Abstract
Background
B7-H6 is a novel co-stimulatory ligand that is detected in most malignancies. However, the significance of B7-H6 in small cell lung cancer (SCLC) remains unknown.
Methods
B7-H6 expression was analyzed by immunohistochemistry (IHC) in 103 collected SCLC samples, and its association with clinicopathological characteristics and prognosis was analyzed. The 2-year survival rates were also investigated.
Results
B7-H6-positive staining was detected in 58 (56.31%) SCLC cases, and found to be localized mainly in the intracellular space of SCLC. Weak staining in lung tissues was observed in 4 (8%) cases. B7-H6 positive staining was significantly related to tumor-node-metastasis stage (P=0.028), age (P=0.001), and distant metastasis (P=0.033), whereas there was no association with smoking status, sex, mass size, limited-stage SCLC/extensive-stage SCLC, Karnofsky performance status, or nodal metastasis status. The 2-year survival rates showed that there were more patients whose survival was shorter than 2 years in the B7-H6-positive group compared with the B7-H6-negative group (P=0.042).
Conclusions
Our findings suggest that B7-H6 is involved in early-stage SCLC and could serve as an early marker to predict human SCLC progression and distant metastasis. B7-H6 may be a valuable therapeutic target with potential clinical applications in the future.
Keywords: B7-H6, immunohistochemistry (IHC), small cell lung cancer (SCLC), prognosis
Introduction
Lung carcinoma still stands as the world’s leading cause of death among human malignancies, and is one of the most commonly diagnosed tumors (1). One of its more common subtypes, small cell lung cancer (SCLC), accounts for 10–20% of lung carcinomas (2). A few of the key hallmarks of SCLC are its rapid development and strong resistance to chemotherapy, thus leading to its overall association with poor prognosis. An effective therapeutic target in SCLC has not been identified to date (3), underscoring the need to identify novel markers and therapeutic targets for the treatment of SCLC.
The immune checkpoint molecules belonging to the B7 family play a vital role in tumor-associated immune responses (4). B7-H6 is a newly identified co-stimulatory ligand that shows similarity to other immune co-stimulatory molecules. B7-H6 as a trans-membrane molecule binds to natural cytotoxicity triggering receptor 3 (NKp30). NKp30 is located mainly on the surface of natural killer cells, which produce cytotoxic chemokines to initiate antitumor responses (5-7). B7-H6-positive expression has been identified in various human malignancies including those of the ovaries and breast, and is associated with tumor progression (8-10). Nevertheless, the clinical significance of B7-H6 expression in SCLC remains unknown.
We thus analyzed B7-H6 staining in SCLC and normal lung tissues by immunohistochemistry (IHC), and investigated the correlation of B7-H6 with the clinical factors of patients having SCLC. We further surveyed the impact of B7-H6 on survival rates at 2 years and the prognosis of SCLC patients. This study’s objective was to determine if B7-H6 could potentially act as a marker for predicting SCLC progression and as a therapeutic target in SCLC. We present the following article in accordance with the REMARK reporting checklist (available at http://dx.doi.org/10.21037/atm-20-2548).
Methods
Specimens
In total, 103 SCLC wax block samples and 50 normal lung wax block specimens (controls) were provided by the Affiliated Hospital of Jiangnan University (Wuxi, China), the Affiliated Jiangyin Hospital of Southeast University Medical College, and the First People’s Hospital of Yancheng Affiliated with Nantong University. In this study, both tumor samples and non-tumor specimens were from patients who were not undergoing chemotherapy or radiotherapy. The median age was 64.21 (range, 33–85) years. The clinicopathological features and survival data of patients were collected. All specimens of patients were taken with informed consent. The International Association for the Study of Lung Cancer (IASLC, 8th edition) was used to evaluate tumor-node-metastasis (TNM) staging of SCLC. The ethics committees of the 3 hospitals approved the study protocol.
IHC for SCLC samples
For IHC staining, wax blocks were sectioned into 4 µm sections, moved to adhesive slides, deparaffinized, and dehydrated. Antigen retrieval was performed as follows: slides with tissue were placed in citrate buffer for 30 min at 100 °C. To hinder the endogenous peroxidase activity, sections were blocked with hydrogen peroxide for 30 min, followed by washes with phosphate buffered saline. Furthermore, B7-H6 polyclonal antibody (1:500 dilution, Abcam, UK) was added to the sections and incubated overnight at 4 °C, and rabbit IgG Ab 1 antibody (1:1,000 dilutions, Merck & Millipore) was used as a control. Next, sections were incubated with horseradish peroxide conjugated goat anti-rabbit IgG as secondary antibody (Abcam, UK) for 15 min. Diaminobenzidine was used to stain the sections and an Olympus BX43light microscope (Olympus, Japan) was used to capture images.
Evaluation of B7-H6 staining in SCLC
Two independent pathologists examined and evaluated the stained sections using clinical diagnosis and staining scores. B7-H6 expression score was calculated based on staining intensity and the staining percentage within tumors. Staining percentage was assessed as follows: 0%=0; 1–10%=1; 11–33%=2; 34–66%=3; 67–100%=4. Staining intensity was evaluated as follows: no staining =0; light yellow indicating weak staining =1; yellowish brown indicating moderate staining =2; brown indicating strong staining =3. The score was determined as follows: 0= negative; 1–4= weakly positive; 5–8= moderately positive; 9–12= strongly positive. B7-H6-positive and -negative samples were divided as follows (11): a negative group (score ≤2) and a positive group (score >2).
Statistical analysis
In this study, data were analyzed by SPSS 17.0. The correlation of B7-H6 staining with clinical factors was evaluated with the Chi-square test. Statistical significance was determined at P values <0.05.
Results
B7-H6 staining in SCLC samples
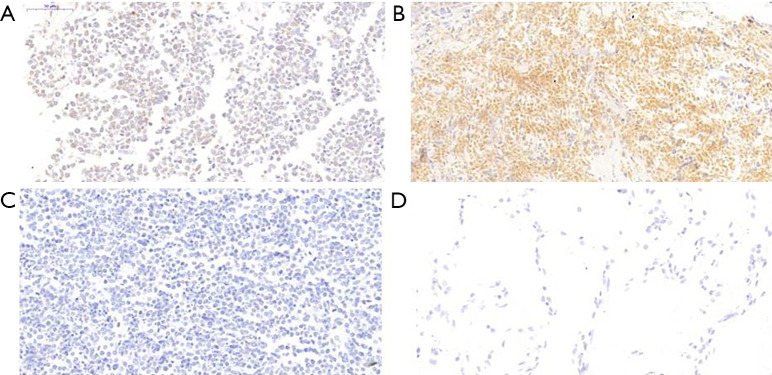
B7-H6 expression was analyzed by IHC in 103 SCLC clinical specimens and 50 normal lung tissue specimens (Table 1). B7-H6-positive staining was mainly localized within the nucleus and cytoplasm of the cells (Figure 1). B7-H6 positivity was detected in 58 (56.31%) SCLC cases. Moderate staining or strong staining was not observed in normal lung specimens (Figure 1), whereas weak staining was observed in 4 (8%) normal lung tissue specimens.
Table 1. B7-H6 expression in SCLC tissues and normal lung tissues.
| Group | Cases | B7-H6 | Positive cases (%) | |
|---|---|---|---|---|
| Positive cases | Negative cases | |||
| SCLC tissues | 103 | 58 | 45 | 56.31 |
| Lung tissues | 50 | 4 | 46 | 8 |
SCLC, small cell lung cancer.
Figure 1.
B7-H6 immunohistochemical staining in SCLC and lung tissues. (A) Weak staining at a magnification of ×400 in SCLC. (B) Moderate staining at a magnification of ×400 in SCLC. (C) Negative staining at a magnification of ×400 in SCLC. (D) Negative staining at a magnification of ×400 in lung tissues. SCLC, small cell lung cancer.
Relations among B7-H6 expression, clinical characteristics, and prognosis
We investigated the correlation of B7-H6 staining with clinicopathologic factors of SCLC patients. The results showed a positive correlation between B7-H6 positivity and clinical parameters such as TNM stage (P=0.028), distant metastasis (P=0.033), and age (P=0.001) (Table 2). B7-H6 positivity was not correlated with mass size, smoking status, sex, Karnofsky performance status, limited-stage SCLC/extensive-stage SCLC, and lymph node metastasis status. The 2-year survival rates of B7-H6-positive and -negative staining groups were also investigated. Significant differences in 2-year survival rates were observed in patients with B7-H6-positive and -negative expression. The SCLC group with B7-H6-positive staining had a higher proportion of patients with a survival time of <2 years (Table 2). The IHC staining results suggested that B7-H6 is correlated with TNM stage and distant metastasis, as B7-H6 expression was increased in early-stage patients with no distant metastasis. SCLC patients with B7-H6 positivity may have a higher degree of malignancy and a poorer prognosis than patients with B7-H6 negativity.
Table 2. Association between B7-H6 expression and the clinicopathological parameters of SCLC patients.
| Characteristic | Cases | B7-H6 | P value | |
|---|---|---|---|---|
| Positive cases | Negative cases | |||
| Gender | ||||
| Male | 80 | 47 | 33 | 0.352 |
| Female | 23 | 11 | 12 | |
| Age (years) | ||||
| <65 | 44 | 33 | 11 | 0.001 |
| ≥65 | 59 | 25 | 34 | |
| Smoking | 0.575 | |||
| Yes | 49 | 29 | 20 | |
| No | 54 | 29 | 25 | |
| Tumor size (cm) | ||||
| <5 | 53 | 33 | 20 | 0.210 |
| ≥5 | 50 | 25 | 25 | |
| LS/ES-SCLC | ||||
| LS-SCLC | 76 | 47 | 29 | 0.058 |
| ES-SCLC | 27 | 11 | 16 | |
| KPS | ||||
| <70 | 11 | 5 | 6 | 0.655 |
| ≥70 | 92 | 53 | 39 | |
| TNM stage | ||||
| I + II | 19 | 15 | 4 | 0.028 |
| III + IV | 84 | 43 | 41 | |
| Nodal metastasis | ||||
| N0 | 10 | 9 | 1 | 0.054 |
| N1 + N2 + N3 | 93 | 49 | 44 | |
| Distant metastasis | ||||
| M0 | 75 | 47 | 28 | 0.033 |
| M1 | 28 | 11 | 17 | |
| Survival time | ||||
| ≤2 years | 64 | 41 | 23 | 0.042 |
| >2 years | 39 | 17 | 22 | |
SCLC, small cell lung cancer; LS, limited stage; ES, extensive stage; TNM, tumor-node-metastasis; KPS, Karnofsky performance status score.
Discussion
B7-H6 is a co-stimulatory ligand that plays a fundamental role in the immune microenvironment and is expressed in human malignancies and involved in tumor progression (5). B7 family checkpoint molecules such as B7-H4 and B7-H1 are co-stimulatory ligands that interact with their receptors on the T-cell surface and function as inhibitory immune checkpoints in various human tumors (12). B7 family members are correlated with tumor progression and prognosis (13). Programmed cell death 1 ligand (PD-L1), B7-H4, and B7-H3 have been detected in SCLC patients (14). The development of therapies based on targeting PD-1 and PD-L1 has progressed in recent years. In the treatment of non-small cell lung cancer (NSCLC), pembrolizumab (MSD Carlow, Ireland) combined chemotherapy and nivolumab (Bristol-Myers Squibb) combined chemotherapy have been used as first- and second-line therapies, respectively (15-18). Atezolizumab (Roche Holding Ltd., China) combined chemotherapy is considered as first-line therapy in extensive-stage (ES)-SCLC (19). These treatments have extended the overall survival of NSCLC and SCLC patients. The clinical importance and commitment to targeting these checkpoint molecules has become evident during the treatment of these malignancies (20).
B7-H6 is a novel B7 family checkpoint molecule. B7-H6 interacts with its receptor NKp30, triggering tumor-associated immune responses of NK cells, and contributes to preventing tumor cells from escaping immune surveillance in the tumor microenvironment (21-23). B7-H6 shows a significant association with human malignancies (24), whereas it is not expressed in non-tumor tissues. Chen et al. (25) observed B7-H6 positivity in human hepatocellular carcinoma (HCC), and the relationship of B7-H6 positivity with tumor size has been noted. The negative relationship of B7-H6 expression with hepatocellular carcinoma (HCC) patient survival time has also been reported, while B7-H6 staining in breast cancer tissues was additionally observed (8). The increased expression of B7-H6 is significantly associated with a high rate of lymph node metastasis and short-term survival, and the human epidermal growth factor receptor 2, which is associated with metastasis and poor prognosis, is correlated with high B7-H6 expression. Sieviläinen et al. (26) investigated the expression of immune co-stimulatory molecules in oral squamous cell cancer (OSCC), and found that the short-term survival of OSCC patients was related to immune checkpoint molecules including B7-H6. In a previous study, we showed that the differentiation status in NSCLC is correlated with B7-H6 expression, whereas NSCLC patient prognosis is not related to B7-H6 expression (27). Rusakiewicz et al. (28) detected the levels of soluble B7-H6 (sB7-H6) in gastrointestinal stromal tumor (GIST) patients, and found that GIST patients with metastasis have higher B7-H6 expression than patients with localized GIST, and B7-H6 levels decreased after imatinib mesylate therapy, suggesting that B7-H6 levels in plasma reflect tumor burden. However, the clinical significance of B7-H6 in SCLC has received little attention.
Here, we examined B7-H6 expression in SCLC specimens by IHC. We investigated the impact of B7-H6 on clinical features, and explored B7-H6 as a potential predictor of early SCLC progression and poor prognosis. B7-H6 staining was observed mainly in the intracellular space within tumor cells. High B7-H6 expression was not observed in normal lung specimens. B7-H6 positivity in SCLC was correlated with distant metastasis, TNM stage, and age, as higher B7-H6 staining was observed in early-stage SCLC and non-distant metastasis groups. The 2-year survival rates were significantly different between positive B7-H6 and negative expression types, and these rates showed that the group with B7-H6-positive expression had a higher proportion of patients with a survival time shorter than 2 years. The patients with B7-H6-positive staining had a shorter survival time than those with B7-H6-negative staining. The IHC results demonstrate that B7-H6 may be involved in SCLC progression. Despite the inclusion of a limited number of SCLC patients, the results indicate that B7-H6 plays a vital role in early stage tumor progression and has potential prognostic significance. B7-H6 may serve as an early marker for predicting human SCLC progression and metastasis.
The current study identified the potential clinical value of B7-H6 for SCLC patients. B7-H6 could serve as an early marker to predict human SCLC progression and distant metastasis and has potential to be a valuable immune target in future SCLC therapy.
Supplementary
The article’s supplementary files as
Acknowledgments
All authors would like to thank International Science Editing (http://www.internationalscienceediting.com) for the editing of this manuscript.
Funding: This work was supported by the Natural Science Foundation of Jiangsu Province of China (No. BK20161141) and the Revitalize and Defend the Key Talent’s Subsidy Project in Science and Education of the Department of Public Health of Jiangsu Province, China (No. QNRC2016156).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All charts and tables are original. The ethics committees of 3 hospitals including the Affiliated Hospital of Jiangnan University (Wuxi, China), the Affiliated Jiangyin Hospital of Southeast University Medical College (Jiangyin, China), and the First People’s Hospital of Yancheng Affiliated with Nantong University (Yancheng, China) approved the study protocol. All specimens of patients in this retrospective study were acquired with signed consent.
Footnotes
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-2548
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-2548
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-2548). The authors have no conflicts of interest to declare.
References
- 1.Lin CC. Challenges of the phase I drug development in non-small cell lung cancer. Chin Clin Oncol 2019;8:25. 10.21037/cco.2019.06.03 [DOI] [PubMed] [Google Scholar]
- 2.Fang S, Shen Y, Chen B, et al. H3K27me3 induces multidrug resistance in small cell lung cancer by affecting HOXA1 DNA methylation via regulation of the lncRNA HOTAIR. Ann Transl Med 2018;6:440. 10.21037/atm.2018.10.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu WH, Zhao X, Zhu J, et al. Checkpoint Kinase 1 Inhibition Enhances Cisplatin Cytotoxicity and Overcomes Cisplatin Resistance in SCLC by Promoting Mitotic Cell Death. J Thorac Oncol 2019;14:1032-45. 10.1016/j.jtho.2019.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parra ER, Villalobos P, Zhang J, et al. Immunohistochemical and Image Analysis-Based Study Shows That Several Immune Checkpoints are Co-expressed in Non-Small Cell Lung Carcinoma Tumors. J Thorac Oncol 2018;13:779-91. 10.1016/j.jtho.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 5.Ni L, Dong C. New B7 Family Checkpoints in Human Cancers. Mol Cancer Ther 2017;16:1203-11. 10.1158/1535-7163.MCT-16-0761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaifu T, Escaliere B, Gastinel LN, et al. B7-H6/NKp30 interaction: a mechanism of alerting NK cells against tumors. Cell Mol Life Sci 2011;68:3531-9. 10.1007/s00018-011-0802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Wang Q, Mariuzza RA. Structure of the human activating natural cytotoxicity receptor NKp30 bound to its tumor cell ligand B7-H6. J Exp Med 2011;208:703-14. 10.1084/jem.20102548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun J, Tao H, Li X, et al. Clinical significance of novel costimulatory molecule B7-H6 in human breast cancer. Oncol Lett 2017;14:2405-9. 10.3892/ol.2017.6417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y, Xu Y, Chen L, et al. B7-H6 expression correlates with cancer progression and patient's survival in human ovarian cancer. Int J Clin Exp Pathol 2015;8:9428-33. [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Jin X, Liu J, et al. The prognostic value of B7-H6 protein expression in human oral squamous cell carcinoma. J Oral Pathol Med 2017;46:766-72. 10.1111/jop.12586 [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Shen Y, Miao Y, et al. Co-expression of uPAR and CXCR4 promotes tumor growth and metastasis in small cell lung cancer. Int J Clin Exp Pathol 2014;7:3771-80. [PMC free article] [PubMed] [Google Scholar]
- 12.Ceeraz S, Nowak EC, Noelle RJ. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol 2013;34:556-63. 10.1016/j.it.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Xie Q, Wang Z, et al. Assessment of combined expression of B7-H3 and B7-H4 as prognostic marker in esophageal cancer patients. Oncotarget 2016;7:77237-43. 10.18632/oncotarget.12628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carvajal-Hausdorf D, Altan M, Velcheti V, et al. Expression and clinical significance of PD-L1, B7-H3, B7-H4 and TILs in human small cell lung Cancer (SCLC). J Immunother Cancer 2019;7:65. 10.1186/s40425-019-0540-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reck M. Pembrolizumab as first-line therapy for metastatic non-small-cell lung cancer. Immunotherapy 2018;10:93-105. 10.2217/imt-2017-0121 [DOI] [PubMed] [Google Scholar]
- 16.Borghaei H, Brahmer J. Nivolumab in Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2016;374:493-4. [DOI] [PubMed] [Google Scholar]
- 17.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 18.Wu YL, Lu S, Cheng Y, et al. Nivolumab Versus Docetaxel in a Predominantly Chinese Patient Population With Previously Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical Trial. J Thorac Oncol 2019;14:867-75. 10.1016/j.jtho.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 19.Horn L, Mansfield AS, Szczesna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. 10.1056/NEJMoa1809064 [DOI] [PubMed] [Google Scholar]
- 20.Ni L, Dong C. New checkpoints in cancer immunotherapy. Immunol Rev 2017;276:52-65. 10.1111/imr.12524 [DOI] [PubMed] [Google Scholar]
- 21.Matta J, Baratin M, Chiche L, et al. Induction of B7-H6, a ligand for the natural killer cell-activating receptor NKp30, in inflammatory conditions. Blood 2013;122:394-404. 10.1182/blood-2013-01-481705 [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Mo J, Jia X, et al. The B7 Family Member B7-H6: a New Bane of Tumor. Pathol Oncol Res 2018;24:717-21. 10.1007/s12253-017-0357-5 [DOI] [PubMed] [Google Scholar]
- 23.Bjornsen EG, Thiruchelvam-Kyle L, Hoelsbrekken SE, et al. B7H6 is a functional ligand for NKp30 in rat and cattle and determines NKp30 reactivity toward human cancer cell lines. Eur J Immunol 2019;49:54-65. [DOI] [PubMed] [Google Scholar]
- 24.Jiang T, Wu W, Zhang H, et al. High expression of B7-H6 in human glioma tissues promotes tumor progression. Oncotarget 2017;8:37435-47. 10.18632/oncotarget.16391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Feng J, Xu B, et al. B7-H6 expression in human hepatocellular carcinoma and its clinical significance [corrected]. Cancer Cell Int 2018;18:126. 10.1186/s12935-018-0627-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sieviläinen M, Almahmoudi R, Al-Samadi A, et al. The prognostic value of immune checkpoints in oral squamous cell carcinoma. Oral Dis 2019;25:1435-45. 10.1111/odi.12991 [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Zhang G, Qin Y, et al. B7-H6 expression in non-small cell lung cancers. Int J Clin Exp Pathol 2014;7:6936-42. [PMC free article] [PubMed] [Google Scholar]
- 28.Rusakiewicz S, Perier A, Semeraro M, et al. NKp30 isoforms and NKp30 ligands are predictive biomarkers of response to imatinib mesylate in metastatic GIST patients. Oncoimmunology 2017;6:e1137418. 10.1080/2162402X.2015.1137418 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as