This cross-sectional study assesses the feasibility of linking neuropathology data to social determinants of health by evaluating the association between neighborhood disadvantage and Alzheimer disease–related neuropathology among decedents in Wisconsin and California.
Key Points
Question
Can neighborhood disadvantage, a social determinant of health, be incorporated into existing brain bank data to evaluate the risk of biological outcomes, such as Alzheimer disease neuropathology?
Findings
In this cross-sectional study using autopsy samples from 447 decedents, living in a disadvantaged neighborhood at the time of death was associated with an increased risk of presence of Alzheimer disease neuropathology when adjusting for age, sex, and year of death.
Meaning
These findings suggest that neighborhood disadvantage can be geolinked to brain bank repositories and may be a marker for Alzheimer disease neuropathology.
Abstract
Importance
Social determinants of health, such as income, education, housing quality, and employment, are associated with disparities in Alzheimer disease and health generally, yet these determinants are rarely incorporated within neuropathology research.
Objective
To establish the feasibility of linking neuropathology data to social determinants of health exposures using neighborhood disadvantage metrics (the validated Area Deprivation Index) and to evaluate the association between neighborhood disadvantage and Alzheimer disease–related neuropathology.
Design, Setting, and Participants
This cross-sectional study consisted of decedents with a known home address who donated their brains to 1 of 2 Alzheimer disease research center brain banks in California and Wisconsin between January 1, 1990, and December 31, 2016. Neither site had preexisting social metrics available for their decedents. Neuropathologic features were obtained from each site for data collected using the standardized Neuropathology Data Set form and from autopsy reports. Data were analyzed from June 7 to October 10, 2019.
Exposures
Geocoded decedent addresses linked to neighborhood disadvantage as measured by the Area Deprivation Index calculated for the year of death.
Main Outcomes and Measures
Presence of Alzheimer disease neuropathology. The association between neighborhood disadvantage and Alzheimer disease neuropathology was evaluated via logistic regression, adjusting for age, sex, and year of death.
Results
The sample consisted of 447 decedents (249 men [56%]; mean [SD] age, 80.3 [9.5] years; median year of death, 2011) spanning 24 years of donation. Fewer decedents (n = 24 [5.4%]) originated from the top 20% most disadvantaged neighborhood contexts. Increasing neighborhood disadvantage was associated with an 8.1% increase in the odds of Alzheimer disease neuropathology for every decile change on the Area Deprivation Index (adjusted odds ratio, 1.08; 95% CI, 1.07-1.09). As such, living in the most disadvantaged neighborhood decile was associated with a 2.18 increased odds of Alzheimer disease neuropathology (adjusted odds ratio, 2.18; 95% CI, 1.99-2.39).
Conclusions and Relevance
The findings of this cross-sectional study suggest that social determinants of health data can be linked to preexisting autopsy samples as a means to study sociobiological mechanisms involved in neuropathology. This novel technique has the potential to be applied to any brain bank within the United States. To our knowledge, this is the first time Alzheimer disease neuropathology has been associated with neighborhood disadvantage.
Introduction
When it comes to health, context matters.1,2 Social determinants of health (SDOH) are the “conditions in the environments in which people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks.”3 They are fundamental mechanisms within frameworks of health and health disparities,3,4,5,6 yet practical means to connect biological end points to contextual factors are not widely available, particularly for a majority of existing biobanks. The few biobanks that are linked to longstanding cohort studies are a notable exception. These biobanks have contributed greatly to knowledge about associations between social factors and biological outcomes but are too few in number.
This situation is now changing. With recent advances in precision public health, it is possible to capture SDOH at more granular geographic levels (eg, neighborhood conditions), improving the ability to understand how interactions between contextual and biological mechanisms drive health.7 Neighborhood disadvantage metrics, which reflect underlying SDOH of a precise geographic region, are now widely available through the National Institutes of Health (NIH)–funded Neighborhood Atlas.7,8 Furthermore, the NIH is encouraging mechanistic-focused health disparities research through targeted funding announcements and frameworks, such as the National Institute on Aging health disparities framework.9
Within the field of Alzheimer disease (AD) and related dementias, health disparities disproportionally affect minority and socially disadvantaged populations.10,11,12,13,14,15 Little is known about the fundamental biological processes that drive these associations, and few existing AD studies link brain tissue neuropathology to SDOH metrics. If more widely available, such linkages could allow for new insight into the extent to which SDOH may affect risk for AD dementia and neuropathology. These links may also unlock new vantage points for fundamental mechanistic discovery and therapeutic development.
Precision medicine necessitates not only a characterization of unique tissue and genomic factors but also an understanding of how context—environmental, sociocultural, and behavioral factors—combines with biological factors to affect our health.16,17,18 Precision medicine holds the promise of identifying individuals at increased risk in an effort to provide tailored treatment and prevention efforts to improve health.18 But this promise may not be fully realized if tissue is considered apart from context; both must be considered as part of the whole.
The first step in realizing this goal is to prove the feasibility of linking SDOH to biological data where these SDOH were not previously measured. With this in mind, the objective of this study is to employ precision public health data technologies to characterize SDOH exposures for existing brain tissue from biobanks (eFigure in the Supplement). Specifically, we examine the extent to which neighborhood disadvantage exposure is associated with AD neuropathology. By doing so, we aim to provide an illustrative example of how to determine the association between SDOH contextual risk factors and health and show the feasibility of a novel method that could potentially be applied to any biorepository in the United States and its territories. Because geographic accessibility is a key factor driving access to many research resources,19 the work presented here also enables us to examine the geographic alignment of the Alzheimer Disease Research Center (ADRC) brain bank network as a whole with the geographic location of disadvantaged US neighborhoods using geosimulation.
Methods
Study Sample
This study is a retrospective, cross-sectional analysis of decedents who donated their brains to 1 of 2 ADRC brain bank repositories. Decedents were included if they died between January 1, 1990, and December 31, 2016, had a noninstitutional address, and had complete information on the presence of diffuse and neuritic plaques (n = 447) as determined by a neuropathologist. This study was reviewed by the University of Wisconsin and University of California San Diego institutional review boards and was determined to be not human subjects research; as such, informed consent was not required. Data use agreements between the 2 sites complied with protected health information under HIPAA regulations. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline was followed.
Precision Public Health Metrics
Social determinants of health were measured by the Area Deprivation Index (ADI) determined at the neighborhood-level.7 The ADI is a validated, neighborhood-level composite index reflecting 17 SDOH dimensions captured in the American Community Survey and US Census Survey data via principal components analysis methodology. The resulting factor score is then transformed into a percentile ranking, facilitating comparisons across time to prior and future ADI builds and to external measures of socioeconomic disadvantage. The ADI state rankings range from 1 to 10, with least disadvantaged neighborhood conditions designated by lower scores and most disadvantaged by higher scores. Examples of neighborhood-level SDOH factors incorporated within the ADI include education level, income, housing, and employment characteristics. For this study, the ADI state decile ranking denoting the decedent’s neighborhood SDOH conditions associated with their last known residence was used. For deaths from 1990 through 2009, the ADI is based on that decade’s Decennial Census data (eg, the ADI ranking for someone who died during the 1990s is built from the 1990 Census). For 2010 and after, additional forms of Census data are available, so yearly versions of the ADI could be generated using the same dimensions via the Census’ American Community Survey.
The decedent’s address at the time of death was linked to its corresponding Census Block Group and ADI value via geoanalytic methods. Census block groups represent neighborhood geographic regions, containing a mean of 1500 people.20 Because census block groups are updated every decade, addresses were linked to the census block group for the decade in which the death occurred. All ADRC locations with brain bank donation were geocoded using ArcGIS 10.7 (Environmental Systems Research Institute). Distance between the ADRC brain bank and the decedent’s address at the time of death were calculated using network analysis via ArcGIS using ESRI Business Analyst (Environmental Systems Research Institute) data.
Neuropathology Data
We relied on 2 methods to extract neuropathologic features. For each ADRC site, we used data collected via the Neuropathology Data Set form (a National Alzheimer Coordinating Center standardized data collection form).21 If Neuropathology Data Set data were not available from the site for a particular decedent (typically for the years prior to 2005), abstractions of the same elements were derived directly from digital scans of autopsy reports. An abstraction guide was generated using previous methods and based on expert knowledge from neuropathologists (including M.S.S.) at both centers.22 All autopsy reports were dually abstracted for the same data by 2 independent, trained abstractors (J.L.L., L.V.). Interrater reliability was strong, with the κ statistic ranging from 0.8 to 1.0 for all abstracted variables. Any discrepancies between abstractors were discussed and corrected via abstraction team consensus. The abstracted data from autopsy reports mirrored those of the derived variables in the Neuropathology Data Set data.
Utilizing an existing methodology as per Hassenstab et al23 and Monsell et al,24 presence of AD neuropathology was defined as the presence of either diffuse plaques or neuritic plaques, following the National Institute of Aging and the Alzheimer Association criteria for neuropathology plaque presence.25 For sensitivity analyses, we also used a stricter definition, which required the presence of both diffuse plaques and neuritic plaques.5 We used this stricter definition in sensitivity analyses to ensure robustness of the overall results. The use of this stricter definition did not change the overall results (see eTable 1 in the Supplement). In order to be consistent with existing studies, we used the methodology as per Hassenstab et al23 and Monsell et al24 within the analyses below.
Brain Donation Catchment Simulation
We generated a nationwide geographic simulation of catchment-area subject pools for all ADRC brain banks using US census population data.26 Because geographic accessibility is a key factor driving access to many research resources,19 the goal of this simulation was to estimate geographic alignment of the ADRC brain bank network as a whole with the geographic location of disadvantaged US neighborhoods using geosimulation. All ADRC locations with brain bank donation were geocoded using ArcGIS. Once the locations were determined, the geographic analysis tools were applied to create a 100-mile circular radius around each ADRC—an estimate of a reasonable geographic accessible area for most typical participants interested in brain donation and consistent with the observed geo-reach of each brain bank donation program within our sample data (approximately 90% of decedents in the cross-sectional study lived within 100 miles). US Census block groups that intersect each buffer were then assessed. However, we recognize that some brain banks may have access that reaches beyond the 100-mile buffer used for this geosimulation. Also, this approach does not account for more motivated participants who are willing to engage with ADRCs at a great distance, which may occur more often in certain populations, such as frontotemporal dementia patients traveling great distances to seek diagnosis and care at world-renowned centers.27
Statistical Analysis
Descriptive statistics for demographic, SDOH, and neuropathologic features were calculated and are reported in Table 1 and Table 2. To evaluate the association between SDOH (via the ADI) and neuropathology features, we employed a generalized linear model, modeling the logit of the binomial AD neuropathology outcome, with clustered robust standards errors to address site-level clustering. To address the concern of possible sparse data bias, corrected coefficients using rare event modeling methods that provide more conservative estimates of risk are reported.28 The key explanatory variable was the ADI state ranking with covariate adjustments for age, sex, and year of death. Odds ratios (ORs) and 95% CIs of resulting models are reported. Decedents without the necessary information to categorize AD neuropathology (n = 6) were removed from the analysis and were significantly younger (mean [SD] age, 62.3 [10.0] years [95% CI, 51.9-72.8 years] vs 80.3 [9.5] years [95% CI, 79.4-81.2 years]; P < .001 with independent, 2-sided t test at α = .05) but otherwise not different on mean ADI, sex, or year of death. Modeling was conducted using Stata/SE 15.1 (StataCorp LLC). The analysis was conducted from June 7 to October 10, 2019.
Table 1. Demographic Characteristics of Decedent Sample.
| Characteristic | No. (%)a | ||
|---|---|---|---|
| Overall sample (N = 447) | AD (n = 394) | No AD (n = 53) | |
| Neighborhood disadvantage, mean (SD) decile | 3.8 (2.3) | 3.8 (2.4) | 3.4 (2.1) |
| Age, mean (SD), y | 80.3 (9.5) | 80.6 (9.3) | 78.4 (10.6) |
| Age group, y | |||
| <65 | 23 (5) | 19 (5) | 4 (8) |
| 65-69 | 47 (11) | 38 (10) | 9 (17) |
| 70-74 | 41 (9) | 36 (9) | 5 (9) |
| 75-79 | 71 (16) | 65 (16) | 6 (11) |
| 80-84 | 95 (21) | 81 (21) | 14 (26) |
| 85-89 | 98 (22) | 90 (23) | 8 (15) |
| ≥90 | 72 (16) | 65 (16) | 7 (13) |
| Sex | |||
| Female | 198 (44) | 177 (45) | 21 (40) |
| Male | 249 (56) | 217 (55) | 32 (60) |
| Year of death | |||
| 1990-1999 | 4 (1) | 4 (1) | 0 |
| 2000-2009 | 184 (41) | 163 (41) | 21 (40) |
| 2010-2016 | 259 (58) | 227 (58) | 32 (60) |
Abbreviation: AD, Alzheimer disease.
Percentages have been rounded and may not equal 100.
Table 2. Neuropathologic Features of Decedent Sample.
| Neuropathologic feature | Overall sample (N = 447) |
|---|---|
| AD neuropathology, No. (%) | |
| No | 53 (12) |
| Yes | 394 (88) |
| Diffuse plaque, No. (%) | |
| Not present | 73 (16) |
| Present | 374 (84) |
| Neuritic plaque, No. (%) | |
| Not present | 69 (15) |
| Present | 378 (85) |
Abbreviation: AD, Alzheimer disease.
Results
Distribution of Disadvantage
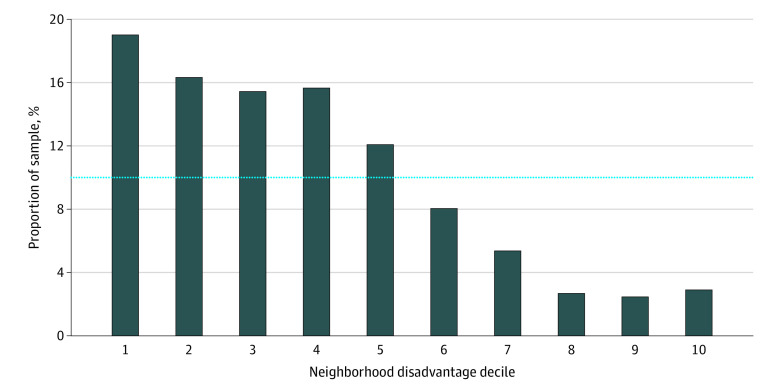
The final sample comprised autopsy data from 447 decedents (198 women [44%] and 249 men [56%]; mean [SD] age, 80.3 [9.5] years; median year of death, 2011) spanning 24 years of collection. Sociodemographic characteristics and neuropathologic features are noted in Table 1 and Table 2, respectively. The distribution of neighborhood disadvantage was right-skewed, such that most sample decedents originated from the most affluent (ie, least disadvantaged) neighborhoods, with 24 decedents (5.4%) from the most disadvantaged quintile (top 20%) neighborhoods (Figure 1). Assessing neuropathology of the sample revealed a high rate of AD neuropathology (n = 394 [88%]), which is consistent with the sample sources.
Figure 1. Neighborhood Disadvantage Within Decedent Sample.
Neighborhood disadvantage measured by Area Deprivation Index percentile rankings, ranging from 1 (least disadvantaged decile neighborhoods) to 10 (most disadvantaged decile neighborhoods). Bars denote the proportion within each neighborhood disadvantage category for the study sample. The dotted line represents the proportion within each category for the United States.
Neighborhood Disadvantage
Decedents originating from the most disadvantaged neighborhoods had the highest risk of AD neuropathology. Adjusted model results demonstrated that with each decile increase in neighborhood disadvantage (by ADI), there was an estimated 8.1% increase in the expected odds for AD neuropathology (eTable 2 in the Supplement) (adjusted OR, 1.08; 95% CI, 1.07-1.09). As such, living in the most disadvantaged neighborhood decile was associated with 2.18 higher odds (95% CI, 1.99-2.39) of AD neuropathology. Decedents within the current study resided a median of 19 miles (interquartile range, 10-31) from their respective ADRC brain bank.
Brain Donation Catchment Simulation
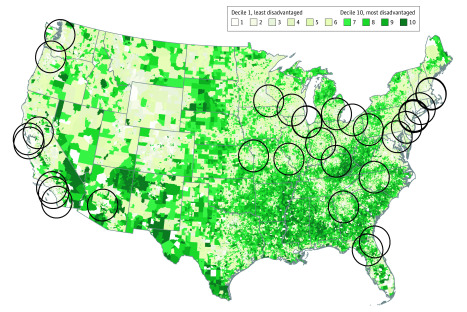
Based on geosimulation modeling, including the applied assumptions, 56% of the total US population lives within 100-miles of an ADRC29 and would potentially have easier geographic access to brain bank donation services (Figure 2). For the other 44% of the US population, access to ADRC brain bank donation may be more geographically difficult. Of the population within the 100-mile radius of an ADRC brain bank—ie, those with easier access—only 13% live within the top quintile of neighborhood disadvantage (the most disadvantaged neighborhoods), whereas 30% live in the most wealthy quintile (least disadvantaged) neighborhoods in the United States. This distribution of geographic access skews toward the most wealthy neighborhoods in a manner that does not reflect the general US population.
Figure 2. Geosimulation of Alzheimer Disease Research Center (ADRC) Catchment Areas.
Recruitment catchment within 100 miles of each ADRC, with darker green areas denoting neighborhoods with the highest levels of neighborhood disadvantage. The list of existing ADRCs is drawn from information listed by the National Institute on Aging.29
Discussion
In this cross-sectional study, we showed the feasibility of using precision public health techniques to characterize SDOH exposures for autopsy samples in 2 ADRC brain banks. These are brain banks that did not otherwise have SDOH data linked to their biospecimens. To our knowledge, this study is the first time that precision public health approaches have been applied in this way. This novel technique has the potential to link contextual factors to autopsy tissue for any biorepository within the United States. Furthermore, as an initial use example, we used this linkage to evaluate the association between SDOH and AD neuropathology, finding that increasing neighborhood disadvantage is associated with increased odds of AD neuropathology. However, additional studies are needed to replicate this finding.
Precision medicine holds great promise to revolutionize diagnostics and therapeutics. However, biobank data should not be considered in isolation from context. A complete approach to precision medicine necessitates characterization of tissues and genes as well as employing precision public health techniques to more fully characterize contextual factors and, synergistically, to allow for a more complete study of how all factors combine to affect health. Precision public health promises “the right intervention to the right population at the right time.”17(p1399) With an eye toward this promise, using the methods demonstrated in this study, SDOH can be linked to existing biobanks and tissue repositories as a means to catalyze the study of sociobiological mechanisms—that is, the biological mechanisms that potentially are at play in health disparities. The SDOH data within the Neighborhood Atlas that facilitate this linkage are free, easily available online, NIH supported, and accessible to all.7
Within this study, decedents rarely originated from the most disadvantaged neighborhoods; decedents from wealthy neighborhoods were more common. This is a pattern that likely is common within biorepositories across the United States,30 but additional study is needed to confirm as well as identify where representation is most lacking. Recruitment of diverse and disadvantaged participants is a known challenge within the AD research field.31,32 Yet improvements are actively being made by many leaders in the field.33,34,35,36,37,38 Some brain banks focus intensely on these populations to great success, but more science on how best to recruit and resources to fund recruitment science are needed. However, in the geosimulations conducted within the present study, even if an ADRC brain bank was able to achieve an optimally population-representative recruitment of its catchment population, the sampled population would still likely skew toward the wealthier neighborhoods. Many people within the United States still do not have easy geographic access to ADRC services, and ADRCs tend to demonstrate tight clustering within certain US regions. Additional efforts to establish ADRCs, ADRC-affiliated branch sites, or ADRC-related services within other geographic regions of the United States could be considered or explored as a potential way to increase easy geographic access to resources and to facilitate broader population samplings for research.
Limitations
This study should be considered in light of a number of limitations. Although we utilized all available samples and employed relatively broad inclusion criteria, decedents from disadvantaged neighborhoods were not as well represented within the study sample. Selection bias may be a factor in brain donation. Therefore, these results need to be replicated in a larger, more generalizable sample, and the present interpretation and generalization must be limited. Assessments of SDOH exposure relied on place of residence at the time of death, which may not accurately reflect SDOH exposure across a life course. Additional in-depth study is underway to develop new methods to address this limitation and to provide a more robust life-course perspective to the sample. However, given the intense resource and time requirements of historical life-course assessments of brain bank decedents, these data will not be available for some time. Definitions of AD neuropathology have changed over time; however, we used a current set of criteria to mitigate this limitation to an extent.
The biosocial pathway of the association between neighborhood disadvantage and AD remains largely unclear. Much more research is needed in this area. Possible factors related to the of amyloid plaques include stress; sleep disruption; lifestyle factors, such as diet and exercise; pollution; and cardiovascular risk factors, such as diabetes.39,40,41,42,43,44,45 These were factors that could not be assessed within the current study, as such data were not collected on these existing brain specimens. In general, linkages to such factors within existing brain bank neuropathology resources are too few. Significant research is needed to better understand the association among these factors and outcomes and to understand how the association between ADI and AD neuropathology varies within key subgroups (eg, race/ethnicity, apolipoprotein E4).
Conclusions
This study demonstrates how accessibility to precision public health data technologies can place brain tissue in context. This linkage offers promising ways to better elucidate the population representativeness of existing samples as well as new tools to investigate mechanisms associated with disparities and AD neuropathology.
eFigure. Putting Tissue in Context: Linking Neighborhood Social Determinants of Health to Alzheimer’s Disease Neuropathology
eTable 1. Unadjusted and Adjusted Main and Sensitivity Model Results
eTable 2. Unadjusted and Adjusted Results with Neighborhood Disadvantage Placed into Tertile Groupings Based on Study Sample
References
- 1.Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995;(Spec No):80-94. doi: 10.2307/2626958 [DOI] [PubMed] [Google Scholar]
- 2.Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities: theory, evidence, and policy implications. J Health Soc Behav. 2010;51(suppl):S28-S40. doi: 10.1177/0022146510383498 [DOI] [PubMed] [Google Scholar]
- 3.Office of Disease Prevention and Health Promotion Healthy People 2020: Social Determinants of Health. Updated May 7, 2020. Accessed September 30, 2019. https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health
- 4.Anderson NB. Levels of analysis in health science. A framework for integrating sociobehavioral and biomedical research. Ann N Y Acad Sci. 1998;840:563-576. doi: 10.1111/j.1749-6632.1998.tb09595.x [DOI] [PubMed] [Google Scholar]
- 5.Frieden TR. A framework for public health action: the health impact pyramid. Am J Public Health. 2010;100(4):590-595. doi: 10.2105/AJPH.2009.185652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill CV, Pérez-Stable EJ, Anderson NA, Bernard MA. The National Institute on Aging Health Disparities Research Framework. Ethn Dis. 2015;25(3):245-254. doi: 10.18865/ed.25.3.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible—The Neighborhood Atlas. N Engl J Med. 2018;378(26):2456-2458. doi: 10.1056/NEJMp1802313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.University of Wisconsin School of Medicine and Public Health, Department of Medicine. Neighborhood Atlas. Accessed September 30, 2019. https://www.neighborhoodatlas.medicine.wisc.edu/
- 9.National Institute on Aging Health Disparities Framework. Accessed May 10, 2019. https://www.nia.nih.gov/research/osp/framework
- 10.Alzheimer’s Association 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15(3):321-387. doi: 10.1016/j.jalz.2019.01.010 [DOI] [Google Scholar]
- 11.Barnes LL, Bennett DA. Alzheimer’s disease in African Americans: risk factors and challenges for the future. Health Aff (Millwood). 2014;33(4):580-586. doi: 10.1377/hlthaff.2013.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper C, Tandy AR, Balamurali TB, Livingston G. A systematic review and meta-analysis of ethnic differences in use of dementia treatment, care, and research. Am J Geriatr Psychiatry. 2010;18(3):193-203. doi: 10.1097/JGP.0b013e3181bf9caf [DOI] [PubMed] [Google Scholar]
- 13.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216-224. doi: 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaffe K, Falvey C, Harris TB, et al. ; Health ABC Study . Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ. 2013;347:f7051. doi: 10.1136/bmj.f7051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeki Al Hazzouri A, Haan MN, Kalbfleisch JD, Galea S, Lisabeth LD, Aiello AE. Life-course socioeconomic position and incidence of dementia and cognitive impairment without dementia in older Mexican Americans: results from the Sacramento area Latino study on aging. Am J Epidemiol. 2011;173(10):1148-1158. doi: 10.1093/aje/kwq483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.What is precision medicine? Genetics Home Reference; U.S National Library of Madison. Published 2019. Updated April 16, 2019. Accessed April 25, 2019. https://ghr.nlm.nih.gov/primer/precisionmedicine/definition
- 17.Chowkwanyun M, Bayer R, Galea S. “Precision” public health–between novelty and hype. N Engl J Med. 2018;379(15):1398-1400. doi: 10.1056/NEJMp1806634 [DOI] [PubMed] [Google Scholar]
- 18.Khoury MJ, Bowen MS, Clyne M, et al. . From public health genomics to precision public health: a 20-year journey. Genet Med. 2018;20(6):574-582. doi: 10.1038/gim.2017.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilmore-Bykovskyi AL, Jin Y, Gleason C, et al. . Recruitment and retention of underrepresented populations in Alzheimer’s disease research: A systematic review. Alzheimers Dement (N Y). 2019;5:751-770. doi: 10.1016/j.trci.2019.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Census Bureau Glossary: block groups. Accessed May 11, 2020. https://www.census.gov/programs-surveys/geography/about/glossary.html#par_textimage_4
- 21.Research Support Group at the National Alzheimer’s Coordinating Center NACC Researchers Data Dictionary: The Neuropathology (NP) Data Set. University of Washington; 2014. [Google Scholar]
- 22.Polnaszek B, Gilmore-Bykovskyi A, Hovanes M, et al. . Overcoming the challenges of unstructured data in multisite, electronic medical record-based abstraction. Med Care. 2016;54(10):e65-e72. doi: 10.1097/MLR.0000000000000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassenstab J, Monsell SE, Mock C, et al. . Neuropsychological markers of cognitive decline in persons with Alzheimer disease neuropathology. J Neuropathol Exp Neurol. 2015;74(11):1086-1092. doi: 10.1097/NEN.0000000000000254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monsell SE, Mock C, Roe CM, et al. . Comparison of symptomatic and asymptomatic persons with Alzheimer disease neuropathology. Neurology. 2013;80(23):2121-2129. doi: 10.1212/WNL.0b013e318295d7a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyman BT, Phelps CH, Beach TG, et al. . National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8(1):1-13. doi: 10.1016/j.jalz.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US Census Bureau. Data. Accessed May 11, 2020. https://www.census.gov/data.html
- 27.Boxer AL, Gold M, Feldman H, et al. . New directions in clinical trials for frontotemporal lobar degeneration: Methods and outcome measures. Alzheimers Dement. 2020;16(1):131-143. doi: 10.1016/j.jalz.2019.06.4956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King G. Zeng L. Logistic regression in rare events data. Polit Anal. 2001;9(2):137-163. doi: 10.1093/oxfordjournals.pan.a004868 [DOI] [Google Scholar]
- 29.National Institute on Aging. Alzheimer’s Disease Research Centers. Reviewed July 11, 2019. Accessed October 8, 2019. https://www.nia.nih.gov/health/alzheimers-disease-research-centers
- 30.Popejoy AB, Fullerton SM. Genomics is failing on diversity. Nature. 2016;538(7624):161-164. doi: 10.1038/538161a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grill JD, Galvin JE. Facilitating Alzheimer disease research recruitment. Alzheimer Dis Assoc Disord. 2014;28(1):1-8. doi: 10.1097/WAD.0000000000000016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyles CR, Lunn MR, Obedin-Maliver J, Bibbins-Domingo K. The new era of precision population health: insights for the All of Us Research Program and beyond. J Transl Med. 2018;16(1):211. doi: 10.1186/s12967-018-1585-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA. The Minority Aging Research Study: ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res. 2012;9(6):734-745. doi: 10.2174/156720512801322627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25(4):163-175. doi: 10.1159/000087446 [DOI] [PubMed] [Google Scholar]
- 35.Howell JC, Watts KD, Parker MW, et al. . Race modifies the relationship between cognition and Alzheimer’s disease cerebrospinal fluid biomarkers. Alzheimers Res Ther. 2017;9(1):88-88. doi: 10.1186/s13195-017-0315-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manly JJ, Bell-McGinty S, Tang MX, Schupf N, Stern Y, Mayeux R. Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Arch Neurol. 2005;62(11):1739-1746. doi: 10.1001/archneur.62.11.1739 [DOI] [PubMed] [Google Scholar]
- 37.Morris MC, Evans DA, Bienias JL, et al. . Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA. 2002;287(24):3230-3237. doi: 10.1001/jama.287.24.3230 [DOI] [PubMed] [Google Scholar]
- 38.Williams MM, Meisel MM, Williams J, Morris JC. An interdisciplinary outreach model of African American recruitment for Alzheimer's disease research. Gerontologist. 2011;51(suppl 1):S134-S141. doi: 10.1093/geront/gnq098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong H, Csernansky JG. Effects of stress and stress hormones on amyloid-beta protein and plaque deposition. J Alzheimers Dis. 2009;18(2):459-469. doi: 10.3233/JAD-2009-1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roh JH, Huang Y, Bero AW, et al. . Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med. 2012;4(150):150ra122. doi: 10.1126/scitranslmed.3004291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berti V, Walters M, Sterling J, et al. . Mediterranean diet and 3-year Alzheimer brain biomarker changes in middle-aged adults. Neurology. 2018;90(20):e1789-e1798. doi: 10.1212/WNL.0000000000005527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25(17):4217-4221. doi: 10.1523/JNEUROSCI.0496-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calderón-Garcidueñas L, Kavanaugh M, Block M, et al. . Neuroinflammation, hyperphosphorylated tau, diffuse amyloid plaques, and down-regulation of the cellular prion protein in air pollution exposed children and young adults. J Alzheimers Dis. 2012;28(1):93-107. doi: 10.3233/JAD-2011-110722 [DOI] [PubMed] [Google Scholar]
- 44.Gottesman RF, Schneider AL, Zhou Y, et al. . Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017;317(14):1443-1450. doi: 10.1001/jama.2017.3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5(1):64-74. doi: 10.1016/S1474-4422(05)70284-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Putting Tissue in Context: Linking Neighborhood Social Determinants of Health to Alzheimer’s Disease Neuropathology
eTable 1. Unadjusted and Adjusted Main and Sensitivity Model Results
eTable 2. Unadjusted and Adjusted Results with Neighborhood Disadvantage Placed into Tertile Groupings Based on Study Sample