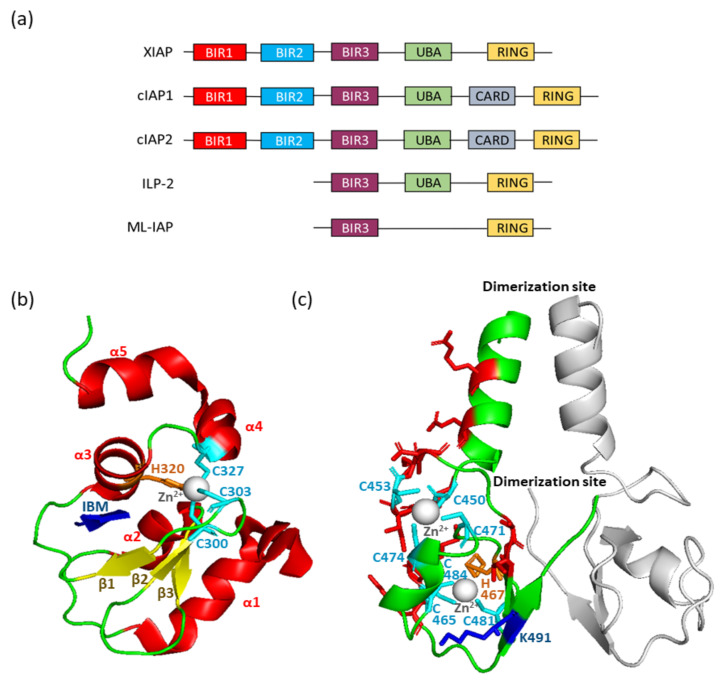
Figure 1.
(a) Structure of Inhibitor of Apoptosis (IAP) E3-ubiquitine ligases. BIR: Baculovirus IAP repeat; UBA: ubiquitin associated; CARD: caspase activation and recruitment domain; RING: really interesting gene. (b) Ribbon diagram of the cIAP1 BIR3 domain in contact with the IAP binding motif (IBM) of caspase 9. The sheets (β) are shown as yellow ribbons and helixes (α) as red ribbons. β-strands and the third α-helix form a deep hydrophobic groove stabilized by zinc atom (grey spheres) that is coordinated by three conserved Cysteine (cyan) and one Histidine (orange) residues. The IBM is represented in blue. (c) Ribbon diagram of an X-linked IAP (XIAP)-RING domain homodimer. The zinc ions are shown as grey spheres, the cysteine residues interacting with zinc as cyan sticks, the histidine as orange sticks, the ubiquitin-interacting site as blue sticks, and the E2-interacting sites as red sticks.