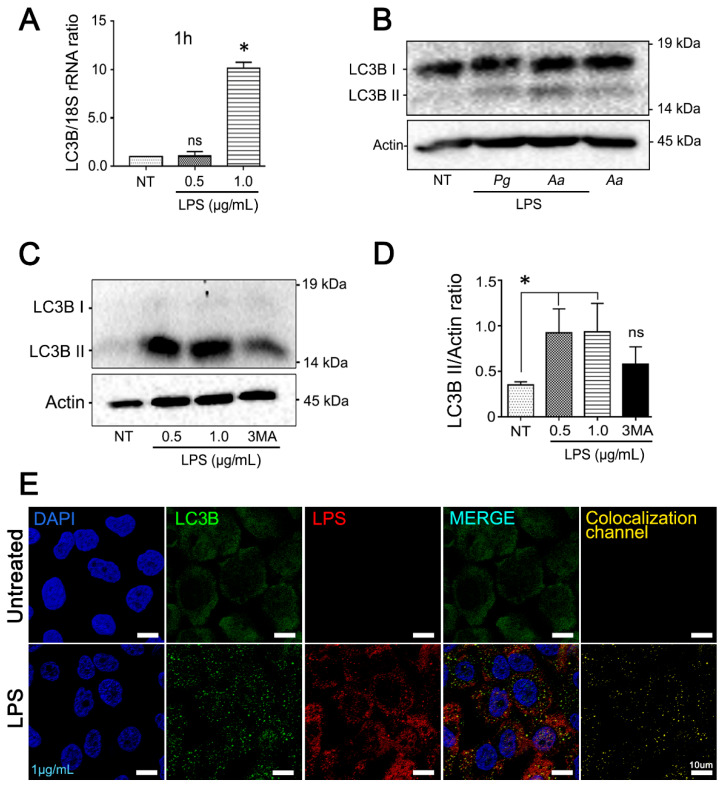
Figure 5.
Increased expression of autophagy markers in JEKs stimulated with purified LPS from A. actinomycetemcomitans. OKF6/TERT-2 cells were incubated with 0.5 or 1 µg/mL of purified LPS from A.a. (A) Quantitative real-time PCR of LC3B transcripts abundance normalized to 18S rRNA levels after 1 h of LPS stimulation. (B) Immunoblotting of protein extracts from OKF6/TERT-2 cells stimulated for 3 h with LPS or whole A.a bacteria incubated with antibodies specific for the autophagy-related protein LC3B. LPS purified from Porphyromonas gingivalis (Pg) was used as positive control. β-actin protein was employed as a loading control. The results are shown as the average of the actin normalized data from three independent experiments. (C) Conversion of LC3B-I to LC3B-II in LPS-stimulated cells. JEKs were pretreated with 3-MA (3 mM) or mock-treated for 3 h before stimulation with LPS. (D) Densitometric quantitation of actin-normalized protein bands is indicated on the right. 3-MA used as control. (E) Confocal image of JEKs stimulated with 1 µg/mL of LPS. Blue: DNA rich structures stained with DAPI; green: LC3B protein labeled with anti-LC3B antibody; red: LPS purified from A.a labeled with anti-LPS antibody; merge: digital overlap of blue, green and red channels; yellow: colocalized voxels from merged file. Note the puncta profile of LC3B-labeling in LPS-stimulated cells. Scale bar 10 µm. * p < 0.05.