Dear Editor,
Coronavirus disease 2019(COVID-19) is an infectious disease that has spread worldwide.1 Carcinoembryonic antigen (CEA) is a known tumor marker for many common cancers2 , 3 and reported to be high expressed in the serum of patients with severe pneumonia.4 , 5 In this study, we found that CEA was highly expressed in the serum of COVID-19 patients without cancer. During the epidemic, the serum levels of CEA in 433 out of 1876 (23.08%) patients infected with COVID-19 were found to be higher than the normal level (5 ng/mL) at Jinyintan Hospital; however, no difference in alpha-fetoprotein levels was detected. Here, we aimed to summarize the clinical significance of CEA in predicting the prognosis of COVID-19 using Nomograms analysis.
We detected the serum levels of CEA in 161 patients with confirmed COVID-19 who were admitted to Jinyintan Hospital in Wuhan, China from January 24 to April 15, 2020. All patients were confirmed to be positive for COVID-19 based on typical chest computed tomography (CT) manifestations, evidence of etiology, and epidemiological history6 (excluding the presence of tumors). The clinical characteristics of these patients are shown in Table 1 .
Table 1.
The clinical characteristics of 161 patients infected with COVID-19.
| Characteristic | Number | CEA levels | P value | CRP | WBC | L | P value |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Male | 91 | 13.55±1.22 | 0.481 | 78.62±7.59 | 9.71±0.70 | 0.79±0.06 | 0.221*; 0.572&; 0.052# |
| Female | 70 | 14.96±1.48 | 64.74±8.39 | 10.33±0.85 | 1.02±0.10 | ||
| Age (years) | |||||||
| <65 | 71 | 11.99±1.13 | 0.033 | 63.35±8.01 | 8.80±0.74 | 0.93±0.07 | 0.184*; 0.074&; 0.695# |
| ≥65 | 90 | 16.23±1.51 | 78.57±7.78 | 10.79±0.75 | 0.88±0.08 | ||
| The admission classification | |||||||
| Moderate | 48 | 11.44±1.45 | 0.094@ | 43.49±8.05 | 6.139±0.51 | 1.10±0.08 | 0.003*@;0.002&@;0.115#@ |
| Severe | 74 | 15.38±1.63 | 0.815$ | 79.46±8.05 | 10.399±0.76 | 0.87±0.09 | 0.048*$;0.167&$;0.106#$ |
| Critically severe | 39 | 16.01±1.94 | 0.058§ | 111.2 ± 12.1 | 12.58±1.50 | 0.60±0.08 | <0.001*§;<0.001&§; <0.001#§ |
| The final classification | |||||||
| Moderate | 21 | 5.82±0.16 | 0.006@ | 41.16±10.35 | 6.57±0.81 | 1.08±0.09 | 0.472*@;0.386&@;0.697#@ |
| Severe | 51 | 10.28±1.00 | <0.001$ | 56.42±8.9 | 7.70±0.60 | 0.98±0.08 | 0.001*$;0.001&$;0.169#$ |
| Critically severe | 89 | 18.58±1.54 | <0.001§ | 98.28±8.00 | 12.34±0.82 | 0.79±0.10 | 0.001*§;0.001&§;0.024#§ |
| Total | 161 | 14.35±1.00 | 72.11±5.64 | 9.92±0.54 | 0.90±0.06 |
Note: Symbol * presents comparative analysis of CRP levels; Symbol & presents comparative analysis of WBC counts; Symbol # presents comparative analysis of L counts. Symbol @ presents moderate patients vs severe patients; Symbol $ presents severe patients vs critically severe patients; Symbol § presents moderate patients vs critically severe patients. P<0.05 indicates that the difference was statistically significant.
The serum CEA levels and other inflammatory markers, including C-reactive protein (CRP) levels, white blood cell (WBC) count, and lymphocyte (L) count were assessed to estimate the progression of COVID-19.7, 8 – 9 The independent prognostic factors were performed by univariate analysis and multivariate Cox regression. Nomograms for OS probability was analyzed by the R software (Version 3.4.4). The receiver operating characteristic (ROC) curve was used to evaluate the sensitivity and specificity of CEA serum level, which used to predict the severity of pneumonia.
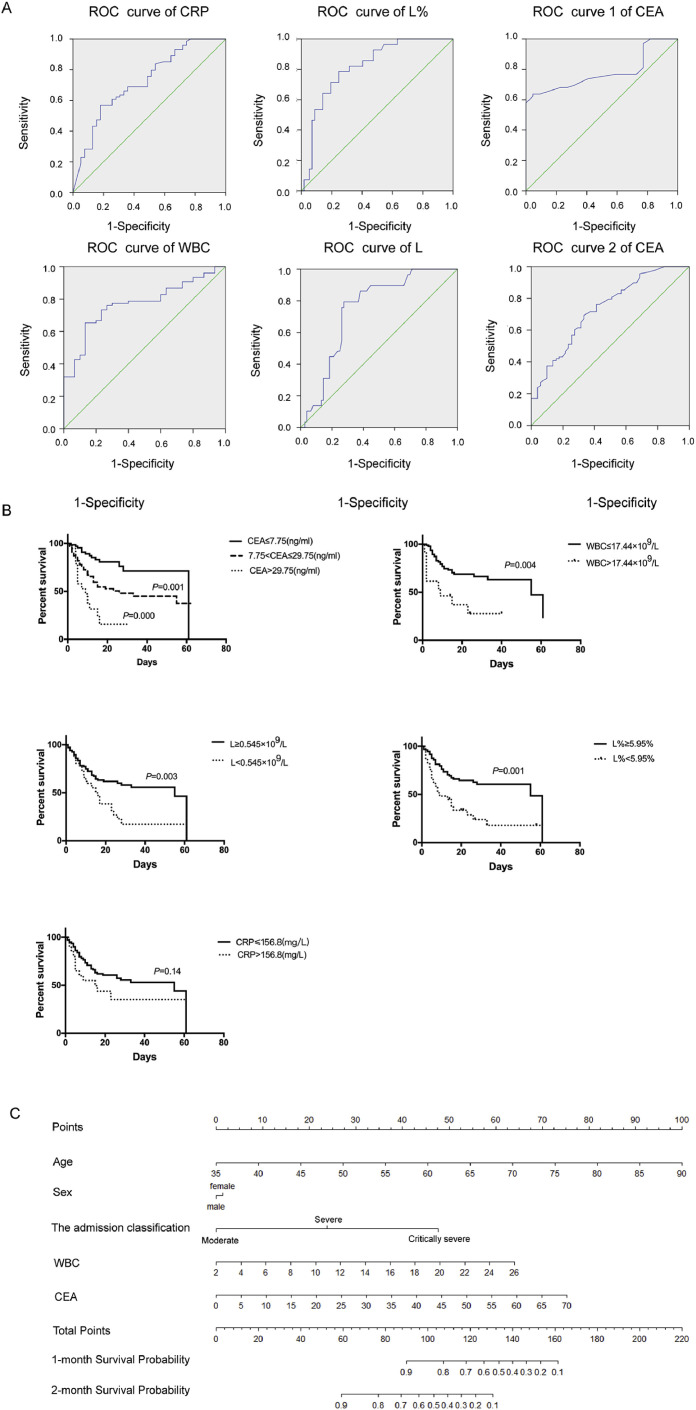
The ROC curves for CRP, WBC count, L%, and L count to assess disease classification and for CEA to assess disease progression are shown in Fig. 1 (A). The area under the curve (AUC) values were evaluated for these markers, as follows (high to low): L% [0.8186(0.728–0.910)]> WBC [0.768(0.676–0.861)]> L [0.743(0.647–0.839)]> CRP [0.724(0.625–0.823)]. The AUC for CEA was 0.741(0.644–0.839). The critical value for CEA to predict severe disease type was over 7.75 ng/mL, while that for WBC, L, L%, and CRP (for disease classification) was 17.44 × 109/L, 0.545 × 109/L, 5.95%, and 156.8 mg/L, respectively. In the ROC curve analysis, moderate type was regarded as negative, while severe and critically severe type as positive. The critical values were calculated following the method of Jordan Index. In addition, when the initial level of CEA was over 29.75 ng/mL, patients were prone to respiratory failure.
Fig. 1.
(A) The ROC curves for CRP, WBC, and L to assess disease classification and for CEA to assess disease progression. The AUC values for CRP, WBC, L, and L% were 0.724(0.625–0.823), 0.768(0.676–0.861), 0.743(0.647–0.839), and 0.8186(0.728–0.910), respectively, while the critical values were 156.8 mg/L, 17.44 × 109/L, 0.545 × 109/L, and 5.95%, respectively. The ROC curves 1–2 for CEA were analyzed to predict the progression of COVID-19. “1” is the ROC curve for CEA for disease progression from moderate to severe type, while “2” is the curve for disease progression from moderate to critically severe type. The AUC values of these curves were 0.578(0.442–0.715) and 0.741(0.644–0.839), respectively. (B) Survival curves of patients with different initial levels of CEA, WBC, L, and CRP. COVID-19 patients with initial CEA levels of over 7.75 or 29.75 ng/mL had worse outcomes than patients with lower CEA levels. Patients with L counts and L% less than 0.545 G/L and 5.95% had worse outcomes than the patients with higher L counts and L%, respectively. There was no significant difference in outcome between patients with CRP levels of over 156.5 ng/mL and patients with lower CRP levels. (C) Construction of nomogram with CEA and other significant indicators that predicted the probability of COVID-19 patients for overall survival. For using the nomogram, the value of individual patients with COVID-19 is located on each variable axis, and a line is depicted upward to determine the number of points received for each variable value. The sum of these numbers is located on the Total Point axis, and a line is drawn downward to the survival axes to predict the likelihood of 1-, 2-month survival of OS.
Next, we investigated the survival curves of patients with COVID-19 with different CEA levels and WBC and L counts at the time of admission, which were shown in Fig. 1(B). We found the patients with initial CEA levels of over 7.75 and 29.75 ng/mL had worse prognosis than patients with lower levels of CEA (Fig. 1(B)). Similar prognosis was reported for patients with initial WBC counts of over 17.44 × 109/L and L counts less than 0.545 × 109/L (L%<5.95%) (Fig. 1(B)). However, patients with CRP levels over 156.8 mg/L showed no significant difference in prognosis (Fig. 1(B)). The univariate and multivariate Cox regression analysis about above markers affecting Overall survival (OS) estimated that CEA, WBC and L(L%) were independent prognostic risk factors for COVID-19 patients.
The prognostic nomogram for overall survival of COVID-19 patients was depicted by independent indicators in the multivariate analysis (Fig. 1(C)). The prognostic value of CEA on outcome in patients with COVID-19 was more significant than other factors (P = 0.001). The next important indicator was WBC(P = 0.015), while L(L%) and CRP had no significant differences on the nomogram model (P>0.05). Each predictor in the nomogram was assigned a score (top scale). And the sum of these scores implied the probability of 1-, 2-months OS (bottom scale). The c-index for the nomogram of OS was 0.85(95%CI, 0.79–0.91), indicating discriminative ability of the models (Admission classification+ WBC+CEA, Fig. 1(C)).
The levels of CEA, CRP, WBC, and L were found to be associated with the severity of COVID-19. Thus, when treating patients, the changes in the levels of these indicators should be monitored. Jean Pierre and his team identified serum CEA as a prognostic marker for HIV-related pneumocystis carinii pneumonia (PCP).4 They showed that the CEA levels in patients with PCP and acute respiratory distress were increased.4 Moreover, in patients with PaO2 lower than 50 mmHg, only high concentrations of CEA (>20 ng/mL) were associated with fatal outcomes.4 , 5 Similarly, our results showed that the preliminary CEA levels were associated with patient outcome in COVID-19. Survival curve analysis indicated that COVID-19 patients with CEA levels higher than 20 ng/mL had worse outcomes. The predictive ability of developed nomogram also increased when CEA was incorporated and more important than other indicators. Hence, the prognostic value of initial CEA level should be under consideration as a specific marker for COVID-19 severity. Once identified, patients with high CEA levels and poor outcomes could benefit from effective treatment protocols.
In conclusion, the serum CEA levels were found to be increased in patients with severe or critically severe SARS-CoV-2 infection. Besides, the initial levels of CEA were associated with the prognosis of patients with COVID-19. Initial CEA levels of over 29.75 ng/mL predicted fatal outcomes in patients. Hence, our findings suggest that CEA may serve as a novel prognostic marker of COVID-19. Due to the limited follow-up time, the relationship between CEA levels and treatment effectiveness could not be explored. Therefore, studies with a higher follow-up duration are needed in the future.
Declaration of Competing Interest
The authors declare no conflicts of interest in this letter.
Acknowledgments
This study was supported by grants from the Health and Family Planning Commission Foundation of Hubei Province awarded to Lei Nie (No. WJ2019H194). We would like to thank all medical staffs fighting against COVID-19.
References
- 1.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campos-da-Paz M., Dorea J.G., Galdino A.S. Carcinoembryonic antigen (CEA) and hepatic metastasis in colorectal cancer: update on biomarker for clinical and biotechnological approaches. Recent Pat Biotechnol. 2018;12:269–279. doi: 10.2174/1872208312666180731104244. [DOI] [PubMed] [Google Scholar]
- 3.De Jong C., Deneer V.H.M., Kelder J.C. Association between serum biomarkers CEA and LDH and response in advanced non-small cell lung cancer patients treated with platinum-based chemotherapy. Thorac Cancer. 2020 doi: 10.1111/1759-7714.13449. . Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan C.M., Chu H., Wang Y. Carcinoembryonic Antigen-related cell adhesion molecule 5 is an important surface attachment factor that facilitates entry of middle east respiratory syndrome coronavirus. J Virol. 2016;90:9114–9127. doi: 10.1128/JVI.01133-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedos J.P., Hignette C., Lucet J.C. Serum carcinoembryonic antigen: a prognostic marker in HIV-related Pneumocystis carinii pneumonia. Scand J Infect Dis. 1992;24:309–315. doi: 10.3109/00365549209061336. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z.M., Fu J.F., Shu Q. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr: WJP. 2020 doi: 10.1007/s12519-020-00345-5. . Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qu R., Ling Y., Zhang Y.H. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J Med Virol. 2020 doi: 10.1002/jmv.25767. . Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L., Zhu F., Xie L. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.03.296. . Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian S., Hu N., Lou J. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80:401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]