Abstract
In glucose-stimulated insulin secretion (GSIS) of pancreatic β-cells, the rise of free cytosolic Ca2+ concentration through voltage-gated calcium channels (VGCCs) triggers the exocytosis of insulin-containing granules. Recently, mechanically induced insulin secretion pathways were also reported, which utilize free cytosolic Ca2+ ions as a direct regulator of exocytosis. In this study, we aimed to investigate intracellular Ca2+ responses on the HIT-T15 pancreatic β-cell line upon low-intensity pulsed ultrasound (LIPUS) stimulation and found that ultrasound induces two distinct types of intracellular Ca2+ oscillation, fast-irregular and slow-periodic, from otherwise resting cells. Both Ca2+ patterns depend on the purinergic signaling activated by the rise of extracellular ATP or ADP concentration upon ultrasound stimulation, which facilitates the release through mechanosensitive hemichannels on the plasma membrane. Further study demonstrated that two subtypes of purinergic receptors, P2X and P2Y, are working in a competitive manner depending on the level of glucose in the cell media. The findings can serve as an essential groundwork providing an underlying mechanism for the development of a new therapeutic approach for diabetic conditions with further validation.
Keywords: ultrasound, calcium oscillations, pancreatic β-cells, purinergic signaling, hemichannels, ATP
1. Introduction
Insulin, a peptide hormone secreted by the pancreas, plays a crucial role in maintaining the homeostasis of the blood glucose levels in the human body. It lowers the blood glucose levels by facilitating glucose uptakes in the liver, skeletal muscle, and adipose tissue. Failure to maintain blood glucose levels, also known as hyperglycemia, can give rise to many different severe health conditions, including diabetes. Insulin secretion is stimulated by many different hormones and neurotransmitters such as glucagon-like peptide-1 [1] and carbon monoxide [2], but glucose is a major secretagogue.
Over the years, the glucose-stimulated insulin secretion (GSIS) has been extensively studied, and its mechanism is well documented [3,4]. An increase in blood glucose concentration accelerates metabolism in pancreatic β-cells, leading to an elevated cytoplasmic ATP/ADP ratio. ATP-sensitive K+ channels respond to the elevated ATP/ADP ratio in the cytoplasm by closing their channels, resulting in the depolarization of the cell’s membrane potential. This depolarization opens voltage-dependent Ca2+ channels (VDCCS), allowing a transient influx of Ca2+ from extracellular space. In turn, the elevated free cytosolic Ca2+ concentration triggers the release of insulin granules.
Furthermore, it has been known that the insulin secretion occurs in a pulsatile manner [5], and the oscillatory release of insulin helps to maintain insulin sensitivity in target cells. Without the oscillatory insulin secretion pattern, more insulin is required to achieve the same effect [6,7,8], possibly causing insulin resistance from the recipient cells. In fact, it was reported that insulin oscillations were diminished from patients with type 1 [9] and type 2 diabetes [10]. Interestingly, the oscillatory insulin secretion is not only found in the pancreas but also from isolated individual pancreatic islets, and even from single β-cells [11]. In a single β-cell, oscillations of the cytosolic Ca2+ concentration, which mainly originated from the glucose metabolism, synchronize with the pulsatile insulin secretion.
There have been numerous reports suggesting that GSIS does not rely solely on the KATP channel-dependent mechanism, and mechanosensitive mechanisms may also be involved. There are a few hypotheses on mechanosensitive pathways, such as volume-regulated anion channels [12] or mechanosensitive transient receptor potential (TRP) channels [13]. However, the level of intracellular Ca2+ plays a fundamental role in all suggested pathways. Recently, Castellanos et al. [14] reported the Ca2+-dependent insulin release from rat INS 832/13 β-cells upon ultrasound stimulation, suggesting a possible therapeutic intervention of the diabetic condition using mechanical energy-based modality.
For the last few years, our group has reported that focused ultrasound can induce intracellular Ca2+ elevations in cancer cell lines [15,16], human umbilical vein endothelial cells (HUVECs) [17], and human mesenchymal stem cells (hMSCs) [18]. In the recent report, we suggested the concept of an ultrasound-based intracellular Ca2+ signaling modulator, which possesses the capability to regulate downstream Ca2+-dependent cellular processes non-invasively and remotely. In the present study, we explored the ultrasound-evoked intracellular Ca2+ dynamics of single cells from the clusters of a clonal HIT-T15 pancreatic β-cell line using a 45-MHz focused ultrasound. The results indicate that ultrasound can induce intracellular Ca2+ oscillations via purinergic signaling. Two distinctive oscillatory Ca2+ patterns, fast-irregular and slow-periodic, were found in the cells stimulated by low-intensity ultrasound, and they have shown a competitive manner depending on the level of glucose. Further study revealed that the fast-irregular mode of Ca2+ oscillation depends on both P2X receptors and L-type voltage-dependent Ca2+ channels, while the slow-periodic mode relies on P2Y receptors and store-operated Ca2+ channels.
2. Materials and Methods
2.1. Reagents and Inhibitors
Ham’s F12K special (with 1.5 g/L sodium bicarbonate) was obtained from the USC Norris Comprehensive Cancer Center Core (Los Angeles, CA, USA). Dialyzed donor equine serum was obtained from Rocky Mountain Biologicals (Missoula, MT, USA). Heat-inactivated fetal bovine serum (HI-FBS), Penicillin–Streptomycin, l-glutamine, Fluo-4 AM, and Calcein AM were purchased from Thermo Fisher Scientific (Cambridge, MA). Apyrase, carbenoxolone (CBX), cyclopiazonic acid (CPA), lanthanum (La3+), nifedipine, Pluronic F-127, pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), and suramin were purchased from Sigma-Aldrich (St Louise, MO, USA).
2.2. Cell Preparation
The HIT-T15 cell line was purchased from ATCC (Manassas, VA, USA) and maintained in the complete growth medium with following ingredients: Ham’s F12K with 2 mM l-glutamine, 10% dialyzed donor equine serum, 2.5% HI-FBS, and 1% Penicillin–Streptomycin. The HIT-T15 cells were seeded at a density of 1.3 × 105 cells/cm2 and cultured in 5% CO2 at 37 °C. The flesh complete media was replenished every 2–3 days, and the cells were passaged every week. For this study, only the cells with passage numbers between 20 and 30 were used.
2.3. Ultrasound Stimulation
For this project, a press-focused 45-MHz single element lithium niobate (LiNbO3) transducer was designed and fabricated in house following a protocol previously described [19]. Among various piezoelectric materials, LiNbO3 was selected because it holds many advantages such as good electromechanical coupling, a low dielectric constant, and high longitudinal sound speed [20]. In the design, the size of a cluster of HIT-T15 cells was primarily considered to generate a beamwidth comparable to a single cluster (10–20 cells), and it was achieved by the press-focusing technique (f-number: 2.2). A calibrated capsule type hydrophone with 20 dB preamplifier (HGL-0085/AH-2010, Onda Corp., Sunnyvale, CA) was employed to assess the actual performance of the fabricated transducer. A lateral beam-width (115 μm) was estimated by measuring the distance between the 6 dB points below the maximum. Note that a theoretical lateral resolution for the transducer was 75 μm ( and the measured value might be overestimated since the hydrophone’s electrode aperture size (85 μm) was not optimal to measure high-frequency transducers (>40 MHz) accurately. In this study, the transducer was driven by a fixed 18 V peak-to-peak voltage, pulse repetition frequency at 1 kHz, and duty cycle at 2% (ISPTA: 113.1 mW/cm2) to be in the realm of low-intensity pulsed ultrasound. The cytotoxicity of ultrasound stimulation was examined using a viability dye, calcein AM. No indication of compromised viability was observed up to 60 h after the ultrasound exposure (Supplementary Figure S1).
2.4. Live Intracellular Ca2+ Imaging
The clonal HIT-T15 cells were seeded on 35 mm culture dishes at a density of 2 × 105 cells/cm2 and kept in the CO2 incubator for 48 h before each experiment. For the imaging solutions, mainly modified Hank’s balanced salt solution with Ca2+ and Mg2+ (HBSS+) containing 11.1 mM D(+) glucose was used, but HBSS+ containing 2.8 mM and 5.5 mM D(+) glucose were also used as needed. The HIT-T15 cells on 35 mm culture dish were washed with HBSS+ once and incubated with 2 μM of Fluo-4 AM in room temperature for 30 min for Ca2+ imaging. After the incubation, the dish was washed three times and imaged with an epi-fluorescence inverted microscope (IX71, Olympus America Inc., Center Valley, PA, USA). Fluorescence images were acquired either for 30 min at 0.5 frames per second or for 5 min at 1 frame per second.
2.5. Data Processing and Statistics
Acquired stacked images were processed with CellProfiler image analysis software [21] using a customized pipeline to locate single cells and collect fluorescence intensities automatically. The extracted intensities were loaded in Matlab (Mathworks) for normalization (ΔF/F) and for counting the number of cells showing active Ca2+ dynamics (defined as cells with ΔF/Fmax greater than basal noise level by 2-fold) with and without ultrasound exposure. The percentage of responding cells was calculated by the active cells divided by the total number of cells in each image field. In addition, the period of Ca2+ oscillations was measured and compared in the cells, either bathing in 5.5 mM glucose or inhibitors that suppressed the fast-irregular oscillations. Due to the nature of irregular oscillations, the period of oscillations cannot be measured in the fast oscillations.
3. Results
3.1. Intracellular Ca2+ Dynamics in HIT-T15 Cells upon Various Stimuli
We first investigated intracellular Ca2+ dynamics in HIT-T15 cells using a high K+ (40 mM) extracellular buffer. The high K+ stimulation has been used to depolarize the cell membrane in order to activate VDCCs on the membrane and allow an influx of Ca2+. A sudden increase of intracellular Ca2+ was observed as soon as the imaging solution was replaced by the high K+ buffer (Supplementary Figure S2a). The result indicates that the VDCCs on the membrane were activated by the altered K+ concentration gradient between the inside and outside of the cells and allow an influx of Ca2+ from the outside. Furthermore, the gradual decrease indicates that the cells’ machinery Ca2+ pumps are functioning.
Next, the HIT-T15 cells were stimulated with a high concentration of glucose to monitor the glucose-induced Ca2+ activity. The cells were maintained in HBSS+, and at t = 600 s, it was replaced with high glucose (17 mM) buffer solution. The cells responded to the high glucose with oscillatory Ca2+ signaling (Supplementary Figure S2b). The oscillations in intracellular Ca2+ are known to synchronize with the oscillatory metabolism of the β-cell and in turn create pulsatile secretion of insulin [22]. The pulsatile insulin secretion gives a means of lowering total insulin amount to maintain the blood glucose level compared to a constant rate of secretion [7].
To test whether ultrasound stimulation can also evoke intracellular Ca2+ oscillations from resting cells as shown in the high-glucose stimulation, a cluster of HIT-T15 cells was exposed to 45-MHz pulsed ultrasound. In this study, the power (ISPTA) of the ultrasound was fixed at 113.1 mW/cm2 (input voltage: 60 mV, pulse repetition time: 1 ms, duty factor: 2%) to be in the range of low-intensity and also comparable to our previous reports [16,18]. The ultrasound stimulation setup is illustrated in Figure 1a.
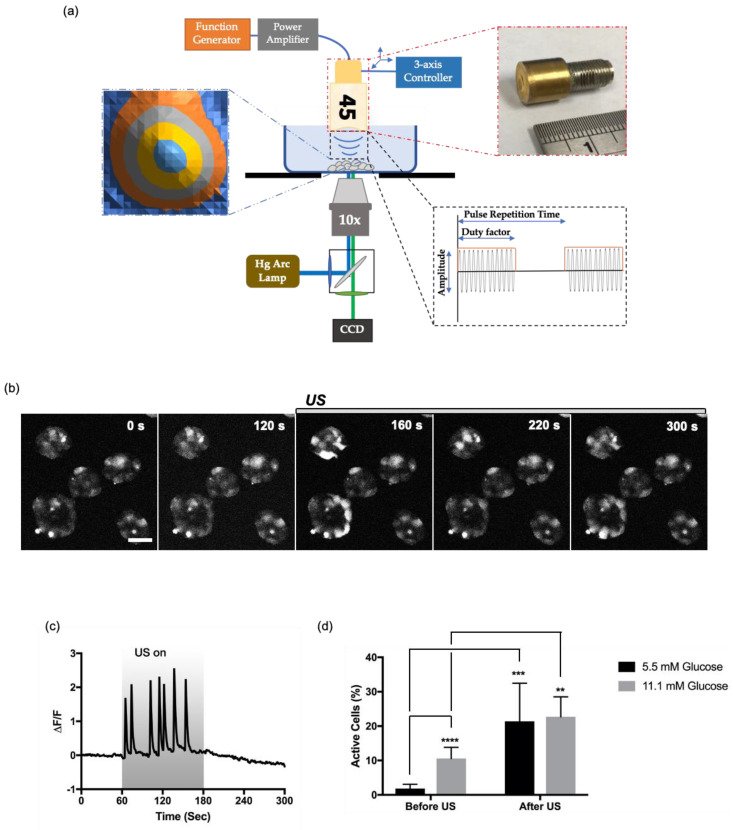
Figure 1.
Ultrasound-induced intracellular Ca2+ dynamics in HIT-T15 pancreatic β-cells. (a) Schematic diagram of an ultrasound stimulation system. A pulsed sine wave was generated by a function generator and amplified by a power amplifier to drive a 45-MHz focused transducer. The position of the transducer was controlled by a three-axis stage controller. The focal distance from the transducer to the surface of culture dish was aligned using a pulser–receiver. Ultrasound-induced intracellular Ca2+ dynamics were monitored by an inverted epifluorescence microscope. (b) Time lapse images of HIT-T15 cells before and after ultrasound stimulation. Scale bar 50 µm. (c) Ultrasound-induced Ca2+ oscillation from a single HIT-T15 cell. The cells were bathed in HBSS+ with 11.1 mM glucose. The gray box indicates the duration of ultrasound exposure. (d) Percentage of cells active in Ca2+ signaling before and after ultrasound stimulation. The cells were adapted to different glucose concentrations, 5.5 mM and 11.1 mM, for 1 h before the imaging. The error bar indicates S.D. **, ***, and **** indicate p-value less than 0.01, 0.001, and 0.0001, respectively.
Ultrasound induced fast Ca2+ oscillations in the HIT-T15 cells (Figure 1c). The periodicity of oscillations was irregular, with the timescale of each Ca2+ spike less than 20 s. The ultrasound-induced Ca2+ dynamics are comparable to fast-irregular oscillations observed in a stepwise increase in glucose concentration that mimics the transition from fasting to feeding states [23].
Although the ultrasound beam was focused on a targeted cluster of cells, only a fraction of cells in the cluster responded. In general, cells on the edges of clusters responded well, while cells on the center did not (Figure 1b). Moreover, ultrasound-induced Ca2+ responses were monitored from non-targeted cells (cells located outside of ultrasound beam) throughout all over the image field, suggesting the involvement of intercellular signaling (Supplementary Video S1). To quantify the number of cells responded to ultrasound, fluorescence imaging was performed for 300 s with the HIT-T15 cells exposed to ultrasound between t = 150 s and t = 300 s. Then, the numbers of cells showing Ca2+ dynamics before and after ultrasound stimulation were compared as percentages. As illustrated in Figure 1d, the percent of cells showing Ca2+ dynamics (defined by active cells) was increased by 10-fold, 1.85 ± 1.22% to 21.40 ± 11.08% (n = 11, p = 0.0002), in the cells bathed with normal HBSS+ containing 5.5 mM glucose. However, when the cells were bathed in HBSS+ containing 11.1 mM glucose (stimulating concentration), the percent of active cells before ultrasound was 10.60 ± 3.21%, and the number was increased by 2-fold with the ultrasound stimulus to 22.72 ± 5.78% (n = 6, p = 0.0025, Figure 1d). Still, the percentages of the active cells were low, which could be due to (1) the heterogeneity of the HIT-T15 cell line [24] and (2) the quantification method, which excluded cells showing Ca2+ dynamics in low amplitude (less than 2-folds from the basal level).
Overall, the results indicate that ultrasound can activate Ca2+ responses from otherwise resting cells both in the basal and stimulating level of glucose.
3.2. Two Distinctive Ultrasound-Induced Ca2+ Oscillations
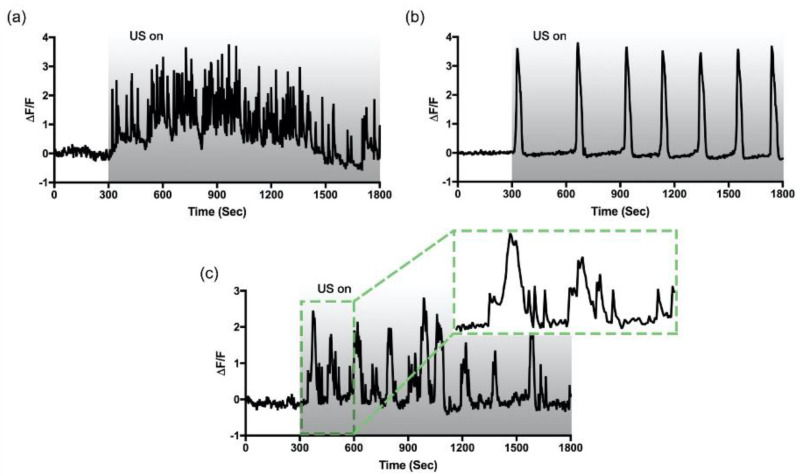
HIT-T15 cells showed the majority of the fast-irregular oscillations in a bath of 11.1 mM glucose (Figure 2a). However, when the HIT-T15 cells were adapted to 5.5 mM glucose, the majority of the fast-irregular oscillations was replaced by single Ca2+ spikes with a longer timescale of 30–50 s. To monitor the Ca2+ dynamic for an extended period of time, we increased the imaging duration to 30 min. Surprisingly, another pattern of oscillatory Ca2+ dynamics was found, which has a slow but periodic oscillatory pattern (period: 231 ± 92 s), as shown in Figure 2b. Leech et al. described the appearance of the slow Ca2+ oscillation in a steady-state concentration of glucose [23]. Seldomly, a third type of oscillation was also found, which can be described as fast oscillations superimposed on the slow oscillation (Figure 2c); this is also known as the mixed type [25]. Aside from the frequency and periodicity differences, it was observed that the baseline for the fast-irregular oscillation fluctuates, while the baseline for the slow-periodic oscillation remains relatively constant. These data suggested that multiple pathways may be involved in ultrasound-induced Ca2+ oscillations, and the appearance of each oscillatory pattern depends on the level of glucose to which the HIT-T15 cells were adapted.
Figure 2.
Different patterns of intracellular Ca2+ oscillations evoked by ultrasound. Clusters of HIT-T15 cells were exposed to low-intensity pulsed ultrasound for 25 min starting at t = 300 s. The gray bar indicates the duration of ultrasound exposure. Fast-irregular (a), and slow-periodic (b) oscillation patterns were dominantly observed from the cells adapted to 11.1 mM (stimulating) and 5.5 mM (non-stimulating) of glucose, respectively. Seldomly, mixed (c) oscillation patterns were also monitored in the both conditions.
3.3. The Involvement of Purinergic P2 Signaling
Purinergic signaling plays a regulatory role in the endocrine system. Many different subtypes of purinergic receptors are expressed in secretory cells to meditate hormone release [26]. In the pancreatic β-cells, ATP is released either from nerve terminals [27,28] or secretory granules [29], and it plays several physiological roles [30]. We hypothesized that ultrasound induces the oscillatory Ca2+ responses by facilitating ATP release via mechanosensitive hemichannels based on our previous study [18].
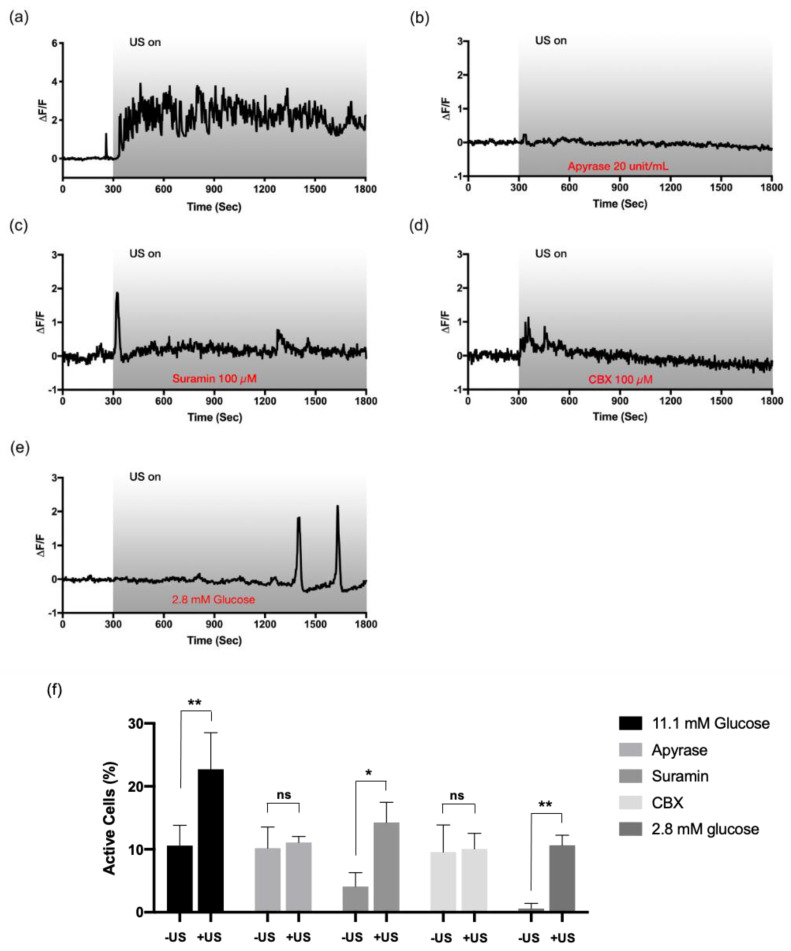
To verify the hypothesis, the HIT-T15 cells were incubated with apyrase (20 units/mL, 10 min), which is known to degrade extracellular ATP. Bathing with apyrase significantly abolished the ultrasound-induced Ca2+ dynamic (Figure 3b,f). A non-selective P2 receptor antagonist, suramin (100 μM, 10 min), also blocked the ultrasound-induced oscillations, suggesting the involvement of P2 receptors in the pathway (Figure 3c,f). Interestingly, the basal Ca2+ activity was also greatly suppressed to 4.09 ± 2.20% with suramin (approximately 10% without the drug) and a single peak appeared upon ultrasound stimulation. As for the source of extracellular ATP, mechanosensitive hemichannels were found to be responsible for the inhibitory effect of CBX (100 μM, 30 min, Figure 3d,f). The overall pathway of ultrasound-induced Ca2+ responses in the HIT-T15 cells was consistent with the findings from the hMSC model [18]. It is worth noting that with the inhibitors, both ultrasound-induced fast-irregular and slow-periodic oscillations disappeared from the cells adapted to 11.1 mM glucose.
Figure 3.
Involvement of purinergic signaling and hemichannels in the ultrasound-evoked Ca2+ mobilization. The HIT-T15 cells pre-incubated (a) without inhibitor (control) or with (b) apyrase (degrades extracellular ATP, 20 unit/mL), (c) suramin (P2 receptor inhibitor, 100 µM), and (d) carbenoxolone (CBX) (hemichannel inhibitor, 100 µM) were stimulated by ultrasound to investigate the underlying pathway. In all cases, ultrasound-induced Ca2+ dynamics were abolished, indicating the involvement of purinergic receptors triggered by ATP released from hemichannels. All experiments were carried out with modified HBSS+ containing 11.1 mM glucose except (e) where HBSS+ with 2.8 mM glucose was used. Delayed Ca2+ oscillations were monitored when the cells were adapted to low glucose. (f) Percentage of cells showing Ca2+ dynamics before and after ultrasound stimulation. Only groups pretreated with suramin and 2.8 mM glucose showed statistically significant increases upon ultrasound, yet they were either single peaks (suramin) or delayed oscillations (low glucose). * and ** indicate p-values less than 0.05 and 0.01, respectively. n = 3 for all groups.
If both modes of the ultrasound-induced Ca2+ oscillations depend on ATP release, the next question was: how do the cells choose one pattern over another? We focused on the fact that two subtypes of purinergic receptors, ionotropic (P2X) and metabotropic (P2Y), have different sensitivity to a range of ATP or ADP concentrations [31]. Since the amount of intracellular ATP would largely determine the amount of ATP release, we bathed the cells in HBSS+ containing 2.8 mM glucose for adaptation and stimulated with ultrasound. Interestingly, the cells adapted to the low glucose lost spontaneous Ca2+ activities before ultrasound exposure, and delayed Ca2+ responses were observed upon ultrasound stimulation; yet, once it appeared, the slow-periodic oscillatory pattern persisted (Figure 3e). The result is consistent with our hypothesis that the level of glucose available in the media, which, in turn, could affect the amount of intracellular ATP in the cells, works as a modulatory signal to determine the patterns.
3.4. Fast and Irregular Oscillation Depends on P2X Receptors Coupled to L-Type Ca2+ Channels
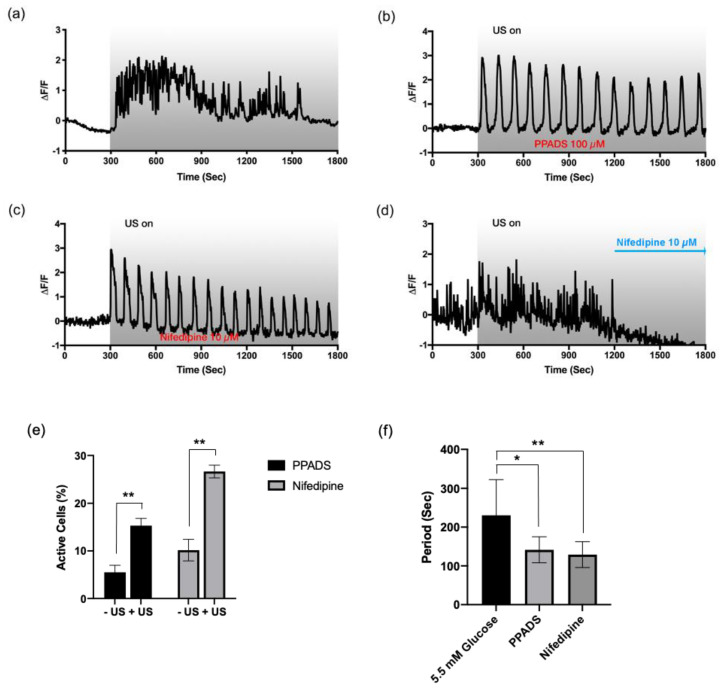
To investigate the subtype of purinergic receptors responsible for the fast-irregular oscillation, we first incubated the HIT-T15 cells with HBSS+ containing 11.1 mM glucose in the presence of PPADS (100 µM, 30 min), which is a selective purinergic P2X receptor blocker at the tested concentration. Interestingly, the fast-irregular oscillations pattern shown in 11.1 mM glucose (Figure 4a) was converted to the slow-periodic oscillatory pattern (period: 142 ± 34 s) under the inhibition of purinergic P2X receptors (Figure 4b,f). This result suggests the correlation of the P2X receptors with the fast-irregular oscillation pattern.
Figure 4.
Fast and irregular Ca2+ oscillations depend on L-type voltage-dependent Ca2+ channels coupled to P2X purinergic receptors. Ultrasound-evoked Ca2+ dynamic profiles from the HIT-T15 cells (a) without drug (control), or in the presence of (b) pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS) (100 μM, pretreated), (c) nifedipine (10 μM, pre-treated), and (d) nifedipine (10 μM, added at 1200 s). Fast and irregular oscillation patterns disappeared with PPADS (b) and nifedipine (c,d); instead, slow-periodic patterns appeared. All experiments were carried out with the modified HBSS+ containing 11.1 mM glucose. (e) Percentage of active cells before and after ultrasound stimulation. n = 3. (f) Oscillation frequency analysis. In comparison to the oscillations found from the cells adapted to 5.5 mM glucose, the oscillations found from the PPADS and nifedipine bathed groups. n = 10. * and ** indicate p-values less than 0.05 and 0.01, respectively.
Although the P2X receptors work as an ion channel allowing the influx of Ca2+ and Na2+, the rapid oscillatory pattern cannot be explained solely by the action of P2X. Previous studies often suggested the role of L-type VDCC in glucose-induced insulin secretion [23,32]. To assess the role of L-type VDCC in the fast Ca2+ oscillation, nifedipine (10 μM) was added to the HBSS+ containing 11.1 mM glucose. When the HIT-T15 cells were pre-bathed with nifedipine, only the periodic Ca2+ oscillations (period: 129 ± 33 s) were observed (Figure 4c,f), similar to with the P2X inhibitor. To confirm, nifedipine was added while imaging, and the fast-irregular Ca2+ oscillation quickly disappeared (Figure 4d). This result strongly suggests the involvement of L-type VDCC in the fast-irregular Ca2+ oscillation induced by ultrasound. It is worth noting that the periods of oscillations found from the cell bathed with both PPADS and nifedipine were smaller (oscillate faster) than the slow-periodic oscillations observed from 5.5 mM glucose groups (Figure 4f). This could be due to the higher ATP level available from the cells bathed with 11.1 mM glucose.
3.5. Slow and Periodic Oscillation Depends on P2Y Receptors and Store-Operated Ca2+ Channels
The expression of both subtypes of purinergic receptors, P2X and P2Y, was reported previously in pancreatic β-cells [33], including HIT-T15 cells [34]. The P2Y receptors are metabotropic receptors coupled to the G-protein, which produces secondary messenger IP3 from PIP2 and in turn initiates the release of Ca2+ via the IP3 receptor. Based on the fact that the presence of apyrase and suramin inhibited both patterns of Ca2+ oscillation (Figure 3b,c), but not with PPADS (Figure 4b), we concluded that the slow-periodic Ca2+ oscillation relies on P2Y receptors.
As to confirm the role of P2Y in the ultrasound-induced slow Ca2+ oscillation, the HIT-T15 cells, adapted to 5.5 mM glucose, were treated with CPA (100 μM, 30 min), a sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) inhibitor, to deplete Ca2+ storage. As shown in Figure 5b, the Ca2+ response was greatly diminished after the first Ca2+ peak, suggesting that the slow-periodic Ca2+ oscillation depends on Ca2+ release from the storage, and without the functioning Ca2+ pump for replenishing the storage, Ca2+ oscillation cannot be sustained.
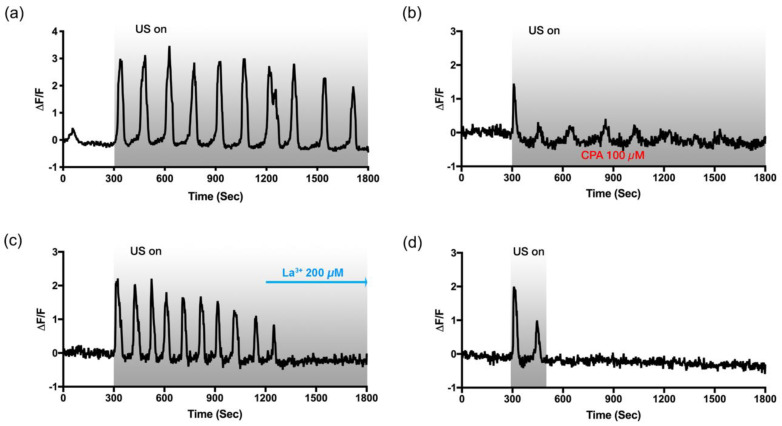
Figure 5.
Slow and periodic Ca2+ oscillations depend on the store-operated Ca2+ entry pathway. Intracellular Ca2+ dynamics in response to ultrasound exposure between t = 300 s and t = 1800 s (a) without drug (control), or bathing with (b) CPA (100 µM, pre-treated), (c) La3+ (200 µM, added at 1200 s). (d) To verify the primary role of P2Y for the Ca2+ release, cells were stimulated for 3 min (300 s–480 s). All experiments were carried out with the regular HBSS+ containing 5.5 mM glucose.
The store-operated Ca2+ channels (SOCs) on the plasma membrane often serve as a Ca2+ replenishing mechanism. When Ca2+ is released from intracellular storage, such as the endoplasmic reticulum (ER), SOCs are activated and allow Ca2+ entry into the cytoplasm and the storage. SOCs also play a critical role in GSIS [35]. La3+, a trivalent cation that inhibits the SOCs, was added while monitoring the ultrasound-induced Ca2+ oscillations to investigate the relevance of SOCs in this pathway. As the SOCs were inhibited by La3+ (200 μM, added at t = 1200 s), the oscillatory Ca2+ response disappeared, as depicted in Figure 5c. Lastly, we stimulated the cells only for 3 min (instead of 25 min) to see if the continuous exposure of ultrasound would be necessary for the sustained oscillations. It was found that the oscillation patterns disappeared as soon as the ultrasound stimulation was stopped (Figure 5d). This indicates that continuous exposure to ultrasound is required to release Ca2+ from the storage, but SOC itself cannot sustain the oscillatory Ca2+ mobilization.
4. Discussion
In the present study, we investigated the cellular responses of the pancreatic HIT-T15 β-cells to 45-MHz low-intensity focused ultrasound by monitoring the ultrasound-induced intracellular Ca2+ dynamics in live cells. The HIT-T15 cell line was initially produced by the SV40 transformation of hamster pancreatic islet cells and has been widely used as the β-cell study model, since it secretes insulin in response to a variety of insulin secretagogues such as glucose, glucagon, and methylxanthine [36]. Upon exposure to ultrasound, it was found that the resting HIT-T15 cells were activated and displayed the oscillatory Ca2+ responses via purinergic signaling, and a mechanosensitive hemichannel was found to be responsible for releasing ATP into extracellular space.
We are not the first to investigate how β-cells respond to ultrasound stimulation. Castellanos et al. [14,37] reported insulin releases from INS-1 832/13 rat insulinoma cell line in response to 800 kHz of ultrasound stimulation (continuous ultrasound, ISATA = 1 W/cm2). In another sequential paper, the authors monitored ultrasound-induced secretary events by carbon fiber amperometry and detected sustained amperometric peaks, which prolong the duration of ultrasound stimulation [14]. In addition, they showed that the ultrasound-induced secretary events are depending on the influx of Ca2+ by showing that chelating extracellular Ca2+ from the imaging buffer significantly reduced the peaks. However, despite the similarity in the concept, the overall experimental design and stimulation parameters used in our study were mostly different. First, the acoustic output utilized in the present study was considerably lower (pulsed ultrasound, ISPTA = 113.1 mW/cm2), which may have resulted in an entirely different Ca2+ mobilizing pathway. More importantly, we aimed attention at spatiotemporal Ca2+ responses using a high-frequency focused ultrasound at 45 MHz, which offered a way to distinguish between directly stimulated cells from their adjacent cells and also allowed us to obtain a better understanding of the underlying mechanism in at the single-cell level. Previously, we have shown that both 3-MHz and 38-MHz ultrasound stimulation can induce Ca2+ responses in cancer cells, indicating that the mechanotransduction pathways reported in this study may not be limited to high-frequency ultrasound [16].
The biological effects of ultrasound can be largely divided into two categories: thermal bioeffects and mechanical bioeffects. However, we excluded the possibility of thermal effects because the intensity level was too low (ISPTA = 113.1 mW/cm2), and acoustic absorption in the cell layer should be minimal, as the absorption correlates with the distance acoustic wave travels [38,39]. Therefore, we believe that the mechanical perturbation created by acoustic radiation force is the dominant factor in our experimental setting. Additionally, the formation of a standing wave could be another possible source of the mechanical perturbation [40]. However, in theory, an adjacent node will be formed 17 µm ( above the culture dish and may not affect directly, as the height of adherent cells are generally considered to be shorter [41,42]. The oscillatory pattern of intracellular Ca2+ has been extensively studied in pancreatic β-cells, since its dynamic is tightly coupled to the exocytosis of insulin-containing vesicles from single cells, and its physiological significances have been documented [43]. Several modes of Ca2+ oscillation have been reported in β-cells through different pathways of Ca2+ entry depending on the concentration of glucose or the presence of other stimuli [44]. To the best of our knowledge, we are the first to report ultrasound-induced intracellular Ca2+ oscillations in the pancreatic β-cells. It was found that exposure to ultrasound induces Ca2+ dynamics even from the cells that did not respond to the stimulating level of glucose. Note that regardless of the glucose levels to which cells were adapted, the percentages of active cells were consistent after the stimulation (approximately 22%, Figure 1d), suggesting that ultrasound-induced Ca2+ responses may not share the same pathway with glucose-stimulated Ca2+ responses.
Interestingly, two very distinct modes of oscillations were dominantly observed where the cells adapted to different levels of glucose (5.6 mM and 11.1 mM). Further investigation with pharmaceutical agents revealed that the two oscillatory Ca2+ responses were originated from the activations of the two subtypes of P2 purinergic receptors. While P2X receptors are known for responding to only ATP and its derivatives, a few P2Y receptors, namely P2Y1, P2Y12, and P2Y13, have generally higher affinity to ADP and its derivatives than ATP [45,46]. Considering β-cell’s intrinsic property that tightly regulates ATP production in response to glucose level fluctuation [47], we believe that the amount of ATP (or ATP/ADP ratio) in cytoplasm plays a pivotal role selecting one pathway over another.
The fast-irregular oscillations found from this study showed very distinctive Ca2+ profiles, an instant rise followed by a relatively slow fall and fluctuations of the baseline, which can be often found from Ca2+ mobilization via VDCCs (Figure 2a). The spiking pattern was abolished or replaced by the slow-periodic oscillations in the presence of P2X inhibitor PPADS and L-type Ca2+ channel blocker, nifedipine, indicating that both channels are required for the specific pattern of Ca2+ entry. Although direct evidence is not presented in this paper, it seems that membrane depolarization is caused by ion exchange through the P2X channels, which in turn activates the VDCC. In fact, the P2X receptor-mediated depolarization was found to be sufficient to open up the VDCC in human pancreatic β-cells [48]. The overall suggested mechanisms of ultrasound-induced Ca2+ oscillations are illustrated in Figure 6.
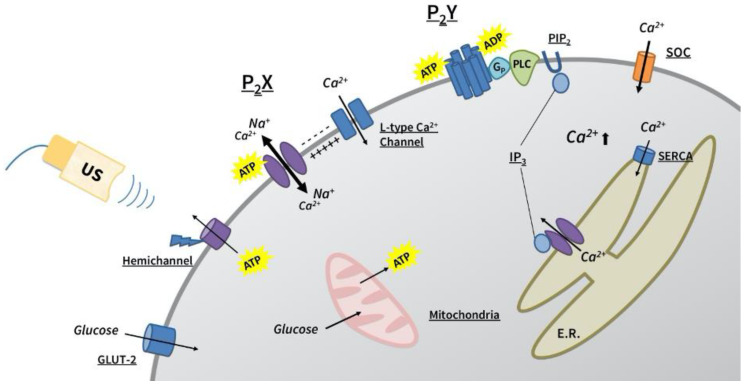
Figure 6.
A schematic drawing representing the proposed pathways of ultrasound-evoked Ca2+ dynamics in the HIT-T15 cells. Mechanosensitive hemichannel releases ATP and/or ADP into extracellular space in response to ultrasound stimulation, which in turn activates both P2 receptors. Depending on the amount of released ATP or ADP, the two subtypes of P2 receptors, P2X and P2Y, will be activated in a competitive manner, evoking either fast-irregular or slow-periodic Ca2+ oscillations, respectively.
The finding that L-type Ca2+ channels are closely linked to the fast-irregular oscillation pattern gives us another insight into the competing aspect of P2X and P2Y activations observed from this study. Gong et al. [49] reported the inhibitory effect of extracellular ATP on L-type Ca2+ channel currents through the P2Y-dependent pathway in rat pancreatic β-cells. A similar line of evidence was also found from myocytes [50,51], and the inhibition was reversed in the presence of protein kinase C (PKC) antagonist, indicating that PKC activation via G-protein-coupled P2Y signaling cascade is the source of the inhibition. It was found that activated PKC phosphorylates two sites on the n terminal of the L-type Ca2+ channel, which leads to the blockage of the channels by changing a net charge [52].
The mechanosensitive characteristic of the non-junctional hemichannels was reported elsewhere [53,54,55], and a recent study proposed that the association with integrin is critical for the sensing [56]. In fact, several lines of evidence support the finding that ATP can be released via mechanosensitive hemichannels. We have previously shown that the connexin 43 hemichannel works as a mechanosensor in hMSC that releases ATP to the extracellular space in response to low-intensity ultrasound [18]. In addition, we recently showed that ultrasound-induced ATP release was reduced significantly when the pannexin 1 hemichannel was either inhibited or knocked down in the prostate PC-3 cancer cells [57]. Ultrasound is a very unique modality that can transmit mechanical energy into tissues deep in the body without an invasive procedure. Thus, the finding that ultrasound induces the release of ATP via endogenous hemichannels in the pancreatic β-cells could pave the way for developing a new therapeutic intervention for diabetes.
In fact, the utilization of β-cell’s endogenous purinergic signaling cascade, as an indirect pathway for ultrasound-induced Ca2+ signaling, gives an edge over the direct activation of Ca2+ influx found from other studies. First, a localized stimulation can unlock the synchronized responses from a targeted pancreas tissue via the diffusion of extracellular ATP. Second, purinergic signaling induces oscillatory, rather than sustained, Ca2+, which mimics β-cell’s native signaling and is also critical for the pulsatile secretion of insulin. Last, the finding that the level of cytoplasmic ATP, which essentially regulates the KATP channel-dependent GSIS pathway, also plays a pivotal role in determining the modes of oscillations in ultrasound-mediated Ca2+ dynamics suggests the means of intrinsic regulation.
In summary, ultrasound-mediated intracellular Ca2+ dynamics were investigated in HIT-T15 pancreatic β-cells using 45-MHz focused ultrasound. The results indicate that low-intensity focused ultrasound is capable of inducing distinctive oscillatory Ca2+ patterns from the HIT-T15 β-cell line through the endogenous purinergic signaling pathway where ATP release from mechanosensitive hemichannels works as a signaling mediator. The mechanotransduction pathway explored in this paper provides meaningful insights into the biophysical basis of ultrasound-induced Ca2+ dynamics in pancreatic β-cells and could stimulate further the development of a novel ultrasound-based therapeutic modality, especially against diabetic conditions. However, to claim the feasibility of the ultrasound-based Ca2+ modulator platform in the diabetic-related therapeutic applications, investigation on pulsatile insulin secretion upon ultrasound exposure, particularly in the context of different Ca2+ oscillation patterns, is necessary. The current ultrasound stimulation system is optimized for imaging live cells within an image field but lacks a populational functional assay capability. Therefore, future works will be focused on building a high-throughput stimulation system that offers consistent stimulation environment and means of systematic assessment for ultrasound-induced secretion events. Additionally, further validation with low-frequency ultrasound (2–6 MHz) is required to be more relevant to clinical settings.
Acknowledgments
We thank Nestor Cabrera Muñoz and Ruimin Chen for their assistance with the transducer fabrication. We thank Madison Zitting for proofreading this manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4409/9/5/1129/s1 [58,59,60,61,62,63,64].
Author Contributions
Conceptualization, C.W.Y., C.Y. and K.K.S.; methodology, C.W.Y., H.G.L.; investigation, C.W.Y., K.M.K., S.M., K.G.; resources, H.G.L., H.J.; data curation, C.W.Y.; writing—original draft preparation, C.W.Y.; writing—review and editing, N.S.L., H.G.L., K.K.S.; supervision, N.S.L., K.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institutes of Health under grant No. P41-EB002182 to K. Kirk Shung and in part by the National Research Foundation of Korea (NRF) grants funded by the Ministry of Science and ICT, Korea (MIST) under Grant No. 2018R1D1A1A02085904 to Hae Gyun Lim.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Nathan D.M., Schreiber E., Fogel H., Mojsov S., Habener J.F. Insulinotropic action of glucagonlike peptide-I-(7-37) in diabetic and nondiabetic subjects. Diabetes Care. 1992;15:270–276. doi: 10.2337/diacare.15.2.270. [DOI] [PubMed] [Google Scholar]
- 2.Lundquist I., Alm P., Salehi A., Henningsson R., Grapengiesser E., Hellman B. Carbon monoxide stimulates insulin release and propagates Ca2+ signals between pancreatic beta-cells. Am. J. Physiol. Endocrinol. Metab. 2003;285:E1055–E1063. doi: 10.1152/ajpendo.00498.2002. [DOI] [PubMed] [Google Scholar]
- 3.Ashcroft F.M., Rorsman P. ATP-sensitive K+ channels: A link between B-cell metabolism and insulin secretion. Biochem. Soc. Trans. 1990;18:109–111. doi: 10.1042/bst0180109. [DOI] [PubMed] [Google Scholar]
- 4.Newsholme P., Gaudel C., McClenaghan N.H. Nutrient regulation of insulin secretion and beta-cell functional integrity. Adv. Exp. Med. Biol. 2010;654:91–114. doi: 10.1007/978-90-481-3271-3_6. [DOI] [PubMed] [Google Scholar]
- 5.Lang D.A., Matthews D.R., Peto J., Turner R.C. Cyclic oscillations of basal plasma glucose and insulin concentrations in human beings. N. Engl. J. Med. 1979;301:1023–1027. doi: 10.1056/NEJM197911083011903. [DOI] [PubMed] [Google Scholar]
- 6.Bratusch-Marrain P.R., Komjati M., Waldhäusl W.K. Efficacy of pulsatile versus continuous insulin administration on hepatic glucose production and glucose utilization in type I diabetic humans. Diabetes. 1986;35:922–926. doi: 10.2337/diab.35.8.922. [DOI] [PubMed] [Google Scholar]
- 7.Matthews D.R., Lang D.A., Burnett M.A., Turner R.C. Control of pulsatile insulin secretion in man. Diabetologia. 1983;24:231–237. doi: 10.1007/BF00282705. [DOI] [PubMed] [Google Scholar]
- 8.Paolisso G., Sgambato S., Torella R., Varricchio M., Scheen A., D’Onofrio F., Lefèbvre P.J. Pulsatile insulin delivery is more efficient than continuous infusion in modulating islet cell function in normal subjects and patients with type 1 diabetes. J. Clin. Endocrinol. Metab. 1988;66:1220–1226. doi: 10.1210/jcem-66-6-1220. [DOI] [PubMed] [Google Scholar]
- 9.O’Meara N.M., Sturis J., Herold K.C., Ostrega D.M., Polonsky K.S. Alterations in the patterns of insulin secretion before and after diagnosis of IDDM. Diabetes Care. 1995;18:568–571. doi: 10.2337/diacare.18.4.568. [DOI] [PubMed] [Google Scholar]
- 10.Lang D.A., Matthews D.R., Burnett M., Turner R.C. Brief, irregular oscillations of basal plasma insulin and glucose concentrations in diabetic man. Diabetes. 1981;30:435–439. doi: 10.2337/diab.30.5.435. [DOI] [PubMed] [Google Scholar]
- 11.Hagren O.I., Tengholm A. Glucose and insulin synergistically activate phosphatidylinositol 3-kinase to trigger oscillations of phosphatidylinositol 3,4,5-trisphosphate in beta-cells. J. Biol. Chem. 2006;281:39121–39127. doi: 10.1074/jbc.M607445200. [DOI] [PubMed] [Google Scholar]
- 12.Best L., Miley H.E., Yates A.P. Activation of an anion conductance and beta-cell depolarization during hypotonically induced insulin release. Exp. Physiol. 1996;81:927–933. doi: 10.1113/expphysiol.1996.sp003993. [DOI] [PubMed] [Google Scholar]
- 13.Casas S., Novials A., Reimann F., Gomis R., Gribble F.M. Calcium elevation in mouse pancreatic beta cells evoked by extracellular human islet amyloid polypeptide involves activation of the mechanosensitive ion channel TRPV4. Diabetologia. 2008;51:2252–2262. doi: 10.1007/s00125-008-1111-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suarez Castellanos I., Singh T., Balteanu B., Bhowmick D.C., Jeremic A., Zderic V. Calcium-dependent ultrasound stimulation of secretory events from pancreatic beta cells. J. Ther. Ultrasound. 2017;5:30. doi: 10.1186/s40349-017-0108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang J.Y., Lee N.S., Lee C., Lam K.H., Kim H.H., Woo J., Lin M.Y., Kisler K., Choi H., Zhou Q., et al. Investigating contactless high frequency ultrasound microbeam stimulation for determination of invasion potential of breast cancer cells. Biotechnol. Bioeng. 2013;110:2697–2705. doi: 10.1002/bit.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weitz A.C., Lee N.S., Yoon C.W., Bonyad A., Goo K.S., Kim S., Moon S., Jung H., Zhou Q., Chow R.H., et al. Functional Assay of Cancer Cell Invasion Potential Based on Mechanotransduction of Focused Ultrasound. Front. Oncol. 2017;7:161. doi: 10.3389/fonc.2017.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang J.Y., Lim H.G., Yoon C.W., Lam K.H., Yoon S., Lee C., Chiu C.T., Kang B.J., Kim H.H., Shung K.K. Non-contact high-frequency ultrasound microbeam stimulation for studying mechanotransduction in human umbilical vein endothelial cells. Ultrasound Med. Biol. 2014;40:2172–2182. doi: 10.1016/j.ultrasmedbio.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon C.W., Jung H., Goo K., Moon S., Koo K.M., Lee N.S., Weitz A.C., Shung K.K. Low-Intensity Ultrasound Modulates Ca2+ Dynamics in Human Mesenchymal Stem Cells via Connexin 43 Hemichannel. Ann. Biomed. Eng. 2017;46:48–59. doi: 10.1007/s10439-017-1949-7. [DOI] [PubMed] [Google Scholar]
- 19.Lam K.H., Hsu H.S., Li Y., Lee C., Lin A., Zhou Q., Kim E.S., Shung K.K. Ultrahigh frequency lensless ultrasonic transducers for acoustic tweezers application. Biotechnol. Bioeng. 2013;110:881–886. doi: 10.1002/bit.24735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cannata J.M., Ritter T.A., Chen W.H., Silverman R.H., Shung K.K. Design of efficient, broadband single-element (20-80 MHz) ultrasonic transducers for medical imaging applications. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2003;50:1548–1557. doi: 10.1109/TUFFC.2003.1251138. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter A.E., Jones T.R., Lamprecht M.R., Clarke C., Kang I.H., Friman O., Guertin D.A., Chang J.H., Lindquist R.A., Moffat J., et al. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung S.K., Kauri L.M., Qian W.J., Kennedy R.T. Correlated oscillations in glucose consumption, oxygen consumption, and intracellular free Ca(2+) in single islets of Langerhans. J. Biol. Chem. 2000;275:6642–6650. doi: 10.1074/jbc.275.9.6642. [DOI] [PubMed] [Google Scholar]
- 23.Leech C.A., Holz G.G., Habener J.F. Voltage-independent calcium channels mediate slow oscillations of cytosolic calcium that are glucose dependent in pancreatic beta-cells. Endocrinology. 1994;135:365–372. doi: 10.1210/endo.135.1.8013370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee N.S., Rohan J.G., Zitting M., Kamath S., Weitz A., Sipos A., Salvaterra P.M., Hasegawa K., Pera M., Chow R.H. A novel dual-color reporter for identifying insulin-producing beta-cells and classifying heterogeneity of insulinoma cell lines. PLoS ONE. 2012;7:e35521. doi: 10.1371/journal.pone.0035521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beauvois M.C., Merezak C., Jonas J.C., Ravier M.A., Henquin J.C., Gilon P. Glucose-induced mixed [Ca2+]c oscillations in mouse beta-cells are controlled by the membrane potential and the SERCA3 Ca2+-ATPase of the endoplasmic reticulum. Am. J. Physiol. Cell Physiol. 2006;290:C1503–C1511. doi: 10.1152/ajpcell.00400.2005. [DOI] [PubMed] [Google Scholar]
- 26.Burnstock G. Purinergic signalling in endocrine organs. Purinergic. Signal. 2014;10:189–231. doi: 10.1007/s11302-013-9396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tahani H.M. The purinergic nerve hypothesis and insulin secretion. Z Ernahrungswiss. 1979;18:128–138. doi: 10.1007/BF02023727. [DOI] [PubMed] [Google Scholar]
- 28.Bertrand G., Chapal J., Loubatieres-Mariani M.M. Potentiating synergism between adenosine diphosphate or triphosphate and acetylcholine on insulin secretion. Am. J. Physiol. 1986;251:E416–E421. doi: 10.1152/ajpendo.1986.251.4.E416. [DOI] [PubMed] [Google Scholar]
- 29.Leitner J.W., Sussman K.E., Vatter A.E., Schneider F.H. Adenine nucleotides in the secretory granule fraction of rat islets. Endocrinology. 1975;96:662–677. doi: 10.1210/endo-96-3-662. [DOI] [PubMed] [Google Scholar]
- 30.Petit P., Lajoix A.D., Gross R. P2 purinergic signalling in the pancreatic beta-cell: Control of insulin secretion and pharmacology. Eur. J. Pharm. Sci. 2009;37:67–75. doi: 10.1016/j.ejps.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Novak I. Purinergic receptors in the endocrine and exocrine pancreas. Purinergic. Signal. 2008;4:237–253. doi: 10.1007/s11302-007-9087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satin L.S., Tavalin S.J., Kinard T.A., Teague J. Contribution of L- and non-L-type calcium channels to voltage-gated calcium current and glucose-dependent insulin secretion in HIT-T15 cells. Endocrinology. 1995;136:4589–4601. doi: 10.1210/endo.136.10.7545106. [DOI] [PubMed] [Google Scholar]
- 33.Bertrand G., Chapal J., Loubatieres-Mariani M.M., Roye M. Evidence for two different P2-purinoceptors on beta cell and pancreatic vascular bed. Br. J. Pharmacol. 1987;91:783–787. doi: 10.1111/j.1476-5381.1987.tb11276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee D.H., Park K.S., Kim D.R., Lee J.W., Kong I.D. Dual effect of ATP on glucose-induced insulin secretion in HIT-T15 cells. Pancreas. 2008;37:302–308. doi: 10.1097/MPA.0b013e318168daaa. [DOI] [PubMed] [Google Scholar]
- 35.Sabourin J., Allagnat F. Store-operated Ca2+ entry: A key component of the insulin secretion machinery. J. Mol. Endocrinol. 2016;57:F35–F39. doi: 10.1530/JME-16-0106. [DOI] [PubMed] [Google Scholar]
- 36.Santerre R.F., Cook R.A., Crisel R.M., Sharp J.D., Schmidt R.J., Williams D.C., Wilson C.P. Insulin synthesis in a clonal cell line of simian virus 40-transformed hamster pancreatic beta cells. Proc. Natl. Acad. Sci. USA. 1981;78:4339–4343. doi: 10.1073/pnas.78.7.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suarez Castellanos I., Jeremic A., Cohen J., Zderic V. Ultrasound Stimulation of Insulin Release from Pancreatic Beta Cells as a Potential Novel Treatment for Type 2 Diabetes. Ultrasound Med. Biol. 2017;43:1210–1222. doi: 10.1016/j.ultrasmedbio.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalecki D. Mechanical bioeffects of ultrasound. Annu. Rev. Biomed. Eng. 2004;6:229–248. doi: 10.1146/annurev.bioeng.6.040803.140126. [DOI] [PubMed] [Google Scholar]
- 39.Tufail Y., Matyushov A., Baldwin N., Tauchmann M.L., Georges J., Yoshihiro A., Tillery S.I., Tyler W.J. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 2010;66:681–694. doi: 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 40.O’Reilly M.A., Huang Y., Hynynen K. The impact of standing wave effects on transcranial focused ultrasound disruption of the blood-brain barrier in a rat model. Phys. Med. Biol. 2010;55:5251–5267. doi: 10.1088/0031-9155/55/18/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato M., Nagayama K., Kataoka N., Sasaki M., Hane K., Kataoka N., Sasaki M., Hane K. Local mechanical properties measured by atomic force microscopy for cultured bovine endothelial cells exposed to shear stress. J. Biomech. 2000;33:127–135. doi: 10.1016/S0021-9290(99)00178-5. [DOI] [PubMed] [Google Scholar]
- 42.Boitor R., Sinjab F., Strohbuecker S., Sottile V., Notingher I. Towards quantitative molecular mapping of cells by Raman microscopy: Using AFM for decoupling molecular concentration and cell topography. Faraday Discuss. 2016;187:199–212. doi: 10.1039/C5FD00172B. [DOI] [PubMed] [Google Scholar]
- 43.Tengholm A., Gylfe E. Oscillatory control of insulin secretion. Mol. Cell Endocrinol. 2009;297:58–72. doi: 10.1016/j.mce.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Grapengiesser E., Gylfe E., Hellman B. Three types of cytoplasmic Ca2+ oscillations in stimulated pancreatic beta-cells. Arch. Biochem. Biophys. 1989;268:404–407. doi: 10.1016/0003-9861(89)90602-4. [DOI] [PubMed] [Google Scholar]
- 45.Khakh B.S., Burnstock G., Kennedy C., King B.F., North R.A., Seguela P., Voigt M., Humphrey P.P., Seguela P., Voigt M., et al. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol. Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- 46.Abbracchio M.P., Burnstock G., Boeynaems J.M., Barnard E.A., Boyer J.L., Kennedy C., Knight G.E., Fumagalli M., Gachet C., Jacobson K.A., et al. International Union of Pharmacology LVIII: Update on the P2Y G protein-coupled nucleotide receptors: From molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka T., Nagashima K., Inagaki N., Kioka H., Takashima S., Fukuoka H., Noji H., Kakizuka A., Imamura H. Glucose-stimulated single pancreatic islets sustain increased cytosolic ATP levels during initial Ca2+ influx and subsequent Ca2+ oscillations. J. Biol. Chem. 2014;289:2205–2216. doi: 10.1074/jbc.M113.499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacques-Silva M.C., Correa-Medina M., Cabrera O., Rodriguez-Diaz R., Makeeva N., Fachado A., Diez J., Berman D.M., Kenyon N.S., Ricordi C., et al. ATP-gated P2 × 3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic beta cell. Proc. Natl. Acad. Sci. USA. 2010;107:6465–6470. doi: 10.1073/pnas.0908935107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gong Q., Kakei M., Koriyama N., Nakazaki M., Morimitsu S., Yaekura K., Tei C. P2Y-purinoceptor mediated inhibition of L-type Ca2+ channels in rat pancreatic beta-cells. Cell Struct. Funct. 2000;25:279–289. doi: 10.1247/csf.25.279. [DOI] [PubMed] [Google Scholar]
- 50.Qu Y., Campbell D.L., Strauss H.C. Modulation of L-type Ca2+ current by extracellular ATP in ferret isolated right ventricular myocytes. J. Physiol. 1993;471:295–317. doi: 10.1113/jphysiol.1993.sp019902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monaghan K.P., Koh S.D., Ro S., Yeom J., Horowitz B., Sanders K.M. Nucleotide regulation of the voltage-dependent nonselective cation conductance in murine colonic myocytes. Am. J. Physiol. Cell Physiol. 2006;291:C985–C994. doi: 10.1152/ajpcell.00112.2006. [DOI] [PubMed] [Google Scholar]
- 52.McHugh D., Sharp E.M., Scheuer T., Catterall W.A. Inhibition of cardiac L-type calcium channels by protein kinase C phosphorylation of two sites in the n-terminal domain. Proc. Natl. Acad. Sci. USA. 2000;97:12334–12338. doi: 10.1073/pnas.210384297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bao L., Sachs F., Dahl G. Connexins are mechanosensitive. Am. J. Physiol. Cell Physiol. 2004;287:C1389–C1395. doi: 10.1152/ajpcell.00220.2004. [DOI] [PubMed] [Google Scholar]
- 54.Garcia M., Knight M.M. Cyclic loading opens hemichannels to release ATP as part of a chondrocyte mechanotransduction pathway. J. Orthop. Res. 2010;28:510–515. doi: 10.1002/jor.21025. [DOI] [PubMed] [Google Scholar]
- 55.Takada H., Furuya K., Sokabe M. Mechanosensitive ATP release from hemichannels and Ca2+ influx through TRPC6 accelerate wound closure in keratinocytes. J. Cell Sci. 2014;127:4159–4171. doi: 10.1242/jcs.147314. [DOI] [PubMed] [Google Scholar]
- 56.Batra N., Burra S., Siller-Jackson A.J., Gu S., Xia X., Weber G.F., DeSimone D., Bonewald L.F., Lafer E.M., Sprague E., et al. Mechanical stress-activated integrin alpha5beta1 induces opening of connexin 43 hemichannels. Proc. Natl. Acad. Sci. USA. 2012;109:3359–3364. doi: 10.1073/pnas.1115967109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee N.S., Yoon C.W., Wang Q., Moon S., Koo K.M., Jung H., Chen R., Jiang L., Lu G., Fernandez A., et al. Focused ultrasound stimulates ER localized mechanosensitive PANNEXIN-1 to mediate intracellular calcium release in invasive cancer cells. BioRxiv. 2020 doi: 10.1101/2020.04.03.024372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Orriss I.R., Key M.L., Hajjawi M.O., Arnett T.R. Extracellular ATP released by osteoblasts is a key local inhibitor of bone mineralisation. PLoS ONE. 2013;8:e69057. doi: 10.1371/journal.pone.0069057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoyle C.H., Knight G.E., Burnstock G. Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br J Pharmacol. 1990;99:617–621. doi: 10.1111/j.1476-5381.1990.tb12979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anselmi F., Hernandez V.H., Crispino G., Seydel A., Ortolano S., Roper S.D., Kessaris N., Richardson W., Rickheit G., Filippov M.A., et al. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc. Natl. Acad. Sci. USA. 2008;105:18770–18775. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flores-Soto E., Reyes-Garcia J., Sommer B., Chavez J., Barajas-Lopez C., Montano L.M. PPADS, a P2X receptor antagonist, as a novel inhibitor of the reverse mode of the Na+/Ca2+ exchanger in guinea pig airway smooth muscle. Eur. J. Pharmacol. 2012;674:439–444. doi: 10.1016/j.ejphar.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 62.Seidler N.W., Jona I., Vegh M., Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J. Biol. Chem. 1989;264:17816–17823. [PubMed] [Google Scholar]
- 63.Nobile M., Monaldi I., Alloisio S., Cugnoli C., Ferroni S. ATP-induced, sustained calcium signalling in cultured rat cortical astrocytes: Evidence for a non-capacitative, P2X7-like-mediated calcium entry. FEBS Lett. 2003;538:71–76. doi: 10.1016/S0014-5793(03)00129-7. [DOI] [PubMed] [Google Scholar]
- 64.Tian C., Du L., Zhou Y., Li M. Store-operated CRAC channel inhibitors: Opportunities and challenges. Future Med. Chem. 2016;8:817–832. doi: 10.4155/fmc-2016-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.