Abstract
Monocytes play an important role in both innate immunity and antigen presentation for specific cellular immune defense. In patients with chronic renal failure, as well as those treated with maintenance hemodialysis, these cells are largely dysregulated. There is a large body of literature on monocyte alterations in such patients. However, most of the publications report on small series, there is a vast spectrum of different methods and the heterogeneity of the data prevents any meta-analytic approach. Thus, a narrative review was performed to describe the current knowledge. Monocytes from patients with chronic renal failure differ from those of healthy individuals in the pattern of surface molecule expression, cytokine and mediator production, and function. If these findings can be summarized at all, they might be subsumed as showing chronic inflammation in resting cells together with limited activation upon immunologic challenge. The picture is complicated by the fact that monocytes fall into morphologically and functionally different populations and population shifts interact heavily with dysregulation of the individual cells. Severe complications of chronic renal failure such as impaired immune defense, inflammation, and atherosclerosis can be related to several aspects of monocyte dysfunction. Therefore, this review aims to provide an overview about the impairment and activation of monocytes by uremia and the resulting clinical consequences for renal failure patients.
Keywords: chronic kidney disease, cytokines, hemodialysis, inflammation, monocytes, uremic toxins
1. Introduction
Monocytes are bone marrow derived cells that circulate in the blood for 1–3 days before differentiating into tissue macrophages or dendritic cells. They are part of the innate immune system and react quickly but unspecifically to any tissue damage or pathogen intrusion. Monocytes can act as phagocytes that take up pathogens. They produce cytokines that regulate inflammatory responses. Among their most important functions are chemotaxis, which describes the approach to sites of tissue damage in response to soluble mediators and cytokines, as well as adhesion and transmigration through the endothelium. The latter is promoted by specific adhesion molecules that bind to endothelial ligands. Monocytes also play an important role as antigen-presenting cells. They can present foreign antigens towards lymphocytes thereby activating the adaptive immune response. In addition, they provide secondary signals to the lymphocytes to regulate the immune reaction. Such secondary signaling can adjust a response towards an antigen from tolerance to cytolytic attack. Monocytes are precursors of dendritic cells, the professional and highly efficient antigen-presenting cells of the immune system.
Monocytes fall into three different subgroups that can be distinguished via the expression pattern of the receptor for lipopolysaccharide (CD14) and the immunoglobulin fc segment receptor CD16 [1]. These populations differ in function and cytokine spectrum [2]. The populations are termed Mo1 (“classical monocytes” expressing high levels of CD14 only), Mo2 (“intermediate monocytes”, CD14++/CD16+), and Mo3 (“nonclassical monocytes, CD14+/CD16++). The functional and morphological heterogeneity of these populations needs to be considered when describing surface marker expression as well as cytokine production by monocytes from patients with chronic renal failure. Differences between healthy individuals and CKD patients may be caused by dysregulation of the cells or by alterations in the relative number of the three different populations in the blood. Unfortunately, this aspect was considered only by a minority of publications in this field.
In the recent 25 years there have been many publications focusing on abnormal structure or function of monocytes in patients with CKD. These studies were done to elucidate the diminished immune defense function that lead to frequent infections in these patients. They also address the causes of chronic inflammation, which is found in the majority of these patients, even in the absence of infection. This review aims firstly to describe the structural and functional abnormalities observed in monocytes from CKD patients, secondly to review the mechanism by which CKD affects monocytes populations and fundamental monocytic functions and thirdly to explain how these alterations may impact both antimicrobial defense and atherosclerosis development in this population.
2. Phenotype of Monocytes in CKD
Most studies describe normal numbers of monocytes in the circulation, while there was a tendency towards lymphopenia [3,4,5]. While the cells are quantitatively available in the blood, there are numerous alterations in their phenotype and function that contribute to immune dysfunction in CKD. Further, the alterations in monocyte function may also be relevant to the pathogenesis of enhanced cardiovascular disease in these patients. In general, uremia shifts monocytes to a CD16-positive phenotype, whereby these cells are smaller in size, inflammatory, adhesive, and senescent [6,7,8].
2.1. Surface Marker Expression
Several studies over the years attempted to characterize monocytes in renal failure patients by detecting the surface expression pattern of typical receptors or membrane bound proteins. The intention was to detect differences in comparison with cells from healthy individuals to draw conclusions on potential functional disruptions. Part of the relevant literature dates back to between 1995 and 2005. Only after that time it became known that monocytes fall into distinct populations and that the quantitative distribution of these populations is affected by CKD. Thus, in the older studies, effects of population distribution cannot be distinguished from alterations of surface marker expression per cell.
Table 1 lists the findings on surface marker expression in patients on hemodialysis in comparison with healthy individuals. It is difficult to draw firm conclusions from these data. First, the studies were done over several decades with changing dialysis technology and treatment modalities. In addition, many of the studies were quite small. With these limitations in mind, a conclusion from the surface marker analysis might be that monocytes show signs of activation, are more prone to react to inflammatory cytokines (e.g., TNF) and oxidized lipoproteins (CD36), and have enhanced ability to adhere and potentially transmigrate the endothelium (CD11b). The enhanced migration capacity of monocytes is suggested by increased chemokine receptor expression of CCR2 and CX3CR1. High expression of ACE may be considered as a further requisite of these cells to produce inflammatory mediators. Enhanced expression of CD40 might be important for cardiovascular diseases.
Table 1.
Comparison of monocyte surface expression density (mean fluorescence intensity) in pre-dialysis samples compared to healthy control persons. Studies that reported % positive cells only without giving expression density are marked with an §. One study measured transcriptional activity for the given protein instead of surface expression by flow cytometry, this study is marked with an #. ↓, down-regulation, ↑, up-regulation, →, unchanged.
| Surface Marker | Function | Comparison to Healthy Control | Quote | Year of Publication |
|---|---|---|---|---|
| CD11b | Integrin, adhesion molecule | ↓ → |
[9] [10,11] |
2000 2000, 2010 |
| CD14 | Endotoxin receptor | ↑ ↓ |
[12] [13] # [10] |
2002 2015 2010 |
| CD16 | Immunoglobulin Fc receptor γIII | → | [13] | 2015 |
| CD31 | PECAM-1 endothelial adhesion | → | [14] | 2001 |
| CD36 | “scavenger” receptor of oxidized lipoproteins | ↑ | [15,16] | 2005 |
| CD40 | Receptor for co-stimulating signals of B-cells, promotes cytokine production in macrophages | ↑ | [17] | 2016 |
| CD68 | Gp110, function? |
↑ | [16] | 2005 |
| CD86 | B7-2, co-stimulation of T-cells | ↓ | [18] | 2001 |
| CD95 | Fas, apoptosis induction |
↑ | [16] | 2005 |
| HLA-DR | Class II tissue antigen, antigen presentation | → ↑ |
[18] [10,19] |
2001 2008, 2010 |
| MAC-1 | CD11b/CD18 dimer, adhesion, complement receptor | ↑ | [14] | 2001 |
| TLR-2 | Toll-like receptor, recognition of bacteria etc. | → ↑ |
[20] [21,22] |
2007 2010, 2011 |
| TLR-4 | Toll-like receptor, LPS-receptor | ↓ → ↑ |
[20] [22] [21] |
2007 2011 2010 |
| TNF-R2 | Receptor for TNF-α | ↑ | [4,23] | 2001, 2005 |
| CX3CR1 | Fractalkine receptor, adhesion molecule | ↑ | [13] # | 2015 |
| CCR2 | C-C chemokine receptor 2 |
↑ | [24] § | 2009 |
| ACE | Angiotensin converting enzyme | ↑ | [25] | 2006 |
| AChR | Receptor for Acetylcholine | ↑ | [26] | 2016 |
2.2. Cytokine and Mediator Secretion
Several studies compared the secretion of cytokines and other mediators by monocytes from patients with CKD with those from healthy individuals. In most cases, these studies purified monocytes by different methods from blood samples, took them into cell culture, and measured the secretion of these mediators into the cell culture supernatant. Table 2 lists the results of these studies.
Table 2.
Cytokine production into the supernatant of cultured monocytes from the blood of hemodialysis patients compared to those from healthy controls. IL = interleukin, TNF = tumor necrosis factor, TGF = transforming growth factor, PTX = pentraxin; * = single cell intracellular measurement. ↓, down-regulation, ↑, up-regulation, →, unchanged.
| Cytokine | Function | Unstimulated | Stimulated by LPS | Quote |
|---|---|---|---|---|
| IL-1ß | Proinflammatory | ↑ | ↓ | [27] |
| IL-6 | Proinflammatory | → * ↑ |
→ * → |
[28] [27,29,30] |
| TNF-α | Proinflammatory | ↑ | → → * ↑ |
[27] [31] [30] |
| TGF-ß | Anti-proliferative, profibrotic | ↑ | [29] | |
| IL-10 | Anti-inflammatory | → * | → | [28] |
| PTX-3 | Pattern recognition, antibacterial defense | ↑ | → | [32] |
A number of earlier studies also described enhanced stimulated secretion of monocyte-derived cytokines, e.g., TNF-α [33], IL-1ß [34,35], IL-1-RA [34], IL-6, and soluble IL-6 receptor [36], TNF-α, IL-8, and the regulatory cytokine IL-10 [37,38]. However, these studies analyzed supernatants from unfractionated leukocytes which makes it difficult to assess the contribution of the monocytes to these findings. These studies will not be considered here in more detail.
Although the literature is ambiguous, it seems safe to conclude that resting monocytes in the circulation of patients with CKD have a higher level of activation than in healthy individuals. Several proinflammatory cytokines are spontaneously secreted into culture supernatant indicating that these cells might contribute to chronic inflammation in the patients. When stimulated by endotoxin (lipopolysaccharide, LPS), these cells cannot enhance cytokine production in the same way as cells from healthy donors do. This might be interpreted as a state of exhaustion.
Again, as with the studies on surface marker expression, monocyte population shifts might contribute to these findings. Studies measuring cytokines in cell culture supernatants of a defined number of monocytes assume that all cells contribute equally. However, later studies showed that this assumption is not true [28,31]. It has been shown by flow-cytometry that the number of cytokine producing monocytes increases while the amount produced by each cell remained unchanged [28].
2.3. Monocyte Function and Expression of Functional Proteins
Monocytes not only secrete cytokines, they also provide several functional proteins that are needed during inflammation to regulate cellular homeostasis. Among these proteins are proinflammatory (ACE, Lp-PLA2) as well as antioxidative substances (SOD1, SOD2). Further cellular systems such as the angiotensin system (ACE, AT1-R, AT2-R) balance functional activation. Table 3 shows the results of studies addressing the expression of functional proteins, most of them measuring transcriptional activation.
Table 3.
Protein content or transcriptional activation for regulatory or anti-inflammatory/antioxidative systems in monocytes from patients with CKD compared to healthy individuals.
| System | Function | Comparison to Healthy Controls | Quote |
|---|---|---|---|
| SOD1 | Superoxide dismutase 1, antioxidative | Protein content low, transcription rate high | [39] |
| SOD2 | Superoxide dismutase 2, antioxidative | Reduced protein content CKD3/4, normal CKD5D; enhanced transcriptional activation all CKD | [40] |
| Rhodanese | Regulation of mitochondrial reactive oxygen species production | Protein content and transcription low | [41] |
| Hsp72 | Heat shock protein 72, protein folding and degradation, cellular damage protection | Protein content and transcription low | [42] |
| SOCS3 | Suppressor of cytokine signaling, modulates intracellular signaling after cytokine-receptor interaction | Enhanced transcription | [43] |
| Lp-PLA2 | Lipoprotein-associated Phospholipase A2, platelet activation, pro-atherogenic | Enhanced transcription | [44] |
| ACE ACE2 AT1-R AT2-R |
Angiotensin converting enzyme Angiotensin converting enzyme type 2 Angiotensin II receptor Type 1 Angiotensin II receptor Type 2 |
Enhanced transcription Reduced transcription Enhanced transcription Normal transcription |
[45] |
CKD and particularly hemodialysis treatment are associated with oxidative stress [6]. The functional alterations regarding antioxidative systems reflect this chronic activation. Such findings can be interpreted as the chronic exhaustion of the protective and regulatory systems with mainly reduced protein content as well as impaired transcription. Interestingly, for several parameters this impairment of protective systems is stronger in dialysis patients than in those with different stages of CKD [39,42]. In contrast, the SOD2 system seems to recover once the patient is on renal replacement therapy [40]. On the other hand, production of reactive oxygen species (ROS) by monocytes is an important mechanism of antibacterial defense by which granulocytes and monocytes respond to Staph. aureus or fungal infections. CKD and dialysis patients are at enhanced risk of staphylococcus sepsis. The studies showed that both ROS production in response to Staphylococcus aureus [38] and phagocytic capacity are impaired in dialysis patients [46]. Thus, the inability of monocytes to mount an adequate response might contribute causally to the high sepsis risk in renal failure patients. With these data, one should keep in mind that monocytes can participate in ROS formation and phagocytosis, however, they are not the “professional” cell type that is quantitatively important for these aspects of antibacterial defense.
Monocytes express several components of the angiotensin system [47], which is mostly known in conjunction with blood pressure and electrolyte regulation. In CKD, monocytes express high levels of the ACE enzyme that converts angiotensin I to angiotensin II. This might be a link between cardiovascular disease and monocyte activation in patients with CKD [25]. However, not only ACE is overexpressed, the entire system of angiotensin modifying enzymes and angiotensin II receptors shows a dysregulated pattern [48]. Treatment with the angiotensin receptor blocker Losartan attenuated monocyte activation in hemodialysis patients [49]. These findings constitute a promising new research area at the boundary between inflammation and atherosclerosis, particularly since the angiotensin system is therapeutically accessible.
Monocytes from patients with CKD and on dialysis treatment show higher apoptosis rates per time than monocytes from healthy controls [50]. This programmed cell death contributes to loss of phagocytic function over time.
3. Monocyte Subpopulations
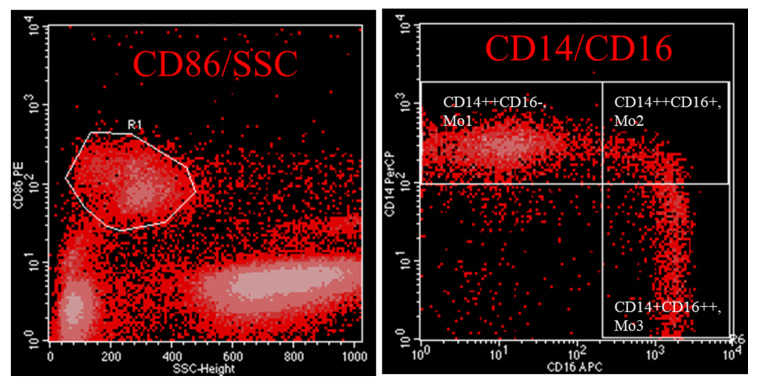
Monocytes are not a homogenous cell type in the circulation. They fall into three distinct populations that have different functions and can be distinguished upon their surface molecule expression pattern. The phenotypic characterization of monocytes can easily be performed by flow-cytometry. Monocytes are staining negative for anti-CD3, -CD19, -CD20, -CD56 and -CD66. According to Ziegler-Heitbrock et al. monocytes are staining positive for HLA-DR and can be subdivided into “classical” (Mo1), “intermediate”, Mo2, or “non-classical”, (Mo3) [1]. An alternative strategy to characterize monocyte subsets favours staining of cells with the pan-monocyte marker CD86 in combination with CD14 and CD16 (Figure 1, [25]). Analysis of gene expression patterns show that Mo2 differ from Mo1 and Mo3 in three predominant aspects: as they express genes encoding MHC class II molecules such as HLA-DR and CD74, Mo2 are predisposed to antigen expression. Further on, Mo2 have a high inflammatory capacity as they express genes such as TGFB1, AIF1 and PTPN6. Finally, since they express the markers Tie2 and CD105, they seem to be involved in angiogenesis [2]. Because of these reasons, it seems justified to label them “inflammatory monocytes”.
Figure 1.
Flow cytometry panel showing gating strategy of monocytes of an uremic patient by anti-CD86 staining and subdivision of monocytes in classical (Mo1), intermediate (Mo2) and non-classical Mo3 according anti-CD14/CD16 staining. R1 includes CD86+ monocytes.
Nockher and Scherberich [51] were the first to point out that there is an expansion of Mo2 cells among the monocytes of patients with CKD. This population accounts for some 8% of the monocytes in healthy individuals while it is expanded up to 18% in CKD. Expansion of the Mo2 population was confirmed by several other groups [31,52,53,54,55,56,57]. The presence of diabetes mellitus seems to accentuate the population expansion in patients with CKD [58].
The expansion of the Mo2 population is associated with cardiovascular disease in dialysis patients [52]. This might be related to the fact that Mo2 cells have particular proinflammatory activity, and systemic inflammation is associated with cardiovascular disease in general. A further interesting finding is that especially CD16+ (Mo2 and Mo3) cells are more prone to attach to endothelial cells in coculture experiments [59]. These cells also appear to be associated with endothelial damage in vivo as Merino et al. found an elevated frequency of CD 16+ monocytes together with increased levels of CD31+Annexin+ microparticles (EMP) in the blood of CKD patients. EMP are considered as an early marker of endothelial damage [60].
The analysis of monocyte populations has revolutionized research on this cell type in CKD. It became obvious that all previous findings on surface marker expression and cytokine secretion need to be reconsidered since they were made with the assumption that monocytes are a homogenous cell type. While up- or downregulation of a marker may be the result of increased or decreased expression per cell, it may well be that this finding merely reflects alterations in monocyte population distribution. To date, several studies have shown clearly that Mo2 cells differ from Mo1 and Mo3 in surface marker expression and function. This was particularly demonstrated for ACE [61], or TLR-9 [62] (both highly expressed on Mo2).
Some studies addressed the question if expansion of the CD14++CD16+ cells can be induced by incubating PBMC in vitro with plasma from patients with CKD [63]. This question cannot be answered yet even though the cited study claimed to see expansion in this setting. Most likely, population shifts occur in the organism by sequestration of cells and release from bone marrow and not by differentiation later on [64]. Nevertheless, uremic toxins may also play a role. At least patients with preserved residual renal function seem to have less expansion of Mo2 cells [65]. Studies on different dialysis modalities found conflicting results. While online-hemodiafiltration (HDF) was reported to reduce the Mo2 population [66,67,68], this could not be demonstrated for high cut-off hemodialysis [69] in prospective trials. In addition to differences in clearance of retention solutes, biophysical aspects of the treatment might be relevant as well. This was suggested by a recent trial comparing mixed vs. postdilution HDF [66]. Potentially, the different hemoconcentration and shear stress contributed to the monocyte population distribution.
4. Causes and Pathogenesis of Monocyte Alterations
Since many of the morphological and functional alterations of monocytes are found in both CKD 3–5 and dialysis patients, uremic toxicity may be one of the important causal factors. However, hemodialysis accentuates some of the monocyte abnormalities. This might be the consequence of inflammatory activation through blood-membrane interactions, and biocompatibility issues may play a role. Further, dialysis can clear several but not all retention solutes from the blood of uremic patients. It is thus reasonable to test different dialysis modalities (HDF, high cut-off dialysis) if they influence monocyte dysfunction. All these aspects have been addressed in clinical or experimental studies.
4.1. Uremic Retention Solutes and Serum Factors
Uremic plasma strongly influences apoptosis rates of monocytes in cell culture [50]. Several studies showed that uremic plasma nearly doubles apoptosis rates [70,71,72]. This effect seems to be attenuated when the plasma donors are treated by high flux compared to low flux dialysis [71].
Plasma from dialysis patients induces several cytokines and activation markers in monocytes in vitro. It was suggested that the plasma content of advanced oxidated protein products and advanced glycated proteins plays a major role in this monocyte activation. This was supported by the incubation of monocytes with artificially modified human serum albumin that led to enhanced oxidative stress and respiratory burst [73].
Incubation of THP1 monocytic cell line with uremic plasma did not only induce a number of cytokines (IL-6, TNF-α) but modified the surface marker pattern similar to what is noticed in monocytes from patients with CKD [48]. Among these surface markers, there is also a marked induction of ACE expression and reduction of ACE2.
While experiments using plasma do not reveal particular mechanisms of monocyte activation, some effects can be tracked down to well defined retention products. Jankowski and coworkers observed a marked inhibition of inducible nitric oxide (NO) synthetase in monocytes incubated with uremic plasma [74]. They fractionated hemofiltrate and submitted it to gas chromatography and mass spectrometry. The inhibitory effect was shown to be caused by phenylacetic acid. This compound could be confirmed to be a uremic toxin that inhibits monocyte phagocytosis [75].
This is a rare example where uremic plasma effects could be tracked down to a particular chemical substance. Most studies describe effects of uremic serum on the whole, e.g., on expression of microRNA-33a and its target genes adenosine triphosphate-binding cassette transporter A1,G1 (ABCA1, ABCG1) in THP-1 macrophages [76].
A recent study showed that serum from patients with renal failure can induce high expression of ACE on monocytes [48]. The effect seems to be closely related to the induction of high level production of the micro-RNA miR-421 by monocytes. Interestingly, miR-421 expression can be induced by the typical uremic toxins indoxyl sulphate, p-cresol (pCS), and p-cresyl sulphate (IS) [77]. pCS and IS are prominent representatives of protein-bound uremic toxins. While pCS induces the basal level of cellular oxidative stress [78], IS spurs leucocyte-endothelial-interactions and induces monocytic inflammation via p38 MAP kinases (mitogen activated protein kinases)—a central pathway triggering inflammatory cytokines such as IL-1ß and TNF-α [79]. The inflammatory nature of IS was demonstrated by other groups. He and colleagues showed that IS induced inflammation via the retinoic acid-inducible gene/NF-κB pathway [80]. Kim et al. proved that monocytes respond to IS by the aryl hydrocarbon receptor followed by production of TNF-α. TNF-α enhanced the expression of the chemokine ligand CX3CR1L by endothelial cells. This, in turn, attracted activated T-cells which induced endothelial apoptosis [81].
Among the water-soluble toxins (<500 dalton) asymmetric dimethylarginine (ADMA) plays an important role in the progression of kidney and cardiovascular diseases. It is regarded as a key modulator of NO metabolism, contributing to oxidative stress and apoptosis [82]. ADMA mediates adhesion of monocytes to the endothelium by up-regulation of chemokine receptors [83]. Another important representative of uremic toxins <500 dalton is homocysteine. Hyperhomocyteinemia (HHcy) is characteristic feature of renal failure patients and linked to cardiovascular diseases. Recently, Yang et al. impressively demonstrated a relationship between HHcy and CD40-positive monocytes. CD40 is a marker that is highly expressed in antigen-presenting cells and interaction of CD40 with its ligand CD40L activates T-cells. In their article, the authors show that CD40+ monocytes are an inflammatory subset similar to intermediate CD14++CD16+. The CD40/CD40L axis is linked to CVD in CKD. A neutralizing antibody against CD40L prevented the differentiation of monocytes towards this inflammatory CD40+monocyte subtype. By this way, the authors illustrate a potential strategy of blocking an inflammatory pathway in CKD patients with cardiovascular disease (CVD) [17].
4.2. The Role of Hemodialysis Treatment on Monocytes Phenotype and Function
Hemodialysis should influence uremic intoxication by removing several solutes in the low to middle molecular weight range. Some studies therefore tested if a hemodialysis session can improve functional parameters in monocytes. However, since clearance of solutes always coincides with blood membrane contact there is the possibility of opposing effects. Contact between blood and dialyzer membranes can induce monocyte activation. Since this activation can be inhibited by treatment with antioxidants, such as superoxide dismutase, it is most likely that oxidative mediators are involved [84].
Carracedo and coworkers studied the role of blood-membrane contacts for the induction of inflammation and apoptosis in monocytes. They incubated THP-1 cells [85] or monocytes [86] in vitro with cuprophane or AN69 dialyzer membranes. This led to different levels of induction of intracellular protein phosphorylation and apoptosis. The activation of caspase-3 is a hallmark of apoptosis. Its activity was raised in cells from patients treated with cellulosic dialyzers. The activation could also be induced by incubating monocytes from healthy individuals in cuprophane mini-dialyzers [87]. Mononuclear cells of patients treated with Cuprophane dialyzer membranes also show a reduced telomer length, which is a characteristic of senescent cells. This feature goes along with increased production of IL-1ß, IL-1RA, and IL-6 [88].
Nowadays the use of biocompatible synthetic membranes is a therapeutic standard in dialysis. When less biocompatible cellulosic membranes were widely used, blood membrane contacts contributed significantly to inflammatory activation of monocytes. Differences between membrane types were described by a number of studies [89,90,91,92,93], among them are also membranes coated with the antioxidant vitamin E [94,95].
Another important feature of dialyzer membranes is their capacity to activate leukocytic adhesion molecules. Adhesion molecules such as CD11b, CD18, and CD62L are a prerequisite for the cells to adhere to the activated vascular endothelium. This is the first step to transendothelial migration. Stavropoulos et al. examined the expression of the adhesion molecules CD11b, CD18, and CD62L before and after a hemodialysis session with either low- or high-flux membranes [96]. While CD62L expression decreased, the other molecules were found at higher expression levels after dialysis. There were no differences between the membrane types.
This study is an example of the difficulties of such experiments: The membranes have different permeability characteristics, however due to their different membrane material, they can also differ in cellular activation signals. Measuring monocytes before and after a dialysis session can be influenced by the dialysis induced sequestration of cells which may heavily change the cell population distribution in peripheral blood. When hemodialysis was introduced as a regular treatment for patients with CKD, profound leukopenia was noted as an immediate complication [97]. Early studies showed that complement activation due to contact of blood with bioincompatible membranes led to granulocyte and monocyte activation. These cell types then adhered to the vascular endothelium, in particular to the capillary endothelium of the lung. Rather few cells may also sequester within the dialyzer module at the membrane [98]. This effect was termed “leukocyte sequestration”, it correlates well with biocompatibility of the dialyzer membrane. Several studies could show that the extent of sequestration is related to the upregulation of adhesion molecules such as P-selectin [99] or CD11b on circulating cells [100,101,102,103].
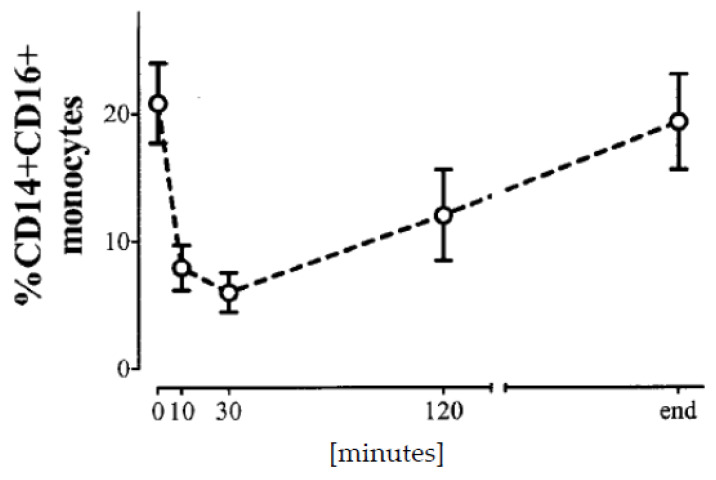
Nowadays, granulocyte sequestration no longer occurs since the membranes are much more biocompatible. Nevertheless, there is still some sequestration of monocytes [5], in particular the proinflammatory populations [104]. Obviously, the Mo2 and Mo3 cell populations are much more likely to be removed from circulation during dialysis [64,105] than Mo1 cells. This effect has been suggested as a measure of dialyzer membrane biocompatibility [106]. As a consequence of sequestration, the cells that remain in the circulation during dialysis produce less cytokines compared to cells from blood samples drawn before a dialysis session [107]. The Mo3 cells reach a nadir at about 15–30 min of a dialysis session and return to predialysis numbers until the end of treatment at 4–5 h (Figure 2) [105]. It is not known whether sequestered cells are released from the endothelium or new cells are spilled out of reservoirs such as the spleen. This sequestration effect has to be considered in all studies that follow cellular or cytokine parameters during a dialysis treatment.
Figure 2.
Time course of circulating Mo3 cell numbers during dialysis sessions (Data from: [105]).
These influences can hardly be controlled experimentally. Therefore, interpretation of pre-post studies has to be done carefully. This holds also true for a study that did not find differences in expression of the proto-oncogene bcl-2 in monocytes [108]. Since the expression increased in lymphocytes (thus indicating inflammatory activation) the lack of effect on monocytes might be caused by sequestration of the activated cells from the circulation. A study on the effect of a dialysis session on monocyte HLA-DR and other markers cannot be interpreted at all since at least part of the findings are likely caused by sequestration [5].
Given these difficulties, all study protocols should measure cytokine expression, monocyte population distribution or other markers preferably before a dialysis session. The effects of different membrane characteristics could then be evaluated without influences from sequestration. Studies comparing the use of high cut-off versus high-flux dialysis membranes on the cytokine production profile [109] and the angiotensin-system [110] found less inflammation and a modification of the ACE/ACE2 relation on monocytes towards the normal situation. This might be taken as a hint that the alterations in dialysis patients may be improved by dialysis technology that removes middle molecules (15–45 kD molecular weight).
4.3. Further Factors That Influence Monocyte Activation
A plethora of further factors have been described that may contribute to the particular phenotype and function of monocytes in CKD. Among them are potential infectious stimulation by colonized central venous catheters [111], cell free DNA [112], or endotoxin that enters the blood stream through the dialyzer membrane from dialysis fluid [113].
The role of therapeutically applied iron is controversial, activation of monocytes is at least possible [114,115], however, seems to be dose-dependent and avoidable [116]. Uremia leads to several modifications of lipoproteins, among them oxidization. Oxidized LDL is associated with activated monocytes and macrophages [117]. The altered HDL composition in plasma also influences monocyte activation [118].
The molecular effects of vitamin D are a further controversial field in terms of monocyte activation. Vitamin D appears to be able to modulate the immune response by down-regulation of HLA-DR expression [119]. Another study demonstrated that vitamin D exerts anti-inflammatory effects in diabetic nephropathy via TLR/NF-κB signaling pathways [120]. Observations in a small patient number with vitamin D deficiency suggested that repletion might influence monocyte activity as serum levels of inflammatory leucocyte-derived cytokines, such as IL-8, IL-6, and TNF-α, were decreased [121]. Meanwhile, a small 12-week prospective trial showed reduction of IL-6 production via supplementation of vitamin D deficiency with 25OH-vitamin D [122], another placebo-controlled study with the same approach neither showed changes in cytokine production nor monocyte CD14/CD16 population distribution [123].
5. Consequences of Monocyte Alterations
5.1. Antimicrobial Defense
Multiple phenotypic and functional observations contribute to the explanation of the clinical aspects of monocyte dysfunction in patients with CKD. These clinical consequences are a profound immune defect leading to a high morbidity from infectious diseases, and progressive atherosclerosis. Many aspects of immunologic dysfunction have been discussed above, e.g., the reduced signaling of monocytes during T-lymphocyte activation. This is in part caused by diminished expression of the signaling molecule B7-2 [18] and contributes to impaired vaccination results in these patients against hepatitis B or influenza. Other examples are reduced phagocytosis [46] that might be important in defense against bacterial infection, or monocyte antiviral activity [124].
Circulating monocytes are not a professional antimicrobial cell type. However, after tissue invasion through the endothelium, they differentiate into dendritic cells (DC). These cells are highly important for activation and regulation of the antigen-specific immune defense. A few studies have tested the capacity of monocytes from patients with renal failure to differentiate into DC in vitro. They found that these cells differentiate more quickly to DC [125], however they also suggest that this differentiation may not entirely follow the functional path of cells from healthy individuals. Choi et al. provided information about the stimulatory capacity of mature mo-derived dendritic cells (mo-DCs). They demonstrated that an inflammatory cocktail consisting of IL-1ß, Il-6, TNF-α and prostaglandin E2 led to significant higher IL-6 production in HD patients compared to healthy controls [126]. Further on, incubation with uremic sera decreased endocytosis and increased IL-12p70 production in mo-DC from healthy donors. Mo-DC isolated from HD patients and incubated with healthy sera showed reduced endocytosis and produced higher amounts of IL-12p70 than mo-DC from healthy donors [127]. Regarding mo-DC, the impairment of these cells have profound clinical consequences. This abnormality is related to the impaired Hepatitis B vaccination, probably because of an impairment of mo-DCs to stimulate antigen-specific T cells [128]. This is very plausible since DCs are involved in tissue antigen presentation after intramuscular injection of vaccine.
5.2. Monocyte Activation Contributes to Atherosclerosis
Monocytes and macrophages strongly contribute to the pathogenesis of atherosclerosis. These cells invade the vessel wall at sites of endothelial lesions and participate in the formation of foam cells, the deposition of lipids in the intima, and the growth of the atheromatous plaque [129]. Macrophages within vascular plaques express inflammatory markers [130]. These observations prompted the study of a relationship between the activation of circulating monocytes in the blood of dialysis patients and their risk of atherosclerotic vascular disease. Investigators found many hints that such relation exists. Studies in dialysis patients described correlations between monocyte expression of IL-1 and IL-1RA and cardiovascular events [131]. The relative frequency of CD14++/16+ Mo2 cells relates to this morbidity in different cohorts with CKD including HD, PD and CKD patients stages 1–5 [52,132,133]. A high expression of ACE on monocytes appears to be associated with cardiovascular disease in HD and PD patients [25,61,134]. In addition, the intensity of monocyte sequestration during a dialysis session as a surrogate for the activation level of this cell type is predictive for cardiovascular events as well [135]. Nevertheless, negative studies must be noted as well. The number of TLR-4 positive monocytes in the circulation was not predictive for cardiovascular mortality in CKD patients stage 5 [136].
While these associations do not establish causality, some findings at least suggest that there could be direct an involvement of the cell type in the pathogenesis of atherosclerosis. Monocytes from dialysis patients highly express the scavenger receptor CD36 [137] or at least show high transcription for this molecule [138]. The scavenger receptor is important for the uptake of LDL-cholesterol into monocytes and macrophages which leads to foam cell formation. Monocytes from renal failure patients differentiate into highly activated, inflammatory and cytokine producing foam cells in vitro [139]. Monocytes are also more prone to adhere to endothelium and to transmigrate in chamber experiments [140]. These findings are closely associated with ACE expression on this cell type [45]. Finally, markers of monocyte activation could also be shown in the vessel wall itself in dialysis patients who had vascular surgery for atherosclerotic artery disease [141]. Vessel invading monocytes were positive for the markers anti-CD14 and ant-CD68. Further on, monocytes expressed the post-translationally modified acetylated form of the Y-box binding protein-1 (YB-1). This protein is known to regulate the transcription of the C-C chemokine 5 (CCL5/Rantes) gene which serves as a chemoattractant during inflammation and atherosclerosis.
While monocytes may contribute to ischemic vascular disease by promoting atherosclerotic plaque growth, the cells can also enhance platelet activation and support thrombotic vessel occlusion. Platelets and monocytes seem to form aggregates in the circulation and the activation of platelets parallels that of monocytes. The formation of these aggregates was higher in HD compared to PD patients [142,143]. In one study the extent of such activation correlated with the cardiovascular event risk in hemodialysis patients [142]. These aggregates between monocytes and platelet are formed during dialysis while platelet P-selectin expression increases in HD patients [144]. However, not all groups could confirm these findings [145].
6. Conclusions and Perspectives
Regarding monocytes, impairment and activation are two sides of the same coin—uremia. A decrease of phagocytic capabilities, impairment of antigen presentation function on the one side and elevation of inflammatory markers and specialized monocyte subsets on the other side characterize the monocyte momentum in CKD. Functionally, the cells are equipped with a machinery for production of inflammatory cytokines and adhesive/migratory receptors, thus being able to enhance and transfer inflammation to sites of vascular damage. This can result in initiation and progression of cardiovascular diseases, most prominently atherosclerosis. Since dialysis contributed to monocyte alterations, the improvement of biocompatibility of the treatment has been a valid approach in the past. However, CKD itself, including the retention of middle molecules and uremic toxins, remains a major pathogenetic cause of inflammation and impaired immune defense. Several studies address the potential of anti-inflammatory intervention to reduce atherosclerotic cardiovascular disease [146]. Such studies should be accompanied by the monitoring of monocyte function in the future.
7. Methods
Since the available literature is highly heterogenic in choice of methods and reporting, the form of a narrative review was chosen. Literature was identified using the following PubMed query:
(“Macrophages”[Mesh] OR “Monocytes”[Mesh]) AND (“Kidney Failure, Chronic”[Mesh] OR “Renal Dialysis”[Mesh])
The results from this query (n = 948) were filtered using the following filters: with abstract, English language, Humans, time range 01.01.1994–31.12.2019. The resulting 466 entries were read and evaluated. Studies on the pathogenesis of glomerular diseases, infectious complications (mainly peritonitis in peritoneal dialysis), kidney transplantation, renal osteodystrophy, and studies not mentioning monocytes were excluded. We further excluded case reports and review publications not reporting primary data.
Author Contributions
M.G. conceived and designed the review; M.G. and C.U. performed the literature research; M.G., B.T. and C.U. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by intramural resources of the Department of Internal Medicine II of the Martin-Luther-University Halle-Wittenberg, Germany.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
Structural and functional abnormalities of monocytes from patients with chronic kidney disease have been described in a large number of publications. This review aimed to collect and summarize these findings. Data obtained to date demonstrate that monocytes from chronic kidney disease (CKD) patients show a high level of basal inflammation combined with impaired activation upon infectious challenge. These phenomena might contribute to the high morbidity observed in these patients.
References
- 1.Ziegler-Heitbrock L., Ancuta P., Crowe S., Dalod M., Grau V., Hart D.N., Leenen P.J.M., Liu Y.-J., MacPherson G., Randolph G.J., et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 2.Zawada A.M., Rogacev K.S., Rotter B., Winter P., Marell R.-R., Fliser D., Heine G.H. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 2011;118:e50–e61. doi: 10.1182/blood-2011-01-326827. [DOI] [PubMed] [Google Scholar]
- 3.Le Meur Y., Lorgeot V., Aldigier J.C., Wijdenes J., Leroux-Robert C., Praloran V. Whole blood production of monocytic cytokines (IL-1beta, IL-6, TNF-alpha, sIL-6R, IL-1Ra) in haemodialysed patients. Nephrol. Dial. Transplant. 1999;14:2420–2426. doi: 10.1093/ndt/14.10.2420. [DOI] [PubMed] [Google Scholar]
- 4.Van Riemsdijk-Van Overbeeke I.C., Baan C.C., Knoop C.J., Loonen E.H., Zietse R., Weimar W. Quantitative flow cytometry shows activation of the TNF-alpha system but not of the IL-2 system at the single cell level in renal replacement therapy. Nephrol. Dial. Transpl. 2001;16:1430–1435. doi: 10.1093/ndt/16.7.1430. [DOI] [PubMed] [Google Scholar]
- 5.Braun N. Expression of adhesion molecules and activation markers on lymphocytes and monocytes during hemodialysis. Blood Purif. 1997;15:61–76. doi: 10.1159/000170318. [DOI] [PubMed] [Google Scholar]
- 6.Liakopoulos V., Roumeliotis S., Zarogiannis S., Eleftheriadis T., Mertens P.R. Oxidative stress in hemodialysis: Causative mechanisms, clinical implications, and possible therapeutic interventions. Semin. Dial. 2019;32:58–71. doi: 10.1111/sdi.12745. [DOI] [PubMed] [Google Scholar]
- 7.Borges Bonan N., Schepers E., Pecoits-Filho R., Dhondt A., Pletinck A., de Somer F., Vanholder R., van Biesen W., Moreno-Amaral A., Glorieux G. Contribution of the uremic milieu to an increased pro-inflammatory monocytic phenotype in chronic kidney disease. Sci. Rep. 2019;9:10236. doi: 10.1038/s41598-019-46724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu Y.-L., Shu K.-H., Yang F.-J., Chou T.-Y., Chen P.-M., Lay F.-Y., Pan S.-Y., Lin C.-J., Litjens N.H.R., Betjes M.G.H., et al. A comprehensive characterization of aggravated aging-related changes in T lymphocytes and monocytes in end-stage renal disease: The iESRD study. Immun. Ageing. 2018;15:27. doi: 10.1186/s12979-018-0131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobson S.H., Thylén P., Lundahl J. Three monocyte-related determinants of atherosclerosis in haemodialysis. Nephrol. Dial. Transpl. 2000;15:1414–1419. doi: 10.1093/ndt/15.9.1414. [DOI] [PubMed] [Google Scholar]
- 10.Pereira R., Costa E., Gonçalves M., Miranda V., do Sameiro Faria M., Quintanilha A., Belo L., Lima M., Santos-Silva A. Neutrophil and monocyte activation in chronic kidney disease patients under hemodialysis and its relationship with resistance to recombinant human erythropoietin and to the hemodialysis procedure. Hemodial. Int. 2010;14:295–301. doi: 10.1111/j.1542-4758.2010.00450.x. [DOI] [PubMed] [Google Scholar]
- 11.Thylén P., Lundahl J., Fernvik E., Grönneberg R., Halldén G., Jacobson S.H. Impaired monocyte CD11b expression in interstitial inflammation in hemodialysis patients. Kidney Int. 2000;57:2099–2106. doi: 10.1046/j.1523-1755.2000.00060.x. [DOI] [PubMed] [Google Scholar]
- 12.Kawabata K., Nakai S., Miwa M., Sugiura T., Otsuka Y., Shinzato T., Hiki Y., Tomimatsu I., Ushida Y., Hosono F., et al. Changes in Mac-1 and CD14 expression on monocytes and serum soluble CD14 level during push/pull hemodiafiltration. Nephron. 2002;90:273–281. doi: 10.1159/000049063. [DOI] [PubMed] [Google Scholar]
- 13.Schepers E., Houthuys E., Dhondt A., de Meyer G., Neirynck N., Bernaert P., van den Bergh R., Brouckaert P., Vanholder R., Glorieux G. Transcriptome analysis in patients with chronic kidney disease on hemodialysis disclosing a key role for CD16+CX3CR1+ monocytes. PLoS ONE. 2015;10:e0121750. doi: 10.1371/journal.pone.0121750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawabata K., Nakai S., Miwa M., Sugiura T., Otsuka Y., Shinzato T., Hiki N., Tomimatsu I., Ushida Y., Hosono F., et al. CD31 expression on leukocytes is downregulated in vivo during hemodialysis. Nephron. 2001;89:153–160. doi: 10.1159/000046062. [DOI] [PubMed] [Google Scholar]
- 15.Chmielewski M., Bryl E., Marzec L., Aleksandrowicz E., Witkowski J.M., Rutkowski B. Expression of scavenger receptor CD36 in chronic renal failure patients. Artif. Organs. 2005;29:608–614. doi: 10.1111/j.1525-1594.2005.29097.x. [DOI] [PubMed] [Google Scholar]
- 16.Wu C.-C., Chen J.-S., Lin S.-H., Chu P., Lin Y.-F., Lin S.-M., Liao T.-N. Aberrant activation of the TNF-alpha system and production of Fas and scavenger receptors on monocytes in patients with end-stage renal disease. Artif. Organs. 2005;29:701–707. doi: 10.1111/j.1525-1594.2005.29110.x. [DOI] [PubMed] [Google Scholar]
- 17.Yang J., Fang P., Zhang L., Zhang D., Yang W.Y., Bottiglieri T., Kunapuli S.P., Yu J., Choi E.T., Ji Y., et al. Chronic Kidney Disease Induces Inflammatory CD40+ Monocyte Differentiation via Homocysteine Elevation and DNA Hypomethylation. Circ. Res. 2016;11:1226–1241. doi: 10.1161/CIRCRESAHA.116.308750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girndt M., Sester M., Sester U., Kaul H., Köhler H. Defective expression of B7-2 (CD86) on monocytes of dialysis patients correlates to the uremia-associated immune defect. Kidney Int. 2001;59:1382–1389. doi: 10.1046/j.1523-1755.2001.0590041382.x. [DOI] [PubMed] [Google Scholar]
- 19.De Cal M., Cruz D.N., Corradi V., Nalesso F., Polanco N., Lentini P., Brendolan A., Tetta C., Ronco C. HLA-DR expression and apoptosis: A cross-sectional controlled study in hemodialysis and peritoneal dialysis patients. Blood Purif. 2008;26:249–254. doi: 10.1159/000122110. [DOI] [PubMed] [Google Scholar]
- 20.Kuroki Y., Tsuchida K., Go I., Aoyama M., Naganuma T., Takemoto Y., Nakatani T. A study of innate immunity in patients with end-stage renal disease: Special reference to toll-like receptor-2 and -4 expression in peripheral blood monocytes of hemodialysis patients. Int. J. Mol. Med. 2007;19:783–790. doi: 10.3892/ijmm.19.5.783. [DOI] [PubMed] [Google Scholar]
- 21.Gollapudi P., Yoon J.-W., Gollapudi S., Pahl M.V., Vaziri N.D. Leukocyte toll-like receptor expression in end-stage kidney disease. Am. J. Nephrol. 2010;31:247–254. doi: 10.1159/000276764. [DOI] [PubMed] [Google Scholar]
- 22.Koc M., Toprak A., Arikan H., Odabasi Z., Elbir Y., Tulunay A., Asicioglu E., Eksioglu-Demiralp E., Glorieux G., Vanholder R., et al. Toll-like receptor expression in monocytes in patients with chronic kidney disease and haemodialysis: Relation with inflammation. Nephrol. Dial. Transpl. 2011;26:955–963. doi: 10.1093/ndt/gfq500. [DOI] [PubMed] [Google Scholar]
- 23.Wu C.C., Liao T.N., Lu K.C., Chen J.S., Chu P., Lin S.H., Chuang C.H., Lin Y.F. Apoptotic markers on lymphocytes and monocytes are unchanged during single hemodialysis sessions using either regenerated cellulose or polysulfone membranes. Clin. Nephrol. 2005;64:198–204. doi: 10.5414/CNP64198. [DOI] [PubMed] [Google Scholar]
- 24.Okumoto S., Taniguchi Y., Nakashima A., Masaki T., Ito T., Ogawa T., Takasugi N., Kohno N., Yorioka N. C-C chemokine receptor 2 expression by circulating monocytes influences atherosclerosis in patients on chronic hemodialysis. Ther. Apher. Dial. 2009;13:205–212. doi: 10.1111/j.1744-9987.2009.00658.x. [DOI] [PubMed] [Google Scholar]
- 25.Ulrich C., Heine G.H., Garcia P., Reichart B., Georg T., Krause M., Köhler H., Girndt M. Increased expression of monocytic angiotensin-converting enzyme in dialysis patients with cardiovascular disease. Nephrol. Dial. Transpl. 2006;21:1596–1602. doi: 10.1093/ndt/gfl008. [DOI] [PubMed] [Google Scholar]
- 26.Seibert E., Zohles K., Ulrich C., Kluttig A., Nuding S., Kors J.A., Swenne C.A., Werdan K., Fiedler R., Girndt M. Association between autonomic nervous dysfunction and cellular inflammation in end-stage renal disease. BMC Cardiovasc. Disord. 2016;16:210. doi: 10.1186/s12872-016-0385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malaponte G., Bevelacqua V., Fatuzzo P., Rapisarda F., Emmanuele G., Travali S., Mazzarino M.C. IL-1beta, TNF-alpha and IL-6 release from monocytes in haemodialysis patients in relation to dialytic age. Nephrol. Dial. Transpl. 2002;17:1964–1970. doi: 10.1093/ndt/17.11.1964. [DOI] [PubMed] [Google Scholar]
- 28.Girndt M., Sester U., Kaul H., Köhler H. Production of proinflammatory and regulatory monokines in hemodialysis patients shown at a single-cell level. J. Am. Soc. Nephrol. 1998;9:1689–1696. doi: 10.1681/ASN.V991689. [DOI] [PubMed] [Google Scholar]
- 29.Mege J.L., Capo C., Purgus R., Olmer M. Monocyte production of transforming growth factor beta in long-term hemodialysis: Modulation by hemodialysis membranes. Am. J. Kidney Dis. 1996;28:395–399. doi: 10.1016/S0272-6386(96)90497-7. [DOI] [PubMed] [Google Scholar]
- 30.Asmis R., Stevens J., Begley J.G., Grimes B., van Zant G., Fanti P. The isoflavone genistein inhibits LPS-stimulated TNFalpha, but not IL-6 expression in monocytes from hemodialysis patients and healthy subjects. Clin. Nephrol. 2006;65:267–275. doi: 10.5414/CNP65267. [DOI] [PubMed] [Google Scholar]
- 31.Kim H.W., Yang H.-N., Kim M.G., Choi H.M., Jo S.-K., Cho W.Y., Kim H.K. Microinflammation in hemodialysis patients is associated with increased CD14CD16(+) pro-inflammatory monocytes: Possible modification by on-line hemodiafiltration. Blood Purif. 2011;31:281–288. doi: 10.1159/000321889. [DOI] [PubMed] [Google Scholar]
- 32.Malaponte G., Libra M., Bevelacqua Y., Merito P., Fatuzzo P., Rapisarda F., Cristina M., Naselli G., Stivala F., Mazzarino M.C., et al. Inflammatory status in patients with chronic renal failure: The role of PTX3 and pro-inflammatory cytokines. Int. J. Mol. Med. 2007;20:471–481. doi: 10.3892/ijmm.20.4.471. [DOI] [PubMed] [Google Scholar]
- 33.Balakrishnan V.S., Jaber B.L., Natov S.N., Cendoroglo M., King A.J., Schmid C.H., Pereira B.J. Interleukin-1 receptor antagonist synthesis by peripheral blood mononuclear cells in hemodialysis patients. Kidney Int. 1998;54:2106–2112. doi: 10.1046/j.1523-1755.1998.00185.x. [DOI] [PubMed] [Google Scholar]
- 34.Donati D., Degiannis D., Mazzola E., Gastaldi L., Raskova J., Raska K., Camussi G. Interleukin-1 receptors and receptor antagonist in haemodialysis. Nephrol. Dial. Transpl. 1997;12:111–118. doi: 10.1093/ndt/12.1.111. [DOI] [PubMed] [Google Scholar]
- 35.Momoi T., Ono M., Takagi T., Sugiura S., Ogawa H., Saito A. The effects of hemodialysis (HD) membranes on interleukin 1-beta (IL-1 beta) production from peripheral blood mononuclear cells (PBMC) Clin. Nephrol. 1995;44:S24–S28. [PubMed] [Google Scholar]
- 36.Memoli B., Grandaliano G., Soccio M., Postiglione L., Guida B., Bisesti V., Esposito P., Procino A., Marrone D., Michael A., et al. In vivo modulation of soluble “antagonistic” IL-6 receptor synthesis and release in ESRD. J. Am. Soc. Nephrol. 2005;16:1099–1107. doi: 10.1681/ASN.2004080628. [DOI] [PubMed] [Google Scholar]
- 37.Morita Y., Yamamura M., Kashihara N., Makino H. Increased production of interleukin-10 and inflammatory cytokines in blood monocytes of hemodialysis patients. Res. Commun. Mol. Pathol. Pharmacol. 1997;98:19–33. [PubMed] [Google Scholar]
- 38.Sardenberg C., Suassuna P., Watanabe R., Cruz Andreoli M.C., Aparecida Dalboni M., Faria Seabra V., Draibe S.A., Cendoroglo Neto M., Jaber B. Balance between cytokine production by peripheral blood mononuclear cells and reactive oxygen species production by monocytes in patients with chronic kidney disease. Ren. Fail. 2004;26:673–681. doi: 10.1081/JDI-200037122. [DOI] [PubMed] [Google Scholar]
- 39.Scholze A., Krueger K., Diedrich M., Räth C., Torges A., Jankowski V., Maier A., Thilo F., Zidek W., Tepel M. Superoxide dismutase type 1 in monocytes of chronic kidney disease patients. Amino Acids. 2011;41:427–438. doi: 10.1007/s00726-010-0763-4. [DOI] [PubMed] [Google Scholar]
- 40.Krueger K., Shen J., Maier A., Tepel M., Scholze A. Lower Superoxide Dismutase 2 (SOD2) Protein Content in Mononuclear Cells Is Associated with Better Survival in Patients with Hemodialysis Therapy. Oxid. Med. Cell. Longev. 2016;2016:7423249. doi: 10.1155/2016/7423249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krueger K., Koch K., Jühling A., Tepel M., Scholze A. Low expression of thiosulfate sulfurtransferase (rhodanese) predicts mortality in hemodialysis patients. Clin. Biochem. 2010;43:95–101. doi: 10.1016/j.clinbiochem.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Marzec L., Zdrojewski Z., Liberek T., Bryl E., Chmielewski M., Witkowski J.M., Rutkowski B. Expression of Hsp72 protein in chronic kidney disease patients. Scand. J. Urol. Nephrol. 2009;43:400–408. doi: 10.3109/00365590903089489. [DOI] [PubMed] [Google Scholar]
- 43.Rastmanesh M.M., Bluyssen H.A.R., Joles J.A., Boer P., Willekes N., Braam B. Increased expression of SOCS3 in monocytes and SOCS1 in lymphocytes correlates with progressive loss of renal function and cardiovascular risk factors in chronic kidney disease. Eur. J. Pharmacol. 2008;593:99–104. doi: 10.1016/j.ejphar.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 44.Ulrich C., Trojanowicz B., Fiedler R., Kohler F., Wolf A.-F., Seibert E., Girndt M. Differential Expression of Lipoprotein-Associated Phospholipase A2 in Monocyte Subsets: Impact of Uremia and Atherosclerosis. Nephron. 2017;135:1–11. doi: 10.1159/000454778. [DOI] [PubMed] [Google Scholar]
- 45.Trojanowicz B., Ulrich C., Kohler F., Bode V., Seibert E., Fiedler R., Girndt M. Monocytic angiotensin-converting enzyme 2 relates to atherosclerosis in patients with chronic kidney disease. Nephrol. Dial. Transpl. 2017;32:287–298. doi: 10.1093/ndt/gfw206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muniz-Junqueira M.I., Braga Lopes C., Magalhães C.A.M., Schleicher C.C., Veiga J.P.R. Acute and chronic influence of hemodialysis according to the membrane used on phagocytic function of neutrophils and monocytes and pro-inflammatory cytokines production in chronic renal failure patients. Life Sci. 2005;77:3141–3155. doi: 10.1016/j.lfs.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 47.Kitazono T., Padgett R.C., Armstrong M.L., Tompkins P.K., Heistad D.D. Evidence that angiotensin II is present in human monocytes. Circulation. 1995;91:1129–1134. doi: 10.1161/01.CIR.91.4.1129. [DOI] [PubMed] [Google Scholar]
- 48.Trojanowicz B., Ulrich C., Seibert E., Fiedler R., Girndt M. Uremic Conditions Drive Human Monocytes to Pro-Atherogenic Differentiation via an Angiotensin-Dependent Mechanism. PLoS ONE. 2014;9:e102137. doi: 10.1371/journal.pone.0102137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merino A., Alvarez-Lara M.A., Ramirez R., Carracedo J., Martin-Malo A., Aljama P. Losartan prevents the development of the pro-inflammatory monocytes CD14+CD16+ in haemodialysis patients. Nephrol. Dial. Transpl. 2012;27:2907–2912. doi: 10.1093/ndt/gfr767. [DOI] [PubMed] [Google Scholar]
- 50.Heidenreich S., Schmidt M., Bachmann J., Harrach B. Apoptosis of monocytes cultured from long-term hemodialysis patients. Kidney Int. 1996;49:792–799. doi: 10.1038/ki.1996.110. [DOI] [PubMed] [Google Scholar]
- 51.Nockher W.A., Scherberich J.E. Expanded CD14+ CD16+ monocyte subpopulation in patients with acute and chronic infections undergoing hemodialysis. Infect. Immun. 1998;66:2782–2790. doi: 10.1128/IAI.66.6.2782-2790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heine G.H., Ulrich C., Seibert E., Seiler S., Marell J., Reichart B., Krause M., Schlitt A., Köhler H., Girndt M. CD14(++)CD16+ monocytes but not total monocyte numbers predict cardiovascular events in dialysis patients. Kidney Int. 2008;73:622–629. doi: 10.1038/sj.ki.5002744. [DOI] [PubMed] [Google Scholar]
- 53.Saionji K., Ohsaka A. Expansion of CD4+CD16+ blood monocytes in patients with chronic renal failure undergoing dialysis: Possible involvement of macrophage colony-stimulating factor. Acta Haematol. 2001;105:21–26. doi: 10.1159/000046528. [DOI] [PubMed] [Google Scholar]
- 54.Kim H.W., Woo Y.S., Yang H.N., Choi H.M., Jo S.K., Cho W.Y., Kim H.-K. Primed monocytes: Putative culprits of chronic low-grade inflammation and impaired innate immune responses in patients on hemodialysis. Clin. Exp. Nephrol. 2011;15:258–263. doi: 10.1007/s10157-010-0379-8. [DOI] [PubMed] [Google Scholar]
- 55.Liakopoulos V., Jeron A., Shah A., Bruder D., Mertens P.R., Gorny X. Hemodialysis-related changes in phenotypical features of monocytes. Sci. Rep. 2018;8:13964. doi: 10.1038/s41598-018-31889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramirez R., Carracedo J., Berdud I., Carretero D., Merino A., Rodríguez M., Tetta C., Martín-Malo A., Aljama P. Microinflammation in hemodialysis is related to a preactivated subset of monocytes. Hemodial. Int. 2006;10:S24–S27. doi: 10.1111/j.1542-4758.2006.01186.x. [DOI] [PubMed] [Google Scholar]
- 57.Kawanaka N., Nagake Y., Yamamura M., Makino H. Expression of Fc gamma receptor III (CD16) on monocytes during hemodialysis in patients with chronic renal failure. Nephron. 2002;90:64–71. doi: 10.1159/000046316. [DOI] [PubMed] [Google Scholar]
- 58.Carmona A., Agüera M.L., Luna-Ruiz C., Buendía P., Calleros L., García-Jerez A., Rodríguez-Puyol M., Arias M., Arias-Guillen M., de Arriba G., et al. Markers of endothelial damage in patients with chronic kidney disease on hemodialysis. Am. J. Physiol. Renal Physiol. 2017;312:F673–F681. doi: 10.1152/ajprenal.00013.2016. [DOI] [PubMed] [Google Scholar]
- 59.Ramírez R., Carracedo J., Merino A., Soriano S., Ojeda R., Alvarez-Lara M.A., Martín-Malo A., Aljama P. CD14+CD16+ monocytes from chronic kidney disease patients exhibit increased adhesion ability to endothelial cells. Contrib. Nephrol. 2011;171:57–61. doi: 10.1159/000327134. [DOI] [PubMed] [Google Scholar]
- 60.Merino A., Portolés J., Selgas R., Ojeda R., Buendia P., Ocaña J., Bajo M.A., del Peso G., Carracedo J., Ramírez R., et al. Effect of different dialysis modalities on microinflammatory status and endothelial damage. Clin. J. Am. Soc. Nephrol. 2010;5:227–234. doi: 10.2215/CJN.03260509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ulrich C., Heine G.H., Seibert E., Fliser D., Girndt M. Circulating monocyte subpopulations with high expression of angiotensin-converting enzyme predict mortality in patients with end-stage renal disease. Nephrol. Dial. Transpl. 2010;25:2265–2272. doi: 10.1093/ndt/gfq012. [DOI] [PubMed] [Google Scholar]
- 62.Merino A., Nogueras S., García-Maceira T., Rodríguez M., Martin-Malo A., Ramirez R., Carracedo J., Aljama P. Bacterial DNA and endothelial damage in haemodialysis patients. Nephrol. Dial. Transpl. 2008;23:3635–3642. doi: 10.1093/ndt/gfn308. [DOI] [PubMed] [Google Scholar]
- 63.Bonan N.B., Steiner T.M., Kuntsevich V., Virzì G.M., Azevedo M., Nakao L.S., Barreto F.C., Ronco C., Thijssen S., Kotanko P., et al. Uremic Toxicity-Induced Eryptosis and Monocyte Modulation: The Erythrophagocytosis as a Novel Pathway to Renal Anemia. Blood Purif. 2016;41:317–323. doi: 10.1159/000443784. [DOI] [PubMed] [Google Scholar]
- 64.Nockher W.A., Wiemer J., Scherberich J.E. Haemodialysis monocytopenia: Differential sequestration kinetics of CD14+CD16+ and CD14++ blood monocyte subsets. Clin. Exp. Immunol. 2001;123:49–55. doi: 10.1046/j.1365-2249.2001.01436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Sequera P., Corchete E., Bohorquez L., Albalate M., Perez-Garcia R., Alique M., Marques M., García-Menéndez E., Portolés J., Ramirez R. Residual Renal Function in Hemodialysis and Inflammation. Ther. Apher. Dial. 2017;21:592–598. doi: 10.1111/1744-9987.12576. [DOI] [PubMed] [Google Scholar]
- 66.Bolasco P., Spiga P., Arras M., Murtas S., La Nasa G. Could there be Haemodynamic Stress Effects on Pro-Inflammatory CD14+CD16+ Monocytes during Convective-Diffusive Treatments? A Prospective Randomized Controlled Trial. Blood Purif. 2019;47:385–394. doi: 10.1159/000494711. [DOI] [PubMed] [Google Scholar]
- 67.Carracedo J., Merino A., Nogueras S., Carretero D., Berdud I., Ramírez R., Tetta C., Rodríguez M., Martín-Malo A., Aljama P. On-line hemodiafiltration reduces the proinflammatory CD14+CD16+ monocyte-derived dendritic cells: A prospective, crossover study. J. Am. Soc. Nephrol. 2006;17:2315–2321. doi: 10.1681/ASN.2006020105. [DOI] [PubMed] [Google Scholar]
- 68.Ariza F., Merino A., Carracedo J., Alvarez de Lara M.A., Crespo R., Ramirez R., Martín-Malo A., Aljama P. Post-dilution high convective transport improves microinflammation and endothelial dysfunction independently of the technique. Blood Purif. 2013;35:270–278. doi: 10.1159/000350611. [DOI] [PubMed] [Google Scholar]
- 69.Fiedler R., Neugebauer F., Ulrich C., Wienke A., Gromann C., Storr M., Böhler T., Seibert E., Girndt M. Randomized controlled pilot study of 2 weeks’ treatment with high cutoff membrane for hemodialysis patients with elevated C-reactive protein. Artif. Organs. 2012;36:886–893. doi: 10.1111/j.1525-1594.2012.01479.x. [DOI] [PubMed] [Google Scholar]
- 70.Andrikos E., Buoncristiani E., D’Intini V., Bordoni V., Bonello M., Levin N., Buoncristiani U., Pappas M., Ronco C. Effect of daily hemodialysis on monocytes apoptosis. Blood Purif. 2005;23:79–82. doi: 10.1159/000082015. [DOI] [PubMed] [Google Scholar]
- 71.Bordoni V., Piroddi M., Galli F., de Cal M., Bonello M., Dimitri P., Salvatori G., Ranishta R., Levin N., Tetta C., et al. Oxidant and carbonyl stress-related apoptosis in end-stage kidney disease: Impact of membrane flux. Blood Purif. 2006;24:149–156. doi: 10.1159/000089452. [DOI] [PubMed] [Google Scholar]
- 72.D’Intini V., Bordoni V., Bolgan I., Bonello M., Brendolan A., Crepaldi C., Gastaldon F., Levin N.W., Bellomo R., Ronco C. Monocyte apoptosis in uremia is normalized with continuous blood purification modalities. Blood Purif. 2004;22:9–12. doi: 10.1159/000074918. [DOI] [PubMed] [Google Scholar]
- 73.Witko-Sarsat V., Friedlander M., Nguyen Khoa T., Capeillere-Blandin C., Nguyen A.T., Canteloup S., Dayer J.M., Jungers P., Drueke T., Descamps-Latscha B. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J. Immunol. 1998;161:2524–2532. [PubMed] [Google Scholar]
- 74.Jankowski J., van der Giet M., Jankowski V., Schmidt S., Hemeier M., Mahn B., Giebing G., Tolle M., Luftmann H., Schluter H., et al. Increased plasma phenylacetic acid in patients with end-stage renal failure inhibits iNOS expression. J. Clin. Investig. 2003;112:256–264. doi: 10.1172/JCI200315524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmidt S., Westhoff T.H., Krauser P., Ignatius R., Jankowski J., Jankowski V., Zidek W., van der Giet M. The uraemic toxin phenylacetic acid impairs macrophage function. Nephrol. Dial. Transpl. 2008;23:3485–3493. doi: 10.1093/ndt/gfn266. [DOI] [PubMed] [Google Scholar]
- 76.Wang J.-M., Zhou J.-J., Zheng Q., Gan H., Wang H. Dialysis method alters the expression of microRNA-33a and its target genes ABCA1, ABCG1 in THP-1 macrophages. Ther. Apher. Dial. 2014;18:44–50. doi: 10.1111/1744-9987.12040. [DOI] [PubMed] [Google Scholar]
- 77.Trojanowicz B., Imdahl T., Ulrich C., Fiedler R., Girndt M. Circulating miR-421 Targeting Leucocytic Angiotensin Converting Enzyme 2 Is Elevated in Patients with Chronic Kidney Disease. Nephron. 2019;141:61–74. doi: 10.1159/000493805. [DOI] [PubMed] [Google Scholar]
- 78.Schepers E., Meert N., Glorieux G., Goeman J., van der Eycken J., Vanholder R. P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol. Dial. Transpl. 2007;22:592–596. doi: 10.1093/ndt/gfl584. [DOI] [PubMed] [Google Scholar]
- 79.Ito S., Higuchi Y., Yagi Y., Nishijima F., Yamato H., Ishii H., Osaka M., Yoshida M. Reduction of indoxyl sulfate by AST-120 attenuates monocyte inflammation related to chronic kidney disease. J. Leukoc. Biol. 2013;93:837–845. doi: 10.1189/jlb.0112023. [DOI] [PubMed] [Google Scholar]
- 80.He T., Xiong J., Huang Y., Zheng C., Liu Y., Bi X., Liu C., Han W., Yang K., Xiao T., et al. Klotho restrain RIG-1/NF-κB signaling activation and monocyte inflammatory factor release under uremic condition. Life Sci. 2019;231:116570. doi: 10.1016/j.lfs.2019.116570. [DOI] [PubMed] [Google Scholar]
- 81.Kim H.Y., Yoo T.-H., Hwang Y., Lee G.H., Kim B., Jang J., Yu H.T., Kim M.C., Cho J.-Y., Lee C.J., et al. Indoxyl sulfate (IS)-mediated immune dysfunction provokes endothelial damage in patients with end-stage renal disease (ESRD) Sci. Rep. 2017;7:3057. doi: 10.1038/s41598-017-03130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Böger R.H., Bode-Böger S.M., Tsao P.S., Lin P.S., Chan J.R., Cooke J.P. An endogenous inhibitor of nitric oxide synthase regulates endothelial adhesiveness for monocytes. J. Am. Coll. Cardiol. 2000;36:2287–2295. doi: 10.1016/S0735-1097(00)01013-5. [DOI] [PubMed] [Google Scholar]
- 83.Chen M.-F., Li Y.-J., Yang T.-L., Lou B., Xie X.-M. Losartan inhibits monocytic adhesion induced by ADMA via downregulation of chemokine receptors in monocytes. Eur. J. Clin. Pharmacol. 2009;65:457–464. doi: 10.1007/s00228-008-0607-2. [DOI] [PubMed] [Google Scholar]
- 84.Bhaskaran M., Radhakrishnan N., Patni H., Singh P., Chaudhary A.N., Singhal P.C. Dialysis membrane-induced oxidative stress: Role of heme oxygenase-1. Nephron Exp. Nephrol. 2007;105:e24–e32. doi: 10.1159/000097016. [DOI] [PubMed] [Google Scholar]
- 85.Carracedo J., Ramírez R., Martín-Malo A., Rodríguez M., Madueño J.A., Aljama P. Role of adhesion molecules in mononuclear cell apoptosis induced by cuprophan hemodialysis membranes. Nephron. 2001;89:186–193. doi: 10.1159/000046066. [DOI] [PubMed] [Google Scholar]
- 86.Carracedo J., Ramírez R., Martin-Malo A., Rodríguez M., Aljama P. Nonbiocompatible hemodialysis membranes induce apoptosis in mononuclear cells: The role of G-proteins. J. Am. Soc. Nephrol. 1998;9:46–53. doi: 10.1159/000051190. [DOI] [PubMed] [Google Scholar]
- 87.Carracedo J., Ramírez R., Soriano S., Martín-Malo A., Rodríguez M., Aljama P. Caspase-3-dependent pathway mediates apoptosis of human mononuclear cells induced by cellulosic haemodialysis membranes. Nephrol. Dial. Transpl. 2002;17:1971–1977. doi: 10.1093/ndt/17.11.1971. [DOI] [PubMed] [Google Scholar]
- 88.Carracedo J., Ramirez R., Soriano S., Alvarez de Lara M.A., Rodriguez M., Martin-Malo A., Aljama P. Monocytes from dialysis patients exhibit characteristics of senescent cells: Does it really mean inflammation? Contrib. Nephrol. 2005;149:208–218. doi: 10.1159/000085542. [DOI] [PubMed] [Google Scholar]
- 89.Girndt M., Heisel O., Köhler H. Influence of dialysis with polyamide vs haemophan haemodialysers on monokines and complement activation during a 4-month long-term study. Nephrol. Dial. Transpl. 1999;14:676–682. doi: 10.1093/ndt/14.3.676. [DOI] [PubMed] [Google Scholar]
- 90.Lin Y.F., Chang D.M., Shaio M.F., Lu K.C., Chyr S.H., Li B.L., Sheih S.D. Cytokine production during hemodialysis: Effects of dialytic membrane and complement activation. Am. J. Nephrol. 1996;16:293–299. doi: 10.1159/000169012. [DOI] [PubMed] [Google Scholar]
- 91.Mandolfo S., Tetta C., David S., Gervasio R., Ognibene D., Wratten M.L., Tessore E., Imbasciati E. In vitro and in vivo biocompatibility of substituted cellulose and synthetic membranes. Int. J. Artif. Organs. 1997;20:603–609. doi: 10.1177/039139889702001102. [DOI] [PubMed] [Google Scholar]
- 92.Marchant A., Tielemans C., Husson C., Gastaldello K., Schurmans T., de Groote D., Duchow J., Vanherweghem L., Goldman M. Cuprophane haemodialysis induces upregulation of LPS receptor (CD14) on monocytes: Role of complement activation. Nephrol. Dial. Transpl. 1996;11:657–662. doi: 10.1093/oxfordjournals.ndt.a027355. [DOI] [PubMed] [Google Scholar]
- 93.Pertosa G., Simone S., Soccio M., Marrone D., Gesualdo L., Schena F.P., Grandaliano G. Coagulation cascade activation causes CC chemokine receptor-2 gene expression and mononuclear cell activation in hemodialysis patients. J. Am. Soc. Nephrol. 2005;16:2477–2486. doi: 10.1681/ASN.2004070621. [DOI] [PubMed] [Google Scholar]
- 94.Dhondt A., Vanholder R., Glorieux G., Waterloos M.A., de Smet R., Lesaffer G., Lameire N. Vitamin E-bonded cellulose membrane and hemodialysis bioincompatibility: Absence of an acute benefit on expression of leukocyte surface molecules. Am. J. Kidney Dis. 2000;36:1140–1146. doi: 10.1053/ajkd.2000.19824. [DOI] [PubMed] [Google Scholar]
- 95.Girndt M., Lengler S., Kaul H., Sester U., Sester M., Köhler H. Prospective crossover trial of the influence of vitamin E-coated dialyzer membranes on T-cell activation and cytokine induction. Am. J. Kidney Dis. 2000;35:95–104. doi: 10.1016/S0272-6386(00)70307-6. [DOI] [PubMed] [Google Scholar]
- 96.Stavroulopoulos A., Petras D., Kakavas I., Agroyannis I., Stamatelou K., Vyssoulis G., Papadakis I.T., Stefanadis C. Monocyte expression of adhesion molecules during low- and high-flux polysulfone hemodialysis and the effect of atorvastatin administration. Blood Purif. 2010;29:274–279. doi: 10.1159/000274462. [DOI] [PubMed] [Google Scholar]
- 97.Kaplow L.S., Goffinet J.A. Profound neutropenia during the early phase of hemodialysis. JAMA. 1968;203:1135–1137. doi: 10.1001/jama.1968.03140130047014. [DOI] [PubMed] [Google Scholar]
- 98.Schouten W.E.M., Grooteman M.P.C., Schoorl M., van Houte A.J., Nubé M.J. Monocyte activation in peripheral blood and dialyser eluates: Phenotypic profile and cytokine release. Nephron. 2002;91:646–653. doi: 10.1159/000065026. [DOI] [PubMed] [Google Scholar]
- 99.Stuard S., Carreno M.P., Poignet J.L., Albertazzi A., Haeffner-Cavaillon N. A major role for CD62P/CD15s interaction in leukocyte margination during hemodialysis. Kidney Int. 1995;48:93–102. doi: 10.1038/ki.1995.272. [DOI] [PubMed] [Google Scholar]
- 100.Tabor B., Geissler B., Odell R., Schmidt B., Blumenstein M., Schindhelm K. Dialysis neutropenia: The role of the cytoskeleton. Kidney Int. 1998;53:783–789. doi: 10.1046/j.1523-1755.1998.00813.x. [DOI] [PubMed] [Google Scholar]
- 101.Thylén P., Fernvik E., Haegerstrand A., Lundahl J., Jacobson S.H. Dialysis-induced serum factors inhibit adherence of monocytes and granulocytes to adult human endothelial cells. Am. J. Kidney Dis. 1997;29:78–85. doi: 10.1016/S0272-6386(97)90011-1. [DOI] [PubMed] [Google Scholar]
- 102.Thylén P., Fernvik E., Lundahl J., Hed J., Jacobson S.H. Cell surface receptor modulation on monocytes and granulocytes during clinical and experimental hemodialysis. Am. J. Nephrol. 1995;15:392–400. doi: 10.1159/000168872. [DOI] [PubMed] [Google Scholar]
- 103.Thylén P., Fernvik E., Lundahl J., Hed J., Jacobson S.H. Modulation of CD11b/CD18 on monocytes and granulocytes following hemodialysis membrane interaction in vitro. Int. J. Artif. Organs. 1996;19:156–163. doi: 10.1177/039139889601900304. [DOI] [PubMed] [Google Scholar]
- 104.Girndt M., Kaul H., Leitnaker C.K., Sester M., Sester U., Köhler H. Selective sequestration of cytokine-producing monocytes during hemodialysis treatment. Am. J. Kidney Dis. 2001;37:954–963. doi: 10.1016/s0272-6386(05)80011-3. [DOI] [PubMed] [Google Scholar]
- 105.Sester U., Sester M., Heine G., Kaul H., Girndt M., Köhler H. Strong depletion of CD14(+)CD16(+) monocytes during haemodialysis treatment. Nephrol. Dial. Transpl. 2001;16:1402–1408. doi: 10.1093/ndt/16.7.1402. [DOI] [PubMed] [Google Scholar]
- 106.Griveas I., Visvardis G., Sakellariou G., Passadakis P., Thodis I., Vargemezis V., Pavlitou A., Fleva A. Biocompatibility study based on differential sequestration kinetics of CD14+CD16+ blood monocyte subsets with different dialyzers. Ren. Fail. 2006;28:493–499. doi: 10.1080/08860220600781336. [DOI] [PubMed] [Google Scholar]
- 107.Kino K., Akizawa T., Koshikawa S. Effects of membrane characteristics on cytokine production by mononuclear cells in regular haemodialysis patients. Nephrol. Dial. Transpl. 1995;10:29–33. doi: 10.1093/ndt/10.supp3.29. [DOI] [PubMed] [Google Scholar]
- 108.Koliousi E., Vartholomatos G., Katopodis K.P., Kolaitis N., Siamopoulos K.C. Effect of the hemodialysis session on bcl-2 expression in peripheral blood mononuclear cells in vivo. Blood Purif. 2006;24:542–547. doi: 10.1159/000097077. [DOI] [PubMed] [Google Scholar]
- 109.Trojanowicz B., Ulrich C., Fiedler R., Storr M., Boehler T., Martus P., Pawlak M., Glomb M.A., Henning C., Templin M., et al. Impact of serum and dialysates obtained from chronic hemodialysis patients maintained on high cut-off membranes on inflammation profile in human THP-1 monocytes. Hemodial. Int. 2017;21:348–358. doi: 10.1111/hdi.12494. [DOI] [PubMed] [Google Scholar]
- 110.Trojanowicz B., Ulrich C., Fiedler R., Martus P., Storr M., Boehler T., Werner K., Hulko M., Zickler D., Willy K., et al. Modulation of leucocytic angiotensin-converting enzymes expression in patients maintained on high-permeable haemodialysis. Nephrol. Dial. Transpl. 2018;33:34–43. doi: 10.1093/ndt/gfx206. [DOI] [PubMed] [Google Scholar]
- 111.Colì L., Donati G., Cappuccilli M.L., Cianciolo G., Comai G., Cuna V., Carretta E., La Manna G., Stefoni S. Role of the hemodialysis vascular access type in inflammation status and monocyte activation. Int. J. Artif. Organs. 2011;34:481–488. doi: 10.5301/IJAO.2011.8466. [DOI] [PubMed] [Google Scholar]
- 112.Atamaniuk J., Kopecky C., Skoupy S., Säemann M.D., Weichhart T. Apoptotic cell-free DNA promotes inflammation in haemodialysis patients. Nephrol. Dial. Transpl. 2012;27:902–905. doi: 10.1093/ndt/gfr695. [DOI] [PubMed] [Google Scholar]
- 113.Pertosa G., Gesualdo L., Bottalico D., Schena F.P. Endotoxins modulate chronically tumour necrosis factor alpha and interleukin 6 release by uraemic monocytes. Nephrol. Dial. Transpl. 1995;10:328–333. [PubMed] [Google Scholar]
- 114.Eleftheriadis T., Pissas G., Remoundou M., Filippidis G., Antoniadi G., Oustampasidou N., Liakopoulos V., Stefanidis I. Ferroportin in monocytes of hemodialysis patients and its associations with hepcidin, inflammation, markers of iron status and resistance to erythropoietin. Int. Urol. Nephrol. 2014;46:161–167. doi: 10.1007/s11255-013-0497-9. [DOI] [PubMed] [Google Scholar]
- 115.Sonnweber T., Theurl I., Seifert M., Schroll A., Eder S., Mayer G., Weiss G. Impact of iron treatment on immune effector function and cellular iron status of circulating monocytes in dialysis patients. Nephrol. Dial. Transpl. 2011;26:977–987. doi: 10.1093/ndt/gfq483. [DOI] [PubMed] [Google Scholar]
- 116.Guz G., Glorieux G.L., de Smet R., Waterloos M.-A.F., Vanholder R.C., Dhondt A.W. Impact of iron sucrose therapy on leucocyte surface molecules and reactive oxygen species in haemodialysis patients. Nephrol. Dial. Transpl. 2006;21:2834–2840. doi: 10.1093/ndt/gfl263. [DOI] [PubMed] [Google Scholar]
- 117.Jurek A., Turyna B., Kubit P., Klein A. The ability of HDL to inhibit VCAM-1 expression and oxidized LDL uptake is impaired in renal patients. Clin. Biochem. 2008;41:1015–1018. doi: 10.1016/j.clinbiochem.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 118.Krishnan S., Shimoda M., Sacchi R., Kailemia M.J., Luxardi G., Kaysen G.A., Parikh A.N., Ngassam V.N., Johansen K., Chertow G.M., et al. HDL Glycoprotein Composition and Site-Specific Glycosylation Differentiates Between Clinical Groups and Affects IL-6 Secretion in Lipopolysaccharide-Stimulated Monocytes. Sci. Rep. 2017;7:43728. doi: 10.1038/srep43728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tokuda N., Kano M., Meiri H., Nomoto K., Naito S. Calcitriol therapy modulates the cellular immune responses in hemodialysis patients. Am. J. Nephrol. 2000;20:129–137. doi: 10.1159/000013569. [DOI] [PubMed] [Google Scholar]
- 120.Wu E.L., Cui H.X. Effect of 1,25-(OH)2D3 and lipopolysaccharide on mononuclear cell inflammation in type 2 diabetes mellitus and diabetic nephropathy uremia. Genet. Mol. Res. 2016;15:1–11. doi: 10.4238/gmr.15038553. [DOI] [PubMed] [Google Scholar]
- 121.Stubbs J.R., Idiculla A., Slusser J., Menard R., Quarles L.D. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J. Am. Soc. Nephrol. 2010;21:353–361. doi: 10.1681/ASN.2009040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meireles M.S., Kamimura M.A., Dalboni M.A., Giffoni de Carvalho J.T., Aoike D.T., Cuppari L. Effect of cholecalciferol on vitamin D-regulatory proteins in monocytes and on inflammatory markers in dialysis patients: A randomized controlled trial. Clin. Nutr. 2016;35:1251–1258. doi: 10.1016/j.clnu.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 123.Seibert E., Heine G.H., Ulrich C., Seiler S., Köhler H., Girndt M. Influence of cholecalciferol supplementation in hemodialysis patients on monocyte subsets: A randomized, double-blind, placebo-controlled clinical trial. Nephron Clin. Pract. 2013;123:209–219. doi: 10.1159/000354717. [DOI] [PubMed] [Google Scholar]
- 124.Arena A., Coppolino G., Nostro L., Pavone B., Bonvissuto G., Campo S., Iannello D., Bonina L., Buemi M. Impaired antiviral activity of monocytes from patients on hemodiafiltration. J. Nephrol. 2007;20:560–567. [PubMed] [Google Scholar]
- 125.Dopheide J.F., Zeller G.C., Kuhlmann M., Girndt M., Sester M., Sester U. Differentiation of Monocyte Derived Dendritic Cells in End Stage Renal Disease is Skewed towards Accelerated Maturation. Adv. Clin. Exp. Med. 2015;24:257–266. doi: 10.17219/acem/40463. [DOI] [PubMed] [Google Scholar]
- 126.Choi H.M., Woo Y.S., Kim M.G., Jo S.-K., Cho W.Y., Kim H.K. Altered monocyte-derived dendritic cell function in patients on hemodialysis: A culprit for underlying impaired immune responses. Clin. Exp. Nephrol. 2011;15:546–553. doi: 10.1007/s10157-011-0424-2. [DOI] [PubMed] [Google Scholar]
- 127.Lim W.H., Kireta S., Leedham E., Russ G.R., Coates P.T. Uremia impairs monocyte and monocyte-derived dendritic cell function in hemodialysis patients. Kidney Int. 2007;72:1138–1148. doi: 10.1038/sj.ki.5002425. [DOI] [PubMed] [Google Scholar]
- 128.Verkade M.A., van Druningen C.J., Op de Hoek C.T., Weimar W., Betjes M.G.H. Decreased antigen-specific T-cell proliferation by moDC among hepatitis B vaccine non-responders on haemodialysis. Clin. Exp. Med. 2007;7:65–71. doi: 10.1007/s10238-007-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ross R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 130.Gottsäter A., Forsblad J., Mätzsch T., Persson K., Ljungcrantz I., Ohlsson K., Lindgärde F. Interleukin-1 receptor antagonist is detectable in human carotid artery plaques and is related to triglyceride levels and Chlamydia pneumoniae IgA antibodies. J. Intern. Med. 2002;251:61–68. doi: 10.1046/j.1365-2796.2002.00926.x. [DOI] [PubMed] [Google Scholar]
- 131.Balakrishnan V.S., Schmid C.H., Jaber B.L., Natov S.N., King A.J., Pereira B.J. Interleukin-1 receptor antagonist synthesis by peripheral blood mononuclear cells: A novel predictor of morbidity among hemodialysis patients. J. Am. Soc. Nephrol. 2000;11:2114–2121. doi: 10.1681/ASN.V11112114. [DOI] [PubMed] [Google Scholar]
- 132.Jeng Y., Lim P.S., Wu M.Y., Tseng T.-Y., Chen C.H., Chen H.P., Wu T.-K. Proportions of Proinflammatory Monocytes Are Important Predictors of Mortality Risk in Hemodialysis Patients. Mediators Inflamm. 2017;2017:1070959. doi: 10.1155/2017/1070959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rogacev K.S., Seiler S., Zawada A.M., Reichart B., Herath E., Roth D., Ulrich C., Fliser D., Heine G.H. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur. Heart J. 2011;32:84–92. doi: 10.1093/eurheartj/ehq371. [DOI] [PubMed] [Google Scholar]
- 134.Ulrich C., Seibert E., Heine G.H., Fliser D., Girndt M. Monocyte angiotensin converting enzyme expression may be associated with atherosclerosis rather than arteriosclerosis in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2011;6:505–511. doi: 10.2215/CJN.06870810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rogacev K.S., Ziegelin M., Ulrich C., Seiler S., Girndt M., Fliser D., Heine G.H. Haemodialysis-induced transient CD16+ monocytopenia and cardiovascular outcome. Nephrol. Dial. Transpl. 2009;24:3480–3486. doi: 10.1093/ndt/gfp287. [DOI] [PubMed] [Google Scholar]
- 136.Lorenzen J.M., David S., Richter A., de Groot K., Kielstein J.T., Haller H., Thum T., Fliser D. TLR-4+ peripheral blood monocytes and cardiovascular events in patients with chronic kidney disease—A prospective follow-up study. Nephrol. Dial. Transpl. 2011;26:1421–1424. doi: 10.1093/ndt/gfq758. [DOI] [PubMed] [Google Scholar]
- 137.Ando M., Lundkvist I., Bergström J., Lindholm B. Enhanced scavenger receptor expression in monocyte-macrophages in dialysis patients. Kidney Int. 1996;49:773–780. doi: 10.1038/ki.1996.107. [DOI] [PubMed] [Google Scholar]
- 138.Konishi Y., Okamura M., Konishi M., Negoro N., Yoshida T., Inoue T., Kanayama Y., Yoshikawa J. Enhanced gene expression of scavenger receptor in peripheral blood monocytes from patients on cuprophane haemodialysis. Nephrol. Dial. Transpl. 1997;12:1167–1172. doi: 10.1093/ndt/12.6.1167. [DOI] [PubMed] [Google Scholar]
- 139.Gonçalves M.S.B., Fabris B.A., Brinholi F.F., Bortolasci C.C., Watanabe M.A.E., Oliveira K.B., Delfino V.D.A., Lavado E.L., Barbosa D.S. Increased oxidative stress in foam cells obtained from hemodialysis patients. Hemodial. Int. 2013;17:266–274. doi: 10.1111/j.1542-4758.2012.00736.x. [DOI] [PubMed] [Google Scholar]
- 140.Kliger E., Kristal B., Shapiro G., Chezar J., Sela S. Primed polymorphonuclear leukocytes from hemodialysis patients enhance monocyte transendothelial migration. Am. J. Physiol. Heart Circ. Physiol. 2017;313:H974–H987. doi: 10.1152/ajpheart.00122.2017. [DOI] [PubMed] [Google Scholar]
- 141.Ewert L., Fischer A., Brandt S., Scurt F.G., Philipsen L., Müller A.J., Girndt M., Zenclussen A.C., Lindquist J.A., Gorny X., et al. Cold shock Y-box binding protein-1 acetylation status in monocytes is associated with systemic inflammation and vascular damage. Atherosclerosis. 2018;278:156–165. doi: 10.1016/j.atherosclerosis.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 142.Ashman N., Macey M.G., Fan S.L., Azam U., Yaqoob M.M. Increased platelet-monocyte aggregates and cardiovascular disease in end-stage renal failure patients. Nephrol. Dial. Transpl. 2003;18:2088–2096. doi: 10.1093/ndt/gfg348. [DOI] [PubMed] [Google Scholar]
- 143.Stach K., Karb S., Akin I., Borggrefe M., Krämer B., Kälsch T., Kälsch A.-I. Elevation of Platelet and Monocyte Activity Markers of Atherosclerosis in Haemodialysis Patients Compared to Peritoneal Dialysis Patients. Mediators Inflamm. 2017;2017:8506072. doi: 10.1155/2017/8506072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Becs G., Hudák R., Fejes Z., Debreceni I.B., Bhattoa H.P., Balla J., Kappelmayer J. Haemodiafiltration elicits less platelet activation compared to haemodialysis. BMC Nephrol. 2016;17:147. doi: 10.1186/s12882-016-0364-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Benck U., Stach K., Jung S., Krämer B.K., Kälsch T., Kälsch A.-I. Short- and long-term effects of hemodialysis on platelet and monocyte activity markers of atherosclerosis in patients with end-stage renal disease. Cardiol. J. 2018;25:595–600. doi: 10.5603/CJ.a2017.0152. [DOI] [PubMed] [Google Scholar]
- 146.Libby P., Everett B.M. Novel Antiatherosclerotic Therapies. Arterioscler. Thromb. Vasc. Biol. 2019;39:538–545. doi: 10.1161/ATVBAHA.118.310958. [DOI] [PMC free article] [PubMed] [Google Scholar]