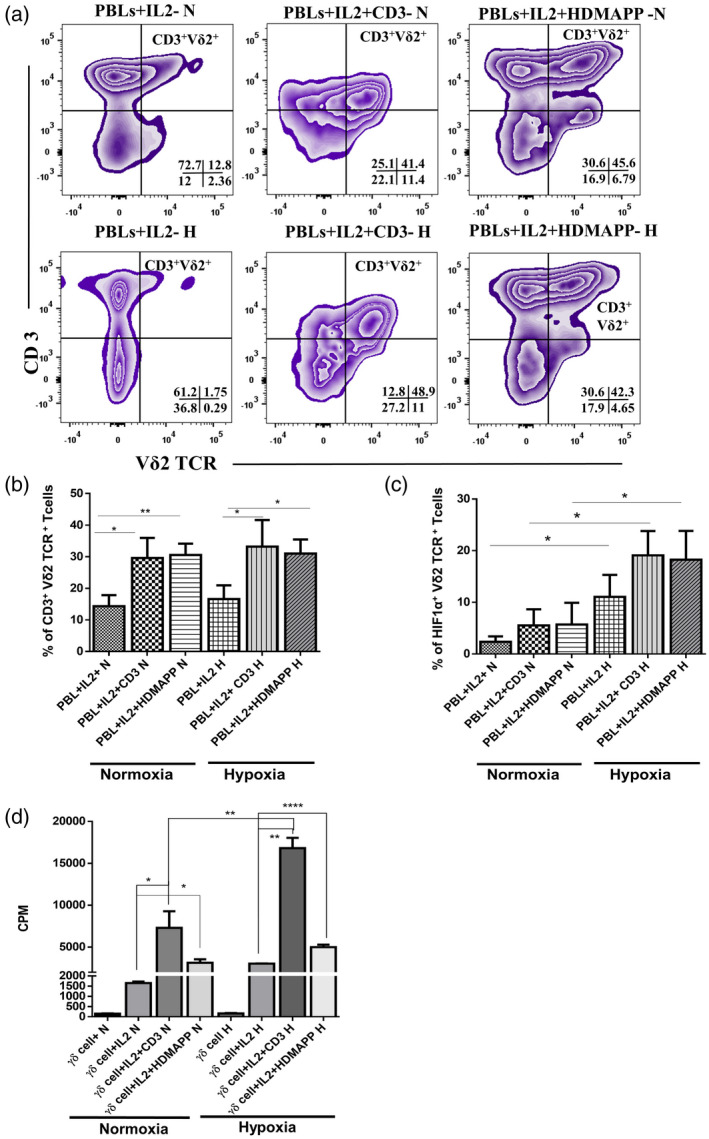
Expansion of gamma delta T (γδT) cells from peripheral blood lymphocytes (PBLs) upon T cell receptor (TCR) stimulation is unaltered under hypoxia. Healthy individuals’ PBLs (HI‐PBL) (n = 5) were expanded with recombinant human interleukin (rhIL‐2) alone, rhIL‐2 + αCD3 or rhIL‐2 + 1‐hydroxy‐2‐methyl‐2‐buten‐4‐yl 4‐diphosphate (HDMAPP) for 12 days under hypoxia (H) or normoxia (N), and assessed for expression of CD3+ Vδ2 TCR+ phenotype (a,b) and hypoxia‐inducible factor 1α (HIF1α) (c). (a) The representative figure of CD3+ Vδ2 TCR+ cells in stimulated HI‐PBLs gating was performed on lymphocytes. The values shown in the figure indicate the percentage‐positive population. (b) Mean ± standard error of the mean (s.e.m.) of the percentage of percentage‐positive CD3+ Vδ2 TCR+cells in HI‐PBLs. (c) Mean ± s.e.m. of the percentage of positive HIF1α+ Vδ2 TCR+cells in HI‐PBLs (gated on CD3+ Vδ2 TCR+ cells; *P < 0·05; **P < 0·01). (d) γδT cells isolated from HI‐PBLs by magnetic‐activated cell sorting (MACS) (n = 3) were stimulated with rhIL‐2, rhIL‐2 + αCD3 or rhIL‐2 + HDMAPP or kept unstimulated and cultured for 72 h in H or N. The proliferation of γδT cells was assessed by [3H]‐thymidine incorporation assay. Results indicated are mean ± s.e.m. of three independent experiments (*P < 0·05; **P < 0·01; ****P < 0·005).
In order to study the effect of hypoxia on the proliferative responses of γδT cells, we sorted γδT cells from HI‐PBLs using MACS. Purified γδT cells were monitored for their proliferative response to rhIL‐2 + αCD3 or rhIL‐2 + HDMAPP cultured under hypoxia and normoxia using tritiated thymidine incorporation assay. A significant increase in proliferation of γδT cells was observed after stimulation with rhIL‐2 + αCD3 or rhIL‐2 + HDMAPP compared to controls (γδT cells and γδT cells + rhIL‐2) under both hypoxia and normoxia. Conversely, the magnitude of the proliferative response of γδT cells to rhIL‐2 + αCD3 under hypoxia was high compared to normoxia. However, this difference in the proliferative response was not observed when γδT cells were stimulated with rhIL‐2 + HDMAPP under hypoxia compared to normoxia (Fig. 2d).
Hypoxia does not alter the activation status and the cytokine secretion of γδT cells
Engagement of TCR with antigen results in the activation of T cells, which includes expression of early and late activation markers and a dramatic increase of intracellular calcium (Ca2+) concentration downstream to T cell antigen receptor ligation. Levels of CD3ζ and activation markers CD69, CD71, CD25 and CD132 were assessed on purified γδT cells after 72 h of culture under hypoxia and normoxia. The median fluorescence intensity (MFI) of CD3ζ and the activation markers were not affected under hypoxia upon stimulation of γδT cells with rhIL‐2 + αCD3 or rhIL‐2 + HDMAPP compared to controls (unstimulated or rhIL‐2‐stimulated γδT cells) (Fig. 3a–e).
Fig. 3.
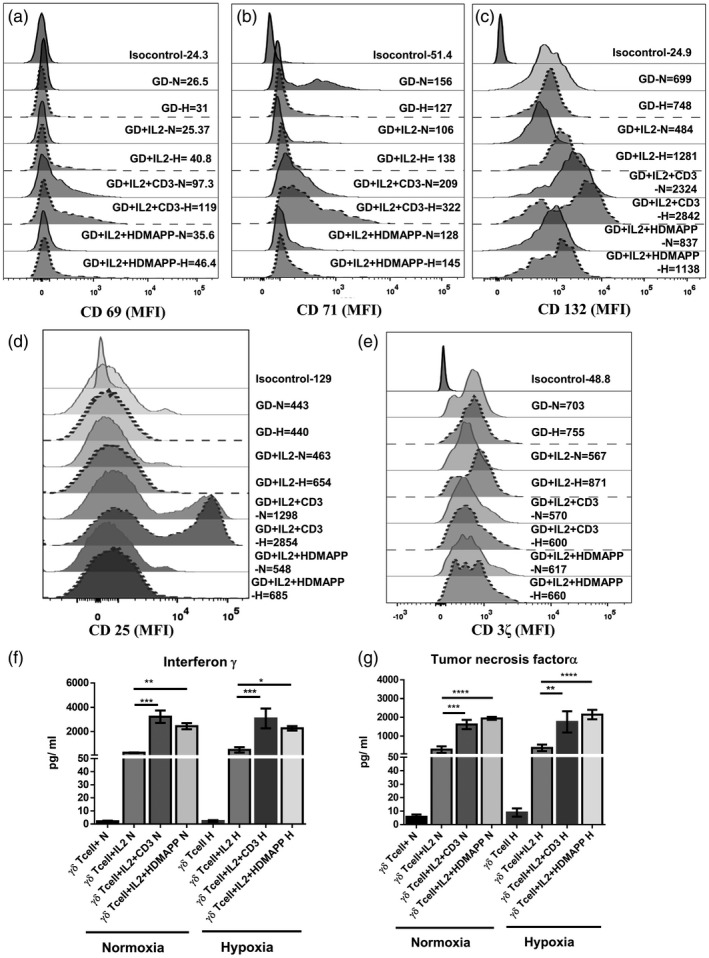
Hypoxia does not alter the activation status and the cytokine secretion of gamma delta T (γδT) cells. γδT cells isolated from healthy individuals’ peripheral blood lymphocytes (HI‐PBL) (n = 3) were stimulated with recombinant human interleukin (rhIL‐2), rhIL‐2+αCD3 or rhIL‐2+1‐hydroxy‐2‐methyl‐2‐buten‐4‐yl 4‐diphosphate (HDMAPP) or kept unstimulated and cultured for 72 h in hypoxia (H) or normoxia (N). The mean fluorescence intensity (MFI) of activation markers (a) CD69, (b) CD71, (c) CD132, (d) CD25 and (e) CD3ζ was assessed using multi‐color flow cytometry. Gating was performed on CD3+ Vδ2 TCR+ T cells. (f,g) γδT cells isolated from HI‐PBLs (n = 3) were stimulated with rhIL‐2, rhIL‐2 + αCD3 or rhIL‐2 + HDMAPP and cultured for 24 h in H or N. The cell‐free supernatants were collected and assessed for secreted cytokines (f) interferon (IFN)‐γ and (g) tumor necrosis factor (TNF)‐α using a cytometric bead array (CBA) kit. All the results indicated are mean ± standard error of the mean (s.e.m.) of three independent experiments (*P < 0·05; **P < 0·01; ***P < 0·005; ****P < 0·001).
Purified γδT cells stimulated with rhIL‐2, rhIL‐2 + αCD3 or rhIL‐2 + HDMAPP or kept unstimulated were cultured under normoxia or hypoxia. After 24 h of stimulation, the cell‐free supernatants were collected and analysed for secreted cytokines. Stimulated γδT cells exposed to hypoxia secreted high levels of IFN‐γ and tumor necrosis factor α (TNF‐α), which were comparable to those secreted under normoxia, indicating that the production of effector cytokines by γδT cells was not altered by hypoxia (Fig. 3f,g). Similarly, elevated levels of proinflammatory cytokine IL‐6 also remained comparable under hypoxia and normoxia upon stimulation of purified γδT cells with rhIL‐2 + αCD3 and rhIL‐2 + HDMAPP compared to controls (unstimulated or rhIL‐2‐stimulated γδT cells) (Supporting information, Fig. S1b).
Hypoxia‐exposed γδT cells exhibit reduced tumor‐directed cytotoxicity
In order to assess the anti‐tumor cytolytic potential of purified γδT cells when exposed to hypoxia, we used aminobisphosphonate‐treated oral tumor cell lines (AW13516 and AW8507) as targets in the 51Cr release cytotoxicity assay. Briefly, purified γδT cells from HI were stimulated with rhIL‐2, rhIL‐2 + αCD3 and rhIL‐2 + HDMAPP or kept unstimulated and cultured in the presence or absence of 1% hypoxia for 72 h. The cytotoxic potential of γδT cells against aminobisphosphonate‐treated tumor cells was determined at a Eff : T ratio of 30 : 1. A marked increase in the cytolytic activity of rhIL‐2 + αCD3 or rhIL‐2 + HDMAPP‐stimulated γδT cells against the oral tumor targets (AW13516 and AW8507) was observed compared to the unstimulated or only rhIL‐2‐stimulated γδT cells used as controls under both normoxia and hypoxia. However, hypoxia‐exposed stimulated γδT cells showed significantly reduced cytotoxicity compared to the normoxia‐exposed γδT cells against the two OC cell lines (AW8507 and AW13516) (Fig. 4a,b).
Fig. 4.
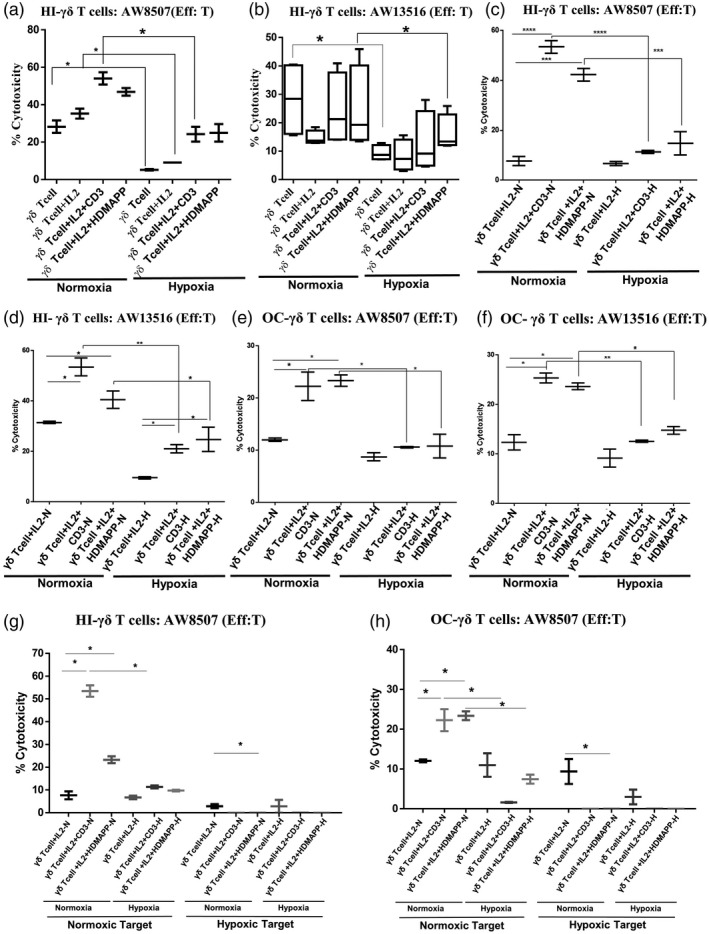
Hypoxia‐exposed gamma delta T (γδT) cells exhibit reduced tumor‐directed cytotoxicity. The zoledronate‐treated oral tumor cell lines (T)‐AW8507 (a,c,e,g,h) and AW13516 (b,d,f) were labeled with 51Cr and used as targets. γδT cells were used as effectors (Eff). (g,h) The zoledronate‐treated oral tumor target (T)‐AW8507 (g,h) was exposed to hypoxia for 48 h and further labeled with 51Cr and was used as the hypoxic target. Co‐culture of γδT cells : tumor cells (Eff : T) was set up at a 30 : ratio for 4 h. (a,b) Purified γδT cells isolated from healthy individuals’ peripheral blood lymphocytes (HI‐PBL) (n = 3) were stimulated with recombinant human interleukin (rhIL‐2), rhIL‐2 + αCD3 or rhIL‐2 + 1‐hydroxy‐2‐methyl‐2‐buten‐4‐yl 4‐diphosphate (HDMAPP) or kept unstimulated and cultured for 72 h in hypoxia (H) or normoxia (N) and co‐cultured with 51Cr‐labeled targets. All the results indicated are represented as box‐and‐whisker plots of three independent experiments (*P < 0·05). (c–h) HI‐PBLs (n = 4) and oral cancer (OC) patient PBLs (n = 4) were stimulated with rhIL‐2, rhIL‐2 + αCD3 or rhIL‐2 + HDMAPP for 12 days under H or N, γδT cells were immunomagnetically purified and used as effectors. (c,d,g) γδT cells isolated from expanded HI‐PBLs (n = 4) and (e,f,h) γδT cells isolated from expanded OC patient PBLs (n = 4) were co‐cultured with 51Cr‐labeled oral tumor targets. All the results indicated are represented as box‐and‐whisker plots of four independent experiments (*P < 0·05; **P < 0·01; ***P < 0·005; ****P < 0·001).
We also wanted to compare the cytolytic ability of γδT cells isolated from OC patients using aminobisphosphonate‐treated oral tumor cells (AW13516 and AW8507) as targets. For obtaining effector γδT cells from OC patients, we adopted a different strategy due to the limitation of acquiring a large volume of blood sample to isolate γδT cells directly from the patient. PBLs from OC patients and HI were expanded using rhIL‐2 + αCD3, rhIL‐2 + HDMAPP or rhIL‐2 alone in independent sets for 12 days. The expanded γδT cells were immunomagnetically purified from both HI and OC patients and used to determine their anti‐tumor cytolytic ability against the oral tumor targets at an Eff : T ratio of 30 : 1. As seen in Fig. 4c,d, exposure of expanded HI‐γδT cells to hypoxia decreased their ability to lyse the oral tumor cells (AW8507 and AW13516) compared to expanded HI‐γδT cells exposed to normoxia. Expanded γδT cells from OC patient PBLs upon stimulation with rhIL‐2 + αCD3 and rhIL‐2 + HDMAPP showed cytotoxicity against AW8507 (Fig. 4e) and AW13516 (Fig. 4f) targets compared to the rhIL‐2‐stimulated control under both normoxic and hypoxic conditions. However, the presence of hypoxia decreased the cytolytic ability of expanded γδT cells from OC patient PBLs (Fig. 4e,f) compared to normoxia.
Although, under normoxia, expanded γδT cells from OC patient PBLs exhibited cytotoxicity against oral tumor targets (AW13516 and AW8507), this was much reduced compared to cytotoxicity exhibited by HI‐PBL‐expanded γδT cells under the same conditions. Exposure to hypoxia further reduced the tumor‐directed cytotoxicity of these γδT cells (Fig. 4c–f).
We then assessed the anti‐tumor cytotoxicity of the γδT cells from HI and OC patients against the hypoxia‐exposed oral tumor target (AW8507). The HI‐γδT cells stimulated under normoxia or hypoxia were unable to lyse oral tumor cells exposed to hypoxia. Conversely, HI‐γδT cells stimulated under normoxia showed increased killing of normoxia‐exposed oral tumors, which was significantly reduced if HI‐γδT cells were stimulated under hypoxia (Fig. 4g). Compared to HI‐γδT cells, the OC‐γδT cells stimulated under hypoxia or normoxia showed a reduced percentage of cytotoxicity against the normoxia‐exposed oral tumor target, which further decreased if oral tumor targets were exposed to hypoxia (Fig. 4h).
We then evaluated the influence of hypoxia on the calcium efflux and expression of degranulation marker LAMP‐1 (CD107a) in γδT cells. Briefly, purified γδT cells were activated with rhIL‐2, rhIL‐2 + αCD3 or rhIL‐2 + HDMAPP stimulation or kept unstimulated for 72 h; these cells were then labeled with Fluo‐3‐AM and restimulated with PMA/ionomycin, and Ca2+ efflux was recorded for 300 s. Upon stimulation with rhIL‐2 + αCD3 and rhIL‐2 + HDMAPP, an increase in the calcium efflux was observed compared to the unstimulated or only rhIL‐2‐stimulated controls under both normoxia and hypoxia. However, hypoxia‐exposed γδT cells showed reduced calcium efflux compared to the normoxia‐exposed cells (Fig. 5a). To further assess the expression of degranulation marker LAMP‐1, purified γδT cells were stimulated with rhIL‐2, rhIL‐2 + αCD3 and rhIL‐2 + HDMAPP or kept unstimulated for 72 h and restimulated with PMA/ionomycin. Upon restimulation with PMA/ionomycin, rhIL‐2 + αCD3 and rhIL‐2 + HDMAPP‐stimulated γδT cells expressed increased MFI values of degranulation marker (CD107a) compared to the unstimulated or only rhIL‐2‐stimulated controls under both normoxia and hypoxia. However, the presence of hypoxia significantly reduced the expression of the degranulation marker in γδT cells (Fig. 5b,c). Collectively, hypoxia reduces the calcium efflux and CD107a expression in γδT cells, which results in decreased anti‐tumor‐cytolytic effector function of γδT cells.
Fig. 5.
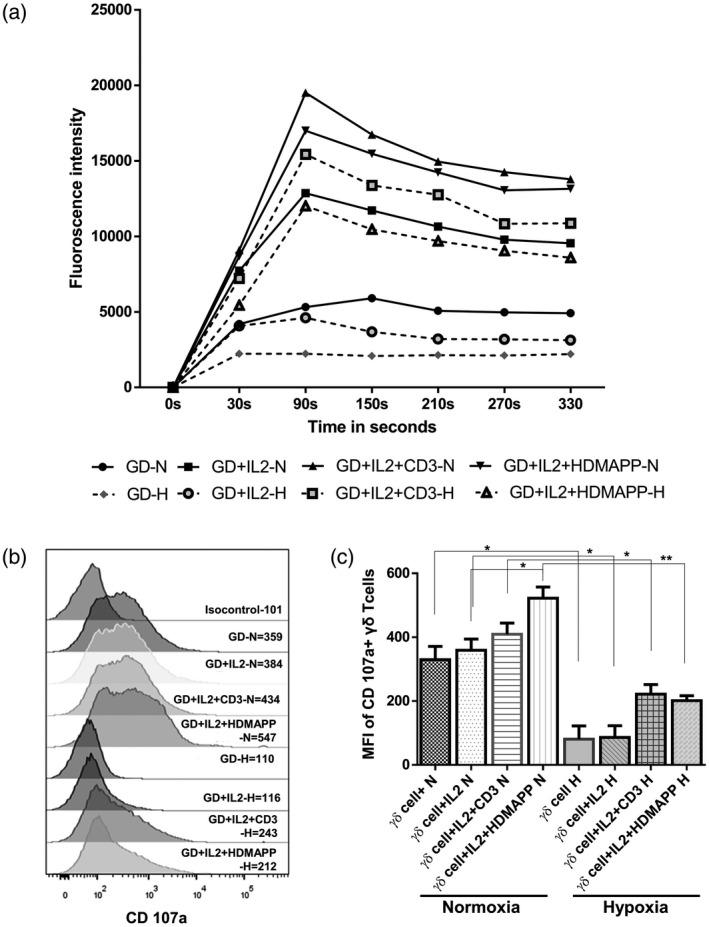
Hypoxia‐exposed gamma delta T (γδT) cells exhibit reduced calcium efflux and degranulation. (a–c) γδT cells isolated from healthy individuals’ peripheral blood lymphocytes (HI‐PBL) (n = 3) were stimulated with recombinant human interleukin (rhIL‐2), rhIL‐2 + αCD3 or rhIL‐2 + 1‐hydroxy‐2‐methyl‐2‐buten‐4‐yl 4‐diphosphate (HDMAPP) or kept unstimulated and cultured for 72 h in hypoxia (H) and normoxia (N). The cells were harvested and (a) calcium efflux was measured in γδT cells labeled with Fluo‐3‐AM and acquired for initial 30 s for baseline values and were restimulated with phorbol myristate acetate (PMA)–ionomycin and acquired for up to 330 s on flow cytometry. Each point on the graph represents the average fluorescence intensity over a range of 60 s each. The graph shows the consolidated data of three independent experiments. (b,c) γδT cells were treated with brefeldin A and monensin and restimulated with PMA–ionomycin and CD107a antibody was added to the culture and maintained for 4 h under N and H conditions. Gating was performed on Vδ2 TCR+ T cells. (b) Representative figure of CD107a expression on Vδ2 TCR+ T cells expressed as mean fluorescence intensity (MFI). (c) Mean ± standard error of the mean (s.e.m.) of MFI values of CD107a expression on Vδ2 TCR+ T cells of three independent experiments (*P < 0·05; **P < 0·01).
γδT17+ cells are enhanced in the presence of hypoxia
Immunophenotyping of oral tumors and peripheral blood lymphocytes showed that the infiltration of IL‐17a‐secreting γδT cells was higher in the tumor tissue compared to peripheral blood in OC patients, whereas IFN‐γ‐secreting γδT cells were reduced in the tumor tissue compared to peripheral blood in OC patients (Fig. 6a–c). The levels of γδT17 in the peripheral blood of OC patients were increased compared to HI. The data indicate that infiltration of γδT17 cells was observed in the oral tumor microenvironment (TME) which harbors hypoxia.
Fig. 6.
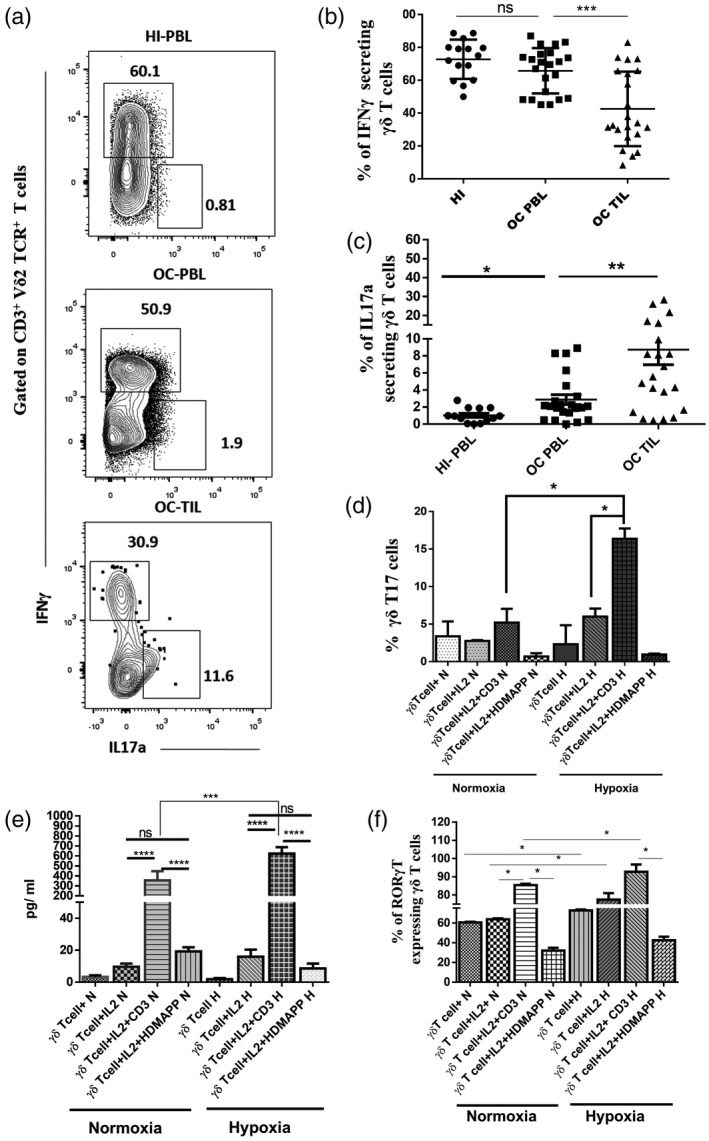
Gamma delta T17+ [γδT cells that produce interleukin (IL)‐17] cells are enhanced in the presence of hypoxia. (a) Representative figure showing the expression of interferon (IFN)‐γ and IL‐17a gated on CD3+ Vδ2 T cell receptor (TCR)+ γδT cells in healthy individuals’ peripheral blood lymphocytes (HI‐PBLs) and PBLs and tumor‐infiltrating lymphocytes (TILs) (paired samples) of the oral cancer (OC) patient. The values shown in the figure indicate the percentage‐positive population. (b,c) Intracellular expression of (b) IFN‐γ and (c) IL‐17a was assessed on a CD3+ Vδ2 TCR+ subset of γδT cells in HI‐PBLs (n = 15) and OC patient paired samples (n = 22) PBLs and TILs. (d,e) γδT cells isolated from HI‐PBLs were stimulated with recombinant human interleukin (rhIL‐2), rhIL‐2 + αCD3 or rhIL‐2 + 1‐hydroxy‐2‐methyl‐2‐buten‐4‐yl 4‐diphosphate (HDMAPP) or kept unstimulated and cultured for 72 h in hypoxia (H) and normoxia (N). γδT cells were assessed for intracellular expression of IL‐17a using multi‐color flow cytometry. (d) Mean ± standard error of the mean (s.e.m.) of the percentage of positive γδT17+ cells and (e) γδT cells were stimulated with rhIL‐2, rhIL‐2 + αCD3 or rhIL‐2 + HDMAPP and cultured for 24 h in the presence or absence of H. The cell‐free supernatants were collected and assessed for the secretion of IL‐17a (pg/ml) using a cytometric bead array (CBA) kit. (f) γδT cells were assessed for intracellular expression of RAR‐related orphan receptor gamma (RORγt) using multi‐color flow cytometry, mean ± s.e.m. of the percentage of positive RORγt+ γδT cells using multi‐color flow cytometry. All the results indicated are mean ± s.e.m. of three independent experiments (*P < 0·05; **P < 0·01; ***P < 0·005; ****P < 0·001).
γδT cells immunomagnetically purified from HI‐PBLs were stimulated with rhIL‐2, rhIL‐2 + αCD3 and rhIL‐2 + HDMAPP or kept unstimulated and were cultured in the presence or absence of 1% hypoxia for 72 h. IL‐17a‐secreting γδT cells were significantly increased upon rhIL‐2 + αCD3 stimulation, but not with rhIL‐2 + HDMAPP stimulation (Fig. 6d) compared to rhIL‐2 alone or unstimulated control under both normoxia and hypoxia. As shown in Fig. 6e, γδT cells stimulated with rhIL‐2 + αCD3 cultured under normoxia secreted IL‐17a cytokine, which was further elevated upon exposure to hypoxia. We further investigated the intracellular expression of transcription factor RORγt, which is important for γδT17 differentiation in γδT cells exposed to normoxia or hypoxia. Protein expression of RORγt was increased upon rhIL‐2 + αCD3 stimulation compared to rhIL‐2 + HDMAPP, rhIL‐2 alone or unstimulated control under both normoxia and hypoxia (Fig. 6f).
Collectively, the data confirm that increased expression of γδT17+ cells observed in TME is driven by hypoxia.
γδT cells induce CD8 T cell apoptosis under hypoxia involving the PD‐1/PD‐L1 axis
The expression of PD‐L1 and its receptor PD‐1 was assessed on γδT, CD4 and CD8 T cells in the peripheral blood of HI and OC patients and paired OC‐TIL samples. We observed that the CD8 T cells showed a significantly high expression of PD1 in OC‐TILs compared to OC‐PBLs. γδT cells and CD4 T cells showed an increase in the levels of PD‐1 in TILs compared to the peripheral lymphocytes of OC patients and HI; however, the difference was not statistically significant (Fig. 7a, Supporting information, Fig. S2).
Fig. 7.
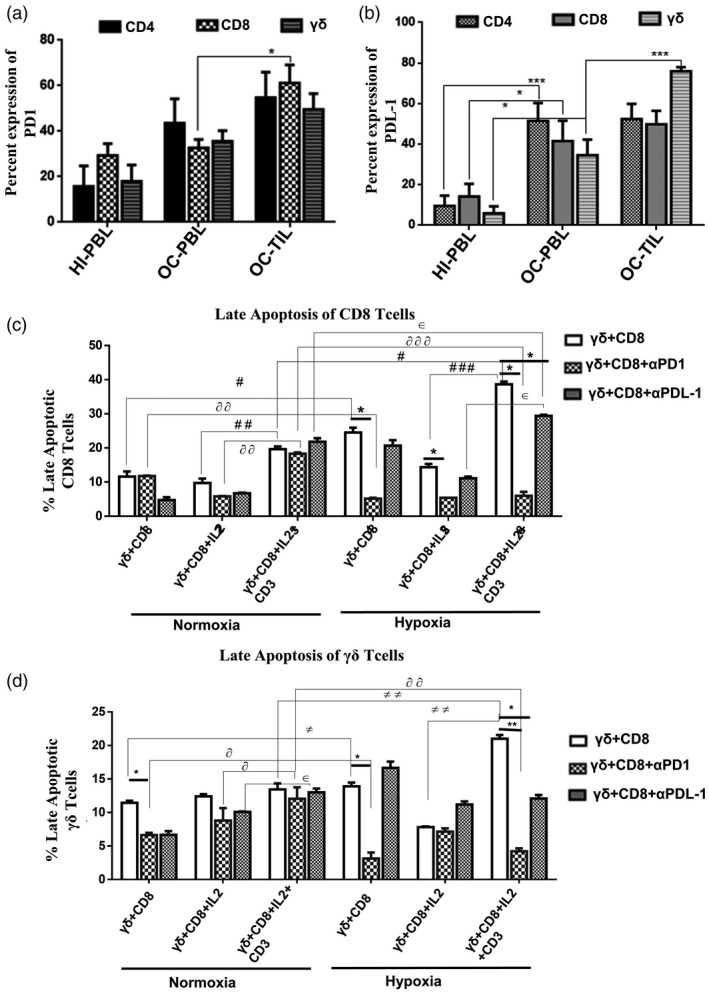
Gamma delta T (γδT) cells induce apoptosis of CD8 T cells under hypoxia mediated through the programmed cell death 1/programmed cell death ligand 1 (PD‐1/PD‐L1) axis. Expression of (a) PD‐1 and (b) PD‐L1 was assessed on CD4 T cells, CD8 T cells and Vδ2 TCR+ γδT cells present in healthy individuals’ peripheral blood lymphocytes (HI‐PBL) (n = 6), paired oral cancer (OC) samples PBLs and TILs (n = 6; *P < 0·05; ***P < 0·01). (c,d) γδT cells and CD8 T cells isolated from healthy individuals’ peripheral blood lymphocytes (HI‐PBL) were co‐cultured and stimulated with recombinant human interleukin (rhIL‐2) (γδ + CD8 + rhIL‐2), rhIL‐2 + αCD3 (γδ + CD8 + rhIL‐2 + αCD3) or kept unstimulated (γδ + CD8) for 72 h under normoxia or hypoxia. Additionally, the co‐cultures were treated with anti‐PD‐1( ) or anti‐PD‐L1(
) or anti‐PD‐L1( ) blockade antibodies or kept untreated(
) blockade antibodies or kept untreated( ), anti‐PD‐1 or anti‐PD‐L1 blockade were performed before 4 h of stimulation in independent experiments. Late apoptosis [annexin V+ and aminoactinomycin D (7‐AAD+)] of (c) CD8 T cells. (d) γδT cells were assessed using annexin V and 7‐AAD, as depicted in the bar graph. Data are represented as mean ± standard error of the mean (s.e.m.) of three independent experiments (*P < 0·05; ***P < 0·01; #
P < 0·01; ##
P < 0·05; ###
P < 0·001; δ
P < 0·02, δδ
P < 0·05; δδδ
P < 0·002; ϵ
P < 0·05).
), anti‐PD‐1 or anti‐PD‐L1 blockade were performed before 4 h of stimulation in independent experiments. Late apoptosis [annexin V+ and aminoactinomycin D (7‐AAD+)] of (c) CD8 T cells. (d) γδT cells were assessed using annexin V and 7‐AAD, as depicted in the bar graph. Data are represented as mean ± standard error of the mean (s.e.m.) of three independent experiments (*P < 0·05; ***P < 0·01; #
P < 0·01; ##
P < 0·05; ###
P < 0·001; δ
P < 0·02, δδ
P < 0·05; δδδ
P < 0·002; ϵ
P < 0·05).
Interestingly, tumor‐infiltrating γδT cells expressed high PD‐L1 compared to the OC patient peripheral compartment. Tumor‐infiltrating CD4 T and CD8 T cells showed a marginal increase in the levels of PD‐L1 compared to the peripheral compartment. The expression of PD‐L1 on CD4, CD8 and γδT cells in the peripheral blood of the OC patients was higher compared to HI‐PBLs (Fig. 7b, Supporting information, Fig. S2).
The relatively high expression of PD‐L1 on γδT cells in TILs led us to hypothesize that PD‐L1high γδT cells might induce apoptosis in PD‐1high CD8 T cells under hypoxia. We isolated CD8 and γδT cells from HI‐PBLs, co‐cultured and stimulated them with rhIL‐2 alone, rhIL‐2 + αCD3 or kept unstimulated and cultured under normoxia and hypoxia for 72 h. Apoptosis of CD8 and γδT cells were analyzed using annexin V and 7‐AAD. Additionally, PD‐1 and PD‐L1 blockade antibodies were used to block the PD‐1/PD‐L1 axis in the co‐culture. CD8 T cells under normoxia stimulated with rhIL‐2 + αCD3 (19·5 ± 1·93%) in the presence of γδT cells showed increased late apoptotic cells (annexin‐V+ 7‐AAD+) compared to γδT cells stimulated with rhIL‐2 alone (9·22 ± 3·22%) or unstimulated controls (11·6 ± 3·6%). A marked increase in the percentage of apoptosis of CD8 T cells was observed when the co‐culture of CD8 T and γδT cells were stimulated with rhIL‐2 + αCD3 (38·65 ± 1·9%) under hypoxia (Fig. 7c, Supporting information, Fig. S3). Although the same effect was observed on γδ T cells, the magnitude of apoptosis in γδT cells was less compared to CD8 T cells (Fig. 7d, Supporting information, Fig. S3). In the co‐culture, a significant reduction in apoptosis of CD8 T cells was observed upon PD‐1 blockade compared to PD‐L1 blockade under hypoxia (Fig. 7c,d, Supporting information, Fig. S3).
Discussion
Earlier studies from our laboratory have shown that γδT cells have the capability to lyse oral tumors and the effector functions of γδT cells are regulated by Notch signaling [27, 28], rendering them unique players in cancer immunotherapy. HIF1α, the first protein to be up‐regulated by hypoxia, acts at the transcriptional and translational level and regulates diverse T cell subsets ranging from inflammatory to immunosuppressive phenotypes [30]. In the present study, we observed an increase in the level of tumor‐infiltrating Vδ2 TCR+ subsets of γδT cells expressing HIF1α compared to the peripheral compartment of the OC patients. Surprisingly, very few reports are available which have investigated the impact of hypoxia on the anti‐tumor effector functions and the differentiation of γδT cells. To establish this, we used γδT cells immediately after isolation from HI‐PBLs or after the in‐vitro expansion of OC‐PBLs and HI‐PBLs using rhIL‐2 + αCD3 or rhIL‐2 + HDMAPP. We analyzed the cytotoxic ability of these γδT cells against aminobisphosphonate‐treated oral tumor cells (AW13516 and AW8507). Our data demonstrated that under hypoxic conditions, γδT cells from HI and OC patients showed significantly reduced ability to lyse oral tumors, which reflects their status in the TME. Sieger et al. showed that the hypoxia‐exposed γδT cells failed to lyse the MCF7 breast tumor cells exposed to hypoxia, which concurs with our observation. They attributed it to the reduced expression of NKG2D and MICA, which is an indirect effect of hypoxia [31]. A recent study demonstrated that oxygen pressure in the tumor microenvironment orchestrates an anti‐ and pro‐tumoral γδT cell equilibrium through tumor‐derived exosomes which inhibited γδT cells proliferation and anti‐tumor cytotoxicity [32].
Calcium efflux is an important regulator of the effector functions of T cells. In our study, hypoxia‐exposed stimulated γδT cells showed reduced calcium efflux. Hypoxia can modulate Ca2+ homeostasis in CD3+ T cells mediated by inhibition of Kv1.3 channel and membrane depolarization [33]. Constitutive activity of HIF‐1α mediates over‐expression of the calcium pump SERCA‐2 and accelerates the influx of Ca2+ from the cytoplasm into intracellular compartments, leading to a distorted Ca2+ response [34]. The cytotoxic function of T cells depends on the amount of degranulation. Exposure to hypoxia decreased the CD107a levels in stimulated γδT cells. Anti‐tumor cytotoxicity in CD8+ αβT cells is largely controlled by their clonal TCRs recognizing specific tumor antigens [14], whereas NKG2D signaling in NK [35] and γδT cells [36] leads to activation resulting in target cell lysis [37, 38]. We observed that γδT cells showed a lower expression of NKG2D and enhanced expression of KIR when exposed to hypoxia (Supporting information, Fig. S1c,d). Reports have shown that low levels of NKG2D induce lower CD107a expression in hypoxia‐exposed NK cells [35, 39]. Further, Ca2+ signaling regulates CD107a expression [40, 41], which explains our observation that decreased anti‐tumor cytotoxicity of γδT cells exposed to hypoxia could be due to reduced CD107a expression regulated by reduced Ca2+ efflux.
Earlier, data from our laboratory and others have reported that tumor‐infiltrating γδT17 cells are pro‐tumorigenic and are associated with poor patient survival [42, 43]. We observed that γδT17 were significantly increased in advanced‐stage oral tumors compared to the peripheral compartment. Lo Presti et al. has reported that IL‐17a‐secreting Vδ2 TCR+ γδT cells were found to be significantly increased in stages III and IV advanced squamous cell carcinoma (SCC) patients compared to stages I and II early disease patients [44], similar to our observation. Advanced tumors have higher hypoxia content compared to early‐stage tumors [45, 46] and Th17 differentiation is reported to be enhanced by HIF1α [16]. Hence, we checked the IL‐17a secretion by γδT cells exposed to hypoxia and the levels of γδT17+ cells in our experimental set‐up. γδT17+ cells were significantly increased upon rhIL‐2 + αCD3 stimulation, which correlated with increased RORγT expression in stimulated γδT cells under hypoxia. Interestingly, HDMAPP stimulation did not show active Th17 differentiation, as observed with αCD3 stimulation. Surprisingly, RORγT expression was not enhanced after HDMAPP stimulation. Similar to our observation, Silva Santos et al. has also reported that activation of naive Vγ9Vδ2 T cells with pyrophosphate agonists in addition to IL‐2 leads to strong IFN‐γ but no IL‐17 production [47].
It is reported that the strength of T cell stimulation determines the capability of Th17 differentiation [48, 49]. The γδ TCR can employ two different binding strategies: an antigen‐like interaction mediating subset‐specific regulation by butyrophilin (BTN) molecules and CDR3‐dependent antibody‐like interactions mediating adaptive γδ T cell responses [50, 51]. Activation of human Vγ9+Vδ2+ T cells by pAgs (i.e. HDMAPP) is mediated by BTN3A1. BTN3A might present pAgs directly to Vγ9Vδ2 T cells via its extracellular immunoglobulin (Ig)V domain or the intracellular B30.2 domain of BTN3A1. The B30.2 intracellular domain of BTN3A1 can directly bind pAg through a positively charged surface pocket under physiological conditions complementary to the negative charge of the pyrophosphate moiety of pAgs which induces subsequent conformational changes, thus activating the Vγ9Vδ2 T cells [26]. It has been reported that another BTN family member, BTN2A2, induces expression of forkhead box protein 3 (FoxP3) [52], which represses expression of the IL‐17 gene and is thus involved in the suppression of Th17 cell differentiation. This hypothesis is consistent with the finding that BTNL1 significantly decreases T cell‐derived cytokines such as IL‐17, but not IFN‐g [53, 54, 55].
Tumor cells express pAgs which, together with butyrophilin 3A1, can activate Vγ9Vδ2 TCR [51]. The γδ TCR is likely to bind to the natural pAg/BTN3A1 ligand [56]. This potential binding stimulates the γδ TCR, but without stabilizing the γδ TCR in its active CD3 conformation [57]. Recruitment of Nck (non‐catalytic region of tyrosine kinase adaptor protein 1) is necessary for the activation of the γδ T cells in the presence of the CD3 antibody, which is not observed with pAg stimulation of γδ T cells [50]. It appears that the BTN expression plays an important role in dictating the IL‐17 versus IFN‐γ dichotomy and explains the increased IL‐17 production following CD3 ligation, which is not observed with HDMAPP. An in‐depth study of how these two different stimuli can affect the differentiation of γδT cells is important to understand the significance of microenvironmental regulation of γδT cells.
Co‐inhibitory signals are transduced by ligation of PD‐L1 with PD‐1; PD‐1 becomes clustered with TCRs and recruits SHP2 [Src homology region 2 (SH2)‐containing protein tyrosine phosphatase 2], resulting in dephosphorylation of the proximal TCR signaling molecules and suppression of T cell activation [58, 59]. High expression of PD‐L1 is associated with decreased survival and treatment outcome of OC [60]. PD‐L1 is reported to be a direct downstream target of HIF1α [61]. We observed that PD‐L1high γδT cells and PD‐1high CD8 T cells infiltrated the tumors. To determine if PD‐L1highγδT cells might be involved in the cell death of PD‐1high CD8 T cells in the hypoxic tumor, CD8 and γδT cell co‐cultures were stimulated with αCD3 and rhIL‐2. Apoptosis of CD8 T cells was elevated under hypoxia compared to normoxia, suggesting that γδT cells can induce cell death in CD8 T cells which was rescued significantly upon blockade of PD‐1. Daley et al. showed that γδT cells were able to induce apoptosis in αβT cells via the PD‐1/PD‐L1 axis, corroborating our results [62]. Thus, hypoxia differentially regulates the T cell population in the tumor microenvironment.
Collectively, our data demonstrate that γδT cells can survive the hypoxic microenvironment, but their anti‐tumor cytotoxic functions are compromised. γδT cells differentiate to form pro‐tumorigenic γδT17 cells, which are known mediators of angiogenesis [42, 63]. PD‐L1high γδT cells represent the exhausted phenotype mediating immune suppression in the hypoxic environment of oral tumors. Thus, a future immunotherapeutic treatment modality for OC may use a combined approach of blocking the PD‐1/PD‐L1 signaling and targeting HIF1α [64], which may help in reversing the hypoxia‐induced immunosuppression in these tumors.
Disclosures
The authors report no conflicts of interest.
Author contributions
S. V. C. conceptualized the study and received funding for the project. S. V. C. and S. K. S. designed the experiments. S. K. S. performed experiments and statistical analysis. S. K. S. and S. V. C. analyzed, interpreted the data and drafted the manuscript. D. C. helped in patient recruitment and provided clinical samples.
Supporting information
Fig. S1. Expression of NKG2D, KIR and IL‐6 on purified γδT cells exposed to hypoxia. (a) γδT cells immunomagnetically sorted from HI‐PBL were assessed for purity.MACS sorted cells were >97% positive.These cells were used for all the experiments. (b) γδT cells isolated from HI‐PBLs were stimulated with rhIL2, rhIL2+αCD3 or rhIL2+HDMAPP or kept unstimulated and cultured for 24 hours in the presence or absence of hypoxia. The cell‐free supernatants were collected and assessed for the secretion of IL‐6 (pg/ml using CBA kit). The results indicated are mean ± SEM of three independent experiments (*P = <0·05, **P = <0·01). (c‐d) γδT cells were stimulated with rhIL2, rhIL2+αCD3 or rhIL2+HDMAPP or kept unstimulated and cultured for 72 hours in hypoxia(H) or normoxia(N). γδT cells were assessed for expression of (c) NKG2D and (d) KIRusing multicolor flow cytometry, (C) representative figure of NKG2D expression on CD3+ Vδ2 TCR+ Tcells expressed as MFI. (D)representative figure of KIR expression on CD3+ Vδ2 TCR+ Tcells expressed as MFI.
Fig. S2. Expression of PD1 and PDL‐1 on T cell subsets‐ (a‐b) Representative figure of the expression of (a) PDL‐1 and (b) PD‐1 assessed on CD4 Tcells, Vδ2 TCR+ γδT cells and CD8 Tcells present in HI‐PBLspaired OC samples‐PBLs and TILs. The gating was done based on the respective FMO controls. The values shown in the figure indicate the percent positive population
Fig. S3. αCD3 stimulated γδT cells induce apoptosis of CD8 Tcells under hypoxia. (a‐b) Representative plots of Annexin V/7‐AAD assessed on (a) CD8 and (b) Vδ2 TCR+ γδT cells stimulated with rhIL2+αCD3 for 72 hours with or without blockade of PD1 under normoxia or hypoxia. The values shown in the figure indicate the percent positive population.
Acknowledgements
We thank The Terry Fox Foundation for the financial support received for our project. We thank the Department of Atomic Energy, Government of India for providing fellowship to S. K. S. We thank all the patients and healthy donors who gave their consent to be a part of this study.
References
- 1. Sharma S, Satyanarayana L, Asthana S, Shivalingesh KK, Goutham BS, Ramachandra S. Oral cancer statistics in India on the basis of first report of 29 population‐based cancer registries. J Oral Maxillofac Pathol 2018; 22:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Del PO, Jorge CC, Oliveira DT, Pereira MC. Hypoxic condition and prognosis in oral squamous cell carcinoma. Anticancer Res 2014; 34:605–12. [PubMed] [Google Scholar]
- 3. Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015; 3:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Graham K, Unger E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int J Nanomed 2018; 13:6049–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu Y, Hu J, Sun W, Duan X, Chen X. Hypoxia‐mediated immune evasion of pancreatic carcinoma cells. Mol Med Rep 2015; 11:3666–72. [DOI] [PubMed] [Google Scholar]
- 6. Schilling D, Tetzlaff F, Konrad S, Li W, Multhoff G. A hypoxia‐induced decrease of either MICA/B or Hsp70 on the membrane of tumor cells mediates immune escape from NK cells. Cell Stress Chaperones 2015; 20:139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Noman MZ, Buart S, Van Pelt J et al The cooperative induction of hypoxia‐inducible factor‐1 alpha and STAT3 during hypoxia induced an impairment of tumor susceptibility to CTL‐mediated cell lysis. J Immunol 2009; 182:3510–21. [DOI] [PubMed] [Google Scholar]
- 8. Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol 2017; 17:559–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jayaprakash P, Ai M, Liu A et al Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. J Clin Invest 2018; 128:5137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Westendorf AM, Skibbe K, Adamczyk A et al Hypoxia enhances immunosuppression by inhibiting CD4+ effector T cell function and promoting Treg activity. Cell Physiol Biochem 2017; 41:1271–84. [DOI] [PubMed] [Google Scholar]
- 11. Huang Y, Yuan J, Righi E et al Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci USA 2012; 109:17561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chiu DK, Tse AP, Xu IM et al Hypoxia inducible factor HIF‐1 promotes myeloid‐derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat Commun 2017; 8:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Larbi A, Zelba H, Goldeck D, Pawelec G. Induction of HIF‐1alpha and the glycolytic pathway alters apoptotic and differentiation profiles of activated human T cells. J Leukoc Biol 2010; 87:265–73. [DOI] [PubMed] [Google Scholar]
- 14. Gropper Y, Feferman T, Shalit T, Salame TM, Porat Z, Shakhar G. Culturing CTLs under hypoxic conditions enhances their cytolysis and improves their anti‐tumor function. Cell Rep 2017; 20:2547–55. [DOI] [PubMed] [Google Scholar]
- 15. Shehade H, Acolty V, Moser M, Oldenhove G. Cutting edge: hypoxia‐inducible factor 1 negatively regulates Th1 function. J Immunol 2015; 195:1372–6. [DOI] [PubMed] [Google Scholar]
- 16. Dang EV, Barbi J, Yang HY et al Control of T(H)17/T(reg) balance by hypoxia‐inducible factor 1. Cell 2011; 146:772–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vuillefroy de Silly R, Ducimetiere L, Yacoub Maroun C, Dietrich PY, Derouazi M, Walker PR. Phenotypic switch of CD8(+) T cells reactivated under hypoxia toward IL‐10 secreting, poorly proliferative effector cells. Eur J Immunol 2015; 45:2263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Daniel SK, Sullivan KM, Labadie KP, Pillarisetty VG. Hypoxia as a barrier to immunotherapy in pancreatic adenocarcinoma. Clin Transl Med 2019; 8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 2015; 15:486–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sceneay J, Chow MT, Chen A et al Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res 2012; 72:3906–11. [DOI] [PubMed] [Google Scholar]
- 21. Berchem G, Noman MZ, Bosseler M et al Hypoxic tumor‐derived microvesicles negatively regulate NK cell function by a mechanism involving TGF‐beta and miR23a transfer. Oncoimmunology 2016; 5:e1062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fisher JPH, Heuijerjans J, Yan M, Gustafsson K, Anderson J. γδ T cells for cancer immunotherapy. OncoImmunology 2014; 3:e27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tanaka Y, Murata‐Hirai K, Iwasaki M et al Expansion of human gammadelta T cells for adoptive immunotherapy using a bisphosphonate prodrug. Cancer Sci 2018; 109:587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buccheri S, Guggino G, Caccamo N, Li Donni P, Dieli F. Efficacy and safety of gammadeltaT cell‐based tumor immunotherapy: a meta‐analysis. J Biol Regul Homeost Agents 2014; 28:81–90. [PubMed] [Google Scholar]
- 25. Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev 2007; 215:59–76. [DOI] [PubMed] [Google Scholar]
- 26. Sandstrom A, Peigne CM, Leger A et al The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vgamma9Vdelta2 T cells. Immunity 2014; 40:490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gogoi D, Dar AA, Chiplunkar SV. Involvement of Notch in activation and effector functions of gammadelta T cells. J Immunol 2014; 192:2054–62. [DOI] [PubMed] [Google Scholar]
- 28. Laad AD, Thomas ML, Fakih AR, Chiplunkar SV. Human gamma delta T cells recognize heat shock protein‐60 on oral tumor cells. Int J Cancer 1999; 80:709–14. [DOI] [PubMed] [Google Scholar]
- 29. Tatake RJ, Rajaram N, Damle RN, Balsara B, Bhisey AN, Gangal SG. Establishment and characterization of four new squamous cell carcinoma cell lines derived from oral tumors. J Cancer Res Clin Oncol 1990; 116:179–86. [DOI] [PubMed] [Google Scholar]
- 30. McNamee EN, Korns Johnson D, Homann D, Clambey ET. Hypoxia and hypoxia‐inducible factors as regulators of T cell development, differentiation, and function. Immunol Res 2013; 55:58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Siegers GM, Dutta I, Lai R, Postovit LM. Functional plasticity of gamma delta T cells and breast tumor targets in hypoxia. Front Immunol 2018; 9:1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li L, Cao B, Liang X et al Microenvironmental oxygen pressure orchestrates an anti‐ and pro‐tumoral gammadelta T cell equilibrium via tumor‐derived exosomes. Oncogene 2019; 38:2830–43. [DOI] [PubMed] [Google Scholar]
- 33. Robbins JR, Lee SM, Filipovich AH et al Hypoxia modulates early events in T cell receptor‐mediated activation in human T lymphocytes via Kv1.3 channels. J Physiol 2005; 564:131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Neumann AK, Yang J, Biju MP et al Hypoxia inducible factor 1α regulates T cell receptor signal transduction. Proc Natl Acad Sci USA 2005; 102:17071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Balsamo M, Manzini C, Pietra G et al Hypoxia downregulates the expression of activating receptors involved in NK‐cell‐mediated target cell killing without affecting ADCC. Eur J Immunol 2013; 43:2756–64. [DOI] [PubMed] [Google Scholar]
- 36. Ribeiro S, Ribot J, Silva‐Santos B. Five layers of receptor signalling in γδ T cell differentiation and activation. Front immunol 2015; 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ebert LM, Meuter S, Moser B. Homing and function of human skin γδ T cells and NK cells: relevance for tumor surveillance. J Immunol 2006; 176:4331–6. [DOI] [PubMed] [Google Scholar]
- 38. Niu C, Jin H, Li M et al In vitro analysis of the proliferative capacity and cytotoxic effects of ex vivo induced natural killer cells, cytokine‐induced killer cells, and gamma‐delta T cells. BMC Immunol 2015; 16:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sarkar S, Germeraad WT, Rouschop KM et al Hypoxia induced impairment of NK cell cytotoxicity against multiple myeloma can be overcome by IL‐2 activation of the NK cells. PLOS ONE 2013; 8:e64835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maul‐Pavicic A, Chiang SCC, Rensing‐Ehl A et al ORAI1‐mediated calcium influx is required for human cytotoxic lymphocyte degranulation and target cell lysis. Proc Natl Acad Sci USA 2011; 108:3324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim KD, Bae S, Capece T et al Targeted calcium influx boosts cytotoxic T lymphocyte function in the tumour microenvironment. Nat Commun 2017; 8:15365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patil RS, Shah SU, Shrikhande SV, Goel M, Dikshit RP, Chiplunkar SV. IL17 producing gammadeltaT cells induce angiogenesis and are associated with poor survival in gallbladder cancer patients. Int J Cancer 2016; 139:869–81. [DOI] [PubMed] [Google Scholar]
- 43. Wu P, Wu D, Ni C et al gammadeltaT17 cells promote the accumulation and expansion of myeloid‐derived suppressor cells in human colorectal cancer. Immunity 2014; 40:785–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lo Presti E, Toia F, Oieni S et al Squamous cell tumors recruit gammadelta T cells producing either IL17 or IFNgamma depending on the tumor stage. Cancer Immunol Res 2017; 5:397–407. [DOI] [PubMed] [Google Scholar]
- 45. Ribeiro M, Teixeira SR, Azevedo MN, Fraga AC Jr, Gontijo AP, Vêncio EF. Expression of hypoxia‐induced factor‐1 alpha in early‐stage and in metastatic oral squamous cell carcinoma. Tumor Biol 2017; 39:1010428317695527. [DOI] [PubMed] [Google Scholar]
- 46. Evans SM, Du KL, Chalian AA et al Patterns and levels of hypoxia in head and neck squamous cell carcinomas and their relationship to patient outcome. Int J Radiat Oncol Biol Phys 2007; 69:1024–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. DeBarros A, Chaves‐Ferreira M, d'Orey F, Ribot JC, Silva‐Santos B. CD70–CD27 interactions provide survival and proliferative signals that regulate T cell receptor‐driven activation of human gammadelta peripheral blood lymphocytes. Eur J Immunol 2011; 41:195–201. [DOI] [PubMed] [Google Scholar]
- 48. Purvis HA, Stoop JN, Mann J et al Low‐strength T‐cell activation promotes Th17 responses. Blood 2010; 116:4829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jensen KD, Su X, Shin S et al Thymic selection determines gammadelta T cell effector fate: antigen‐naive cells make interleukin‐17 and antigen‐experienced cells make interferon gamma. Immunity 2008; 29:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Juraske C, Wipa P, Morath A et al Anti‐CD3 Fab fragments enhance tumor killing by human gammadelta T cells independent of nck recruitment to the gammadelta T cell antigen receptor. Front Immunol 2018; 9:1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Blazquez JL, Benyamine A, Pasero C, Olive D. New insights into the regulation of gammadelta T cells by BTN3A and other BTN/BTNL in tumor immunity. Front Immunol 2018; 9:1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ammann JU, Cooke A, Trowsdale J. Butyrophilin Btn2a2 inhibits TCR activation and phosphatidylinositol 3‐kinase/Akt pathway signaling and induces Foxp3 expression in T lymphocytes. J Immunol 2013; 190:5030–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Harly C, Guillaume Y, Nedellec S et al Key implication of CD277/butyrophilin‐3 (BTN3A) in cellular stress sensing by a major human gammadelta T‐cell subset. Blood 2012; 120:2269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Esser C. A fat story‐antigen presentation by butyrophilin 3A1 to gammadelta T cells. Cell Mol Immunol 2014; 11:5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yamazaki T, Goya I, Graf D, Craig S, Martin‐Orozco N, Dong C. A butyrophilin family member critically inhibits T cell activation. J Immunol 2010; 185:5907–14. [DOI] [PubMed] [Google Scholar]
- 56. Benyamine A, Loncle C, Foucher E et al BTN3A is a prognosis marker and a promising target for Vgamma9Vdelta2 T cells based‐immunotherapy in pancreatic ductal adenocarcinoma (PDAC). Oncoimmunology 2017; 7:e1372080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dopfer EP, Hartl FA, Oberg HH et al The CD3 conformational change in the gammadelta T cell receptor is not triggered by antigens but can be enforced to enhance tumor killing. Cell Rep 2014; 7:1704–15. [DOI] [PubMed] [Google Scholar]
- 58. Granier C, De Guillebon E, Blanc C et al Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open 2017; 2:e000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Seidel JA, Otsuka A, Kabashima K. Anti‐PD‐1 and Anti‐CTLA‐4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol 2018; 8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maruse Y, Kawano S, Jinno T et al Significant association of increased PD‐L1 and PD‐1 expression with nodal metastasis and a poor prognosis in oral squamous cell carcinoma. Int J Oral Maxillofac Surg 2018; 47:836–45. [DOI] [PubMed] [Google Scholar]
- 61. Noman MZ, Desantis G, Janji B et al PD‐L1 is a novel direct target of HIF‐1alpha, and its blockade under hypoxia enhanced MDSC‐mediated T cell activation. J Exp Med 2014; 211:781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Daley D, Zambirinis CP, Seifert L et al gammadelta T cells support pancreatic oncogenesis by restraining alphabeta T cell activation. Cell 2016; 166:1485–99 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wakita D, Sumida K, Iwakura Y et al Tumor‐infiltrating IL‐17‐producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur J Immunol 2010; 40:1927–37. [DOI] [PubMed] [Google Scholar]
- 64. Yu T, Tang B, Sun X. Development of inhibitors targeting hypoxia‐inducible factor 1 and 2 for cancer therapy. Yonsei Med J 2017; 58:489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Expression of NKG2D, KIR and IL‐6 on purified γδT cells exposed to hypoxia. (a) γδT cells immunomagnetically sorted from HI‐PBL were assessed for purity.MACS sorted cells were >97% positive.These cells were used for all the experiments. (b) γδT cells isolated from HI‐PBLs were stimulated with rhIL2, rhIL2+αCD3 or rhIL2+HDMAPP or kept unstimulated and cultured for 24 hours in the presence or absence of hypoxia. The cell‐free supernatants were collected and assessed for the secretion of IL‐6 (pg/ml using CBA kit). The results indicated are mean ± SEM of three independent experiments (*P = <0·05, **P = <0·01). (c‐d) γδT cells were stimulated with rhIL2, rhIL2+αCD3 or rhIL2+HDMAPP or kept unstimulated and cultured for 72 hours in hypoxia(H) or normoxia(N). γδT cells were assessed for expression of (c) NKG2D and (d) KIRusing multicolor flow cytometry, (C) representative figure of NKG2D expression on CD3+ Vδ2 TCR+ Tcells expressed as MFI. (D)representative figure of KIR expression on CD3+ Vδ2 TCR+ Tcells expressed as MFI.
Fig. S2. Expression of PD1 and PDL‐1 on T cell subsets‐ (a‐b) Representative figure of the expression of (a) PDL‐1 and (b) PD‐1 assessed on CD4 Tcells, Vδ2 TCR+ γδT cells and CD8 Tcells present in HI‐PBLspaired OC samples‐PBLs and TILs. The gating was done based on the respective FMO controls. The values shown in the figure indicate the percent positive population
Fig. S3. αCD3 stimulated γδT cells induce apoptosis of CD8 Tcells under hypoxia. (a‐b) Representative plots of Annexin V/7‐AAD assessed on (a) CD8 and (b) Vδ2 TCR+ γδT cells stimulated with rhIL2+αCD3 for 72 hours with or without blockade of PD1 under normoxia or hypoxia. The values shown in the figure indicate the percent positive population.


