Abstract
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a rapidly spreading disease causing increased morbidity and mortality across the globe. There is limited available knowledge regarding the natural history of the SARS-CoV-2 infection. Other factors that are also making this infection spread like a pandemic include global travelers, lack of proven treatment, asymptomatic carriers, potential reinfection, underprepared global health care systems, and lack of public awareness and efforts to prevent further spread. It is understood that certain preexisting medical conditions increase the risk of mortality with COVID-19; however, the outcome of this disease in traditionally vulnerable chronic illnesses such as end-stage renal disease is not well documented. We present a case of a 56-year-old African American lady with end-stage renal disease on the peritoneal dialysis who presented predominantly with nausea, vomiting, and subsequently found to have COVID-19. We use this case to illustrate an atypical presentation of the COVID-19 in a vulnerable patient and discuss the literature.
Keywords: COVID-19, end-stage renal disease, peritoneal dialysis, atypical presentation
Introduction
On December 31, 2019, the World Health Organization’s (WHO) China country office notified of cases of pneumonia of unknown etiology detected in Wuhan city, Hubei province, with subsequent detection of the new strain of coronavirus on January 7, 2020. The virus was subsequently named by the WHO as SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), and the disease caused by it as COVID-19. The severe acute respiratory syndrome caused by the novel coronavirus (SARS-CoV-2) has evolved into a global pandemic as declared by the WHO, on March 11, 2020.1 The disease has since been spreading at an unrelenting pace infecting more than 3.2 million and causing more than 233 000 deaths globally. Currently, the United States leads in the number of cases in the world.2
Published studies have reported that the COVID-19 infects mostly males, the elderly, and patients with comorbidities.3,4 Patients with chronic kidney disease (CKD) and those on renal replacement therapies are potentially susceptible to developing COVID-19 infection, given the concentration of the risk factors and comorbidities.5 Ma et al5 reported that hemodialysis patients with COVID-19 are likely to experience mild disease that does not develop into full-blown pneumonia, probably given reduced function of the immune system and decreased cytokine storms.6 COVID-19 has an alarming case fatality rate of up to 5%; it is highly contagious, has variable symptoms, and can cause cluster outbreaks.7,8 COVID-19 has been described in patients who are on maintenance hemodialysis but not in patients on peritoneal dialysis. In this article, we report one of the first US patients who are on peritoneal dialysis and developed COVID-19. We have also reviewed the available literature relevant to this discussion.
Case Report and Case Presentation
A 56-year-old African American women with a history of end-stage renal disease (ESRD) on peritoneal dialysis (PD; since December 2019), diabetes mellitus with retinopathy, neuropathy, hypertension, heart failure with preserved ejection fraction, obesity, chronic intermittent use of nonsteroidal anti-inflammatories for 10 years presented to the emergency department (ER) with complaints of nausea, vomiting, abdominal pain, and a generalized weakness for the past 4 days along with peritoneal catheter malfunction on the day of arrival. She reported subjective fevers on the morning of arrival with cold-like symptoms, which included dry cough. The patient worked at a group home, active physically with a family history of mother and brother with ESRD. She is a nonsmoker and does not drink alcohol. A week before her presentation, she was switched to continuous cycler-assisted peritoneal dialysis (CCPD) from continuous ambulatory PD, following 2 days of training. She had no recent travel outside of her town. Her home medications included Aspirin, insulin, atorvastatin, gabapentin, labetalol, sevelamer, torsemide, renal vitamin, and omeprazole.
On arrival to the ER, her vitals were notable for the temperature of 98.3 °F, oxygen saturation of 98%, and blood pressure of 150/87 mm Hg. The physical was positive for mild diffuse tenderness at the PD catheter site and pitting edema. The initial laboratory works are presented in Table 1 and, in summary, showed normal white blood cell count, lactate level but mildly elevated procalcitonin level, moderately elevated serum ferritin level, and troponin T level. Her significantly elevated level of glycosylated hemoglobin level was suggestive of her uncontrolled diabetes mellitus.
Table 1.
Laboratory Studies.
| Laboratory study | Patient’s value | Normal range | Unit of measure |
|---|---|---|---|
| White blood cell count | 8.0 | 4.0-10.4 | 103/µL |
| Hemoglobin | 12.4 | 11.6-14.9 | g/dL |
| Platelet count | 355 | 130-400 | 103/µL |
| Serum sodium | 137 | 136-145 | mmol/L |
| Serum potassium | 3.7 | 3.5-5.4 | mmol/L |
| Serum lactate level | 0.8 | 0.5-2.0 | mmol/L |
| Serum troponin T | 166 | 0-13 | ng/mL |
| Serum procalcitonin | 0.22 | <0.10 | ng/mL |
| Hemoglobin A1c | 10.4 | 4.0-5.6 | % |
A chest X-ray showed right-sided pulmonary infiltrate (Figure 1). A nasopharyngeal swab was negative for influenza A and B. Evaluation for atypical pneumonia panel remained negative. Nasopharyngeal swab for the SARS-COV-2 by polymerase chain reaction was obtained and came back positive a day later. Computed tomography scan of chest (Figure 2) and abdomen on admission showed peripheral air space disease in the right pulmonary lobe along with pelvic fluid and PD catheter. A PD nurse evaluated the patient in the ER with an uneventful catheter flush. PD effluent was sent for the cell count, gram stain, and was normal. Her international normalized ratio and prothrombin time were normal.
Figure 1.
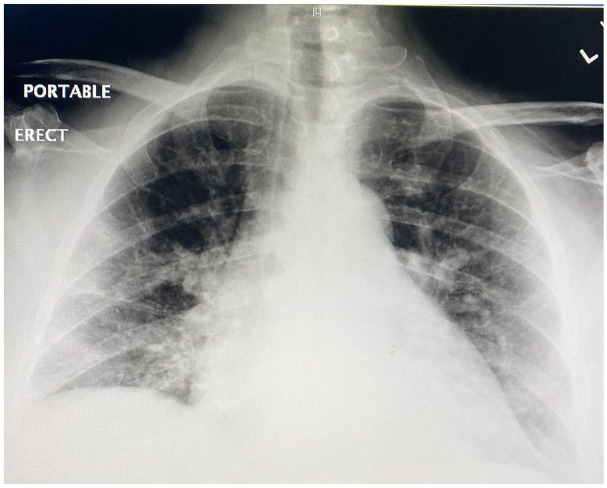
Chest X-ray showed right-sided pulmonary infiltrates.
Figure 2.
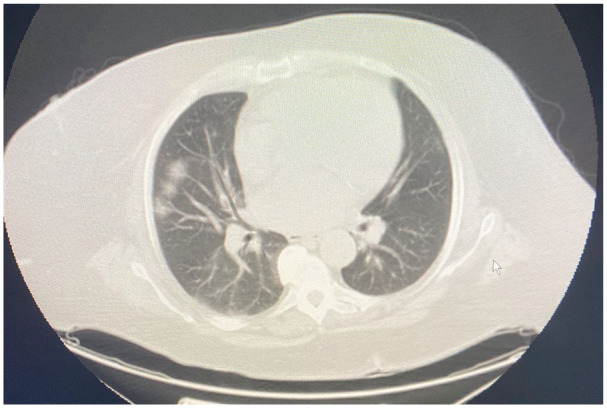
Computed tomography chest with peripheral air space disease in the right pulmonary lobe.
On admission to the hospital, she was started on intravenous ceftriaxone, doxycycline for community-acquired pneumonia, and admitted to the COVID isolation unit until the test came back positive. Following COVID-19 positive result, she was switched to azithromycin from doxycycline. She was started and remained on the CCPD since her arrival. She stayed in the hospital given low-grade fevers (Tmax = 100.8) and a risk of deterioration. Following 7-day hospitalization, she was discharged home. She remained clinically stable 25 days later on follow-up.
Existing data about COVID-19 suggest that it is significantly more common in men and typically associated with high fever in symptomatic patients. Our patient, who is a female, presented mainly with nausea and vomiting. Moreover, mild abdominal pain and low-grade fever in this patient could be otherwise suspicious for peritonitis as she is on PD. Therefore, we felt that her presentation with COVID-19 was overall atypical.
Efforts were made to do contact tracing for this patient as she went to her Home Dialysis unit for CCPD training just a week before the presentation with COVID-19. Her Home Dialysis clinic was alerted regarding possible exposure to other patients or nurses. However, at the time of her recent CCPD training, the Home Dialysis unit already adopted mandatory mask use, hand washing protocol for all in the facility, and no one who came near the patient during the 2 days of her CCPD training developed any symptoms of COVID-19 in the following 2 weeks. The patient also goes to a group home, and authorities were informed there about potential exposure through this patient—further details of measures taken by that facility are not available to the authors. Given the one-on-one care provided in a separate room for CCPD training and universal precautions observed for PD patients even under normal circumstances, the authors felt that the dialysis unit was not the source of her COVID-19. However, it was not possible to determine the exact source of her infection at this time.
Discussion and Conclusion
The SARS-CoV-2 is the third zoonotic infection by a coronavirus that spread on a large scale after SARS-CoV in 2002 and Middle Eastern Respiratory Syndrome (MERS) in 2015. The genomic homology of SARS-CoV 2 is 79%, like SARS-CoV. SARS-CoV2 resembles bat coronavirus very closely. The virus has a spike (S) protein on its surface and enters the human cell through angiotensin-converting enzyme 2 (ACE2) aided by serine proteases. ACE2 is widely distributed in the human body, particularly type 2 pneumocytes in the lung resulting in the respiratory symptoms. In severe infection, acute respiratory distress syndrome (ARDS) ensues, which can result in mortality. The typical clinical symptoms experienced by the patients with COVID are fever, myalgia, sore throat, cough, loss of smell, and shortness of breath. Fatigue and chills have been reported. Few patients present atypically with gastrointestinal symptoms.3,8,9 Our patient presented atypically with gastrointestinal symptoms and none to minimal respiratory symptoms. SARS-CoV-2 was suspected given the patient’s low-grade fever, occupational history, and the infiltrates on the chest X-ray.
Current pandemic with COVID-19 is a rapidly evolving scenario with Centers for the Disease Control and Prevention, International Society of Nephrology, American Society of Nephrology, International Society of Peritoneal dialysis, and the major dialysis providers in the United States publishing and providing adequate guidance given the risk profiles and comorbidities of the patients.1,4,5,10-12 The number of infected and exposed are expected to multiply in the coming days and weeks.9 Limited data are published so far in the CKD and ESRD population with COVID-19 with some case series and case reports.5,13-15 As per the unpublished data from Wuhan city, China, the infection was up to 10% in ESRD patients, with 6.4% of the medical staff.7
The renin-angiotensin-aldosterone system (RAAS) may upregulate ACE2 binding capacity, and thus, it was postulated that being on RAAS blockade may facilitate increased risk of the novel coronavirus infection, especially in patients with diabetes.4 However, this has not been proven beyond doubt, and research is ongoing on this subject. Our patient is diabetic and was not on the RAAS blockade at the time of presentation.
Treatment of COVID-19 is currently evolving as no drug has been proven effective. There have been mixed efficacy reported in decreasing the duration of viremia with chloroquine, hydroxychloroquine, azithromycin, and remdesivir, but these studies were mostly observational and have not been found conclusive. Many clinical trials are underway with experimental drugs such as favipiravir, sarilumab, bevacizumab, darunavir/ritonavir, and ivermectin. Most of the trials excluded CKD and particularly ESRD patients, for a higher possibility of side effects and difficulty with dose adjustment. The nephrologists should be mindful of the renal dosing of any experimental drugs in CKD, hemodialysis, and PD patients. Our patient remained clinically stable and was not subjected to any experimental medications.
The presence of SARS-CoV-2 was not checked in peritoneal effluent before. We do not know the benefit of the effluent testing for COVID-19, given the current lack of testing kits, and unsure if it is likely to change the management. In COVID-positive patient, discarding effluent at home should be in the drain where nobody has access, and handling by the close contacts should be discouraged. Can consider adding 500 mg/L chlorine-containing solution for 1 hour before pouring the effluent into the toilet.11 If the caregiver is involved in providing PD, they should wear personal protective equipment to limit the exposure.
The incidence of acute kidney injury (AKI) has varied widely depending on the studies and is more pronounced in patients with a severe disease with SARS-CoV-2.3,8 Consideration can be given for initiation of urgent start PD in the setting of AKI from COVID-19 if resources for hemodialysis and continuous renal replacement therapy are exhausted.12,16,17 When traditional hemodialysis and continuous renal replacement therapy resources are exhausted for high-volume AKI in hospitals, PD may be regarded as an option to treat AKI patients in need of immediate renal replacement therapy. Urgent start PD in the background of ESRD is gaining momentum recently in the United States. Flexible Tenckhoff peritoneal catheters can be placed through a percutaneous method by an experienced surgeon. A shorter break period for 24 to 48 hours before the use of PD catheters with low volumes will minimize the complications. Both manual and automated PD can be used. PD has the advantage of needing less nursing interventions, preserving vascular access that would be needed for critical care purposes, and avoiding systemic anticoagulation. The disadvantages of PD as renal replacement therapy in AKI are less predictable volume and solute removal, risk of peritonitis, catheter malfunctions, and PD fluid leakage. COVID-19 patients with severe respiratory failure may need aggressive ventilatory support, including prone ventilation. The effect of the PD in a prone patient with ARDS has been discussed by experts but not well studied and potentially could pose immense challenges for the nursing staff. In such patients with ARDS, PD could pose detrimental effects on respiratory biomechanics.
Given the higher risk of exposure at the in-center unit, the urgent start PD can be considered in a new ESRD patient with COVID-19, which can limit the risk of cluster exposure.12 In general, the ESRD patients on home therapies are at relatively less risk of exposure to novel coronavirus compared with in-center patients. The current global scenario is also an opportunity for increasing awareness and, subsequently, the number of patients on home modalities as it is a readily available protective intervention in a rapid pandemic. Moreover, the home modalities place less economic burden with potential improved overall patient outcomes. This is in line with the initiative of “Advancing American Kidney Health” recently launched by the Human Health Services to focus on increasing home dialysis patients and transplantation by 2025.
In the era of COVID-19 pandemic, care must be taken to acknowledge the challenges and unique requirements of ESRD population who are particularly vulnerable for existing high-risk comorbidities and potential exposure to COVID-19 in the process of getting their routine care. During this evolving crisis, ongoing studies, trials, and publication data should be used on an emergent basis to improve patient outcomes and medical staff safety.
In conclusion, we report one of the first cases in the United States of a patient on peritoneal dialysis presenting with COVID-19. ESRD patients with COVID-19 infection may present with atypical symptoms, and a high degree of suspicion is warranted for rapid testing, appropriate use of isolation precautions, careful implementation of available proven treatments, and close monitoring. Although ESRD patients on home therapies, such as PD, are at relatively less risk of exposure to infectious pandemics compared with in-center patients, it is certainly possible and should be suspected under current circumstances of ongoing COVID-19 pandemic.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Verbal informed consent obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iDs: Sreedhar Adapa  https://orcid.org/0000-0001-5608-5654
https://orcid.org/0000-0001-5608-5654
Venu Madhav Konala  https://orcid.org/0000-0003-1953-8815
https://orcid.org/0000-0003-1953-8815
Srikanth Naramala  https://orcid.org/0000-0003-1238-856X
https://orcid.org/0000-0003-1238-856X
Narothama Reddy Aeddula  https://orcid.org/0000-0001-8530-668X
https://orcid.org/0000-0001-8530-668X
References
- 1. World Health Organization. Coronavirus disease (COVID-19) pandemic. Accessed May 16, 2020 https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2. Johns Hopkins University. Coronavirus COVID-19 global cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Accessed May 16 2020. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kliger AS, Silberzweig J. Mitigating risk of COVID-19 in dialysis facilities. Clin J Am Soc Nephrol. 2020;15:707-709. doi: 10.2215/CJN.03340320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma Y, Diao B, Xifeng LV, et al. 2019. novel coronavirus disease in hemodialysis (HD) patients: report from one HD center in Wuhan, China [published online February 27, 2020]. medRxiv. doi: 10.1101/2020.02.24.20027201 [DOI] [Google Scholar]
- 6. Li J, Xu G. Lessons from the experience in Wuhan to reduce risk of COVID-19 infection in patients undergoing long-term hemodialysis. Clin J Am Soc Nephrol. 2020;15:717-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hopkins K. Loss of sense of smell as marker of COVID-19 infection. Accessed May 16, 2020 https://www.entuk.org/sites/default/files/files/Loss of sense of smell as marker of COVID.pdf
- 9. Naicker S, Yang CW, Hwang SJ, Liu BC, Chen JH, Jha V. The novel coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97:824-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention. Interim additional guidance for infection prevention and control recommendations for patients with suspected or confirmed COVID-19 in outpatient hemodialysis facilities. Accessed May 16, 2020 https://www.cdc.gov/coronavirus/2019-ncov/hcp/dialysis.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fhealthcare-facilities%2Fdialysis.html
- 11. International Society for Peritoneal Dialysis. Strategies regarding COVID-19 in PD patients. Accessed May 16, 2020 https://ispd.org/strategies-covid19/
- 12. American Society of Nephrology. COVID-19 information for the providers of dialysis services. https://www.asn-online.org/ntds/resources/Webcast_2020_03_11_COVID-19.mp4
- 13. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state [published online March 19, 2020]. JAMA. doi: 10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rombolà G, Heidempergher M, Pedrini L, et al. Practical indications for the prevention and management of SARS-CoV-2 in ambulatory dialysis patients: lessons from the first phase of the epidemics in Lombardy. J Nephrol. 2020;33:193-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldfarb DS, Benstein JA, Zhdanova O, et al. Impending shortages of kidney replacement therapy for COVID-19 patients [published online April 28, 2020]. Clin J Am Soc Nephrol. doi: 10.2215/CJN.05180420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El Shamy O, Sharma S, Winston J. Peritoneal dialysis during the coronavirus 2019 (COVID-19) pandemic: acute inpatient and maintenance outpatient experiences [published online April 23, 2020]. Kidney Med. doi: 10.1016/j.xkme.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]


