Abstract
Russell body gastritis is an extremely rare gastritis characterized by abundant infiltration of plasma cells with Russell body and eccentric nuclei, known as Mott cells. An 81-year-old Japanese woman with Helicobacter pylori and hepatitis C virus infection complaining of abdominal discomfort underwent upper gastrointestinal endoscopy, which detected an elevated lesion 2 cm in diameter at the anterior wall of the gastric body. A histological examination of the lesion revealed the infiltration of numerous Mott cells with an abundant eosinophilic crystal structure and eccentric nuclei in the lamina propria, resulting in a pathological diagnosis of Russell body gastritis. Endoscopic submucosal dissection (ESD) was performed subsequently. The histological findings of the resected specimen were compatible with those of Russell body gastritis. Upper gastrointestinal endoscopy performed 2 months after endoscopic submucosal dissection revealed the presence of new multiple flat elevated lesions in the antrum up to 1 cm in diameter, distant from the site of endoscopic submucosal dissection. A histological examination revealed a few Mott cells in the biopsy specimens taken from the new lesions. In turn, H. pylori eradication therapy was performed 1 month after the detection of the new lesions. One year after the eradication therapy, follow-up upper gastrointestinal endoscopy revealed that multiple lesions had almost disappeared, and the histological examination of the gastric biopsy specimens confirmed the disappearance of Mott cells. We herein report a case of Russell body gastritis in which multifocal lesions were observed after endoscopic submucosal dissection, and which was subsequently treated by H. pylori eradication therapy.
Keywords: Pathology, gastroenterology/hepatology, Russell body gastritis, endoscopic submucosal dissection, Mott cells
Introduction
Russell body gastritis is a special type of gastritis characterized by the abundant infiltration of plasma cells with Russell body and eccentric nuclei, known as Mott cells, in the lamina propria of the gastric wall. Since the first case was reported by Tazawa and Tsutsumi1 in 1998, 32 cases have been reported in English, to our knowledge. The complication of Russell body gastritis with Helicobacter pylori infection has been mentioned in previous reports.2 In addition, infection with hepatitis C virus, human immunodeficiency virus, or candida has also been reported to be associated with Russell body gastritis.2–7 Infection with these infectious agents has been suspected to cause the over-accumulation of immunoglobulin in plasma cells, leading to the formation of Mott cells in the stomach.
As Russell body gastritis is considered a non-malignant lesion of the stomach, endoscopic excision of the lesion has been rarely performed. Therefore, opportunities to observe the pathological features of the whole lesion of Russell body gastritis are limited.
We herein report a case of Russell body gastritis resected by endoscopic submucosal dissection (ESD) to offer an overview of the lesion and illustrate the pathological details, including the mucosal and submucosal distribution of Mott cells. Of note, the detection of Mott cells in a biopsy performed 2 months after the ESD suggests possible Russell body gastritis had involved the entire stomach latently when ESD was performed.
Case report
An 81-year-old Japanese woman had been observed as an outpatient at our hospital for hepatitis C virus seropositivity for 11 years. No marked changes had been observed in the infectious status of hepatitis C virus without therapy. Although H. pylori infection had been noted 3 years earlier based on the findings of serum H. pylori IgG, no eradication therapy had been provided due to the patient’s disagreement to undergo such therapy.
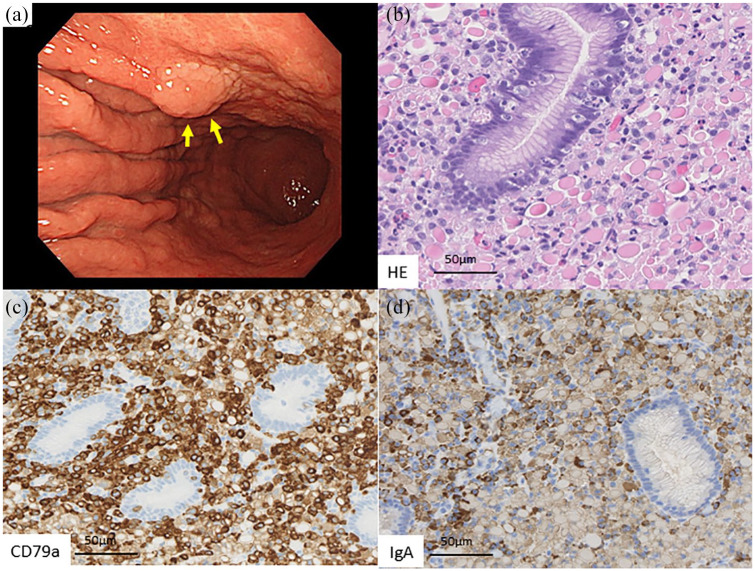
She complained of abdominal discomfort, and upper gastrointestinal endoscopy revealed a flat elevated lesion 2 cm in diameter at the anterior wall of the middle part of the stomach (Figure 1(a)). Due to suspicion of early gastric cancer, a single gastric biopsy was taken from the lesion. The microscopic observation of the specimen revealed the infiltration of numerous cells with abundant eosinophilic crystal structure and eccentric nuclei, infiltrating the lamina propria (Figure 1(b)). Gastric glandular structures were well-preserved among the infiltrating cells, and lymphoepithelial lesions were not observed.
Figure 1.
Representative images of the upper gastrointestinal examination and histopathological examination of the biopsied specimens. (a) A flat, elevated lesion 2 cm in diameter was found at the anterior wall of the middle part of the stomach. (b) Infiltration of numerous cells with abundant eosinophilic crystal structure and eccentric nuclei in the lamina propria. (c) CD79a expression in the infiltrating cells’ cytoplasm. (d) IgA expression in the intracytoplasmic eosinophilic crystal structure.
An immunohistochemical analysis was performed with antibodies against CD20 (L26, dilution 1:150; Leica Biosystems, Wetzlar, Germany), CD79a (JCB117, 1:100; Dako, Glostrup, Denmark), cytokeratin (AE1/AE3, 1:150; Leica), and IgA (polyclonal, 1:12000; Dako). For in situ hybridization analyses, EBER probe (Thermo Fisher Scientific, Waltham, USA), Kappa probe (Thermo Fisher Scientific), and Lambda probe (Thermo Fisher Scientific) were utilized. Immunohistochemically, the infiltrating cells showed CD79a expression and faint CD20 expression, and the intracytoplasmic eosinophilic crystal structure was positive for IgA (Figure 1(c) and (d)). Therefore, the infiltrating cells were considered to be Mott cells. In situ hybridization studies of kappa and lambda light chain did not demonstrate any light chain restriction of immunoglobulin of these Mott cells. Both the gastric epithelium and Mott cells were negative for EBER. Given these findings, the pathological diagnosis of Russell body gastritis was made for the biopsy specimen.
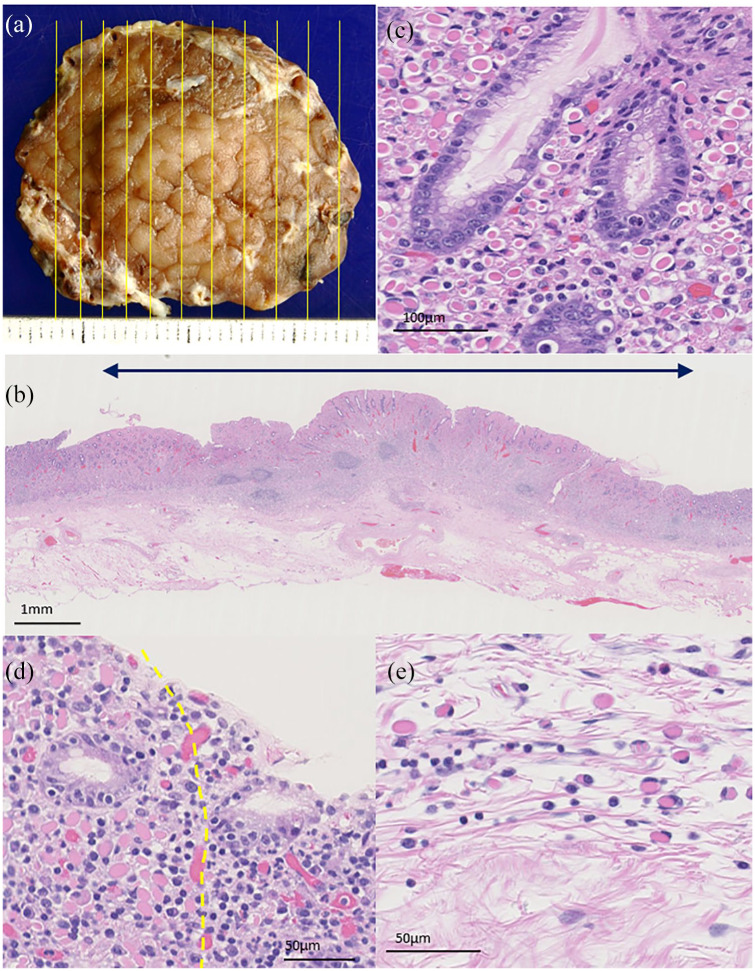
We thought that the lesion was single and localized based on the endoscopic findings. Also, the coexistence of gastric cancer has been reported for Russell body gastritis and the possible presence of extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) was included in the differential diagnosis.8,9 Subsequently, ESD was performed for the lesion based on the comprehensive judgment mentioned above. The resected specimen contained a 20 × 17 mm flat, elevated lesion (Figure 2(a)). A histopathological examination of the resected specimen revealed that the lesion was located mainly in the gastric mucosal layer and extended to the muscularis mucosae and slightly to the submucosal layer, with scattered lymphocytic aggregate without germinal centers (Figure 2(b)). The lesion was clearly demarcated from the background mucosa. Mott cells were abundantly observed in the lamina propria without the destruction of glandular structures (Figure 2(c)). Lymphoepithelial lesions and gastric cancer were not observed throughout the resected specimen. Mott cells were more frequently observed in the lamina propria than in the muscularis mucosae or submucosal layer (Figure 2(d) and (e)). No Mott cells were found at the horizontal or vertical margins.
Figure 2.
A representative image of the specimen resected by ESD and its histopathological findings. (a) A flat, elevated lesion 20 × 17 mm in diameter was observed. The yellow line is the cut line. (b) Mott cells were distributed mainly in the gastric mucosal layer and infiltrated the muscularis mucosae as well as slightly into the submucosal layer, with lymphocytic aggregate. Double arrows indicate the lesion area. (c) Abundant Mott cells were seen in the lamina propria without destruction of the glandular structure. (d) Mott cells formed a relatively clear boundary (yellow dotted line) with the surrounding gastric mucosa at the lateral side of the lesion. (e) In the submucosal layer, Mott cells infiltrated individually, in contrast to the mucosal layer.
Hematoxylin-eosin-stained slides were scanned using a pathology digital imaging system (Nanozoomer Virtual Slide System; Hamamatsu Photonics, Shizuoka, Japan), and the densities of Mott cells, plasma cells, and lymphocytes in the representative area of the gastritis were determined using the acquired digital images. The density of Mott cells was 1172 cells/mm2 in the lamina propria and 218 cells/mm2 in the submucosal layer. The density of non-Mott cell plasma cells and lymphocytes was 730/mm2 in the lamina propria, and 204/mm2 in the submucosal layer.
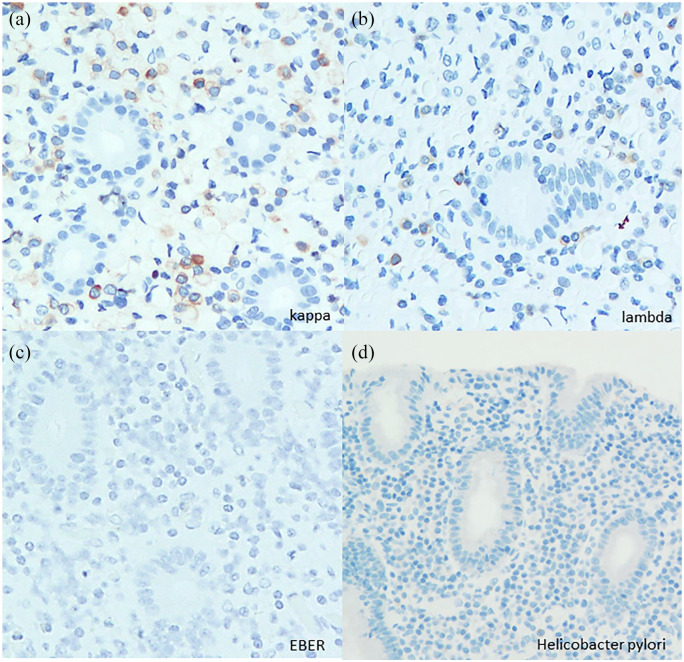
The immunohistochemical and in situ hybridization findings were the same as those of the biopsy specimen. Mott cells showed CD79a expression with neither light chain restriction nor EBER positivity (Figure 3(a)–(c)). An immunohistochemical analysis with the anti–H. pylori antibodies (polyclonal, 1:50; Agilent, California, USA) could not reveal the existence of H. pylori, but all of the pathological findings were compatible with those of Russell body gastritis (Figure 3(d)).
Figure 3.
In situ hybridization analyses showed no light chain restriction or EBER positivity ((a), (b), (c)). An immunohistochemical analysis with anti–H. pylori antibodies could not detect H. pylori (d).
Upper gastrointestinal endoscopy performed 2 months after ESD revealed the presence of multiple new, flat, elevated lesions in the antrum up to about 1 cm in diameter, distant from the site of ESD (Figure 4(a)). Biopsy specimens were taken from the new lesions, and a histopathological examination revealed a few Mott cells in all of the biopsied specimens (Figure 4(b)). The Mott cells showed the same immunohistochemical and in situ hybridization findings as those of the previous biopsy and ESD. H. pylori eradication therapy had not been administered as of the histopathological diagnosis of the new lesions. This clinical course and the findings were not typical of the progression of cancer or the endoscopic findings of cancer. Rather, they suggested the possibility of Russell body gastritis involving the entire stomach. Therefore, H. pylori eradication therapy was performed 1 month after the detection of new lesions. At 1 year after eradication therapy, follow-up upper gastrointestinal endoscopy revealed that multiple lesions had almost disappeared, and the histological examination of a specimen from the antrum wall of the stomach found no Mott cells (Figure 5(a) and (b)). Thus, the present study is the first to report the case of a patient who presented with multifocal lesions after ESD and who was subsequently treated by H. pylori eradication therapy.
Figure 4.

(a) Representative images of the upper gastrointestinal examination 2 months after ESD. ESD scar (arrowhead), multiple new lesions (arrow). (b) A few Mott cells (arrow) were seen in the biopsy specimen taken from the new lesions.
Figure 5.

(a) Representative images from the upper gastrointestinal examination at 1 year after eradication therapy. Multiple elevated lesions had almost disappeared. Arrowhead shows an ESD scar. (b) There were a few plasma cells, but no Mott cells in the biopsy specimen taken from the antrum wall of the stomach.
Discussion
Russell body is a rounded, eosinophilic, crystalloid cytoplasmic structure in plasma cells considered to be condensed immunoglobulin,10 and cells with Russell bodies and eccentric nuclei are called Mott cells. Russell body gastritis is a special type of gastritis characterized by the abundant infiltration of Mott cells, as shown in the present case.
Russell body gastritis is rarely treated with resection of the lesion, as it is regarded as non-neoplastic and benign. In the present case, as the initial lesion was a single elevated and localized, it was considered to be localized Russell body gastritis; ESD was performed due to the possible coexistence of cancer. There was no infiltration of Mott cells at the resection margin. Subsequently, however, lesions with Mott cells presented 2 months after ESD distant from the initial lesion.
To evaluate the prevalence of Mott cells in gastric biopsy samples, we surveyed 221 consecutive gastric biopsy specimens at Akita University Hospital (Akita, Japan), noting seven specimens with a few Mott cells from seven patients. Four of the seven cases with Mott cells were H. pylori-positive, while the H. pylori infection status was unknown in the other three. Considering the low rate of the presence of Mott cells in the stomach, it is unlikely that Mott cells incidentally reemerged after ESD. As mentioned above, previous reports suggest the etiology of Russell body gastritis is due to infectious antigens such as H. pylori. Actually, in this case, the Mott cells disappeared after H. pylori eradication therapy. This clinical course suggests that the etiology of Russell body gastritis is a reaction to pathogens such as H. pylori, and that Russell body gastritis might not represent a localized lesion but may involve the entire stomach. Hence, there were no Mott cells at the margins when ESD was performed, but the reaction of Russell body gastritis might have already latently involved in the entire stomach. Although the clinical significance of Russell body gastritis remains unclear, this case may provide one line of evidence to suggest that Russell body gastritis is a response to persistent H. pylori infection. Alternatively, some specific clone or a group of Mott cells may have migrated from the initial lesion in the middle part of the stomach to the antrum. If a B cell repertoire analysis by next generation sequencing was performed to identify any B cell clonal characterization, the results may have shown whether or not the Mott cells originated from the original lesion.11 But this modality could not be performed in the present case due to the fact that the patient declined approval and the anticipated degradation of the samples by formalin fixation.
In the present case, ESD clearly showed the distribution of Mott cells in the mucosal and submucosal layers of the Russell body gastritis lesion. In the lamina propria, the lesion was well-demarcated and packed with Mott cells, which histologically explained the endoscopic finding of the flat elevated lesion (Figure 2(d)). A gradual decrease in the number of Mott cells was observed from the mucosal to the submucosal layer. In the gastrointestinal mucosal immune system, plasma cell precursors migrate into the lamina propria to mature into plasma cells and secrete secretory-IgA (S-IgA) into the lumen through mucosal epithelial cells.12 Given these features of the gastrointestinal immune system, it is unsurprising that we observed the aggregation of Mott cells positive for IgA more frequently in the lamina propria than in the submucosal layer. Since Russell bodies were observed in the cytoplasm of plasma cells and defined as such, their formation may be correlated with the deregulation of the IgA secretion mechanisms in plasma cells rather than that of the trans-epithelial secretion mechanisms in mucosal epithelial cells.
H. pylori infection is a suspected etiology of Russell body gastritis. Joo M2 reviewed 30 cases of Russell body gastritis, reporting an H. pylori infection rate of 66.7% (20/30). Factors other than infection with H. pylori, hepatitis C virus, or human immunodeficiency virus and Candida esophagitis have been reportedly complicated with Russell body gastritis.2–7 In the present case, the patient was infected with H. pylori and hepatitis C virus, a double infection that might have been a causal factor for the development of Russell body gastritis. The detailed mechanism underlying the development of the disease requires further investigation.
Histopathologically, Mott cells with eccentric nuclei should be differentially diagnosed from signet ring cell carcinoma and extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) with plasma cell differentiation. In the differential diagnosis between Russell body gastritis and signet ring cell carcinoma, combinatorial immunohistochemistry of pan-keratin and CD79a is useful, involving the detection of reciprocal staining patterns between the diseases.8,9 For the differential diagnosis of MALT lymphoma, the detection of the light chain restriction of immunoglobulin, which was not observed in the present case, would favor the diagnosis of MALT lymphoma. However, some cases of Russell body gastritis have reportedly shown the light chain restriction of immunoglobulin.13–15 Therefore, in lesions with Mott cell infiltration with the light chain restriction of immunoglobulin, the histological observation of the destructive invasion of the epithelium and/or the detection of MALT lymphoma-related genetic abnormalities, such as the BIRC3-MALT1 fusion gene, would be required for the differential diagnosis.
Conclusion
We experienced a case of Russell body gastritis that showed multifocal lesions after ESD, and which was subsequently treated by H. pylori eradication therapy.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: The patient had the decisional capacity, and written informed consent was obtained from the patient for their anonymized information to be published in this article.
ORCID iD: Michinobu Umakoshi  https://orcid.org/0000-0001-7013-0503
https://orcid.org/0000-0001-7013-0503
References
- 1. Tazawa K, Tsutsumi Y. Localized accumulation of Russell body-containing plasma cells in gastric mucosa with Helicobacter pylori infection: “Russell body gastritis.” Pathol Int 1998; 48: 242–244. [DOI] [PubMed] [Google Scholar]
- 2. Joo M. Rare gastric lesions associated with Helicobacter pylori infection: a histopathological review. J Pathol Transl Med 2017; 51(4): 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Erbersdobler A, Petri S, Lock G. Russell body gastritis: an unusual, tumor-like lesion of the gastric mucosa. Arch Pathol Lab Med 2004; 128(8): 915–917. [DOI] [PubMed] [Google Scholar]
- 4. Drut R, Olenchuk AB. Images in pathology: Russell body gastritis in an HIV-positive patient. Int J Surg Pathol 2006; 14(2): 141–142. [DOI] [PubMed] [Google Scholar]
- 5. Licci S, Sette P, Del Nonno F, et al. Russell body gastritis associated with Helicobacter pylori infection in an HIV-positive patient: case report and review of the literature. Z Gastroenterol 2009; 47(4): 357–360. [DOI] [PubMed] [Google Scholar]
- 6. Coyne JD, Azadeh B. Russell body gastritis: a case report. Int J Surg Pathol 2012; 20: 69–70. [DOI] [PubMed] [Google Scholar]
- 7. Bhalla A, Mosteanu D, Gorelick S, et al. Russell body gastritis in an HIV positive patient: case report and review of literature. Conn Med 2012; 76: 261–265. [PubMed] [Google Scholar]
- 8. Shinozaki A, Ushiku T, Fukayama M. Prominent Mott cell proliferation in Epstein –Barr virus- associated gastric carcinoma. Hum Pathol 2010; 41(1): 134–138. [DOI] [PubMed] [Google Scholar]
- 9. Wolof EM, Mrak K, Tschmelitsch J, et al. Signet ring cell cancer in a patient with Russell body gastritis: possible diagnostic pitfall. Histophathology 2011; 58: 1178–1180. [DOI] [PubMed] [Google Scholar]
- 10. Portwit A, McCullough J, Eber WN. Normal bone marrow cells: development and cytology. In: Wickramasinghe SN, Porwit A, Erber WN. (eds) Blood and bone marrow pathology. 2nd ed. Philadelphia, PA: Churchill Livingstone, 2011, pp. 35–36. [Google Scholar]
- 11. Katoh H, Komura D, Konishi H, et al. Immunogenetic profiling for gastric cancers identifies sulfated, glycosaminoglycans as major and functional B cell antigens in human malignancies. Cell Rep 2017; 20(5): 1073–1087. [DOI] [PubMed] [Google Scholar]
- 12. Wang X, Hao GL, Wang BY, et al. Function and dysfunction of plasma cells in intestine. Cell Biosci 2019; 9: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Araki D, Sudo Y, Imamura Y, et al. Russell body gastritis showing IgM kappa-type monoclonality. Pathol Int 2013; 63: 565–567. [DOI] [PubMed] [Google Scholar]
- 14. Zhang H, Jin Z, Cui R. Russell body gastritis/duodenitis: a case series and description of immunoglobulin light chain restriction. Clin Res Hepatol Gastroenterol 2014; 38(5): e89–e97. [DOI] [PubMed] [Google Scholar]
- 15. Joo M. Gastric mucosa-associated lymphoid tissue lymphoma masquerading as Russell body gastritis. Pathol Int 2015; 65(7): 396–398. [DOI] [PubMed] [Google Scholar]