Abstract
This prospective study compared exercise test and intravenous fluid challenge in a single right heart catheter procedure to detect latent diastolic heart failure in patients with echocardiographic heart failure with preserved ejection function. We included 49 patients (73% female) with heart failure with preserved ejection function and pulmonary artery wedge pressure ≤15 mmHg. A subgroup of 26 patients had precapillary pulmonary hypertension. Invasive haemodynamic and gas exchange parameters were measured at rest, 45° upright position, during exercise, after complete haemodynamic and respiratory recovery in lying position, and after rapid infusion of 500 mL isotonic solution. Most haemodynamic parameters increased at both exercise and intravenous fluid challenge, with the higher increase at exercise. Pulmonary vascular resistance decreased by –0.21 wood units at exercise and –0.56 wood units at intravenous fluid challenge (p = 0.3); 20% (10 of 49) of patients had an increase in pulmonary artery wedge pressure above the upper limit of 20 mmHg at exercise, and 20% above the respective limit of 18 mmHg after intravenous fluid challenge. However, only three patients exceeded the upper limit of pulmonary artery wedge pressure in both tests, i.e. seven patients only at exercise and seven other patients only after intravenous fluid challenge. In the subgroup of pulmonary hypertension patients, only two patients exceeded pulmonary artery wedge pressure limits in both tests, further five patients at exercise and four patients after intravenous fluid challenge. A sequential protocol in the same patient showed a significantly higher increase in haemodynamic parameters at exercise compared to intravenous fluid challenge. Both methods can unmask diastolic dysfunction at right heart catheter procedure, but in different patient groups.
Keywords: dyspnoea, right heart catheter, exercise, fluid challenge, haemodynamic
Introduction
Chronic dyspnoea has multiple aetiologies; many cases result from heart diseases, and other frequent aetiologies include chronic obstructive pulmonary disease, muscle deconditioning and obesity.1 Differentiation of the cause of dyspnoea is crucial, because possible therapies differ widely. Heart failure as a cause of dyspnoea can be categorised by ejection fraction into systolic failure (with reduced ejection fraction) or diastolic failure (with preserved ejection fraction: HFpEF), with implications for treatment; a combined form (with mid-range ejection fraction) has also been recognised.2 Another decisive factor is the presence or absence of pulmonary hypertension (PH).
Categorisation of patients with chronic heart failure solely by non-invasive measurements is often difficult. In particular, patients at an early stage of heart failure show only minor dysfunction at rest. Additionally, the clinical appearance of PH in heart failure varies widely,3 the separate aetiologies and classes of PH may be part of a disease continuum in practice,4 and higher age and comorbidities may cover up the distinct phenotypes of pulmonary arterial hypertension (PAH).5
Therefore, the diagnostic work-up of patients with dyspnoea or PH requires both non-invasive and invasive measurements.6,7 As the cause of dyspnoea is often unmasked only under exercise conditions, haemodynamic measurements at rest have been increasingly complemented by data from right heart catheterisation (RHC) during exercise for the evaluation of unexplained dyspnoea8,9 and detection of exercise-induced haemodynamic abnormalities.10,11
However, the standardisation of exercise RHC is problematic, with differences in body position (supine vs half-supine), work rate patterns over time (steady state vs incremental exercise) and interpretation of the results.12 Moreover, exercise RHC is technically challenging and requires experienced personnel; it is therefore not ubiquitously available. The search for more practicable alternatives led to the concept of intravenous (i.v.) fluid challenge as a surrogate for exercise. The fluid challenge approach has become an established method for the detection of occult/latent HFpEF in recent years,13 and there appears to be consensus regarding both the protocol for fluid challenge and the interpretation of the results.14 Currently, an increase of pulmonary artery wedge pressure (PAWP) to more than 18 mmHg following the administration of 500 mL saline over 5 min is considered abnormal and indicative of LV dysfunction.15
However, studies comparing exercise and i.v. fluid challenge during RHC for the evaluation of suspected HFpEF are rare.16 The aim of our study was to assess whether the two methods show differences in the detection of latent diastolic heart failure.
Methods
Patients and study design
We prospectively studied 90 patients who underwent echocardiography and RHC at the University Hospital Greifswald between 2014 and 2017. Invasive haemodynamics were evaluated at rest, during exercise and (after recovery to baseline values) following i.v. fluid challenge in the same RHC session. Patients with complete data sets and resting PAWP ≤15 mmHg (n = 49) were included in the analysis. Left ventricular ejection fraction was >50% in all patients. Indications for RHC were (double categorisation possible) collagenosis (n = 18), echocardiography suspicious to PH (n = 16), dyspnoea and atrial fibrillation (n = 11, combined with coronary intervention (n = 7), after coronary artery bypass graft (n = 2) or mitral valve replacement (n = 1)) or dyspnoea and diastolic dysfunction uncertain by echocardiography (n = 10). A subgroup of patients with severe comorbidities (left heart phenotype) was defined by three or more criteria of the following: arterial hypertension, coronary heart disease, diabetes mellitus, obesity with BMI ≥30 kg/m2, left atrial dilation and atrial fibrillation.4–6
The study was approved by the ethics committee of Greifswald University (No. BB 050/18). All participants provided written informed consent.
Right heart catheterisation
RHC was performed in clinically compensated patients. We used digital RHC equipment (evoelement; Schwarzer GmbH, Heilbronn, Germany) with a parameter monitoring package (Argus PB-1000; Schwarzer GmbH). All catheterisations were performed with a two-lumen thermodilution catheter tipped with a latex balloon (Swan-Ganz, 7F, 110 cm; Edwards Lifesciences Services GmbH, Unterschleissheim, Germany). To ensure exact positioning of the catheter in each patient, we used a mobile fluoroscopy X-ray unit (Ziehm-Vision; Fa. Ziehm, Nürnberg, Germany) with documentation of the radiation dose. Venous approaches for the Swan-Ganz catheter were via cubital, jugular or subclavian vein, and the arterial approach was via radial artery with a 20G cannula (Arrow Intern, Reading, USA).
Exercise and fluid challenge protocol
Measurement of haemodynamic and gas exchange parameters at rest (supine position) was performed 10 min after correct positioning of the catheter. The patient was then placed in a partially upright position (with the thorax tilted upwards by 45°) and haemodynamic and gas exchange measurements were repeated (baseline 1). This procedure was followed by the exercise test, which began with unloaded cycling at 45 r/min for 5 min and continued with stepwise increases of workload by 25 W at intervals of 5 min. Haemodynamic and gas exchange parameters were measured at unloaded cycling and at every step of increased workload. Trained patients had an extra 50 W step at the end of exercise. The patients reached a median exercise level of 69% of their individual peak exercise oxygen uptake (peak VO2; determined in a bicycle exercise test according to a modified Jones protocol17 3–5 days before RHC).
The zero pressure point for RHC was set at the mid-thoracic level. PAWP was measured at end-expiration and reported as a mean of 3–5 breathing cycles in accordance with Rosenkranz et al.18 Cardiac output (CO) was calculated by two methods in each patient: thermodilution (the mean of 3–5 cold water injections) and the direct Fick method. The latter was performed after reaching a steady state of VO2 at the respective exercise level. Pulmonary vascular resistance (PVR) was calculated as (mean pulmonary artery pressure (PAPm) – PAWP)/CO. VO2 was measured (10 s average) by a cardiopulmonary exercise test system (SentrySuite; Viasys Healthcare GmbH, Höchberg, Germany) via a face mask. Blood samples for the measurement of mixed-venous and arterial oxygen saturation and arterial partial pressure of oxygen (paO2) and carbon dioxide (paCO2) were taken at the same time as the exhaled gas measurement and analysed immediately (ABL 90; Radiometer, Copenhagen, Denmark).
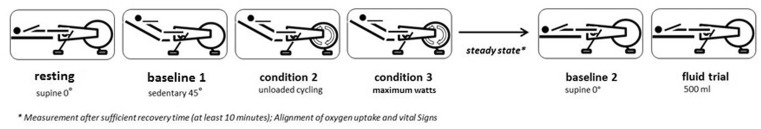
After completion of the exercise protocol, the patients rested until their haemodynamic parameters and VO2 had returned to baseline values. After reaching stable resting conditions, complete haemodynamic and gas exchange measurements were performed in supine position (baseline 2). This was followed by a rapid infusion of 500 mL isotonic (0.9%) NaCl solution within 5–10 min (by pressure cuff, C-Fusor 500, Smiths Medical, Minneapolis, USA). With the infusion completed, haemodynamic and gas exchange measurements were repeated. The entire course of the protocol is shown schematically in Fig. 1. All data were acquired, calculated and saved electronically.
Fig. 1.
Schematic depiction of the study protocol showing the sequence of exercise and fluid challenge during right heart catheterisation. *Measurement after sufficient recovery time (≥10min); alignment of oxygen uptake and vital signs.
Definitions
We defined diastolic dysfunction according to the 2007 guideline of the European Society of Cardiology19 and the revised criteria proposed by Huis in’t Veld et al.20 Patients were classed as having diastolic dysfunction if (a) the ratio of transmitral to mitral annular early diastolic velocity (E/e’) was >15 or (b) E/e’ was between 8 and 15 together with an early to late (atrial) transmitral diastolic velocity ratio (E/A) of <0.5 and an E deceleration time of <280 ms. PH at rest was defined as an invasively measured PAPm ≥25 mmHg.21 A concomitant PAWP ≤15 mmHg signified precapillary PH.
Haemodynamic thresholds indicating abnormal pulmonary circulation during exercise were defined according to Guazzi and Naeije.3 The upper limit of normal of PAPm was 30 mmHg at a CO of 10 L min−1, corresponding to a total PVR (TPVR, calculated as PAPm/CO) of 3 Wood units (WU). Left ventricular dysfunction was defined as PAWP >20 mmHg during exercise or PAWP >18 mmHg following fluid challenge with 500 mL of saline infusion.
Statistics
Continuous variables are reported as median and interquartile range. Categorical variables are reported as absolute numbers and percentages. Differences among groups were verified by Wilcoxon tests (continuous data) and χ2 tests (categorical data), with α < 0.05 considered significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., NC, USA).
Results
Patients
Baseline demographic data and functional parameters are shown in Table 1. Of the 49 patients, 36 (73%) were female; 26 (53%) had precapillary PH in the haemodynamic examination at rest, and of those 13 patients were on PH-specific medication.
Table 1.
Baseline demographic data and functional parameters.
| Characteristics | Patients (n = 49) |
|---|---|
| Female | 36 (73%) |
| Age | 63 (±13)/(range 26–80) |
| Diagnoses | |
| Precapillary PH | 26 (53%) |
| With PAH medication | 13 (50%) |
| Comorbidities | |
| Arterial hypertension | 36 (73%) |
| Coronary heart disease | 12 (24%) |
| Atrial fibrillation | 11 (22%) |
| Obesity (BMI >30 kg/m2) | 7 (14%) |
| Diabetes mellitus | 10 (20%) |
| Chronic renal failure | 13 (26%) |
| Obstructive ventilation disorder | |
| (FEV1/FVC <70%) | 17 (35%) |
| Restrictive ventilation disorder | |
| (VC or TLC <80% predicted) | 13 (27%) |
| Echocardiography | |
| Diastolic dysfunction | 24 (49%) |
| TAPSE | 21.5 (±5.9)/(range 9–36) |
| Right atrial area (n = 26) | 23.8 (±11.7)/(range 10–69) |
| Pericardial effusion (n = 46) | 6 (13%) |
| Pulmonary function | |
| VC% predicted | 94.2 (±21.1)/(range 38.5–135.6) |
| FVC% predicted | 96.4 (±20.8)/(range 54.8–138.6) |
| FEV1% predicted | 86.2 (±20.8)/(range 38.1–130.0) |
| FEV1/FVC% | 73.0 (±9.5)/(range 41.7–92.4) |
| TLC% predicted | 101.8 (±18.7)/(range 68.8–142.9) |
| RV% predicted | 121.1 (±39.4)/(range 67.2–275.1) |
| RV/TLC% | 46.2 (±9.7)/(range 26.9–73.8) |
| DLCO% predicted | 49.7 (±20.9)/(range 23.3–108.2) |
| KCO% predicted | 59.2 (±22.3)/(range 8.6–117.6) |
| Blood gases at rest | |
| paCO2 in mmHg (n = 44) | 34.0 (±4.7)/(range 22.9–43.5) |
| paO2 in mmHg (n = 44) | 67.3 (±12.8) / (range 42.8–93.3) |
| CPET | |
| Maximum work in Watt (n = 46) | 86.7 (±23.7)/(range 36–164) |
| Maximum work in % predicted (n = 46) | 65.1 (±16.2)/(range 33.3–99.3) |
| Anaerobic threshold (VO2@AT) in mL/min (n = 45) | 687.0 (±199.9)/(range 239–1067) |
| Work in Watt at VO2@AT (n = 46) | 45.0 (±14.2)/(range 20–84) |
| PeakVO2 in mL/min/kg (n = 46) | 1110.9 (±400.1)/(range 118–2242) |
| PeakVO2 % predicted (n = 46) | 67.1 (±22.3)/(range 5.5–116.6) |
| VE/VCO2 @ VO2@AT (n = 45) | 40.6 (±9.1)/(range 26.1–59.6) |
| pET CO2@AT (n = 46) | 27.9 (±6.3)/(range 12.3–40.4) |
| VE/VCO2-slope (n = 46) | 42.2 (±17.2)/(range 18–96) |
| VE/VCO2-slope >34 (n = 46) | 31 (67%) |
| VE/MVV in % (n = 45) | 63.2 (±15.2)/(range 31.6–95.3) |
| VE/MVV >80% (n = 45) | 6 (13%) |
| AaDO2 (maximum) (n = 42) | 47.6 (±20.1)/(range 13.4–88.7) |
| AaDO2 >35 (n = 42) | 30 (71%) |
| pa-ETCO2 (maximum) (n = 41) | 7.5 (±3.9)/(range 0.7–15.9) |
| pa-ETCO2 >6 mmHg (n = 41) | 59 (57%) |
Notes: For nominal variables n (%), for continuous variables mean (±standard deviation) are given.
AaDO2: difference of arterial and end tidal pressure of oxygen; AT: anaerobic threshold; BMI: body mass index; DLCO: diffusion capacity of carbon monoxide; KCO: Krogh factor (DLCO per alveolar volume); CPET: cardiopulmonary exercise testing; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; PAH: pulmonary arterial hypertension; paCO2: arterial partial pressure of carbon dioxide; pa-ETCO2: difference of capillary and end tidal pressure of carbon dioxide; paO2: arterial partial pressure of oxygen; peakVO2: maximum oxygen uptake; PH: pulmonary hypertension; PoPH: pulmonary venous occlusive disease with pulmonary hypertension; RV: residual volume; TAPSE: tricuspidal annular plane systolic excursion; TLC: total lung capacity; VE/MVV: ratio of ventilation to maximum voluntary ventilation; VE/VCO2@AT: ratio of ventilation to carbon dioxide output at anaerobic threshold; VE/VCO2: slope of the relation between ventilation and carbon dioxide output; pETCO2: end-tidal pressure of carbon dioxide.
Echocardiography was performed in all patients with 24/49 patients (49%) showing diastolic dysfunction of the left ventricle according to the definitions mentioned above. Detailed echocardiographic results and comorbidities are shown in Supplementary Table E3.
Response to exercise
All patients performed ergometric exercise without complication. Most haemodynamic parameters increased from baseline 1 values during exercise (Supplementary Table E1). However, PVR decreased by a median of 0.21 or 0.20 WU depending on the method of calculation of CO (thermodilution or Fick, respectively). TPVR decreased by a median of 0.30 WU and systemic vascular resistance (SVR) decreased by a median of 7.66 or 4.98 WU (based on thermodilution or the Fick method, respectively). Cardiopulmonary exercise data showed an increase in oxygen pulse (VO2/heart rate) by a median of 4.1 mL and a decrease in paO2 by a median of 7.9 mmHg from baseline 1 values; end-tidal pressure of carbon dioxide (pETCO2), paCO2 and the quotient of ventilation/carbon dioxide output (VE/VCO2) showed no relevant median change from baseline 1 (Supplementary Table E1).
Response to fluid challenge
The rapid saline infusion was performed without complication. Haemodynamic parameters after this fluid challenge showed an increase from baseline 2 for pulmonary artery pressure, right atrial pressure (RAP), PAWP, CO, cardiac index (measured by thermodilution) and stroke volume (SV) (Supplementary Table E1). In contrast, there was a slight decrease in both PVR (median: −0.30 WU by the Fick method and −0.56 WU by thermodilution) and SVR (median: −0.72 WU by the Fick method and −2.32 WU by thermodilution). Gas exchange measurements (paCO2, VE/VCO2 and pETCO2) showed no relevant changes (Supplementary Table E1).
Comparison of exercise and fluid challenge
Haemodynamics and gas exchange
Heart rate, systemic artery pressures, pulmonary pressures, RAP, transpulmonary pressure gradient, diastolic pressure gradient, CO and cardiac index showed significantly higher increases from baseline during exercise compared with fluid challenge (Supplementary Table E1). The change from baseline in PAWP, PVR and SV showed no significant difference between exercise and fluid challenge. By contrast, SVR decreased from baseline to a significantly greater extent during exercise compared with fluid challenge. Gas exchange data showed a significantly greater increase from baseline in VO2 and oxygen pulse and a significantly greater decrease from baseline in paO2 during exercise compared with fluid challenge (Supplementary Table E1).
Detection of exercise PH based on mean PAP
Of the 23 patients with PAPm <25 mmHg and PAWP ≤15 mmHg at rest, 10 (43%) had an increase in PAPm above the upper limit of 30 mmHg during exercise.
Detection of latent diastolic dysfunction based on PAWP
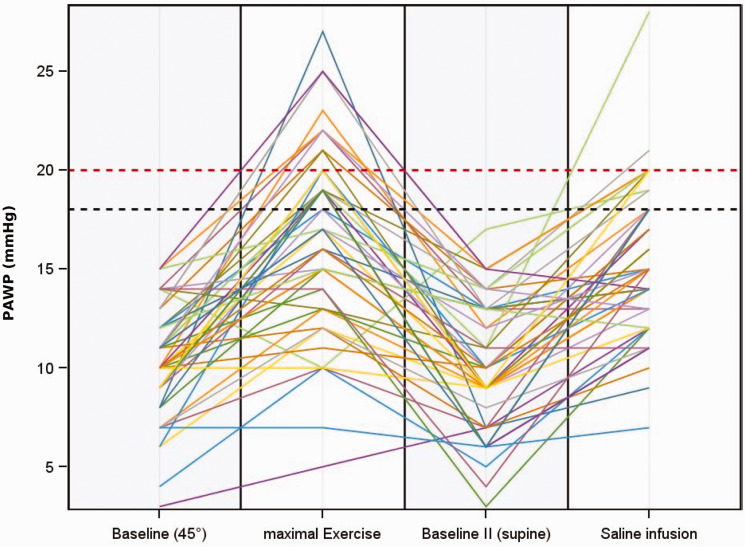
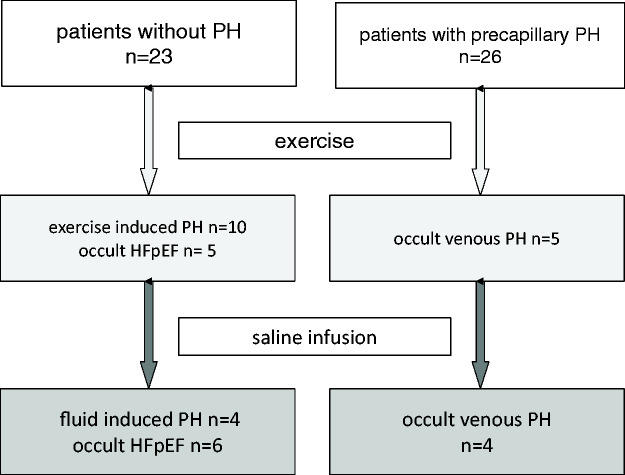
Of the 49 patients with PAWP ≤15 mmHg at rest, 10 (20%) had an increase in PAWP above the upper limit of 20 mmHg (above 25 mmHg in one patient only) during exercise. Similarly, 10 (20%) of the patients had an increase in PAWP above the respective limit of 18 mmHg after the fluid challenge. However, the two methods often gave different results in the same patient (Fig. 2). Only three patients exceeded the upper limit of PAWP in both tests (i.e. >20 mmHg during exercise and >18 mmHg during fluid challenge). Seven patients exceeded the limit during exercise only and seven other patients exceeded the limit after fluid challenge only (Fig. 3).
Fig. 2.
Course of PAWP at rest, during exercise, at second baseline and after fluid challenge in the entire patient group (n = 49).
PAWP: pulmonary artery wedge pressure.
Fig. 3.
Summary of results after exercise and after fluid challenge.
PH: pulmonary hypertension; HFpEF: heart failure with preserved ejection fraction.
A similar result was found in the subgroup of patients with precapillary PH at rest (n = 26). In this subgroup, only two patients exceeded the upper limit of PAWP in both tests, while five patients exceeded the limit only during exercise and four patients exceeded the limit only after fluid challenge only (Supplementary Fig. E1). Detailed data for the PH subgroup are shown in Supplementary Table E2, and the responses of CO, PAWP, PAPm and RAP to exercise and fluid challenge in this subgroup are shown in Supplementary Fig. E2.
Left heart phenotype and PAWP increase
The constellation of a left heart phenotype was found in 10 of the 17 patients (59%) with pathological PAWP increase in one of both tests, in 1 of the 3 patients with increase in both tests, and in 8 of the 32 patients (25%) without PAWP increase in any test.
Discussion
Our data show that the haemodynamic response to exercise (at 69% of previously measured peak VO2) is significantly different from the response to i.v. fluid challenge in patients undergoing RHC for evaluation of haemodynamic status (with or without PH). Each method identified latent diastolic dysfunction in 10 patients (20%), but there was little overlap between the two methods; only three patients had latent diastolic dysfunction identified by both methods. In patients without resting PH, we have detected in 43% of cases an exercise-induced pulmonary hypertension.
Most previously published studies in this field assessed patients with one of the two methods only. Irrespective of the different study populations, previous studies of i.v. fluid challenge detected comparable proportions of patients with left ventricular dysfunction. In patients with PAH associated with systemic sclerosis, 11 of 53 (21%) had an increase of PAWP to >15 mmHg after fluid administration.22 This occult venous pulmonary hypertension occurs comparably often in association with collagenosis, and hence a high proportion of our indications for RHC origins from this diagnosis. In another study of 207 patients with PAH (50% were receiving specific PAH therapy), PAWP increased to >15 mmHg in 46 patients (22%) after fluid challenge.23 D’Alto et al. showed in a large study (n = 190) that fluid challenge increased PAWP by 7 ± 2 mmHg in postcapillary PH and 3 ± 1 mm Hg in both PAH and non-PH patients groups. However, only 6% of patients with PAH and 8% without PH exceeded the PAWP cut-off of 18 mmHg and could be re-classified as occult left ventricular dysfunction.24 A recent study by the same working group showed that also systemic sclerosis (SSc) patients without PH (n = 25) increased both PAPm and PAWP after fluid challenge.25 However, 76% of patients in this study had an impaired DLCO, what might more indicate on interstitial lung disease associated to SSc than on backward transmission of PAWP elevation.
Studies on exercise RHC revealed a higher proportion of left ventricular dysfunction. In a retrospective study, 119 of 619 patients (19%) had exercise-induced pulmonary venous hypertension defined as a maximum PAWP >20 mmHg.9 Another retrospective study of symptom-limited maximum exercise in 66 patients without PH at resting RHC showed exercise-induced postcapillary PH in 26 patients (39%).26 This proportion of about one-third of patients showing occult diastolic dysfunction is in line with a study using a cut-off of 18 mmHg at exercise.27 Interestingly, the latter study showed a correlation of BMI and exercise PAWP, what is discussed in the pathophysiology paragraph below.
To our knowledge, there is only one previously published study that compares exercise and fluid challenge in the invasive diagnosis of left ventricular dysfunction.16 The study was relatively small, and included 14 patients with HFpEF and normal PAWP at rest and 12 control subjects with normal PAWP at rest and during exercise. The control group had a comparable increase in PAWP during exercise and after fluid challenge, whereas in the HFpEF group, the increase in PAWP during exercise was twofold greater than that observed after fluid challenge. The authors concluded that exercise testing is more sensitive than fluid challenge for the detection of haemodynamic changes in patients with HFpEF. However, even in this small cohort, two controls (17%) had PAWP >15 mmHg after fluid challenge but not during exercise.
Pathophysiology
Our study used slightly different limits of PAWP to define left ventricular dysfunction, but confirmed that most pathological increases of PAWP occurred in response to either exercise or fluid challenge but not both in the same patient. This might be explained by the fact that the two methods activate different mechanisms, as described by Guazzi and Naeije.3 Exercise testing activates the sympathetic nervous system, increases intrathoracic pressure and leads to oxygen desaturation of mixed venous blood. By contrast, i.v. fluid challenge also increases interstitial fluid content, with subsequent impairment of gas exchange and activation of J receptors. Heart failure may increase PAPm in the sense of backward failure of left ventricle, i.e. as upstream congestion of an elevating PAWP, whereas hypoxia or pulmonary vascular disease may increase PVR.3,28 Moreover, these pathways will overlap, vary with gender and shift with ageing.29 This might explain that the discrimination of patients by ‘left heart phenotype’ failed to predict an increase in PAWP. This concept of a ‘left heart phenotype PH’ (LH-PH) considers the risk factors diabetes mellitus, arterial hypertension, coronary artery disease and obesity.21 However, the LH-PH constellation did not predict PAWP increase in our tests. Comparable to our data, a recent study by Agrawal et al.30 found no association with these risk factors and PAWP after i.v. fluid challenge. Additionally, studies on PH patients revealed an inverse relationship of resting PVR and ΔPAWP after i.v. fluid challenge31 and exercise.27 An interpretation of this unexpected finding might be that a high PVR prevents a fluid overload of the LV at the expense of higher right heart pressures. We think that the broad variation in pathophysiological reactions finally leads to the different response groups in our study and the impossibility to assign a certain pathology to a certain test.
Due to a lack of standardisation, differences in diagnostic threshold values and weak evidence regarding therapy or prognosis, neither the European Society for Cardiology/European Respiratory Society guidelines6 nor a recent update7 recommended a specific procedure for exercise testing or fluid challenge. There are ongoing efforts to fill this gap of evidence and proposals for standardisation of exercise testing12 and fluid challenge23 have been published. There is emerging consensus that exceeding a PAWP of 18 mmHg after i.v. fluid administration (500 mL in 5–10 min) is a sign of left ventricular dysfunction.24 However, there is currently no consensus in defining an exercise-induced pathology. Some authors regard a PAWP of 20 mmHg as the upper limit of normal during exercise,3 but this limit can be exceeded in elderly individuals.12,32,33 An increase of PAWP above 25 mmHg at exercise is gaining acceptance as a sign of exercise-induced left ventricular dysfunction and consequently as a criterion that defines HFpEF.32 Other authors regard PAWP >15 mmHg as pathological and as a sign of ‘early HFpEF’,20 or a combination of PAWP >20 mmHg and PVR <1.5 WU as a sign of left ventricular dysfunction.10
In our patients, diastolic dysfunction was diagnosed more often by echocardiography than by invasive haemodynamic measurement at rest. This phenomenon has been reported previously in the literature and is related to early forms of left ventricular diastolic dysfunction and therapeutically well-adjusted patients.20 Similarly, patients with PAH being on specific therapy meet the echocardiographic criteria for diastolic dysfunction, but they are not correlated to PAWP.34,35 A recent study suggested a remarkably lower cut-off for E/e’ (>8) in PH patients, but again the number of patients with occult diastolic dysfunction (5 of 52 patients; 10%) remained in the established range.30 However, even with the different cut-off in this study, none of the LH-PH comorbidities despite diabetes mellitus was associated with occult diastolic dysfunction. Another reason for the different proportions of diastolic dysfunction origins in the different clinical state, i.e. the echocardiography preceded other diagnostic procedures and was performed in not optimal recompensated state. In contrast, the RHC was performed under strictly defined conditions and in always recompensated state, what might lead to a pre-test bias of echocardiography and RHC. The summation of rerespectively de-compensated state, comorbidities, specific PAH therapy and early forms of diastolic dysfunction explains that pre-test echocardiography does not provide evidence for the PAWP reaction at exercise or fluid, as shown in Supplementary Table E3.
Limitations
PAWP was measured at end-expiration and reported as the mean of 3–5 breathing cycles at rest and during exercise, which is certainly a compromise18 but is accepted under consideration of the factors that influence PAWP.36–38 Another compromise was the selection of one exercise protocol from the variety of RHC exercise protocols available39,40; we chose a stepwise incremental protocol that enabled us to measure thermodilution and VO2 (for the Fick method) simultaneously. Furthermore, the most reliable parameter for the invasive detection of left ventricular diastolic dysfunction is the left ventricular end-diastolic pressure (LVEDP); although PAWP is not identical to LVEDP, we used PAWP as a reliable surrogate parameter.33,37,38 Finally, we did not randomise the sequence of exercise and fluid challenge, so we cannot exclude the possibility that the exercise influenced the response to the subsequent fluid challenge, even with an intervening 10-min recovery period and normalisation of haemodynamic parameters.
Our results confirm that RHC during both exercise and fluid challenge can contribute to the differentiation of causes of dyspnoea and definition of the haemodynamic state. Using a sequential exercise and fluid challenge protocol during a single RHC procedure, we were able to demonstrate that exercise leads to a significantly greater increase in haemodynamic parameters than i.v. fluid challenge. PVR changed only slightly in response to exercise and fluid challenge, with no significant difference between the two methods. Each method unmasked diastolic dysfunction in different, only slightly overlapping patient groups, consistent with the activation of different pathophysiological pathways by the two methods. Therefore, our study does not indicate superiority of one method over the other for the detection of diastolic dysfunction during RHC. This requires further studies to standardise exercise and fluid challenge protocols and to evaluate patients with different aetiologies of dyspnoea.
Supplemental Material
Supplemental material, sj-pdf-1-pul-10.1177_2045894020917887 for Exercise and fluid challenge during right heart catheterisation for evaluation of dyspnoea by Ralf Ewert, Alexander Heine, Annegret Müller-Heinrich, Tom Bollmann, Anne Obst, Susanna Desole, Christine Knaak, Beate Stubbe, Christian F. Opitz and Dirk Habedank in Pulmonary Circulation
Acknowledgements
We wish to thank our medical assistance staff, especially Mrs Dagmar Fimmel, for the help in performing the functional assessment and the RHC. Editorial assistance was provided by Claire Mulligan, PhD (Beacon Medical Communications Ltd, Brighton, UK).
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This study was funded by the Ernst-Moritz-Arndt University of Greifswald.
ORCID iD
Dirk Habedank https://orcid.org/0000-0003-3403-7146
Supplemental material
Supplemental material for this article is available online.
References
- 1.Pratter MR, Curley FJ, Dubois J, et al. Cause and evaluation of chronic dyspnea in a pulmonary disease clinic. Arch Intern Med 1989; 149: 2277–2282. [PubMed] [Google Scholar]
- 2.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 3.Guazzi M, Naeije R. Pulmonary hypertension in heart failure: pathophysiology, pathobiology, and emerging clinical perspectives. J Am Coll Cardiol 2017; 69: 1718–1734. [DOI] [PubMed] [Google Scholar]
- 4.Opitz CF, Hoeper MM, Gibbs JS, et al. Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum. J Am Coll Cardiol 2016; 68: 368–378. [DOI] [PubMed] [Google Scholar]
- 5.Hoeper MM, Apitz C, Grünig E, et al. Targeted therapy of pulmonary arterial hypertension: updated recommendations from the Cologne Consensus Conference 2018. Int J Cardiol 2018; 272S: 37–45. [DOI] [PubMed] [Google Scholar]
- 6.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 7.Rosenkranz S, Gibbs JS, Wachter R, et al. Left ventricular heart failure and pulmonary hypertension. Eur Heart J 2016; 37: 942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hager WD, Collins I, Tate JP, et al. Exercise during cardiac catheterization distinguishes between pulmonary and left ventricular causes of dyspnea in systemic sclerosis patients. Clin Respir J 2013; 7: 227–236. [DOI] [PubMed] [Google Scholar]
- 9.Oldham WM, Lewis GD, Opotowsky AR, et al. Unexplained exertional dyspnea caused by low ventricular filling pressures: results from clinical invasive cardiopulmonary exercise testing. Pulm Circ 2016; 6: 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maron BA, Cockrill BA, Waxman AB, et al. The invasive cardiopulmonary exercise test. Circulation 2013; 127: 1157–1164. [DOI] [PubMed] [Google Scholar]
- 11.Naeije R, Vanderpool R, Dhakal BP, et al. Exercise-induced pulmonary hypertension: physiological basis and methodological concerns. Am J Respir Crit Care Med 2013; 187: 576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovacs G, Berghold A, Scheidl S, et al. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J 2009; 34: 888–894. [DOI] [PubMed] [Google Scholar]
- 13.Borlaug BA. Invasive assessment of pulmonary hypertension: time for a more fluid approach? Circ Heart Fail 2014; 7: 2–4. [DOI] [PubMed] [Google Scholar]
- 14.Naeije R, Gerges M, Vachiery JL, et al. Hemodynamic phenotyping of pulmonary hypertension in left heart failure. Circ Heart Fail 2017; 10: pii: e004082. [DOI] [PubMed] [Google Scholar]
- 15.Vachiéry J-L, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J 2019; 53: 1801897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen MJ, Olson TP, Melenovsky V, et al. Differential hemodynamic effects of exercise and volume expansion in people with and without heart failure. Circ Heart Fail 2015; 8: 41–48. [DOI] [PubMed] [Google Scholar]
- 17.Jones NL, Makrides L, Hitchcock C, et al. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis 1985; 131: 700–708. [DOI] [PubMed] [Google Scholar]
- 18.Rosenkranz S, Behr J, Ewert R, et al. Right heart catheterization in pulmonary hypertension. Dtsch Med Wochenschr 2011; 136: 2601–2616; quiz 2617–2620. [DOI] [PubMed] [Google Scholar]
- 19.Paulus WJ, Tschope C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007; 28: 2539–2550. [DOI] [PubMed] [Google Scholar]
- 20.Huis In’t Veld AE, De Man FS, Van Rossum AC, et al. How to diagnose heart failure with preserved ejection fraction: the value of invasive stress testing. Neth Heart J 2016; 24: 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenkranz S, Lang IM, Blindt R, et al. Pulmonary hypertension associated with left heart disease: updated recommendations of the Cologne Consensus Conference 2018. Int J Cardiol 2018; 272S: 53–62. [DOI] [PubMed] [Google Scholar]
- 22.Fox BD, Shimony A, Langleben D, et al. High prevalence of occult left heart disease in scleroderma-pulmonary hypertension. Eur Respir J 2013; 42: 1083–1091. [DOI] [PubMed] [Google Scholar]
- 23.Robbins IM, Hemnes AR, Pugh ME, et al. High prevalence of occult pulmonary venous hypertension revealed by fluid challenge in pulmonary hypertension. Circ Heart Fail 2014; 7: 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Alto M, Romeo E, Argiento P, et al. Clinical relevance of fluid challenge in patients evaluated for pulmonary hypertension. Chest 2017; 151: 119–126. [DOI] [PubMed] [Google Scholar]
- 25.D’Alto M, Romeo E, Argiento P, et al. Hemodynamic changes after acute fluid loading in patients with systemic sclerosis without pulmonary hypertension. Pulm Circ 2019; 9: 2045894018816089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keusch S, Bucher A, Muller-Mottet S, et al. Experience with exercise right heart catheterization in the diagnosis of pulmonary hypertension: a retrospective study. Multidiscip Respir Med 2014; 9: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maor E, Grossman Y, Balmor RG, et al. Exercise haemodynamics may unmask the diagnosis of diastolic dysfunction among patients with pulmonary hypertension. Eur J Heart Fail 2015; 17: 151–158. [DOI] [PubMed] [Google Scholar]
- 28.Lewis GD, Bossone E, Naeije R, et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 2013; 128: 1470–1479. [DOI] [PubMed] [Google Scholar]
- 29.Fujimoto N, Borlaug BA, Lewis GD, et al. Hemodynamic responses to rapid saline loading: the impact of age, sex, and heart failure. Circulation 2013; 127: 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agrawal V, D’Alto M, Naeije R, et al. Echocardiographic detection of occult diastolic dysfunction in pulmonary hypertension after fluid challenge. J Am Heart Assoc 2019; 8: e012504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moghaddam N, Swiston JR, Levy RD, et al. Clinical and hemodynamic factors in predicting response to fluid challenge during right heart catheterization. Pulm Circ 2019; 9: 2045894018819803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esfandiari S, Wolsk E, Granton D, et al. Pulmonary arterial wedge pressure at rest and during exercise in healthy adults: a systematic review and meta-analysis. J Card Fail 2019; 25: 114–122. [DOI] [PubMed] [Google Scholar]
- 33.Wolsk E, Kaye D, Komtebedde J, et al. Central and peripheral determinants of exercise capacity in heart failure patients with preserved ejection fraction. JACC Heart Fail 2019; 7: 321–332. [DOI] [PubMed] [Google Scholar]
- 34.Hemnes AR, Opotowsky AR, Assad TR, et al. Features associated with discordance between pulmonary arterial wedge pressure and left ventricular end diastolic pressure in clinical practice: implications for pulmonary hypertension classification. Chest 2018; 154: 1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliveira RK, Ferreira EV, Ramos RP, et al. Usefulness of pulmonary capillary wedge pressure as a correlate of left ventricular filling pressures in pulmonary arterial hypertension. J Heart Lung Transplant 2014; 33: 157–162. [DOI] [PubMed] [Google Scholar]
- 36.Tonelli AR, Mubarak KK, Li N, et al. Effect of balloon inflation volume on pulmonary artery occlusion pressure in patients with and without pulmonary hypertension. Chest 2011; 139: 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LeVarge BL, Pomerantsev E, Channick RN. Reliance on end-expiratory wedge pressure leads to misclassification of pulmonary hypertension. Eur Respir J 2014; 44: 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryan JJ, Rich JD, Thiruvoipati T, et al. Current practice for determining pulmonary capillary wedge pressure predisposes to serious errors in the classification of patients with pulmonary hypertension. Am Heart J 2012; 163: 589–594. [DOI] [PubMed] [Google Scholar]
- 39.Bossone E, Naeije R. Exercise-induced pulmonary hypertension. Heart Fail Clin 2012; 8: 485–495. [DOI] [PubMed] [Google Scholar]
- 40.Bitar A, Selej M, Bolad I, et al. Poor agreement between pulmonary capillary wedge pressure and left ventricular end-diastolic pressure in a veteran population. PLoS One 2014; 9: e87304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pul-10.1177_2045894020917887 for Exercise and fluid challenge during right heart catheterisation for evaluation of dyspnoea by Ralf Ewert, Alexander Heine, Annegret Müller-Heinrich, Tom Bollmann, Anne Obst, Susanna Desole, Christine Knaak, Beate Stubbe, Christian F. Opitz and Dirk Habedank in Pulmonary Circulation