Abstract
Left ventricle thrombus is considered a rare complication of Takotsubo syndrome. However, both a stress condition predisposing to Takotsubo syndrome and coagulation abnormalities coexist in COVID-19. We describe a case of a patient with COVID-19 with Takotsubo syndrome. (Level of Difficulty: Intermediate.)
Key Words: COVID-19, left ventricle thrombus, Takotsubo syndrome
Abbreviations and Acronyms: COVID-19, coronavirus disease-2019; LVEF, left ventricle ejection fraction calculated by Simpson’s biplane method
Graphical abstract
Left ventricle thrombus is considered a rare complication of Takotsubo syndrome. However, both a stress condition predisposing to Takotsubo syndrome…
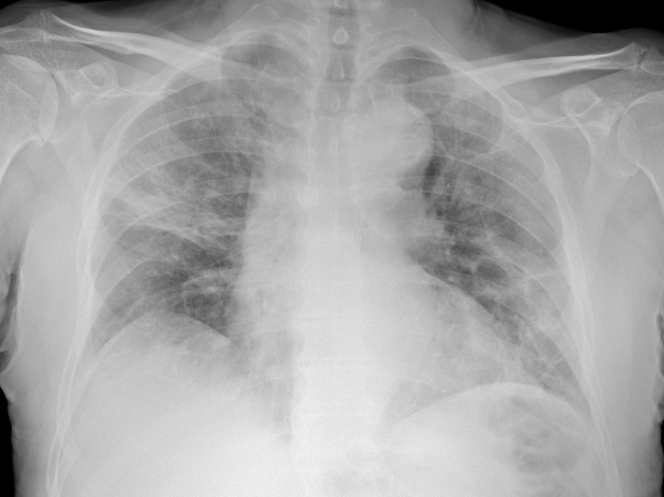
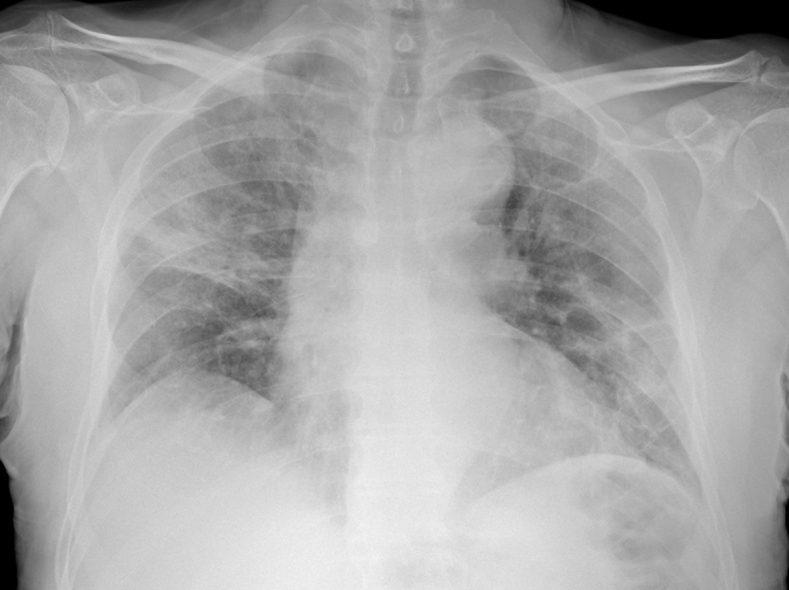
A 74-year-old man presented to the emergency department of a peripheral hospital with fever up to 38°C, dyspnea, and cough. Physical examination showed a blood pressure of 135/85 mm Hg and a heart rate of 95 beats/min. Arterial gas analysis showed a pH of 7.46, oxygen partial pressure of 57 mm Hg, and carbon dioxide partial pressure of 36 mm Hg, underlining respiratory failure. O2 therapy with continuous positive airway pressure was started, and the patient was hospitalized in the internal medicine unit. Chest x-ray was indicative of coronavirus-2019 (COVID-19) pneumonia (Figure 1). Therapy with azithromycin (500 mg once daily), hydroxychloroquine (200 mg twice daily), and dexamethasone (20 mg once daily for 5 days and then 10 mg once daily for 5 days) was started. A nasopharyngeal swab was positive for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) on real-time reverse transcriptase polymerase chain reaction assay.
Learning Objectives
-
•
COVID-19 has extrapulmonary and cardiovascular manifestations.
-
•
COVID-19 may be associated with exaggerated inflammatory response with an abnormal activation of coagulation, so a screening of coagulation setup may be indicated.
-
•
COVID-19 may show up with Takotsubo syndrome.
Figure 1.
Chest Radiograph
Diffuse hazy densities suggesting coronavirus disease pneumonia.
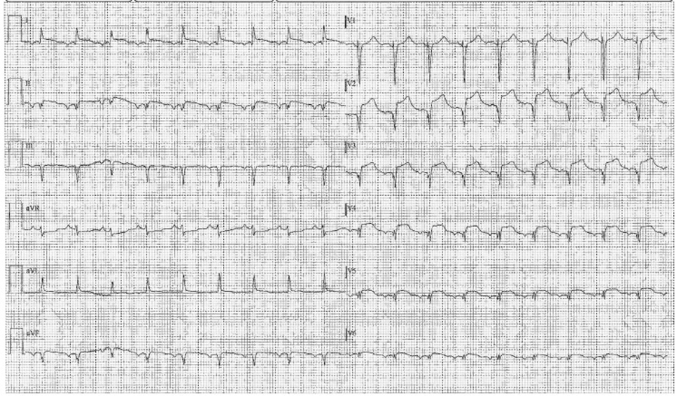
Five days later at his hospitalization, the patient presented with retrosternal typical chest pain. Electrocardiography demonstrated ST-segment elevation in anterolateral leads, suggesting an acute myocardial infarction (Figure 2).
Figure 2.
Electrocardiogram
ST-segment elevation in the anterolateral leads.
Medical History
The patient’s medical history showed arterial hypertension, dyslipidemia, and impaired fasting blood sugar.
Differential Diagnosis
The differential diagnosis included acute myocardial infarction, Takotsubo syndrome, myocarditis, and coronary embolism.
Investigations
The patient was transferred to our center for an urgent coronary angiography, which revealed nonsignificant coronary atherosclerosis.
Blood test results revealed elevated levels of markers of myocyte necrosis (troponin T: 775 ng/l; creatine kinase-myocardial band: 26.8 μg/l), elevated N-terminal pro–B-type natriuretic peptide of 8,999 ng/l, and elevated levels of inflammation indexes (white blood cell count: 12,870/μl; C-reactive protein: 14.2 mg/l; ferritin: 1,580 μg/l). Regarding the coagulation screening, we found a normal fibrinogen level of 282 mg/dl and an international normalized ratio (INR) of 1.1 but also elevated levels of D-dimer at 2,931 ng/ml.
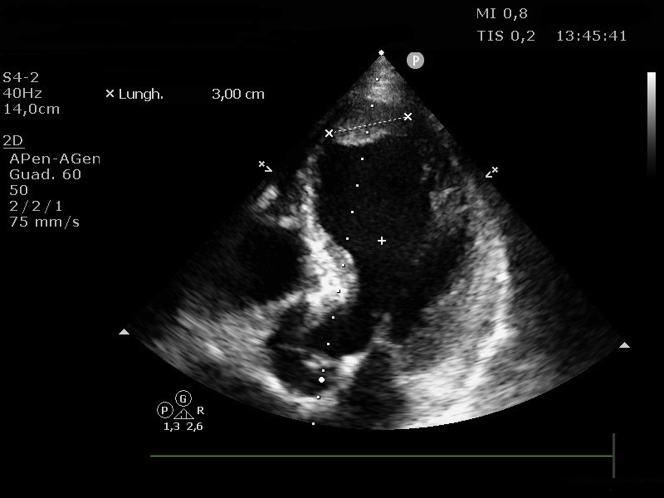
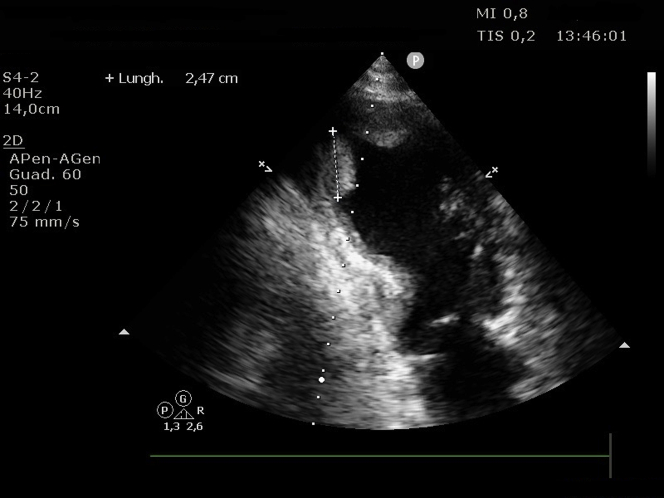
Transthoracic echocardiography revealed a dilated left ventricle with akinesis of the mid and apical ventricle segments with hyperkinesis of the basal segments and severe systolic dysfunction (left ventricle ejection fraction calculated by Simpson’s biplane method [LVEF]: 30%); first grade diastolic dysfunction; partial left ventricle outflow tract obstruction determining a late maximal gradient of 56 mm Hg with systolic anterior motion of the mitral valve and associated moderate to severe mitral regurgitation; and, finally, 2 large apical thrombotic formations: the posterior one was elongated (maximum: 31 mm) and mobile, and the anterior one was wide and oval (Figures 3 and 4, Videos 1 and 2).
Figure 3.
Echocardiographic Image
Four-chamber view showing left ventricle apical thrombus.
Figure 4.
Echocardiographic Image
Two-chamber view showing 2 left ventricle thrombi.
Online Video 1.
Echocardiographic Video
4 chamber apical view showing left ventricle apical thrombus
Online Video 2.
Echocardiographic Video
3 chamber apical view showing left ventricle apical thrombus
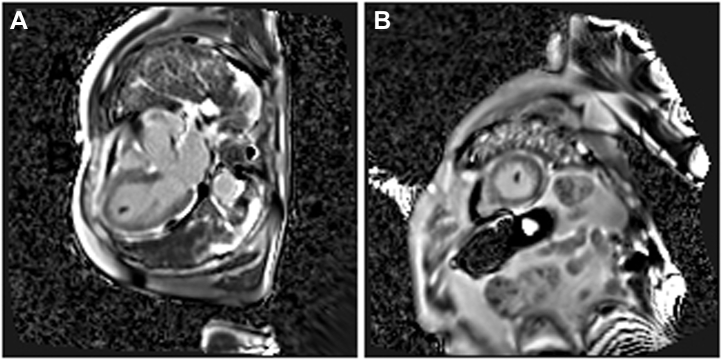
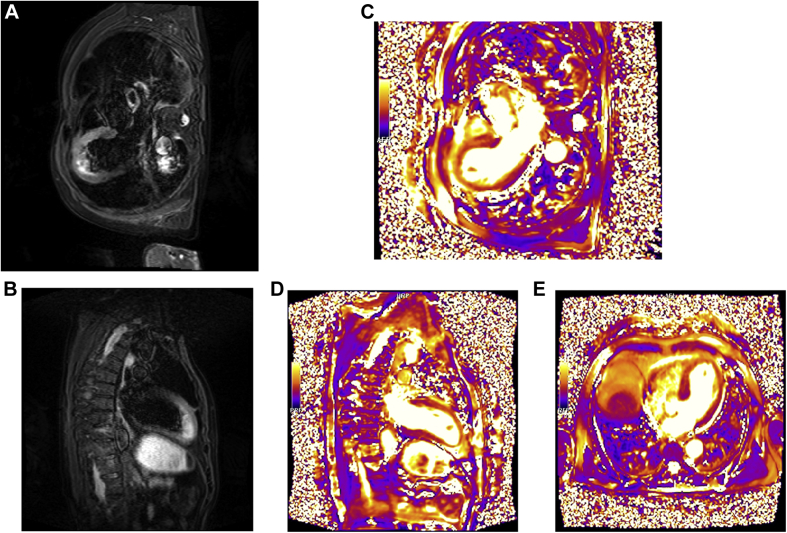
To make a diagnosis, a few days later, we performed cardiac magnetic resonance imaging, which showed an increased telesystolic volume with a severe systolic dysfunction (LVEF: 22%), hypokinesia of the medioapical segments of the left ventricle with the typical apical ballooning pattern, the T2-weighted images (short tau inversion recovery and T2 mapping) showed myocardial edema in the midapical segments of the left ventricle. After gadolinium administration, no areas of late enhancement were found; a thrombus (13 × 7 mm) was visible at the apex of the left ventricle (Figures 5 and 6, Videos 3 and 4).
Figure 5.
Cardiac Magnetic Resonance Image
Phase-sensitive inversion recovery (PSIR) sequences in the (A) short-axis and (B) 3-chamber views: an intracavitary hypointense formation, consistent with thrombus at the apex of the left ventricle and no late gadolinium myocardial enhancement.
Figure 6.
Cardiac Magnetic Resonance Image
Short tau inversion recovery sequences in the (A) 2-chamber and (B) 3-chamber views showed myocardial signal hyperintensity the in left ventricle mid-apical segments, consistent with interstitial edema, confirmed on (C) 2-, (D) 3-, and (E) 4-chamber views of T2 mapping sequences.
Online Video 3.
RM
Balanced steady state free precession B-SSFP Cine sequence in 2-chamber (Video 3) and 3 chamber (Video 4) view showed hypokinesia of apical segments with apical “balloning” of left ventricle. At the apex of left ventricle, an intracavitary mobile hypointense formation is evident, ascribable to with thrombus when compared to PSIR sequences 3-chamber view and in short-axis (image 5).
Online Video 4.
RM
Balanced steady state free precession B-SSFP Cine sequence in 2-chamber (Video 3) and 3 chamber (Video 4) view showed hypokinesia of apical segments with apical “balloning” of left ventricle. At the apex of left ventricle, an intracavitary mobile hypointense formation is evident, ascribable to with thrombus when compared to PSIR sequences 3-chamber view and in short-axis (image 5).
Management
Our priority was to treat the patient with enoxaparin 7,000 IU twice daily as per the patient’s weight. During the first days of hospitalization, and taking into consideration that the patient was hypotensive (systolic blood pressure: 80 mm Hg; mean blood pressure: <65 mm Hg), we treated the patient with intravenous dobutamine at 5 μg/kg/min with a progressive stabilization of pressure and heart rate. Heart-failure-directed treatment was not started because of hypotension.
Discussion
COVID-19 rapidly spread worldwide, with critical challenges for public health systems. The clinical course of this illness is mostly characterized by respiratory tract symptoms, including fever, cough, fatigue, pharyngodynia, and acute respiratory distress syndrome. Even though the presence of both extrapulmonary and other cardiovascular manifestations has been reported previously (1), the coexistence of Takotsubo syndrome and COVID-19 has been reported in only 3 cases to date (2, 3, 4). To our knowledge, this is the first report of a case of symptomatic Takotsubo syndrome complicated by left ventricle thrombi. Ventricle thrombi are a very rare complication of stress cardiomyopathy (5). There is growing evidence that COVID-19 may be associated with exaggerated inflammatory response with an abnormal activation of the coagulation system and signs of small vessel vasculitis and extensive microvascular thrombosis (6). Although the specific mechanism of this response is not fully understood, it can cause profound changes in a patient’s coagulation function; this pattern of presentation is associated with poor prognosis (7). These observations are confirmed by changes in coagulation test results such as increased D-dimer and decreased fibrinogen. Interestingly, cases of small pulmonary embolism are reported in the literature, even in the absence of deep vein thrombosis (8). This evidence has oriented the therapeutic approach, which now includes a parenteral anticoagulant drug (such as unfractionated heparin or low-molecular-weight heparin) as a thromboprophylaxis strategy to reduce hospital stay and mortality (9). Accordingly, a study by Zhou et al. (10) showed a lower 28-day mortality in hospitalized patients who were treated with heparin than in those who were not. Our patient was treated with enoxaparin leading to complete resolution of the thrombi in about 2 weeks. The use of heparin is recommended in patients with COVID-19. On the other hand, the use of direct oral anticoagulants is still being studied in thrombosis of the ventricle, and in general, these drugs are replaced by heparin in patients with COVID (11). Regarding Takotsubo cardiomyopathy, more evidence is needed to find a possible link between stress cardiomyopathy and COVID-19.
Follow-Up
Chest radiography was repeated in the following days and showed progressive reduction of interstitial pneumonia. Also, blood test results revealed an improvement of inflammation indexes (Table 1).
Table 1.
Clinical Laboratory Results
| Test | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 10 | Day 15 |
|---|---|---|---|---|---|---|---|
| HB, g/dl | 13.7 | 13.1 | 12.8 | 12.5 | 12.5 | 10.5 | 9.8 |
| WBCs, ×103/μl | 14.67 | 16.86 | 12.66 | 13.15 | 14.68 | 10.66 | 5.18 |
| Neutrophils, ×103/μl | 11.78 | N/A | 10.37 | 11.14 | N/A | 7.64 | 3.67 |
| Lymphocytes, ×103/μl | 1.83 | N/A | 1.37 | 1.01 | N/A | 1.82 | 1.57 |
| PLT, ×103/μl | 321 | 271 | 254 | 221 | 208 | 162 | 242 |
| Creatinine, mg/dl | 0.93 | 0.89 | 0.91 | 0.79 | 0.76 | 0.91 | 0.86 |
| PT, s | 14.5 | N/A | 12.6 | N/A | 11 | N/A | 12 |
| INR | 1.1 | N/A | 1.0 | N/A | 0.9 | N/A | 0.9 |
| APTT, s | 20.2 | N/A | 22.2 | N/A | 23.8 | N/A | N/A |
| Fibrinogen, mg/dl | 282 | N/A | 162 | N/A | 134 | N/A | N/A |
| D-dimer, ng/ml | 2,931 | 2,883 | 3,044 | 2,810 | 2,729 | N/A | 412 |
| CRP, mg/l | 14.2 | 9.4 | 5.6 | 3.3 | 2.1 | 89.8 | 36.6 |
APTT = activated partial thromboplastin time; CRP = C-reactive protein; HB = hemoglobin; INR = international normalized ratio; N/A = not applicable; PLT = platelets; PT = prothrombin time; WBC = white blood cell.
On day 7 of hospitalization, the nasopharyngeal swab was repeated, with a positive result. The first negative result was registered on day 15.
On the 14th day, we performed another transthoracic echocardiography, which showed the resolution of the 2 thrombi (Figure 7) and a complete restoration of LVEF (57%) (Video 5).
Figure 7.
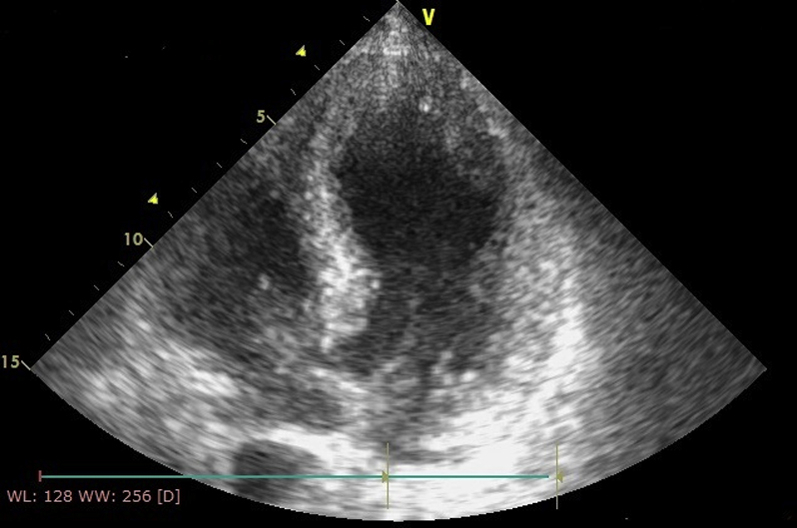
Echocardiographic Image
Four-chamber apical view showing resolution of left ventricle thrombi.
Online Video 5.
Echocardiographic Video
4 chamber apical view showing restoration of left ventricle function
The parenteral anticoagulant was then gradually switched to a long-term oral anticoagulant therapy with warfarin (dosage adjustment according to INR values, with an INR range of 2 to 3). Then, after 3 weeks of hospitalization, the patient, asymptomatic and in good hemodynamic compensation, was discharged.
Conclusions
We consider it clinically relevant to report this case of Takotsubo syndrome accompanying COVID-19; this may improve knowledge about this new disease. Furthermore, coagulation disorders in patients with COVID-19 are very common, and it is important to screen carefully. On the therapeutic side, in this case, low-molecular-weight heparin has proven effective in solving thrombosis.
Footnotes
Prof. Metra has received personal fees from Abbott Vascular, Amgen, Bayer, Edwards Therapeutics, and Vifor Pharma. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, please see the online version of this paper.
References
- 1.Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1096. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dabbagh M.F., Aurora L., D’Souza P., Weinmann A.J., Bhargava P., Basir M.B. Cardiac tamponade secondary to COVID-19. J Am Coll Cardiol Case Rep. 2020 Apr 23 doi: 10.1016/j.jaccas.2020.04.009. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sala S., Peretto G., Gramegna M. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Hear J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer P., Degrauwe S., Delden C.V., Ghadri J.R., Templin C. Typical takotsubo syndrome triggered by SARS-CoV-2 infection. Eur Heart J. 2020;41:1860. doi: 10.1093/eurheartj/ehaa306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nonaka D., Takase H., Machii M., Ohno K. Intraventricular thrombus and severe mitral regurgitation in the acute phase of Takotsubo cardiomyopathy: two case reports. J Med Case Rep. 2019;13:152. doi: 10.1186/s13256-019-2081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thachil J., Tang N., Gando S. (2020), ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;41:1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published correction appears in Lancet 2020;395(10229):1038] Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomasoni D., Sciatti E., Bonelli A., Vizzardi E., Metra M. Direct oral anticoagulants for the treatment of left ventricular thrombus—a new indication? A meta-summary of case reports DOACs in left ventricular thrombosis. J Cardiovasc Pharmacol. 2020;75:530–534. doi: 10.1097/FJC.0000000000000826. [DOI] [PubMed] [Google Scholar]