Abstract
The extracellular matrix (ECM) is a macromolecules network, in which the most abundant molecule is collagen. This protein in triple helical conformation is highly resistant to proteinases degradation, the only enzymes capable of degrading the collagen are matrix metalloproteinases (MMPs). This resistance and maintenance of collagen, and consequently of ECM, is involved in several biological processes and it must be strictly regulated by endogenous inhibitors (TIMPs). The deregulation of MMPs activity leads to development of numerous diseases. This review shows MMPs complexity.
Keywords: matrix metalloproteinases, TIMP, collagen
1. Extracellular Matrix—Collagen
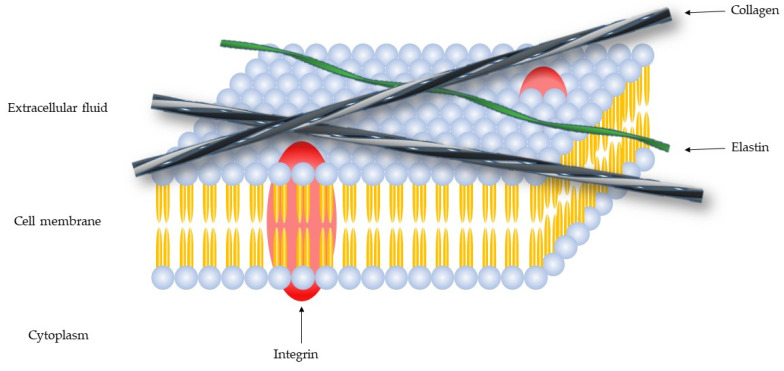
The extracellular matrix (ECM) is a macromolecules network, composed of collagen, enzymes and proteins (Figure 1), that promote a structural and biochemical support.
Figure 1.
Schematic representation of the cell membrane.
The ECM has many components [1]: Fibers (collagen, elastin, laminin, and fibronectin), proteoglycans (syndecan-1 and aggrecan), glycoproteins (tenascin, vitronectin and entactin) and polysaccharides (hyaluronic acid) [2], that regulate cell migration, growth, and differentiation [3].
The collagen is the most abundant protein in ECM which gives structural support for cells [1,3]. Depending of mineralization degree, the tissues can be divided in rigid (bones) or compliant (sinews) or have a gradient between these two states (cartilage) [4]. Collagen can come in two main forms: fibrillar (type I, II, III, V, and XI) and non-fibrillar, the latter includes facit-fibril associated collagens with interrupted triple helix (type IX, XII, XIV, XIX, and XXI); short chain (type VIII and X); basement membrane (type IV); multiplexin (type XV and XVIII); MACIT- membrane associated collagens with interrupted triple-helix (type XIII and XVII) and others types (Type VI, VII, and VIII). The collagen protein consists of three α chains, in triple helix, where two chains are chemically similar (α1 and α2), with approximate dimensions of 300 × 1.5 nm [3,5]. The triple helix is divided into five d-segments with D1–D4 having a length of 67 nm and D5 equal to 0.46 nm [3,5]. The high glycine content is important for collagen helix stabilization as it allows collagen fibers to combine, facilitating hydrogen bridges and cross-link formation [5].
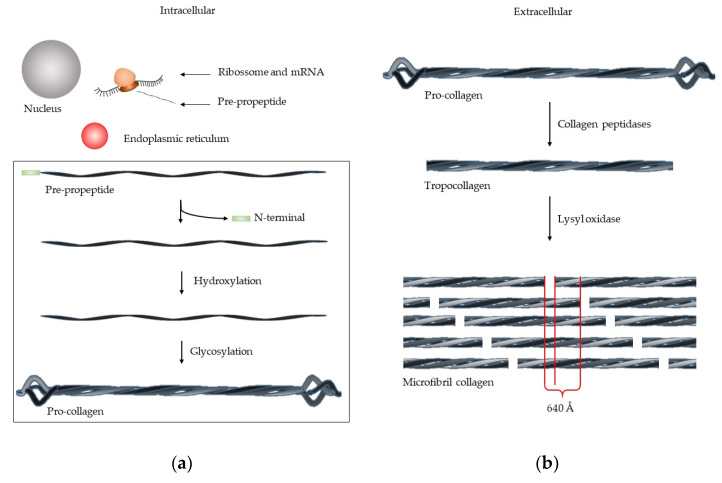
The collagen synthesis involves several steps [5] (Figure 2). The mRNA is transcripted by ribosome, forming pre-propeptide. The following three stages occur in the endoplasmic reticulum: (1) The N-terminal signal sequence is removed; (2) hydroxylation of lysine and proline by prolyl hydroxylase and lysyl hydroxylases takes place, together with (3) glycosylation of lysine, forming the pro-collagen. This structure is a triple helix chain, but with the unwound terminals. The removal of these terminals occurs in extracellular medium, by collagen peptidases, forming tropocollagen. The microfibril collagen is formed by lysyl oxidase that packs together five tropocollagen chain [3], which have a characteristic image. At intervals of 67 nm, one zone has the roll of all tropocollagen and another zone has one less—“gap”. The fibril collagen is composed of various microfibril collagen group and the alternating overlap and gap regions create the characteristic “bright and dark” d-banding pattern [3].
Figure 2.
Synthesis of microfibril collagen. (a) In intracellular medium, the mRNA is transcripted by ribosome, forming the pre-peptide, which is then processed in endoplasmic reticulum, forming pro-collagen. (b) In extracellular medium, the pro-collagen is processed by collagen peptidase, forming tropocollagen. For microfibril collagen formation, the tropocollagen is processed by lysil oxidase.
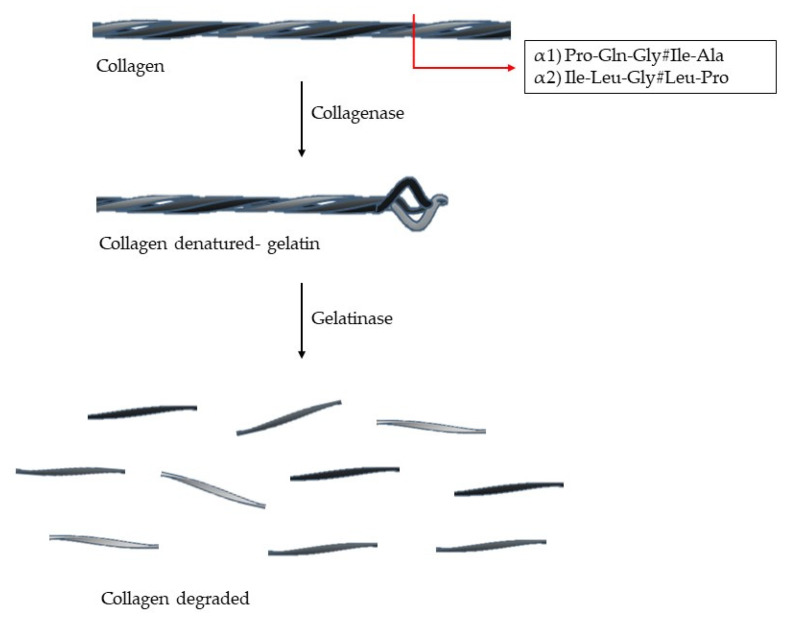
Its triple helix conformation makes collagen resistant to many proteases. The enzymes able to cleave this structure are capthesin K and enzymes with collagenolytic activity (MMPs-1, -2, -8, -13, -14, and -18) [3,4]. The collagen type I, II and III have a specific cleavage sequence: (Gln/Leu)-Gly#(Ile/Leu)-(Ala/Pro), which is located at 3/4 of N-terminal and this is crucial for collagen degradation [6,7] (Figure 3). The denatured collagen is called gelatin, which can be further degraded by gelatinases [4,6]. The collagen degradation is involved in many biological processes, such as embryogenesis, morphogenesis, tissue remodulation, angiogenesis, and wound healing [4].
Figure 3.
Collagen degradation. The enzyme with collagenolytic activity (collagenases) cleaves the triple helix at two fragments: 3/4 N-terminal and 1/4 C-terminal. Each chain (α1 and α2) has a specific cleavage sequence (# represents the cleavage site).
The ECM degradation is also an important process in development, morphogenesis, tissue repair and remodulation [8], and can affect the cellular behavior and phenotype [6,9]. The excessive degradation of ECM leads to metabolic and immune diseases, cancer and cardiovascular disorders (hypertension, atherosclerosis and aneurysm) [2,3,6].
2. Metzincs Superfamily—Matrix Metalloproteinases (MMPs)
The metzincs are a family of multidomain zinc-dependent endopeptidases, where the metalloproteinases such as matrix metalloproteinases (MMPs) [10], the desintegrins and metalloproteins (ADAMS), the ADAMs with thrombospondin motif (ADAMTS), were snapalysins, leishmanolysins, pappalysins belong [1,6,8,11,12,13,14]. All of them have a common conserved domain: HExxHxxGxxH [6,11,12,13,15,16,17,18,19] and, in case for MMPs, this sequence is HEBGHxLGLxHSBMxP [13].
The MMPs were first described in 1949 [20], as depolymerizing enzymes that facilitate tumor growth, by making connective tissue stroma, including small blood vessels that are more fluid [16,20,21]. In 1962, a MMP collagenase was isolated and characterized as an enzyme responsible for tadpole tail resorption [1,2,6,13,14,16,21,22], by Gross and Lapiere [22]. Over the next 20 years, several enzymes were purified, but it was in 1985 that the area has developed more significantly [16,21]. Taken together it has been demonstrated that MMPs are present in virus, archeabacteria, bacteria, plants, nematodes, and animals [6,13].
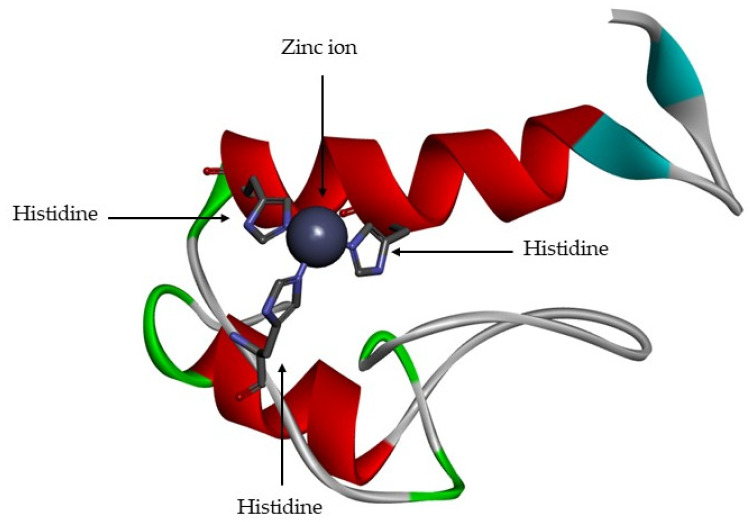
The MMPs are a family of proteolytic enzyme that have different substrates, but which share similar structural characteristics [1,2,11,13,23]. The active site is zinc-dependent [1,2,6,11,13,15,16,17,18,24,25] and is highly conserved, with three histidine residues bound to catalytic zinc [6,11,12,15] (Figure 4). They are functional at neutral pH [9,18].
Figure 4.
Active site of matrix metalloproteinase (MMP)-1. The zinc catalytic is represented by grey ball and the three histidine residues are represented by sticks.
MMPs have been recently recognized as biomarkers in several fields (diagnosis, monitoring, and treatment efficacy) [19], since their overexpression in diseases conditions is specific and elevated [19]. Huang et al. [26] related that MMP-9 represents a potential biomarker which is overexpressed in several types of tumors (colarectal carcinoma, breast, pancreatic, ovaria, cervical, osteosarcoma non-small cell lung cancer (NSCLC), and giant cell tumor of bone (GCTB)), which makes MMP-9 a preferential candidate for the early detection of these diseases [26]. The elevated levels of MMP-9 can be detected in plasma or blood, show triple protein levels compared to healthy patients [19,27,28]. The MMP-9 in contrast with other proposed biomarkers (MMP-1 and -3), also presents increased levels in specific risk group to atherosclerosis [19], as demonstrated by Rohde et al. [29]. Nilsson et al. [30] studied the MMPs-1, -3, -7, -10, and -12 in plasma and demonstrated that MMP-7 and -12 were elevated in type 2 diabetes, which is related to atherosclerosis and coronary events [30].
3. MMPs Functions
The principal biologic function of MMPs is degradation of ECM proteins and glycoproteins [1,2,6,8,11,12,14,16,18,19,24,31], membrane receptors [13,14,24,31], cytokines [11,13,14,31], and growth factors [11,13,14,24,31]. The MMPs are involved in many biologic processes, such as, tissue repair and remodulation [1,2,9,13,14,16,18,24], cellular differentiation [1,2,18], embryogenesis [2,6,9,13,14], morphogenesis [2,24], cell mobility [9,18], angiogenesis [1,2,6,9,14,18,24], cell proliferation [1,2], and migration [1,2], wound healing [1,2,6,9,13,14,15,18], apoptosis [1,2,18], and main reproductive events such as ovulation [13,14] and endometrial proliferation [18].
The deregulation of MMP activity leads to the progression of various pathologies, that can be grouped into [1,6,14,18,19,24,25]: (1) Tissue destruction, (2) fibrosis, and (3) matrix weakening. The overexpression of MMPs is involves in several diseases (Table 1).
Table 1.
Examples of diseases caused by deregulation of MMPs.
| Pathologies | Diseases |
|---|---|
| Tissue destruction | Cancer invasion and metastasis |
| Arthritis | |
| Ulcers | |
| Periodontal diseases | |
| Brain degenerative diseases | |
| Fibroses | Liver cirrhosis |
| Fibrotic lung disease | |
| Otosclerosis | |
| Atherosclerosis | |
| Multiple sclerosis | |
| Weakening of matrix | Dilated cardiomyopathy |
| Aortic aneurysm | |
| Varicose veins |
The MMPs are involved in the development and progression of atherosclerosis, which is correlated with cardiovascular diseases [19]. Their activity promotes the loss of collagen, elastin and other ECM proteins, inducing the necrotic core of atherosclerotic plaque that leads to myocardial infarction or a stroke [19].
Some studies show intracellular localization of MMP-2 in cardiac myocytes and colocalization of MMP-2 with troponin I in cardiac myofilaments [32]. The MMP-2 activity has also been detected in nuclear extracts from human heart and rat liver [32]. Poly ADP-ribose polymerase is a nuclear matrix enzyme involved in DNA repair and is susceptible to cleavage by MMP-2, in vitro its cleavage being blocked by MMPs inhibitors [32]. The presence of MMP-2 in nucleus could play a role in poly ADP-ribose polymerase degradation and affect DNA repair [32].
4. Types of MMPs
In vertebrates, there are 28 different types of MMPs [1,2,8,9,11,12,13,16,17], at least 23 are expressed in human tissue [1]. MMPs can be subdivided according to bioinformatic analysis, in 5 types [23]:
Non-furin regulated MMPs (MMP-1, -3, -7, -8, -10, -12, -13, -20, and -27);
MMPs bearing three fibronectin-like inserts in the catalytic domain (MMP-2 and -9);
MMPs anchored to the cellular membrane by a C-terminal glycosylphosphatidylinositol (GPI) moiety (MMP-11, -17 and -25);
MMPs bearing a transmembrane domain (MMP-14, -15, -16, and -24) and
All the other MMPs (MMP-19, -21, -23, -26 and -28).
But, MMPs can be also subdivided according to substrate specificity, sequential similarity and domain organization (Appendix A) into: Collagenases, gelatinases, stromelysins, metrilysins, membrane-type MMPs, and other MMPs [1,2,8,9,12,13,14,16,17,18,19,25].
Collagenases (Appendix A, Table A1) cleave some ECM proteins and other soluble proteins, but the most important role of this type of MMPs is the cleavage of fibrillar collagen type I, II, III, IV and XI into two characteristic fragments, 1/4 C-terminal and 3/4 N -terminal [1,6,8,9,12,14,17,21]. This process takes place in two steps: first MMP unwind triple helical collagen and then hydrolyze the peptide bonds [1]. The hemopexin domain is essential for cleaving native fibrillar collagen while the catalytic domain can cleave non-collagen substrates [1].
Gelatinases (Appendix A, Table A2) play an important role in many physiological processes, such as ECM degradation and remodeling, osteogenesis and wound healing [9]. Gelatinases degrade gelatin [12,17], collagen type IV [8,17,21], V [8,17], VIII, X, XI [8,17], and XIV, elastin [21], proteoglycan core proteins [8,17], fibronectin [21], laminin [17,21], fibrilin-1, and TNF-α and IL-1b precursor [9], due to the existence of three repeated fibronectin type II domain [1,8,12,17,18], which binds gelatin [1], collagen, and laminin [1]. MMP-2 is primarily a gelatinases, but can acts like collagenase, albeit in a weaker manner [1]. MMP-2 degrades collagen in two steps: first by inducing a weak interstitial collagenase-like collagen degradation and then by promoting gelatinolysis using the fibronectin-like domain [1]. MMP-9 can act as collagenases and gelatinase [1].
Gelatinases are involved in physiological and pathological states, such as, embryonic growth and development, angiogenesis, vascular diseases, inflammatory, infective diseases, degenerative diseases of the brain and tumor progression [1]. Tumor metastasis is a process that involves the release of tumor cells, their migration through blood vessels, penetration into the blood and lymphatic system and their adhesion into the endothelial vessel and extravasation into tissue [11]. The activity of gelatinases is crucial for metastatic cell output and metastasis site entry [11]. Increased expression and activity of gelatinases have been described in malignant diseases such as breast, urogenital, brain, lung, skin and colorectal cancer [11].
Stromelysines (Appendix A, Table A3) have the same domain arrangement as collagenases, but do not cleave interstitial collagen [1]. MMP-3 and -10 are closely related by their structure and substrate specificity [1,8,9,17], while MMP-11 is distantly related [1]. The intracellular activation of MMP-1 is regulated by 10 amino acids insert, localized between the pro- and catalytic domains (RXRXKR), which is recognition by Golgi-associated proteinase furin.
The main characteristic of the matrilysins (Appendix A, Table A4) is the lack of hemopexin domain, present in the other MMPs [9,12,17,18]. This MMP group has a specific feature in the amino acid sequence with a threonine residue adjacent to the Zn2+- binding site [1].
Membrane-type metalloproteinases (MT-MMP; Appendix A, Table A5) contain a furin-like pro-protein convertase recognition site (RX[R/K]R) in their pro-domain C-terminal [1,8,17,18], allowing pro-enzyme activation by proteolytic removal of this domain. They are activated intracellularly and the active enzymes are expressed on the cell surface [1]. This group can be subdivided into: type I transmembrane proteins (MMPs-14, -15, -16, and -24) [1,8,9,17,18] and glycosylphosphatidylinositol (GPI) anchored proteins (MMPs-17 and -25) [1,8,9,17,18]. The type I transmembrane protein have about 20 amino acids long cytoplasmic tail following the transmembrane domain [1]. MT-MMPs have the insert of eight amino acids in the catalytic domain, which in case of MMP-14 consists of PYAYIREG and this sequence can influence on conformation of the active site cleft [1].
5. Structure
Lovejoy et al. reported the first structure of MMP-inhibitor complex [33]. This structure reveals that the active site of MMP is a deep cavity and moreover that the catalytic domains of MMPs share a sequential similarity, where the percentage of similarity ranges between 33% (between MMP-21 and MMP23) and 86% (between MMP-3 and MMP-10) [33]. 3D structures of the catalytic domains of MMP-1 and -8 as well as structures of pro-MMP-3 and MMP-1 followed [33].
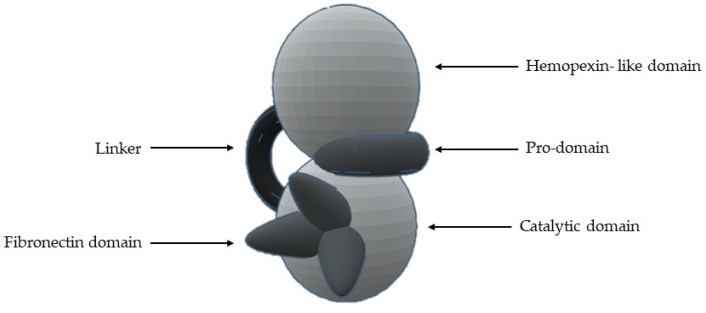
The most common structural features are (Figure 5 and Table 2) [1,2,8,9,13,14,15,16,17,18,19,23]:
-
1-
A signal N-terminal peptide with variable length, that targets the peptide for secretion;
-
2-
A pro-domain (with about 80 aa), which keeps MMP inactive and is removed when the enzyme is proteolytically activated;
-
3-
A catalytic domain (with about 160 aa), with a zinc ion, that consists of five β-sheets, three α-helixes and three calcium ions;
-
4-
A linker of variable length (14–69 aa), which links the catalytic domain to hemopexin-like domain—“hinge region”;
-
5-
A hemopexin-like domain (with about 210 aa) that is characterized by four β-propeller and
-
6-
An additional transmembrane domain with the small cytoplasmatic C-terminal domain, only present in MMPs-14, -15, -16 and -24.
Figure 5.
Schematic representation of the general structure of MMP.
Table 2.
Domain and presence in MMPs.
| Domain | Presence |
|---|---|
| Signal Peptide | All MMPs |
| Pro-domain | All MMPs |
| Catalytic | All MMPs |
| Hemopexin-like | All MMPs, except in MMP-7, -23, and -26 |
| Fibronectin | Only MMP-2 and -9 |
| Vitronectin insert | Only MMP-21 |
| Type I transmembrane | Only MMP-14, -15, -16, and -24 |
| Cytoplasmic | Only MMP-14, -15, -16, and -24 |
| GPI anchor | Only MMP-17 and -25 |
| Cysteine Array Region | Only MMP-23 |
| IgG-like domain | Only MMP-23 |
All MMPs are synthesized with an N-terminal signal sequence, which is removed in the endoplasmic reticulum, producing pro-enzymes [13].
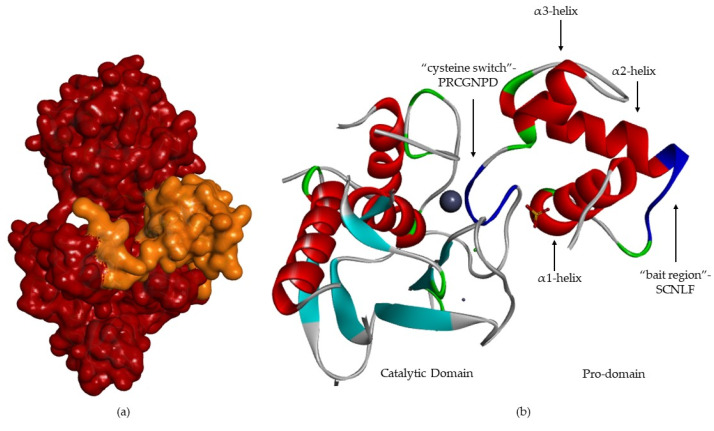
The pro-domain (Figure 6) contains three α-helixes [8,12,13,17,18]. In the case of MMP-1 and -2 [13], the first loop has a cleavage region—“bait-region” [8,17]—which is protease sensitive [12,13,18] (EKRRN for pro-MMP-1 and SCN*LF for pro-MMP-2) [13]. The α(3)-helixes are followed by a “cysteine switch” [2,6,12,18], a very conserved region (PRCGXPD) [1,2,17,18,19,21], where the sulfhydryl group (Cys residue) coordinates the catalytic zinc [1,2,6,8,17,18,21], forming a tetrahedral coordination sphere, thereby blocking enzyme [2,15,17,18,21].
Figure 6.
MMP-2 with pro-domain. (a) Surface of MMP-2, where the pro-domain is represented in orange. (b) Three-dimensional structure of MMP-2, where “bait-region” and “cysteine switch” are represented in blue.
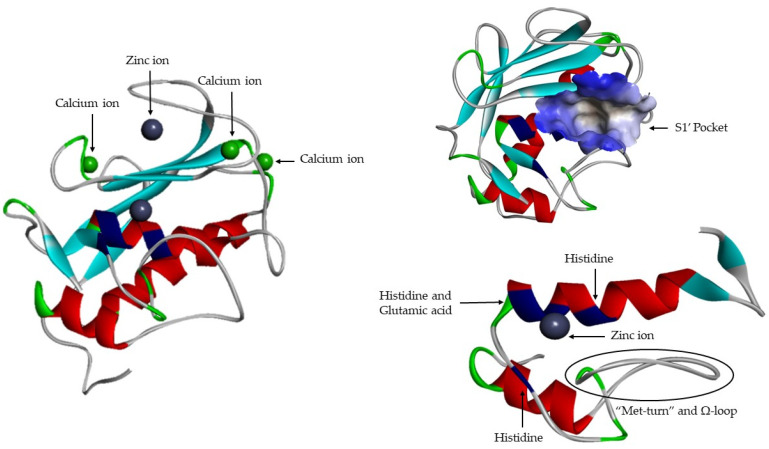
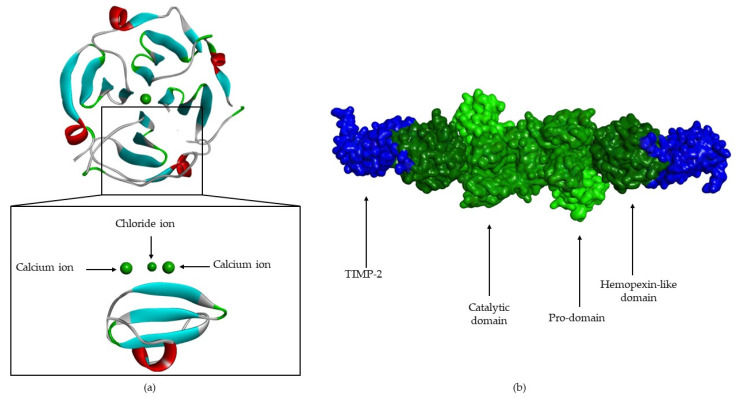
Active site (Figure 7) consists of two regions: a cavity on the surface of the protein that houses the zinc ion in the center and a specific site S1′ [16]. The catalytic domains of the known MMP share the same structural organization: Three α-helixes, five β-sheets (four parallel: β2–β1–β3–β5 and one anti-parallel: β4), connected by eight loops [8,9,12,13,16,17,19,21]. They are sphere shaped, with a diameter of ~40 Å [14]. Catalytic domain is highly conserved and in addition to catalytic zinc ions contain another Zn2+, that has a structural function [1,6,17,19,21], three calcium ions [6,8,9,12,15,17,19,23] and three histidine residue [1,18,19,21] that are part of a highly conserved sequence: VAAHEXGHXXGXXH [1,19,21]. The Ω-loop is found between α2-helix and α3-helix, its length and amino acid composition vary significantly among MMPs [19]. This loop is the least conserved fragment within the catalytic domain, which is most likely responsible for different selectivity of MMPs [19]. In the terminal zone of the catalytic domain, located 8 residues down from the Zn2+ [1], there is a region that forms the outer wall of pocket S1′ [1,19]—“met-turn” (XBMX) [1,13]. The catalytic zinc ion and the “met-turn” are conserved and also are present in ADAM and ADAMTS families, astacins and bacterial serralysins [8]. The second zinc ion and the three calcium ions, that are present is all MMPs, have only a structural function and they been crucial for maintaining protein conformation [23]. Glutamic acid adjacent to the first histidine residue is essential for the catalytic process [1,12,16,21].
Figure 7.
MMP-1 catalytic domain.
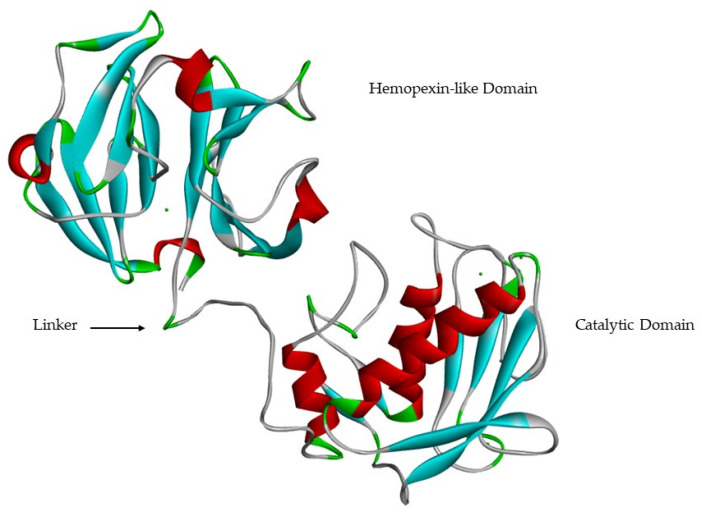
Catalytic and hemopexin-like domains are linked by a proline-rich linker [9,13,17] of variable length [18] that allows inter-domain flexibility [17], enzyme stability [13] and is involved in the hydrolysis of some structurally complex substrates [9,13,23] (Figure 8). Mutations in the linker region in MMP-1 [34] and MMP-8 [35] reduce collagenolytic activity, supporting the hypothesis that correct movement and rearrangement between the catalytic domain and the hemopexin-like domain is important for activity [17].
Figure 8.
MMP-1 catalytic domain, hemopexin-like domain and linker.
The hemopexin-like domain has four β-propeller structural elements [8,12,13,17]. This domain is necessary for collagen triple helix degradation [8,11,17] and is important for substrate specificity [11,12,13,21] (Figure 9). It also may be essential for the recognition and subsequent catalytic degradation of fibrillar collagen, whereas the catalytic domain is sufficient for the degradation of non-collagen substrates [1]. The only MMPs that don’t have the hemopexin-like domain are MMP-7, -23, and -26 [8,15,17]. In the case MMP-23, this domain is substituted by an immunoglobulin-like domain and a cysteine-rich domain [8,15,17], located immediately after the C-terminal of the catalytic domain [17].
Figure 9.
(a) MMP-1 hemopexin-like domain, composed to 4 β-propeller (4 β- sheets and 1 α-helix). (b) Surface of pro-MMP-2-TIMP-2 complex (pro-domain, catalytic domain and hemopexin-like domain are represented in green; TIMP-2 is represented in blue).
S1′ Pocket Selectivity
MMPs possess six pockets (S1, S2, S3, S1′, S2′, and S3′) [19] and the fragments of the substances and inhibitors are consequently named after the pocket that they interact with (P1, P2, P3, P1′, P2′, and P3′) [2,6]. The S1, S2, and S3 pockets are unprimed pockets, which are localized on the right side of zinc ion [19]. The S1′, S2′, and S3′ pockets are the primed pockets, which are localized on the left side of zinc ion [2,19]. It has been demonstrated that the S1′ is the most variable pocket in MMPs [1,6,8,15,16], followed by S2, S3′, S1, and S3 pockets with an equivalent degree of variance, while the S2′ has the lowest variability. The S2′ and S3′ pockets are shallower than S1′ and are more exposed to solvent [1]. The S3 pocket may also contribute to substrate specificity [1].
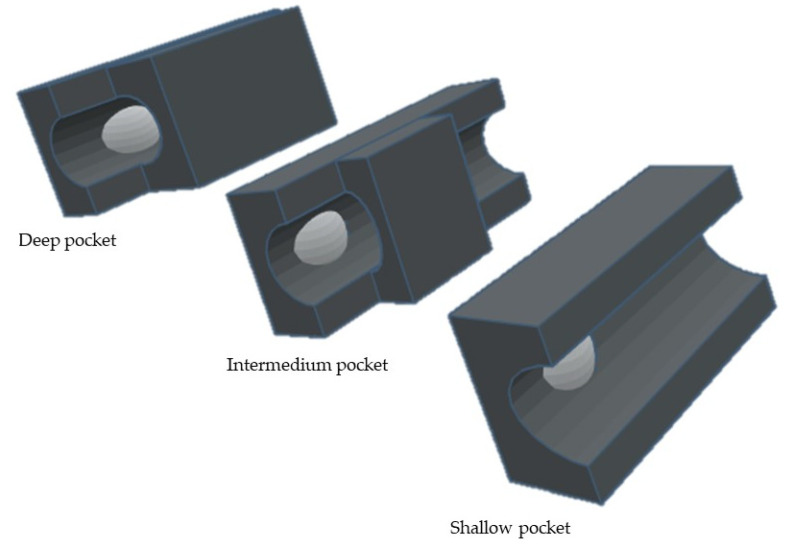
S1′ pocket is the most important since it is a determining factor for substrate specificity [1,2,8,16], Its cavity [1,19], formed by the Ω-loop [19] is a highly hydrophobic. By analyzing the depth of the different S1′ subsites, MMPs can be divided into three different subgroups [1,2,17,19,36] (Figure 10): the shallow, the intermedium and the deep pockets. This differentiation is related to the size of amino acids: MMP-1 and -7 have an Arg214 and Tyr214 residue, respectively [13,36], instead of a leucine present in MMP-2, -3, -8, -9, -10, -12, -13, and -14 [16,36], giving origin to shallow S1′ pocket [15].
Figure 10.
Schematic representation of different subgroups of S1′ pocket.
6. MMP Activity Regulation
MMP expression is closely controlled for transcription, secretion, activation, and inhibition of the activated enzyme [1,2,9,14,18]. Some MMPs are not constitutively expressed by cells, in vivo, instead their expression is induced by exogenous signals such as cytokines [1,14,16], growth factors [1,14,16], hormones [1,16], and changes in cell–matrix and cell–cell interactions [8,9,15].
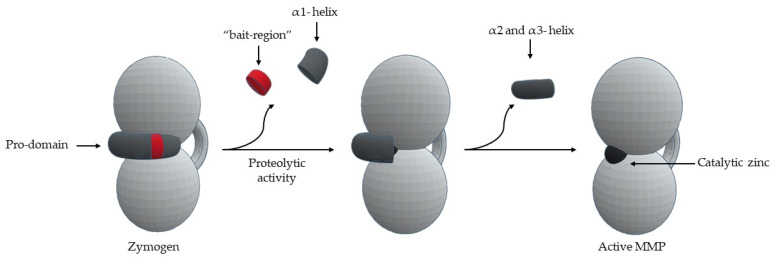
MMPs are produced by various tissues and cells [1]. MMPs are secreted by connective tissue, pro-inflammatory and uteroplacental cells, such as fibroblast, endothelial cells, osteoblasts, vascular smooth muscle, macrophages, lymphocytes, cytotrophoblasts, and neutrophils [1]. MMPs are synthesized as pre-proMMPs, from which the signal peptide is removed during translation to generate proMMPs or zymogens [1]. MMPs are secreted in the form of latent precursors- “zymogens”, which are proteolytically activated in extracellular medium [1,2,6,13,14,16,17,18,19,24]. The important activation mechanism is the proteolytic removal of the pro-domain by endopeptidases, such as, other MMPs, serine proteases, plasmin or furin [1,11,12,13,14,15,16,21]. The first proteolytic attack occurs in the “bait region” [8,19,24] and the specificity of cleavage is dictated by a sequence found in each MMP [9,12,18]. This leads to the removal of part of the pro-domain, destabilizing the rest of the pro-domain [13], including cysteine switch-zinc interaction [17,24], which allows intramolecular processing [8,9,12,18]—“stepwise activation” [8,18] (Figure 11). One question that remains unanswered is how a simple bait-region cleavage can result in destabilization of the pro-domain and cause conformational changes in the zone between the pro-domain and the catalytic domain [13].
Figure 11.
Schematic representation of MMP activation.
Pro-MMP-2 forms a complex with TIMP-2 through interactions of the hemopexin-like domain with the non-inhibitory C-terminal domain of TIMP-2 [1,2,8,17,18,25]. Formation of this complex is essential for pro-MMP-2 activation by MT1-MMP [8,17,18]. The complex then reaches the cell surface where it binds to the active site of MT1-MMP [1], via the free inhibitory N-terminal of TIMP-2 [2], orienting the pro-MMP-2 pro-domain adjacent to MT1-MMP [8,17,18]. With the aid of a second MT1-MMP [2], interactions between the MT1-MMP occur through their hemopexin-like domains, forming a quaternary tetrameric complex [1,17]: an MT1-MMP acts as a receptor for the pro-MMP-2-TIMP-2 complex and the other as a pro-MMP-2 activator [2,8,18]. Excess of TIMP-2 prevents this activation process by inhibiting the second MT1-MMP [2,8].
The MMP activation can also occur by physiochemical agents, such as heat, low pH, chaotropic agents and thiol-modifying agents (4-aminophenylmercurin acetate, mercury chloride and N-ethylmaleimide) [1], which cause disruption of the cysteine-Zn2+ coordination [1].
Pro-enzymes and active forms of MMP are controlled by the specific stereochemical binding of metalloproteinase-specific inhibitors (TIMPs: -1, -2, -3, and -4) [1,2,6,9,13,14,16,18,21,25] and by non-specific proteinase inhibitors such as the α1-proteinase inhibitor and α2-macroglobulin [8,9,13,14,16,17,18,21,25].
7. Catalytic Mechanism
Over the past 30 years, the detailed structural characterization of different MMPs allowed disentangling of the individual catalytic steps that occur at the active site during proteolysis [23].
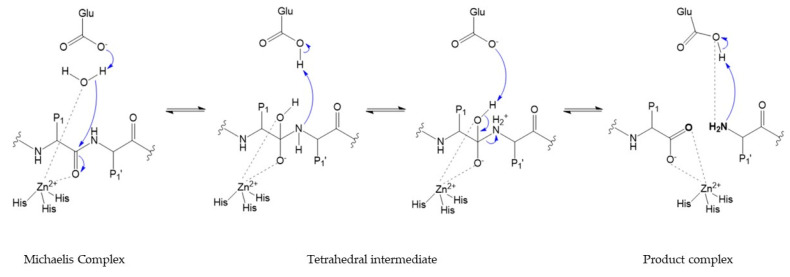
The catalytic activity requires catalytic zinc ion and a water molecule flanked by three conserved histidine and a conserved glutamate, with a conserved methionine acting as a hydrophobic base to support the structure surrounding the catalytic zinc ion [1,2,14] (Figure 12). In the initial transition states of the MMP-substrate interaction, Zn2+ is penta-coordinated with a substrate’s carbonyl oxygen atom, one oxygen atom from the glutamate-bound water and the three conserved histidine residues [1]. Hydrolysis of the peptide bond begins with the nucleophilic attack of the water coordinated with zinc to the carbonyl carbon of the substrate [13,14,17,18], subsequently proton transfer to the amine nitrogen occurs through the glutamic acid residue [2,8,12,13,15,16,18,21], promoting a gem-diol reaction intermediate [9,11,23] with a tetrahedral geometry [1,2,13]. This results in the breakdown of the substrate and the release of a water molecule [1]. The peptide is stabilized at the active site, by interaction between N-terminal residues and S1′ pocket [1,12], and by new hydrogen bonds formed between N-terminal, glutamate and water [2,9,11,13,15,16,18,23]. The two key steps in the catalytic process involve a structural rearrangement of the active site and the fate of the two obtained peptides [23]. Particularly relevant to the catalytic mechanism is the flexibility of the loop that forms the exterior of the S1′ pocket [23]. The internal flexibility of the catalytic domain plays an important role for enzymatic activity, but it is also the cause of drawbacks related to inhibitor selectivity.
Figure 12.
Catalytic mechanism.
8. Conclusions
MMPs are the most important enzymes for ECM maintenance. They are also involved in several biological and pathological diseases. However, MMPs remain a challenge for science, due to their high complexity, both in terms of regulation and activity. To that end, understanding of their role in the development of diseases and their mode of action require further studies. The development synthetic inhibitors in particular, critically depend on the full understanding of the structural details about S1′ pocket in MMPs and its interaction with the substrate.
Appendix A
Table A1.
| MMP | Name | Substrate | Production | Diseases | Other Information |
|---|---|---|---|---|---|
| 1 | Collagenase-1; Interstitial or Fibroblast collagenase | Collagen type I, II, III, VII, VIII, X and XI, gelatin, entactin, tenascin, aggrecan, fibronectin, vitronectin, myelin basic protein, ovostatin, casein | Sensitive to oxidative stress. Cells: fibroblasts, keratinocytes, endothelial cells, macrophages, hepatocytes, chondrocytes, platelets and osteoblasts. | Rheumatoid arthritis, atherosclerosis, pulmonary emphysema, fibrosis, autoimmune disease, wound healing and cancer | Identified in 1962. MMP-1 cleaves pro-MMP-2 and -9 into its active form. MMP-1 expression is increased by inflammatory cytokines (TNF-α and IL-1). |
| 8 | Collagenase-2; Neutrophil collagenase | Collagen type I, II and III, fibronectin, aggrecan and ovostatin. | Cells: chondrocytes, endothelial cells, activated macrophages and smooth muscle cells. | Rheumatoid arthritis, asthma, wound healing, periodontitis and cancer. | Identified in 1990 and it was discovered in cDNA library constructed from mRNA extracted from peripheral leukocytes of a patient with chronic granulocytic leukemia. MMP-8 is secreted by pro-MMP-8 form and it is activated by MMP-3 and -10 |
| 13 | Collagenase-3 | Collagen type I, II, III, IV, IX, X and XIV, tenascin C isoform, fibronectin, laminin, aggrecan core protein, gelatin, plasminogen, osteonectin, casein, fibrillin-1 and serine proteinases inhibitors | Connective tissue (cartilage and developing bone) Cells: epithelial and neuronal cells. | Osteoarthritis, lung diseases (lung injury, viral infection and chronic obstructive pulmonary disease), liver fibroses, cancer and metastasis | MMP-13 have gelatinolytic activity. MMP-13 active the pro-MMP-2 and -9. |
| 18 | Collagenase-4 | Collagen and gelatin | Organs: mammary glands, placenta, lung, pancreas, ovary, intestine, spleen, thymus, prostate, colon and heart | MMP-18 has not been directly linked to a specific pathological condition. | Identified in 1990s, in sequence similarity studies. Show closest identity with MMPs-1, -3, -10 and -11. MMP-18 differs from other MMPs in its amino acids sequence contains two cleavage sites for activation. |
Table A2.
| MMP | Name | Substrate | Production | Diseases | Other Information |
|---|---|---|---|---|---|
| 2 | Gelatinase A | Collagen typeI, III, IV, V, VII and X, gelatin, some glycoprotein of ECM, fibronectin, laminin, aggrecan, elastin, tenascin, myelin basic protein and vitronectin | Cells: dermal fibroblasts, keratinocytes, endothelial cells, chondrocytes, osteoblasts, leukocytes, platelets and monocytes | Promotion and inhibition of inflammation, asthma, fibrosis, cardiovascular diseases and cancer | MMP-2 expression is constitutive and TNF-α and -β stimulate its production, but IFN-τ suppresses its production. |
| 9 | Gelatinase B | Collagen type IV, V, and XI, cytokines, elastin, aggrecan, decorin, laminin, entactin, myelin basic protein, casein, chemokines, IL-8 and IL-1β | Cells: neutrophils, macrophages, polymorphonuclear leucocytes, osteoblasts, epithelial cells, fibroblasts, dendritic cells, granulocytes, T-cells and keratinocytes. | Cardiovascular diseases, inflammation and esophageal cancer | Identified such as neutrophil in 1974. MMP-9 has a strongly O-glycosylated collagen type V insert |
Table A3.
| MMP | Name | Substrate | Production | Diseases | Other Information |
|---|---|---|---|---|---|
| 3 | Stromelysin-1 | Collagen type I, II, III, IV, V, X and IX, fibronectin, gelatin, laminin, aggrecan, vitronectin, entactin, tenascin, decorin, myelin basic protein, ovostatin, casein, osteonectin elastin and proteoglycans. | Cells: fibroblasts and platelets MMP-3 has been detected in the nucleus, and it may function as a trans-regulator of connective tissue growth factor. | Arthritis, osteoarthritis, asthma, aneurism, atherosclerosis, coronary artery diseases, periodontitis, wound healing, Alzheimer diseases and cancer. | Identified in 1985. MMP-3 is secreted as inactive enzyme and actives pro-MMP-1, pro-MMP-13 and gelatinases. MMP-3 retains protease capability regardless of metal center, but this replace leads to sensitivity changes for different substrates. MMP-3 has a unique deep active site that transverses the length of the enzyme. |
| 10 | Stromelysin-2 | Collagen type III, IV, V, IX and X, proteoglycans, gelatin, fibronectin, laminin, elastin, aggrecan, cassein and fibrilin-10 | Cells: keratinocytes, macrophages and epithelium | Wound healing, arthritis, fibrosis, idiopathic pulmonary fibrosis, peripheral arterial disease and cancer. | MMP-10 has 82% MMP-3 homologous sequence. MMP-10 is secreted as pro-MMP-10. Actives others pro-MMPs, such as pro-collagenases. MMP-10 plays a role in liver regeneration. |
| 11 | - | No protein of major relevance to ECM can be degraded by MMP-11 but it degrades laminin receptor and serine proteinases inhibitors, α1-proteinases and α1-antitrypsin inhibitors. | Cells: fibroblasts Organs: uterus, placenta and mammary glands | Would healing, progression of epithelial malignancies and cancer | Identified in 1990, in stromal cells surrounding invasive breast carcinoma.MMP-11 is activated intracellularly by furin, since it has a furin recognition sequence (RXRXKR) and secreted in active form. |
Table A4.
| MMP | Name | Substrate | Production | Diseases | Other Information |
|---|---|---|---|---|---|
| 7 | - | Faz-ligand, pro-TNF-α, E-cadherin, syndecan-1, fibronectin, laminin, elastin, casein, gelatin type I, II, IV and V, collagen type I and IVm vitronectin, entactin, tenascin, aggrecan, myelin, and proteoglycans | Cells: epithelia cells, mammalian glands, liver, pancreas, prostate and skin | Idiopathic pulmonary fibrosis, cancer, metastasis and inflammatory processes. | MMP-7 was described such as putative uterine metalloprotease- 1 in 1988. MMP-7 acts intracellularly in the intestine to process procryptidins to bactericidal forms. |
| 26 | Matrilysin-2 or endometase | (in vitro): collagen type IV, fibronectin, fibrinogen, gelatin, vitronectin, α1-antipripsin, β-casein, α2-macroglobulin and IGFBP-1 | Cancer cells of epithelial origin | Carcinomas of the lung, prostate and breast, angiogenesis and tumor progression. | MMP-26 contains a signal sequence for secretion and a prodomain with an unusual cysteine switch for latency preservation. Active pro-MMP-9. MMP-26 is negatively regulated by TIMP-2 and -4, with TIMP-4 being more potent inhibitor. |
Table A5.
| MMP | Name | Substrate | Production | Diseases | Other Information |
|---|---|---|---|---|---|
| 14 | MT1-MMP (Membrane-type) | Collagen type I, II and III; gelatin, fibronectin, laminin-1, vitronectin, cartilage proteoglycans, fibrilin-1, tenascin, entactin, aggrecan, α1-proteinase inhibitor and α2-macroglobulin. | Cells: fibroblasts, platelets and osteoblasts | Cancer | Identified in 1994. MMP-14 actives the MMP-2, -8 and -13 latent forms. MMP-14 activates pro-MMP-13 on the cell surface. |
| 15 | MT2-MMP (Membrane-type) | Laminin, fibronectin, entactin, aggrecan, gelatin, vibronectin and tenascin. | Organs: placenta, heart and brain | Cancer (glioblastoma, ovarian and breast carcinoma) | Identified in 1995. MMP-15 can active MMP-2 and -13 latent forms. |
| 16 | MT3-MMP (Membrane-type) | Gelatin, casein, collagen type III, laminin and fibronectin. | Organs: lungs, placenta, kidney, ovaries, intestine, prostate, spleen, heart and skeletal muscle Cells: cardiomyocytes progenitor cells | Tumor invasion | Identified in 1997. MMP-16 can activate MMP-2 and -9. |
| 17 | MT4-MMP (GPI-anchored) | Gelatin, fibrinogen and fibrin | Cells: leucocytes Organs: brain, colon, ovaries and testicles | Inflammatory processes, cancer and tumor progression. | In the mid-1990s, MMP-17 was cloned from a human breast carcinoma cDNA library. ADAMTS-4 activator. MMP-17 cannot active pro-MMP-2. |
| 24 | MT5-MMP (Membrane-type) | Fibronectin, gelatin and proteoglycans | Organs: brain, kidney, pancreas and lung | Brain tumor (astrocytomas and glioblastomas) and tumor progression and angiogenesis. | Identified in 1999 and cloned from a human brain cDNA library. MMP-24 can activate MMP-2 latent form. MMP-24 is neuro-specific and contribute to neuronal circuit formation and plasticity. It has a role in the development of dermal neuro-immune synapses. |
| 25 | MT6-MMP (GPI-anchored) | Collagen type IV, fibronectin, gelatin and proteoglycans. | Cells: leucocytes and cancer tissue Organs: testicles, kidney and skeletal muscle | Cancer | The stem region contains three cysteine residues which may contribute to dimerization by inter- and intramolecular disulfide bond. MMP-25 cannot degrade laminin-1. MMP-25 actives pro-MMP-2, but differently than the other MT-MMPS. |
Table A6.
| MMP | Name | Substrate | Production | Diseases | Other Information |
|---|---|---|---|---|---|
| 12 | Macrophage metalloelastase | Gelatin type I, elastin, fibronectin, laminin, vitronectin, proteoglycans, elastin, collagen type I, IV and V, entactin, osteonectin, aggrecan, myelin, fibrinogen and α1-antitripsin | Cells: chondrocytes, macrophages and other stromal cells, osteoblasts. Organs: placenta | Chronic pulmonary disease, atherosclerosis, emphysema and lung cancer. | MMP-12 may affect the blood-brain barrier after cerebral ischemia. |
| 19 | RASI-1 or stromelysin-4 | Collagen type I and IV, laminin and nidogen, tenascin-C isoform, entactin, aggrecan, fibronectin and gelatin type I, in vitro | Cells: leucocytes Organs: colon, intestine, ovary, testis, prostate, thymus, spleen, pancreas, kidney, skeletal muscle, liver, lung, placenta, brain and heart | Wound healing and arthritic disease. | The MMP-19 can activate pro-MMP-9, but cannot activate other latent forms (MMP-1, -2, -3, -13 and -14, in vitro) |
| 20 | Enamelysin | Ameloblasts, aggrecan, odontoblasts and amelogenin | Organs: dental tissue (enamel) | Tooth development | MMP-20 is a tooth-specific MMP expressed in newly formed tooth enamel. MMP-20 contains a very basic hinge region compared to the hinge region of stromelysins (hydrophobic) or MMP-19 (acidic) |
| 21 | Xenopus-MMP | - | Cells: leucocytes, macrophages, fibroblasts, basal and squamous cell Organs: ovary, kidney, lung, placenta, intestine, neuroectoderm, skin and brain. | Embryogenesis, pancreatic cancer and tumor progression | - |
| 22 | Chicken-MMP | - | - | - | MMP-22 catalytic domain is closely related to stromelysin-3. |
| 23 | Cysteine array (CA)-MMP | Gelatin | Organs: ovary, testicles and prostate | - | MMP-23 lacks a signal sequence, it has a short pro-domain and the C-terminal domain is considerable shortened and shows no sequence similarity to hemopexin.MMP-23 is the only one that lacks the hemopexin domain, having a cysteine rich immunoglobulin-like domain.MMP-23 lacks the cysteine switch motif in propeptide. |
| 27 | - | Gelatin | Cells: B-lymphocytes Organs: testicles, intestine, lung and skin. | Ovarian or peritoneal endometriotic lesions, breast cancer development and tumor progression. | MMP-27 is classified as stromelysin and holds 51.6% structural homology with MMP-10. |
| 28 | Epilysin | Casein | Cells: basal keratinocytes Organs: epidermis. High levels- testis. Low levels-lungs heart, intestine, colon, placenta and brain. | Tissue homeostasis and repair, osteoarthritis and rheumatoid arthritis. | - |
Funding
This research was funded by A Molecular View of Dental Restoration, grant number PTDC/SAU-BMA/122444/2010 and by Molecular Design for Dental Restauration, grant number SAICT-POL/24288/2016.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Cui N., Hu M., Khalil R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017;147:1–73. doi: 10.1016/bs.pmbts.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J., Khalil R.A. Matrix Metalloproteinase Inhibitors as Investigational and Therapeutic Tools in Unrestrained Tissue Remodeling and Pathological Disorders. Prog. Mol. Biol. Transl. Sci. 2017;148:355–420. doi: 10.1016/bs.pmbts.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu J., Hoop C.L., Case D.A., Baum J. Cryptic binding sites become accessible through surface reconstruction of the type I collagen fibril. Sci. Rep. 2018;8:16646. doi: 10.1038/s41598-018-34616-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung L., Dinakarpandian D., Yoshida N., Lauer-Fields J.L., Fields G.B., Visse R., Nagase H. Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis. EMBO J. 2004;23:3020–3030. doi: 10.1038/sj.emboj.7600318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricard-Blum S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011;3:a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer T., Senn N., Riedl R. Design and Structural Evolution of Matrix Metalloproteinase Inhibitors. Chemistry. 2019;25:7960–7980. doi: 10.1002/chem.201805361. [DOI] [PubMed] [Google Scholar]
- 7.Yu Z., Visse R., Inouye M., Nagase H., Brodsky B. Defining requirements for collagenase cleavage in collagen type III using a bacterial collagen system. J. Biol. Chem. 2012;287:22988–22997. doi: 10.1074/jbc.M112.348979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagase H., Visse R., Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Amălinei C., Căruntu I.D., Bălan R.A. Biology of metalloproteinases. Rom. J. Morphol. Embryol. 2007;48:323–334. [PubMed] [Google Scholar]
- 10.Goodwin L. A Closer Look At Metalloproteinases. Nova Science Publisher Inc.; Hauppauge, NY, USA: 2019. p. 310. [Google Scholar]
- 11.Klein T., Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41:271–290. doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visse R., Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 13.Maskos K. Crystal structures of MMPs in complex with physiological and pharmacological inhibitors. Biochimie. 2005;87:249–263. doi: 10.1016/j.biochi.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Tallant C., Marrero A., Gomis-Rüth F.X. Matrix metalloproteinases: Fold and function of their catalytic domains. Biochim. Biophys. Acta. 2010;1803:20–28. doi: 10.1016/j.bbamcr.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen J.A., Major Jourden J.L., Miller M.T., Cohen S.M. To bind zinc or not to bind zinc: An examination of innovative approaches to improved metalloproteinase inhibition. Biochim. Biophys. Acta. 2010;1803:72–94. doi: 10.1016/j.bbamcr.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Verma R.P., Hansch C. Matrix metalloproteinases (MMPs): Chemical-biological functions and (Q)SARs. Bioorg. Med. Chem. 2007;15:2223–2268. doi: 10.1016/j.bmc.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Murphy G., Nagase H. Progress in matrix metalloproteinase research. Mol. Aspects Med. 2008;29:290–308. doi: 10.1016/j.mam.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mannello F., Medda V. Nuclear localization of matrix metalloproteinases. Prog. Histochem. Cytochem. 2012;47:27–58. doi: 10.1016/j.proghi.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Rangasamy L., Geronimo B.D., Ortín I., Coderch C., Zapico J.M., Ramos A., de Pascual-Teresa B. Molecular Imaging Probes Based on Matrix Metalloproteinase Inhibitors (MMPIs) Molecules. 2019;24:E2982. doi: 10.3390/molecules24162982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gersh I., Catchpole H.R. The organization of ground substance and basement membrane and its significance in tissue injury disease and growth. Am. J. Anat. 1949;85:457–521. doi: 10.1002/aja.1000850304. [DOI] [PubMed] [Google Scholar]
- 21.Whittaker M., Floyd C.D., Brown P., Gearing A.J. Design and therapeutic application of matrix metalloproteinase inhibitors. Chem. Rev. 1999;99:2735–2776. doi: 10.1021/cr9804543. [DOI] [PubMed] [Google Scholar]
- 22.Gross J., Lapiere C.M. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proc. Natl. Acad. Sci. USA. 1962;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerofolini L., Fragai M., Luchinat C. Mechanism and Inhibition of Matrix Metalloproteinases. Curr. Med. Chem. 2019;26:2609–2633. doi: 10.2174/0929867325666180326163523. [DOI] [PubMed] [Google Scholar]
- 24.Tokuhara C.K., Santesso M.R., Oliveira G.S.N., Ventura T.M.D.S., Doyama J.T., Zambuzzi W.F., Oliveira R.C. Updating the role of matrix metalloproteinases in mineralized tissue and related diseases. J. Appl. Oral Sci. 2019;27:e20180596. doi: 10.1590/1678-7757-2018-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandenbroucke R.E., Dejonckheere E., Libert C. A therapeutic role for matrix metalloproteinase inhibitors in lung diseases? Eur. Respir. J. 2011;38:1200–1214. doi: 10.1183/09031936.00027411. [DOI] [PubMed] [Google Scholar]
- 26.Huang H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors. 2018;18:E3249. doi: 10.3390/s18103249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zajkowska M., Zbucka-Krętowska M., Sidorkiewicz I., Lubowicka E., Będkowska G.E., Gacuta E., Szmitkowski M., Ławicki S. Human Plasma Levels of Vascular Endothelial Growth Factor, Matrix Metalloproteinase 9, and Tissue Inhibitor of Matrix Metalloproteinase 1 and Their Applicability as Tumor Markers in Diagnoses of Cervical Cancer Based on ROC Analysis. Cancer Control. 2018;25:1073274818789357. doi: 10.1177/1073274818789357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanco-Prieto S., Barcia-Castro L., Páez de la Cadena M., Rodríguez-Berrocal F.J., Vázquez-Iglesias L., Botana-Rial M.I., Fernández-Villar A., De Chiara L. Relevance of matrix metalloproteases in non-small cell lung cancer diagnosis. BMC Cancer. 2017;17:823. doi: 10.1186/s12885-017-3842-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silvello D., Narvaes L.B., Albuquerque L.C., Forgiarini L.F., Meurer L., Martinelli N.C., Andrades M.E., Clausell N., dos Santos K.G., Rohde L.E. Serum levels and polymorphisms of matrix metalloproteinases (MMPs) in carotid artery atherosclerosis: Higher MMP-9 levels are associated with plaque vulnerability. Biomarkers. 2014;19:49–55. doi: 10.3109/1354750X.2013.866165. [DOI] [PubMed] [Google Scholar]
- 30.Goncalves I., Bengtsson E., Colhoun H.M., Shore A.C., Palombo C., Natali A., Edsfeldt A., Dunér P., Fredrikson G.N., Björkbacka H., et al. Elevated Plasma Levels of MMP-12 Are Associated With Atherosclerotic Burden and Symptomatic Cardiovascular Disease in Subjects With Type 2 Diabetes. Arterioscler. Thromb. Vasc. Biol. 2015;35:1723–1731. doi: 10.1161/ATVBAHA.115.305631. [DOI] [PubMed] [Google Scholar]
- 31.Young D., Das N., Anowai A., Dufour A. Matrix Metalloproteases as Influencers of the Cells’ Social Media. Int. J. Mol. Sci. 2019;20:E3847. doi: 10.3390/ijms20163847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwan J.A., Schulze C.J., Wang W., Leon H., Sariahmetoglu M., Sung M., Sawicka J., Sims D.E., Sawicki G., Schulz R. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J. 2004;18:690–692. doi: 10.1096/fj.02-1202fje. [DOI] [PubMed] [Google Scholar]
- 33.Lovejoy B., Cleasby A., Hassell A.M., Longley K., Luther M.A., Weigl D., McGeehan G., McElroy A.B., Drewry D., Lambert M.H. Structure of the catalytic domain of fibroblast collagenase complexed with an inhibitor. Science. 1994;263:375–377. doi: 10.1126/science.8278810. [DOI] [PubMed] [Google Scholar]
- 34.Tsukada H., Pourmotabbed T. Unexpected crucial role of residue 272 in substrate specificity of fibroblast collagenase. J. Biol. Chem. 2002;277:27378–27384. doi: 10.1074/jbc.M201367200. [DOI] [PubMed] [Google Scholar]
- 35.Knäuper V., Docherty A.J., Smith B., Tschesche H., Murphy G. Analysis of the contribution of the hinge region of human neutrophil collagenase (HNC, MMP-8) to stability and collagenolytic activity by alanine scanning mutagenesis. FEBS Lett. 1997;405:60–64. doi: 10.1016/S0014-5793(97)00158-0. [DOI] [PubMed] [Google Scholar]
- 36.Gimeno A., Beltrán-Debón R., Mulero M., Pujadas G., Garcia-Vallvé S. Understanding the variability of the S1’ pocket to improve matrix metalloproteinase inhibitor selectivity profiles. Drug Discov. Today. 2019;25:38–57. doi: 10.1016/j.drudis.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Bar-Or A., Nuttall R.K., Duddy M., Alter A., Kim H.J., Ifergan I., Pennington C.J., Bourgoin P., Edwards D.R., Yong V.W. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain. 2003;126:2738–2749. doi: 10.1093/brain/awg285. [DOI] [PubMed] [Google Scholar]