Publisher's Note: There is a Blood Commentary on this article in this issue.
Key Points
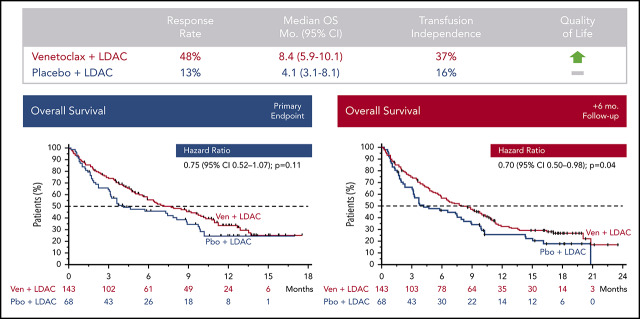
Venetoclax plus LDAC improves response rate, transfusion independence, and patient-reported outcomes vs LDAC alone in older AML patients.
Median OS for patients receiving venetoclax plus LDAC was 8.4 months vs 4.1 months for those receiving LDAC alone.
Abstract
Effective treatment options are limited for patients with acute myeloid leukemia (AML) who cannot tolerate intensive chemotherapy. Adults age ≥18 years with newly diagnosed AML ineligible for intensive chemotherapy were enrolled in this international phase 3 randomized double-blind placebo-controlled trial. Patients (N = 211) were randomized 2:1 to venetoclax (n = 143) or placebo (n = 68) in 28-day cycles, plus low-dose cytarabine (LDAC) on days 1 to 10. Primary end point was overall survival (OS); secondary end points included response rate, transfusion independence, and event-free survival. Median age was 76 years (range, 36-93 years), 38% had secondary AML, and 20% had received prior hypomethylating agent treatment. Planned primary analysis showed a 25% reduction in risk of death with venetoclax plus LDAC vs LDAC alone (hazard ratio [HR], 0.75; 95% confidence interval [CI], 0.52-1.07; P = .11), although not statistically significant; median OS was 7.2 vs 4.1 months, respectively. Unplanned analysis with additional 6-month follow-up demonstrated median OS of 8.4 months for the venetoclax arm (HR, 0.70; 95% CI, 0.50-0.98; P = .04). Complete remission (CR) plus CR with incomplete blood count recovery rates were 48% and 13% for venetoclax plus LDAC and LDAC alone, respectively. Key grade ≥3 adverse events (venetoclax vs LDAC alone) were febrile neutropenia (32% vs 29%), neutropenia (47% vs 16%), and thrombocytopenia (45% vs 37%). Venetoclax plus LDAC demonstrates clinically meaningful improvement in remission rate and OS vs LDAC alone, with a manageable safety profile. Results confirm venetoclax plus LDAC as an important frontline treatment for AML patients unfit for intensive chemotherapy. This trial was registered at www.clinicaltrials.gov as #NCT03069352.
Visual Abstract
Introduction
Older adults and patients with significant comorbidities are often ineligible for intensive chemotherapy. Median age at diagnosis of acute myeloid leukemia (AML) is >68 years; therefore, a large portion of patients diagnosed with AML have limited effective treatment options.1,2 Less intense frontline treatments, such as hypomethylating agents (HMAs; eg, azacitidine or decitabine), are often used and provide complete remission (CR) plus CR with incomplete blood count recovery (CRi) rates of <30%.3-5 Response rates to low-dose cytarabine (LDAC) as frontline therapy in older patients with AML are similarly poor (CR/CRi, 11%-19%), with median survival times ≤6 months.5-7 These results highlight the lack of highly effective, well-tolerated treatment options for older adults with AML, particularly those who are ineligible to receive intensive chemotherapy.
B-cell leukemia/lymphoma-2 (BCL2) family members, including BCL2, BCL-XL, and MCL1, mediate cancer cell survival by sequestering proapoptotic proteins, and BCL2 activity promotes chemotherapy resistance and enhances survival of leukemic progenitor and blast cells.8,9 Venetoclax is a potent and selective small-molecule BCL2 inhibitor that has been studied in several hematologic malignancies both as monotherapy and in combination with other agents.10-16 Resistance to venetoclax may be mediated by other prosurvival proteins, such as MCL1 and BCL-XL, that sequester endogenous BH3-only proteins released by venetoclax upon BCL2 binding. Cytotoxic drugs, including cytarabine, synergize with venetoclax by enhancing BH3-only activity and/or suppressing MCL1 to promote apoptosis in preclinical models of AML.17-19 Translating these preclinical observations, a phase 2 study of venetoclax combined with LDAC in AML resulted in a CR/CRi rate of 54%, with median overall survival (OS) of ∼10 months,16 comparing favorably with historical response rates and survival outcomes previously reported for LDAC monotherapy in AML. Notably, responses were achieved rapidly and with low early mortality, suggesting the addition of venetoclax to LDAC may represent a useful clinical advance for older patients currently receiving LDAC alone.
This study compared the safety and efficacy of treatment with venetoclax coadministered with LDAC with placebo plus LDAC in previously untreated patients with AML, either age ≥75 years or with comorbidities precluding intensive chemotherapy.
Methods
Study design
This randomized double-blind placebo-controlled phase 3 study enrolled patients between May 2017 and November 2018. The study was conducted globally across 76 sites, including in North and South America, Europe, Asia, Africa, and Australia (complete list of countries in the supplemental Appendix, available on the Blood Web site). Data cutoff for this initial analysis was 15 February 2019; cutoff for additional follow-up time was 15 August 2019. The primary objective was to evaluate whether venetoclax, when coadministered with LDAC, improved the overall survival (OS) of patients compared with placebo plus LDAC. Secondary objectives were to compare the following measures between treatment arms: complete remission (CR); CR plus CR with partial hematologic recovery (CRh); CR plus CR with incomplete hematologic recovery (CRi); proportion of patients with CR/CRi and CR/CRh by initiation of therapy cycle 2; rate of transfusion independence; event-free survival (EFS); minimal residual disease (MRD), response rates and OS in the subsets of patients with mutations in NPM1, IDH1/2, FLT3, or TP53; and fatigue, global health status, and quality of life based on patient-reported outcomes. A detailed list of objectives is outlined in the supplemental Appendix.
Patients
Patients age ≥18 years with previously untreated AML (as defined by the World Health Organization20) who were ineligible for intensive chemotherapy were enrolled. Patients were considered ineligible for intensive induction chemotherapy if they were age ≥75 years or age ≥18 to 74 years and fulfilled at least 1 criterion associated with lack of fitness for intensive induction chemotherapy, including: Eastern Cooperative Oncology Group (ECOG) performance status of 2 to 3, cardiac history of congestive heart failure requiring treatment or ejection fraction ≤50% or chronic stable angina, diffusion capacity of the lung for carbon monoxide ≤65% or first second of forced expiration ≤65%, creatinine clearance ≥30 to <45 mL/min, moderate hepatic impairment with total bilirubin >1.5 to ≤3.0 × upper limit of normal, or any other comorbidity that was physician judged to be incompatible with conventional intensive chemotherapy. The supplemental Appendix contains a complete list of eligibility criteria. Patients with secondary AML with or without prior treatment with HMAs for myelodysplastic syndrome were included; those with secondary AML from underlying myeloproliferative neoplasms were not. Exclusion criteria included prior therapy for AML (except hydroxyurea before or during the first cycle of study treatment) and any previous exposure to cytarabine for any indication. Local ethics committee approval was obtained, and patients provided written informed consent. The study was conducted in accordance with the International Conference on Harmonisation, Good Clinical Practice guidelines, and the Declaration of Helsinki.
Patient randomization
Patients were randomized 2:1 via interactive response technology to receive either venetoclax plus LDAC or placebo plus LDAC. Randomization was stratified by AML status (secondary vs de novo), age (18 to <75 vs ≥75), and region (United States, Europe, China, Japan, or rest of the world).
Treatment
Patients were hospitalized for tumor lysis syndrome (TLS) evaluation and prophylaxis during the venetoclax ramp-up period (4 days) in treatment cycle 1 until 24 hours after the target venetoclax dose was reached. Prophylaxis for TLS included a uric acid–reducing agent and oral or IV hydration. Venetoclax was administered orally, once daily, with food. Venetoclax dosing began at 100 mg on day 1 and increased stepwise over 4 days to reach the target dose of 600 mg (100, 200, 400, and 600 mg); dosing was continued at 600 mg per day from day 4 through day 28. In all subsequent 28-day cycles, venetoclax was commenced at the target dose. For patients randomized to the placebo arm, placebo (identical appearance tablet) was administered in the same fashion as venetoclax. For patients in both arms, LDAC (20 mg/m2) was administered by subcutaneous injection once daily on days 1 to 10 in all cycles. Patients could continue receiving treatment until progression or until study treatment discontinuation criteria were met (supplemental Appendix). Patients remained on study for OS assessment and follow-up, even if they initiated additional lines of treatment. Because venetoclax is a CYP3A and P-glycoprotein (P-gp) substrate, protocol-recommended dose modifications for patients receiving these inhibitors were applied: venetoclax dose was reduced to 50% if coadministered with moderate CYP3A inhibitors or P-gp inhibitors and reduced to 50 mg if coadministered with strong CYP3A inhibitors.21 If a patient was on multiple inhibitors, venetoclax dose adjustment was based on the strongest inhibitor.
Study assessments
Safety
Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03).22 Treatment-emergent AEs, including clinical TLS, were defined as those that occurred between the first dose of study drug until 30 days after the last dose of study drug. Laboratory TLS was defined as previously reported by Howard et al.23
Efficacy
Response assessments were performed after cycle 1 (patients with resistant disease after cycle 1 had repeat assessments after cycle 2 or 3 to assess initial CR/CRi response) and every 3 cycles thereafter (starting at the end of cycle 4 and continuing until disease progression) until 2 consecutive samples confirmed stable achievement of CR or CRi. Assessments were also performed if there was suspected relapse and/or at the final study visit. Criteria for evaluation of disease assessment are outlined in the data supplement. Clinical responses were defined according to modified International Working Group response criteria for AML (supplemental Methodology).24 Progressive disease was defined per European LeukemiaNet recommendations.25 Treatment failure was defined as failure to achieve morphologic leukemia-free state or higher response (CR, CRi, partial remission) after at least 6 cycles of treatment. EFS was defined as the number of days from randomization to disease progression, confirmed relapse, treatment failure, or death.
Pharmacokinetics
Blood samples were collected for pharmacokinetic analysis in cycles 1, 2, 4, and 8. Details on pharmacokinetic sample time points are found in the supplemental Appendix.
Other assessments
Assessment of cytogenetic risk followed the National Comprehensive Cancer Network guideline for AML (version 2.2016). Fatigue and global health status/quality of life were assessed via the Patient-Reported Outcomes Measurement Information System Fatigue SF7a and European Organisation for Research and Treatment of Cancer QLQ-C30 patient outcomes scale, respectively.
Statistical analyses
The planned sample size was 210 patients randomized 2:1; 133 events were required to be observed at the time of analysis. The study was designed to detect a 45.5% reduction in mortality with 90% power and a significance level with 2-sided α of 0.05. An interim analysis was planned when 75% of death events occurred. The O’Brien-Fleming boundary was used to control the type 1 error rate at 0.05 (2 sided). Information on end point analyses is detailed in the supplemental Appendix.
Results
Overall, 211 patients were enrolled and 210 were treated; 68 patients were randomized to the placebo arm (placebo plus LDAC), and 143 were randomized to the venetoclax arm (venetoclax plus LDAC; 1 of these patients never received treatment). The CONSORT diagram in supplemental Figure 1 shows the flow of patients through the trial. In the venetoclax arm (as of 15 February 2019), median treatment duration was 3.9 months (range, 0-17 months) and median number of cycles delivered was 4. In the placebo arm, median treatment duration was 1.7 months (range, 0.1-14 months) and median number of treatment cycles delivered was 2. Median time on study (observation time for event-free patients) was 12 months for both arms. Poststudy therapy was received by 33 (23%) of 143 patients in the venetoclax plus LDAC arm and 30 (44%) of 68 in the placebo arm. No patients went on to stem cell transplantation after study treatment. Primary reasons for discontinuation of study drug (venetoclax plus LDAC vs placebo plus LDAC) were: treatment failure (12% vs 19%), progressive disease (11% vs 16%), death (12% for both), withdrawal of consent (6% vs 10%), AEs not related to disease progression (9% for both), AEs related to disease progression (4% for both), physician decision (5% vs 12%), morphologic relapse (13% vs 4%), and other (4% vs 3%).
Patient demographics and clinical characteristics
Baseline demographics are shown in Table 1, separated by treatment arm. Across all patients, median age was 76 years, 32% had poor cytogenetic risk, 38% had secondary AML, 20% had prior HMA exposure, and baseline mutations in TP53, FLT3, IDH1/2, or NPM1 were detected in 19%, 18%, 20%, and 15% of patients (in whom data were available), respectively. A majority of baseline characteristics had similar frequencies across the randomized arms, except rates of secondary AML (41% vs 34%), which were more frequent in the venetoclax arm.
Table 1.
Patient demographics and clinical characteristics
| Characteristic | n/N (%) | |
|---|---|---|
| Placebo + LDAC (n = 68) | Venetoclax + LDAC (n = 143) | |
| Age, y | ||
| Median | 76 | 76 |
| Range | 41-88 | 36-93 |
| ≥75 | 40 (59) | 82 (57) |
| Male sex | 39 (57) | 78 (55) |
| AML type | ||
| De novo | 45 (66) | 85 (59) |
| Secondary | 23 (34) | 58 (41) |
| Secondary AML type | ||
| Therapy related | 4/23 (17) | 6/58 (10) |
| Prior hematologic disorder | 19/23 (83) | 52/58 (90) |
| ECOG performance status | ||
| 0 | 11 (16) | 22 (15) |
| 1 | 23 (34) | 52 (36) |
| 2 | 25 (37) | 63 (44) |
| 3 | 9 (13) | 6 (4) |
| Bone marrow blast count, % | ||
| <30 | 18 (27) | 42 (29) |
| ≥30 to <50 | 22 (32) | 36 (25) |
| ≥50 | 28 (41) | 65 (46) |
| Antecedent hematologic disorder | 17 (25) | 47 (33) |
| Prior HMA treatment | 14 (21) | 28 (20) |
| Cytogenetic risk category | ||
| Favorable | 3 (4) | 1 (1) |
| Intermediate | 43 (63) | 90 (63) |
| Poor | 20 (29) | 47 (33) |
| No mitosis/missing | 2 (3) | 5 (3) |
| Somatic mutation* | ||
| TP53 | 9 (17) | 22 (20) |
| FLT3 | 9 (17) | 20 (18) |
| IDH1/2 | 12 (23) | 21 (19) |
| NPM1 | 7 (14) | 18 (16) |
| Transfusion dependent at baseline† | ||
| Red blood cells | 53 (78) | 104 (73) |
| Platelets | 24 (35) | 53 (37) |
Mutation data missing for 16 and 31 patients in placebo and venetoclax arms, respectively; percentage calculated based on n of patients with data.
Underwent transfusion within 8 wk before first dose of study drug.
Safety profile
A total of 210 patients (venetoclax arm, n = 142; placebo arm, n = 68) were evaluated for safety. Median dose intensity across all patients, accounting for dose reductions resulting from planned drug-drug interactions, was 96.7%. Overall, a total of 141 patients (99%) in the venetoclax arm and 67 (99%) in the placebo arm experienced at least 1 AE. A summary of treatment-emergent AEs is shown in Table 2. Consistent with expectations and prior studies in AML, the most frequently reported grade ≥3 AEs, irrespective of cause, were hematologic in nature and included (venetoclax plus LDAC vs placebo plus LDAC): febrile neutropenia (32% vs 29%), neutropenia (46% vs 16%), thrombocytopenia (45% vs 37%), and anemia (25% vs 22%). The most common nonhematologic AEs of any grade (venetoclax plus LDAC vs placebo plus LDAC) were: nausea (42% vs 31%), hypokalemia (28% vs 22%), diarrhea (28% vs 16%), and constipation (18% vs 31%). Serious AEs (any grade) were reported in 66% and 62% of patients in the venetoclax and placebo arms, respectively; serious AEs common to patients with AML included febrile neutropenia (16% vs 18%), pneumonia (13% vs 10%), and sepsis (6% in both arms). There were no other serious AEs observed in ≥10% of patients in either arm. Although there was a higher percentage of grade 3+ bleeding AEs in patients receiving venetoclax plus LDAC (n = 15; 11%) compared with placebo plus LDAC (n = 5; 7%), incidence of fatal bleeding events was similar in both arms (venetoclax plus LDAC, 1.4%; placebo plus LDAC, 1.5%). There were 8 patients (6%) with TLS reported in the study (4 clinical and 4 laboratory), all in the venetoclax arm. Of these 8 cases, 2 were reported as serious AEs related to TLS; both patients received TLS prophylaxis as per protocol.
Table 2.
Summary of treatment-emergent AEs by frequency across all patients
| AE | n (%) | |
|---|---|---|
| Placebo + LDAC (n = 68) | Venetoclax + LDAC (n = 142) | |
| Hematologic (grade ≥3)* | ||
| Thrombocytopenia | 25 (37) | 64 (45) |
| Neutropenia | 11 (16) | 66 (46) |
| Febrile neutropenia | 20 (29) | 45 (32) |
| Anemia | 15 (22) | 36 (25) |
| Nonhematologic (any grade)* | ||
| Nausea | 21 (31) | 60 (42) |
| Hypokalemia | 15 (22) | 40 (28) |
| Diarrhea | 11 (16) | 40 (28) |
| Constipation | 21 (31) | 26 (18) |
| Vomiting | 9 (13) | 36 (25) |
| Pneumonia | 11 (16) | 29 (20) |
| Edema peripheral | 14 (21) | 19 (13) |
| Selected key AML serious AEs | ||
| Febrile neutropenia | 12 (18) | 23 (16) |
| Pneumonia | 7 (10) | 18 (13) |
| Sepsis | 4 (6) | 8 (6) |
| Thrombocytopenia | 2 (3) | 7 (5) |
| Anemia | 0 | 4 (3) |
| Neutropenia | 0 | 4 (3) |
AEs shown were reported in ≥20% of patients in either treatment arm.
Overall, a similar percentage of patients in both arms had AEs leading to death (33 [23%] and 14 patients [21%], respectively). The 30-day mortality rates were 13% (n = 18) and 16% (n = 11) in the venetoclax and placebo arms, respectively.
Thirty-six patients (25%) treated with venetoclax plus LDAC and 16 (24%) treated with placebo plus LDAC had AEs leading to treatment discontinuation. Sixty-three percent and 53% of patients in the venetoclax and placebo arms, respectively, had dose interruptions because of AEs; dose reductions because of AEs occurred in 9% and 6% of patients, respectively. The most common AEs (≥5% of patients) leading to dose interruption or reduction were (venetoclax plus LDAC vs placebo plus LDAC): neutropenia (18% vs 7%), thrombocytopenia (15% vs 9%), febrile neutropenia (6% vs 7%), and pneumonia (6% vs 7%).
Venetoclax exposure during preplanned dose modifications for coadministration with CYP3A or P-gp inhibitors was generally comparable to the exposure of patients when not receiving these inhibitors (supplemental Figure 1).
Efficacy
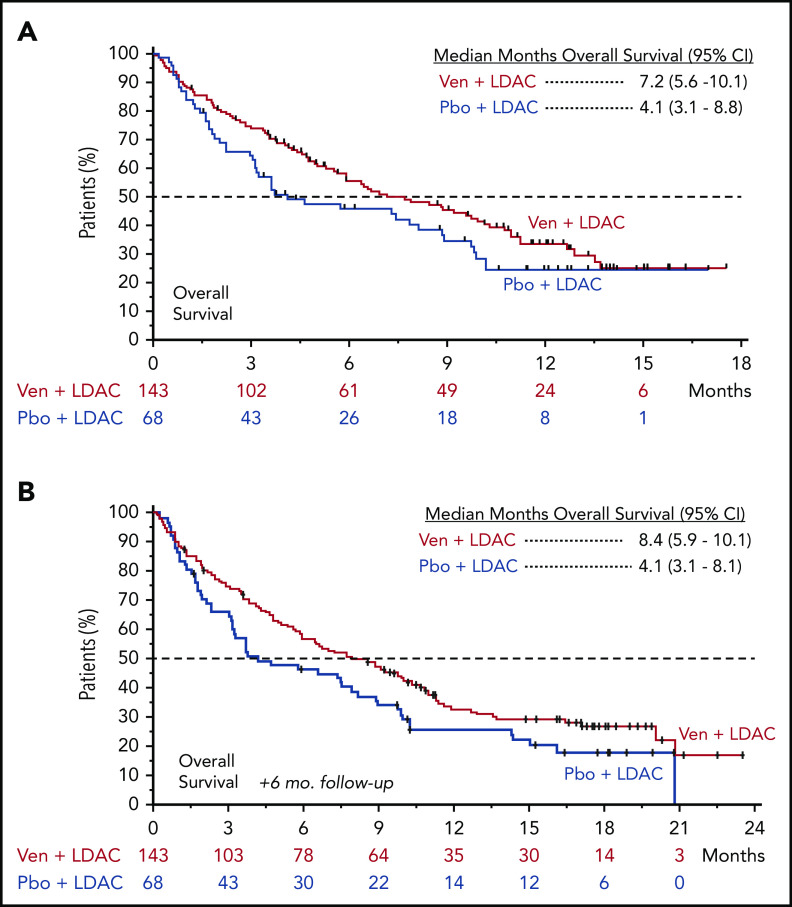
At the preplanned primary analysis, median follow-up time was 12.0 months in both the venetoclax (range, 0.1-17.6 months) and placebo arms (range, 0.2-17.0 months). At the time of the preplanned analysis, 40% (57 of 143) and 31% (21 of 68) of patients remained alive in the venetoclax and placebo arms, respectively. Median OS for patients treated with venetoclax plus LDAC was 7.2 months (95% confidence interval [CI], 5.6-10.1), compared with 4.1 months (95% CI, 3.1-8.8) for those treated with placebo plus LDAC, with a hazard ratio (HR) between the 2 treatment arms (venetoclax vs placebo) of 0.75 (95% CI, 0.52-1.07; P = .11; Figure 1A). To determine the independent effect of venetoclax on OS and identify baseline prognostic factors that may have influenced OS, a post hoc stepwise multivariate Cox proportional hazards model was used. This analysis identified AML status (de novo vs secondary), cytogenetic risk (intermediate vs poor), ECOG performance status (<2 vs ≥2), and age (<75 vs ≥75 years) as significantly correlated with OS (Table 3) and demonstrated that, when controlling for baseline prognostic factors, the adjusted HR (venetoclax vs placebo) for the venetoclax arm was 0.67 (95% CI, 0.47-0.96; P = .03). Additionally, at the time of the preplanned OS analysis, there was greater censoring of patients in the venetoclax arm, because more patients had not yet reached the median OS by the study cutoff date (15 February 2019) for the primary analysis. With an additional 6 months of follow-up, a majority of patients had passed the median survival time in both arms, demonstrating a median OS of 8.4 months (95% CI, 5.9-10.1) for patients treated with venetoclax plus LDAC, compared with 4.1 months (95% CI, 3.1-8.1) for those receiving placebo plus LDAC (Figure 1B). HR between the 2 treatment arms (venetoclax vs placebo) after this additional follow-up was 0.70 (95% CI, 0.50-0.99), with a nominal P value of .04.
Figure 1.
Overall survival. Kaplan-Meier plots showing OS rate of all patients over time, separated by treatment arm; patients at risk at each time point are shown below graph. Tick marks indicate censored data. (A) Preplanned OS analysis. (B) OS analysis with 6 months of additional follow-up. Pbo, placebo; Ven, venetoclax.
Table 3.
Multivariable Cox regression of preplanned OS analysis
| Covariate | HR (95% CI) | P |
|---|---|---|
| Treatment arm (venetoclax vs placebo) | 0.67 (0.47-0.96) | .03 |
| Age (<75 vs ≥75 y) | 0.56 (0.37-0.84) | .005 |
| AML status (de novo vs secondary) | 0.59 (0.41-0.85) | .004 |
| ECOG performance status (<2 vs ≥2) | 0.48 (0.33-0.70) | <.001 |
| Cytogenetic risk (intermediate vs poor) | 0.57 (0.40-0.82) | .003 |
CR/CRi was achieved in 48% (95% CI, 39%-56%) of patients in the venetoclax arm, compared with 13% (95% CI, 6%-24%) in the placebo arm, with CR achieved in 27% and 7% of patients, respectively. Responses were also achieved more rapidly with addition of venetoclax, with CR/CRi before initiation of cycle 2 observed in 34% (95% CI, 27%-43%) of patients in the venetoclax arm, compared with only 3% (95% CI, 0%-10%) of patients in the placebo arm (Table 4). Concordantly, higher rates of remission in the venetoclax arm were accompanied by a longer median EFS of 4.7 months (95% CI, 3.7-6.4) vs 2.0 months (95% CI, 1.6-3.1) in the placebo arm (HR, 0.58; 95% CI, 0.42-0.82; Table 4). Rates of transfusion independence were also significantly higher for patients treated with venetoclax plus LDAC, with 37% (95% CI, 29%-46%) of patients achieving red blood cell and platelet transfusion independence, compared with 16% (95% CI, 8%-27%) of those treated with LDAC alone. Upon confirmation of morphologic CR or CRi, 6% (8 of 143) of those in the venetoclax arm and 1% (1 of 68) of those in the placebo arm had a flow cytometric MRD level of <0.1%.
Table 4.
Summary of response rates and efficacy end points
| End point | % (95% CI) | P | |
|---|---|---|---|
| Placebo + LDAC (n = 68) | Venetoclax + LDAC (n = 143) | ||
| Remission rate | |||
| CR* | 7 (2-16) | 27 (20-35) | <.001 |
| CR/CRi† | 13 (6-24) | 48 (39-56) | <.001 |
| By initiation of cycle 2 | 3 (0-10) | 34 (27-43) | <.001 |
| CR/CRh‡ | 15 (7-25) | 47 (39-55) | <.001 |
| By initiation of cycle 2 | 4 (1-12) | 31 (23-39) | <.001 |
| Other | |||
| EFS, mo | .002 | ||
| Median | 2.0 | 4.7§ | |
| 95% CI | 1.6-3.1 | 3.7-6.4 | |
| Transfusion independence | |||
| Red blood cells | 18 (10-29) | 41 (32-49) | .001 |
| Platelets | 32 (22-45) | 48 (39-56) | .040 |
| Both | 16 (8-27) | 37 (29-46) | .002 |
Bone marrow with <5% blasts, absence of circulating blasts and blasts with Auer rods, absence of extramedullary disease, absolute neutrophil count ≥103/μL, platelets ≥100 × 109/L, and red cell transfusion independence.
All criteria for CR except absolute neutrophil count <103/μL or platelets <100 × 109/L ± red cell transfusion independence.
‡Bone marrow with <5% blasts, absence of circulating blasts and blasts with Auer rods, absence of extramedullary disease, absolute neutrophil count >0.5 × 103/μL, and platelets >50 × 109/L.
HR, 0.58 (95% CI, 0.42-0.82).
Response rates and OS times were also determined for subsets of patients with baseline intermediate or poor cytogenetic risk, somatic mutations in TP53, IDH1/2, FLT3, or NPM1, and key baseline prognostic factors. Response rates for these subgroups are shown in supplemental Table 1. Across all patient subgroups, those treated with venetoclax plus LDAC had increased rates of remission (CR, CRi, CRh) compared with those treated with placebo plus LDAC. Similarly, patient survival showed a trend toward longer median OS in those treated with venetoclax compared with placebo across most patient subgroups, except those with mutant FLT3 (supplemental Table 2). FLT3 mutations were detected in 29 patients: 9 patients in the placebo arm and 20 in the venetoclax arm (1 patient was randomized but never treated). Among the FLT3 mutation–positive (FLT3+) patients in the placebo arm, 3 had coexisting NPM1 mutations, and all 3 (100%) such patients achieved CR/CRi; 1 of the remaining 6 (17%) FLT3+ patients without NPM1 mutations achieved CRi. Among the 20 FLT3+ patients in the venetoclax arm, 6 had coexisting NPM1 mutations (not treated, n = 1), and all 5 (100%) who received therapy achieved CR/CRi; 4 of the remaining 14 (29%) FLT3+ patients without NPM1 mutations achieved CR/CRi, with a median OS of 2.2 months. In contrast, median OS for patients with coexisting FLT3 and NPM1 mutations was 10.2 months in the placebo arm and not yet reached in the venetoclax arm.
Patients in the placebo plus LDAC arm showed only marginally improved fatigue by cycle 5 (mean change from baseline, −0.3), with additional improvements over time that were sustained by cycle 9 (mean change from baseline, −3.5; supplemental Table 3). In contrast, fatigue scores changed rapidly in the venetoclax plus LDAC arm, with improvements already evident by cycle 3 (mean change from baseline, −2.9) and a trend toward greater improvements in fatigue scores (P = .13) compared with the LDAC alone arm that were sustained by cycle 9 (mean change from baseline, −5.1). Similar improvements were recorded in global health status and quality-of-life (P = .09) scores compared with the placebo arm (supplemental Table 3).
Discussion
To evaluate venetoclax in combination with LDAC, a multinational randomized placebo-controlled phase 3 study involving 25 countries was conducted, allocating patients in a 2:1 ratio to treatment with venetoclax plus LDAC or placebo plus LDAC. A 25% reduction in the risk of death was observed with the addition of venetoclax, showing that venetoclax plus LDAC was associated with a clinically meaningful improvement in median OS (7.2 vs 4.1 months). Despite this finding, the primary end point was not met (HR, 0.75; 95% CI, 0.52-1.07; P = .11) at the time of planned analysis. It was observed that several imbalances in baseline characteristics (secondary AML, prior hematologic disorder–related secondary AML, and poor cytogenetic risk) between the randomized arms, as well as increased administrative censoring in the venetoclax arm before the median OS time, may have affected the analyses. To evaluate the impact of the imbalances on baseline characteristics, a post hoc multivariate Cox proportional hazards analysis was performed; this analysis indicated that venetoclax added significant clinical benefit in OS over placebo when controlling for those baseline prognostic factors (AML type, cytogenetic risk, ECOG performance status, and age). Regarding the high number of administratively censored patients, trial enrollment was ongoing as recently as 3.4 months before the preplanned OS analysis, and administrative censoring of patients still alive at the time of analysis occurred more frequently in the venetoclax plus LDAC arm than in the placebo plus LDAC arm (17 [12%] vs 4 [6%] patients within the first 6 months). However, a longer-term analysis with an additional 6 months of patient follow-up, now with a majority of patients censored in both arms beyond the median OS time, demonstrated a clinically meaningful increase in median OS in the venetoclax arm, with an HR of 0.70 (95% CI, 0.50-0.99) and nominal P value of .04. Concordant with these findings, all secondary end points were robustly in favor of the venetoclax-based combination as well, including higher rates of response, earlier remissions, increased transfusion independence, and longer EFS. Achievement and prolongation of remission are key determinants of quality of life for patients with AML. In line with this, the higher rates of response and transfusion independence, as well as increased median OS, in the venetoclax arm correlated with strong improvements in patient-reported outcomes, such as fatigue and quality of life.
Venetoclax plus LDAC was well tolerated and manageable, with treatment exposure longer for patients in the venetoclax arm (median, 4 vs 2 cycles in the placebo plus LDAC arm). Although the venetoclax arm showed modest increases in hematologic AEs, the rate of AEs leading to treatment discontinuation (24% vs 25%) and the rate of serious AEs such as pneumonia (13% vs 10%) or sepsis (6% in each arm) were nearly identical between venetoclax plus LDAC and placebo plus LDAC, respectively. Although there was a numerically higher rate of grade ≥3 bleeding events in the venetoclax arm (11% vs 7%), incidence of fatal bleeding events was similar in the venetoclax and placebo arms (1.4% and 1.5%, respectively). The safety profile of the combination of venetoclax plus LDAC is a reassuring factor in the treatment of elderly or unfit AML patients, because it is known that this patient population has limited effective treatment options, with the increased risk of treatment-related toxicity associated with intensive chemotherapy a deterring factor for those considering this option.25
Because concomitant use of venetoclax with strong or moderate CYP3A inhibitors increases venetoclax exposure, the study protocol prospectively recommended venetoclax dose modifications when coadministered with these inhibitors. Pharmacokinetic analyses showed that recommended venetoclax dose adjustments while receiving concomitant moderate or strong CYP3A inhibitors resulted in venetoclax exposures comparable to the exposures when patients were not receiving these inhibitors, supporting the dose-modification scheme used in this study.
This study enrolled a challenging AML population, with nearly 60% age ≥75 years and a high proportion of patients with secondary disease (38%), prior HMA treatment (20%), poor cytogenetic risk (32%), and TP53 mutations (15%), which are known factors associated with dismal prognosis in AML. Although the rate of patients who achieved an MRD value <0.1% appears lower in the current study (6% [8 of 143]) compared with the prior phase 1b/2 study of venetoclax plus LDAC (21% [17/82] of all patients),26 the MRD assessments from the phase 2 and 3 studies cannot be directly compared; in the present study, bone marrow MRD assessment was not required after confirmation of CR (contrary to the phase 2 study). For this reason, the phase 2 study was likely more proficient in capturing the deeper responses achieved over time in patients with CR/CRi. In our recent phase 1b/2 study combining venetoclax with low-dose cytarabine in 82 treatment-naïve older patients with AML, CR/CRi responses (54%) were achieved, with a short median time to first response (1.4 months).16 Among the CR/CRi population, median duration of remission was 8.1 months and median OS for all patients was 10.1 months. Interestingly, a survival plateau beyond 18 months was observed, with ∼25% of patients demonstrating extended and ongoing long-term survival. Survival outcome was particularly promising for patient subgroups with NPM1- (median OS, not reached) and IDH1/2-mutant AML (median OS, 19.4 months).
In the current study, induction of disease remission was notable in the venetoclax arm, with a CR/CRi rate of 48% (including 27% CR rate), compared with only 13% in the placebo arm. Glasdegib plus LDAC, also available for frontline AML treatment, recently showed a CR/CRi rate of 27%.27 Venetoclax plus LDAC also performed well in patients with IDH mutations; studies have demonstrated a CR/CRh rate of 42% for those treated with ivosidenib for IDH1-mutant AML, whereas those with IDH1/2 mutations treated with venetoclax plus LDAC resulted in a 57% CR/CRi rate.28 In addition, the CR rate observed in this study is in line with previously reported rates for venetoclax combined with azacitidine (37%).16,29
In this study, LDAC plus placebo was associated with CR/CRi rates of 16% and 10% for patients with intermediate- and poor-risk karyotypes, respectively, which is on par with historical response rates in the literature.30,31 In comparison, patients treated with LDAC plus venetoclax had CR/CRi rates of 56% and 28% for intermediate and poor risk, respectively.
In summary, the combination of venetoclax plus LDAC demonstrated a well-tolerated and manageable safety profile, together with clinically meaningful benefits in OS, rates of remission, EFS, transfusion requirements, and patient-reported outcomes, compared with LDAC alone, in previously untreated patients with AML who were ineligible for intensive chemotherapy. The rapid induction of remission and favorable benefit-risk profile suggest that the addition of venetoclax to LDAC may provide an important treatment option for patients not suitable for intensive chemotherapy.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
Medical writing and assistance was provided by Ryan J. Bourgo of AbbVie. AbbVie and the authors thank all the patients who participated in the included studies and their families. They also thank all of the participating study investigators and coordinators.
This study was sponsored by AbbVie, which contributed to design, collection, analysis, and interpretation of the data and participated in the writing, review, and approval of the abstract. All authors had access to relevant data. Venetoclax (ABT-199/GDC-0199) is being developed in collaboration between AbbVie and Genentech.
Footnotes
The clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided after review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: All authors had access to the data and participated in data collection and interpretation, especially A.H.W., C.D.D., J.B., P.P., W.M., and J.H.; initial analysis was performed by B.C., S.G., and Q.J., with all other authors contributing thereafter; and all authors contributed to manuscript revision and gave final approval of the manuscript.
Conflict-of-interest disclosure: A.H.W. has been a consultant for AbbVie, Celgene, Roche, Janssen, Astellas, Novartis, Amgen, MacroGenics, and Servier; has received research funding from AbbVie, Novartis, Celgene, and Servier; and is a former employee of the Walter and Eliza Hall Institute of Medical Research, which receives royalties in relation to venetoclax, and A.H.W. is entitled to a fraction of these payments. P.M. has received grants/research support from Astellas, Celgene, Daiichi Sankyo, Janssen, Karyopharm, Novartis, Pfizer, and Teva; has been a speaker/advisor for AbbVie, Celgene, Daiichi Sankyo, Incyte, Janssen, Karyopharm, Novartis, Pfizer, Teva, and Tolero; and has been a consultant for Agios, Astellas, Celgene, Daiichi Sankyo, Oryzon, and Tolero. V.I., I.K., D.A.S., O.S., Y.H., J.-Z.H., and A.M. have been investigators in AbbVie-sponsored clinical trials. C.D.D. has received grants/research support from AbbVie, Agios, Bayer, Celgene, and MedImmune and been a consultant for Agios. J.N. has been a consultant/advisor for Novartis, Roche, Pfizer, Amgen, and Takeda and received travel expenses from Amgen and Janssen. K.L. has received grants/research support from AbbVie, Novartis, Takeda, Roche, and Sandoz and received honoraria or speaker’s bureau/personal fees from AbbVie, Novartis, Takeda, Roche, Sandoz, Celgene, Jansen, and Amgen. I.K. has been an investigator in AbbVie-sponsored clinical trials. W.F. has served on the board of directors/advisory board for Amgen, ARIAD/Incyte, Pfizer, Novartis, Jazz Pharmaceuticals, and Celgene; received patents/royalties from Amgen; received support for meeting attendance from Amgen, Gilead, Jazz Pharmaceuticals, Servier, and Daiichi Sankyo; and received research funding from Amgen and Pfizer; M.P. has been a speaker/advisor for AbbVie, Amgen, Astellas, Genesis, Janssen, Novartis, and Pfizer. A.A. has received institutional research support (clinical trials) from AbbVie, Celgene, Sanofi, Takeda, Amgen, Pharmacyclics, Acerta, and Novartis. J.B. has been a consultant for Pfizer, Jazz, Novartis, Amgen, and Astellas and received travel expenses from Amgen and Novartis. V.M. has provided conference support attendance for Abbvie, Celgene, Novartis, Takeda, and Janssen and been a consultant for Celgene, Novartis, and Janssen. T.Y. has received research support/honoraria or been an advisor for Pfizer, Otsuka, Takeda, Astellas, Solasia, Gilead Sciences, and SymBio. P.P. has received grants/research support from AbbVie, Roche, Novartis, and Genesis and honoraria from AbbVie, Janssen, Roche, Novartis, Gilead, and Genesis. B.C., S.G., Q.J., W.M., and J.H. are employees of AbbVie and may hold stock or have stock options.
Correspondence: Andrew H. Wei, Department of Haematology, The Alfred Hospital and Monash University, Commercial Rd, Melbourne VIC 3004 Australia; e-mail: a.wei@alfred.org.au.
REFERENCES
- 1.Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113(18):4179-4187. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute Surveillance, Epidemiology, and End Results Program: Cancer stat facts: leukemia—acute myeloid leukemia. Available at: https://seer.cancer.gov/statfacts/html/amyl.html. Accessed 3 September 2019.
- 3.Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972-979. [DOI] [PubMed] [Google Scholar]
- 4.Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnett AK, Milligan D, Prentice AG, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109(6):1114-1124. [DOI] [PubMed] [Google Scholar]
- 7.Dennis M, Hills RK, Russell NH, et al. An evaluation of 17 years of low dose cytarabine as therapy for AML patients not fit for intensive treatment, including patients with adverse cytogenetics, shows improving survival, potential underutilisation and highlights the need for new therapy [abstract]. Blood. 2017;130(1). Abstract 3874. [Google Scholar]
- 8.Del Poeta G, Venditti A, Del Principe MI, et al. Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML). Blood. 2003;101(6):2125-2131. [DOI] [PubMed] [Google Scholar]
- 9.Lagadinou ED, Sach A, Callahan K, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12(3):329-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer K, Al-Sawaf O, Fink AM, et al. Venetoclax and obinutuzumab in chronic lymphocytic leukemia [published correction appears in Blood. 2017;130(2):232]. Blood. 2017;129(19):2702-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar S, Kaufman JL, Gasparetto C, et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood. 2017;130(22):2401-2409. [DOI] [PubMed] [Google Scholar]
- 12.Moreau P, Chanan-Khan A, Roberts AW, et al. Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood. 2017;130(22):2392-2400. [DOI] [PubMed] [Google Scholar]
- 13.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107-1120. [DOI] [PubMed] [Google Scholar]
- 15.Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17(6):768-778. [DOI] [PubMed] [Google Scholar]
- 16.Wei AH, Strickland SA Jr., Hou JZ, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. 2019;37(15):1277-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogenberger JM, Delman D, Hansen N, et al. Ex vivo activity of BCL-2 family inhibitors ABT-199 and ABT-737 combined with 5-azacytidine in myeloid malignancies. Leuk Lymphoma. 2015;56(1):226-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niu X, Zhao J, Ma J, et al. Binding of released Bim to Mcl-1 is a mechanism of intrinsic resistance to ABT-199 which can be overcome by combination with daunorubicin or cytarabine in AML cells. Clin Cancer Res. 2016;22(17):4440-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teh TC, Nguyen NY, Moujalled DM, et al. Enhancing venetoclax activity in acute myeloid leukemia by co-targeting MCL1. Leukemia. 2018;32(2):303-312. [DOI] [PubMed] [Google Scholar]
- 20.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal SK, DiNardo CD, Potluri J, et al. Management of venetoclax-posaconazole interaction in acute myeloid leukemia patients: evaluation of dose adjustments. Clin Ther. 2017;39(2):359-367. [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.03. Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed 27 August 2019.
- 23.Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364(19):1844-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheson BD, Bennett JM, Kopecky KJ, et al. ; International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia . Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia [published correction appears in J Clin Oncol. 2004;22(3):576]. J Clin Oncol. 2003;21(24):4642-4649. [DOI] [PubMed] [Google Scholar]
- 25.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei AH, Strickland SA, Hou JZ, Fiedler W, Lin H, Walter RB. Venetoclax with low-dose cytarabine induces rapid, deep, and durable responses in previously untreated older adults with AML ineligible for intensive chemotherapy Paper presented at the 60th Annual Meeting of the American Society of Hematology; 2 December 2018; San Diego, CA; 2018. [Google Scholar]
- 27.Cortes JE, Douglas Smith B, Wang ES, et al. Glasdegib in combination with cytarabine and daunorubicin in patients with AML or high-risk MDS: phase 2 study results. Am J Hematol. 2018;93(11):1301-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386-2398. [DOI] [PubMed] [Google Scholar]
- 29.DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cashen AF, Schiller GJ, O’Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28(4):556-561. [DOI] [PubMed] [Google Scholar]
- 31.Döhner H, Lübbert M, Fiedler W, et al. Randomized, phase 2 trial of low-dose cytarabine with or without volasertib in AML patients not suitable for induction therapy. Blood. 2014;124(9):1426-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.