Abstract
Background
Metabolic risk factors including obesity, insulin resistance, dyslipidemia, metabolic syndrome (MS), and diabetes are associated with nonalcoholic fatty liver disease (NAFLD). γ-Glutamyl transpeptidase (GGT) and high-density lipoprotein cholesterol (HDL-C) are associated with insulin resistance, dyslipidemia, oxidative stress, and obesity. We investigated the associations between GGT/HDL-C ratio and prevalence of NAFLD in a Chinese population.
Methods
The study included 1,813 NAFLD (526 females, 1,287 males) and 4,513 non-NAFLD (3,077 females, 1,436 males) participants. The diagnosis of NAFLD was based on ultrasonography.
Results
Participants with NAFLD had higher GGT/HDL-C ratio, BMI, WC, TG, TC, and HOMA-IR, but lower HDL-C than participants without NAFLD. GGT/HDL-C ratio was significantly associated with prevalence of NAFLD. Specifically, for each 1 unit increase in GGT/HDL-C ratio, the prevalence of NAFLD will increase by 0.3%. As GGT/HDL-C ratio quartiles increased, prevalence of NAFLD/MS in Q4 (highest GGT/HDL-C ratio quartile) was 6.362/3.968 times higher than that in Q1 (lowest GGT/HDL-C ratio quartile). The AUC [0.799 (0.788–0.810)] for GGT/HDL-C ratio was significantly higher than those for GGT and HDL-C alone.
Conclusions
The present results suggest that GGT/HDL-C ratio can be used as a predictive factor for prevalence of NAFLD after adjustment for confounding variables.
Keywords: Nonalcoholic fatty liver disease (NAFLD), γ-glutamyl transpeptidase (GGT), high-density lipoprotein cholesterol (HDL-C), metabolic syndrome (MS)
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease worldwide, with an estimated global prevalence of 15–40% that continues to increase rapidly (1). NAFLD encompasses a wide spectrum of conditions from benign accumulation of fat in hepatocytes to nonalcoholic steatohepatitis (NASH), cirrhosis, and end-stage liver disease (2,3). Metabolic risk factors like obesity, insulin resistance (IR), dyslipidemia, and diabetes are associated with NAFLD (4-6), while the pathogenesis of NASH remains unclear. Several theories for NAFLD have been proposed, from the Two Hit Theory to the Multiple Hit Theory, and involve widespread metabolic dysfunction through interactions of genetic and environmental factors as well as crosstalk among the adipose tissue, pancreas, gut, and liver (2,7). A biopsy is the gold standard test for NAFLD, but is not feasible for epidemiological studies aiming to screen for NAFLD in healthy populations (7,8). Therefore, it is imperative to identify novel predictors for liver damage in NAFLD, with the aims of preventing progression from simple fatty liver to NASH and formulating early intervention strategies.
Recently, studies have indicated associations of γ-glutamyl transpeptidase (GGT) and high-density lipoprotein cholesterol (HDL-C) with NAFLD. Cruz et al. (9) found that GGT was more strongly associated with severity of fatty liver than alanine aminotransferase (ALT). Mansour-Ghanaei et al. (10) and Novakovic et al. (11) demonstrated a significant relationship between increased GGT and increased degree of NAFLD. Alam et al. (12) showed that serum ALT and aspartate aminotransferase (AST) levels were unable to predict NASH, while serum GGT level was significantly higher in NASH patients than in simple steatosis patients, with sensitivity of 45% and specificity of 68%, in a Bangladesh population. HDL-C has anti-inflammatory, antioxidant, and antithrombotic properties and is associated with IR, dyslipidemia, atherogenic indices, and obesity (13,14). Decreased HDL-C concentration is one of the characteristics of metabolic syndrome (MS) (15). IR may be an underlying mechanism leading to dyslipidemia featuring decreased HDL-C among MS components. NAFLD is strongly associated with MS (8,16). It can be seen from the above literature that single increase in GGT can be used as an indicator of steatosis in liver cells, while single decrease in HDL-C is associated with IR and dyslipidemia. However, the prognostic value of single GGT and single HDL-C measurements is limited. Given that GGT and HDL-C are both associated with NAFLD, we calculated their ratio, and speculated that GGT/HDL-C ratio may combine both functions to indicate NAFLD. The objectives of the present study were to investigate the predictive value of GGT/HDL-C ratio for NAFLD and to evaluate the diagnostic efficacy of GGT/HDL-C ratio in NAFLD in a Chinese general population.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-19-4516).
Methods
Study population
A total of 7,882 consecutive participants who underwent a general health checkup at the Health Care Centre in the First Affiliated Hospital of Medical College of Zhejiang University between July 2014 and November 2017 were initially enrolled. The personal history examined during the health checkup included alcohol consumption, history of liver disease, hypertension, and diabetes, and medication use for hypertension, hyperlipidemia, and diabetes. Among the 7,882 participants, 1,556 were excluded for one or more of the following criteria: alcohol consumption >30 g/day for men and >20 g/day for women (n=705); viral hepatitis or history of liver disease, including liver cirrhosis, chronic hepatitis, and autoimmune hepatitis (n=761); history of malignancy (n=45); presence of pregnancy (n=30); and missing laboratory data or incomplete participant information (n=15). The final sample size was 6,326 participants. We divided the 6,326 participants into two groups: NAFLD group (n=1,813), comprising 526 females (age: 53.1±9.4 years) and 1,287 males (age: 48.2±9.3 years); and non-NAFLD group (n=4,513), comprising 2,551 females (46.3±10.1 years) and 1,962 males (age: 48.1±10.5 years). This work was approved by the Ethics Committee of the First Affiliated Hospital of Medical College at Zhejiang University (Ethics Approval Ref: 2019-1486) and informed consent was obtained from participants.
Diagnostic criteria
NAFLD was diagnosed according to the guidelines established for the diagnosis and treatment of NAFLD issued by the Chinese National Consensus Workshop on Nonalcoholic Fatty Liver Disease (17). The diagnosis of NAFLD was based on ultrasonography findings of hepatic steatosis associated with characteristic echo patterns using a Toshiba Nemio 20 sonography machine with a 3.5-MHz probe (Toshiba, Tokyo, Japan). The hepatic ultrasound examinations were performed by experienced doctors. The characteristics of the echo patterns for hepatic steatosis included ultrasound beam attenuation, diffuse hyperechogenicity of liver, and poor visualization of intrahepatic structures.
MS was diagnosed in participants with three or more of the following criteria (18): (I) abdominal obesity (central obesity): waist circumstance (WC) ≥90 cm in men or ≥85 cm in women; (II) hyperglycemia: fasting plasma glucose (FPG) ≥6.1 mmol/L or plasma glucose 2 h after breakfast ≥7.8 mmol/L and/or confirmed diabetes under treatment; (III) hypertension: blood pressure ≥130/85 mmHg and/or on antihypertensive therapy; (IV) fasting triglyceride (TG) ≥1.70 mmol/L and/or on anti-hyperlipidemic therapy; (V) fasting HDL-C <1.0 mmol/L in males or <1.3 mmol/L in females.
Assessment of clinical and biochemical variables
All participants underwent a physical examination that included anthropometry, blood pressure measurement, and health habit inventory. The physical examination was performed by trained doctors. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured by an automated sphygmomanometer with the subject in the sitting position. Body mass index (BMI) was calculated as measured weight (kg) divided by height squared (m2). According to the criteria for Chinese people, normal weight was defined as BMI ≥18.5 and <24 kg/m2, overweight as BMI ≥24 and <28 kg/m2, and obesity as BMI ≥28 kg/m2. All venous blood samples were obtained in the morning after a 12-h fast.
The following biochemical parameters were determined in all participants: ALT, AST, alkaline phosphatase (ALP), TG, total cholesterol (TC), HDL-C, low-density lipoprotein cholesterol (LDL-C), GGT, FPG, creatinine (Cr), uric acid (UA), and insulin (INS). ALT, AST, TG, TC, GGT, HDL-C, LDL-C, Cr, UA, and FPG levels were measured by a Hitachi DDP autoanalyzer (Hitachi Corp., Ibaragi, Japan) using assay-specific Roche reagents (Roche Diagnostics, Indianapolis, IN, USA). Hepatitis B virus surface antigen, anti-hepatitis C virus antibody, and INS were determined by electrochemiluminescence immunoassay using an ARCHITECT i4000 (Abbott Diagnostics, Abbott Park, IL, USA). IR was assessed by the homeostasis model assessment of insulin resistance (HOMA-IR) according to the following equation: HOMA-IR (mIU·mmol/L) = fasting insulin (mIU/L) × fasting glucose (mmol/L)/22.5. All biochemical parameters were conducted in the same laboratory and the laboratory quality control is within the control range.
Statistical analysis
Statistical analyses were performed using SPSS version 22 software (SPSS, Chicago, IL, USA). One-sample Kolmogorov-Smirnov tests were used to assess the normality of data distributions. Data were presented as mean ± standard deviation when the distribution was normal and median (interquartile range) when the distribution was skewed. Categorical data were displayed as absolute and relative frequencies. Differences between/among groups were analyzed by Student’s t-test/one-way analysis of variance (ANOVA) or the Mann-Whitney U test/Kruskal-Wallis H test for continuous variables, and the chi-square test for categorical variables. Comparisons of prevalence of MS and NAFLD by GGT/HDL-C ratio quartiles were carried out by the Cochran-Armitage trend test. Spearman correlation analysis was used to examine the correlations between GGT/HDL-C ratio and clinical and laboratory parameters. Binary logistic regression was carried out to evaluate the association between GGT/HDL-C ratio and NAFLD after adjustment for clinical and biochemical variables. Receiver-operating characteristic (ROC) curve analyses were performed to assess the diagnostic accuracy of GGT/HDL-C ratio to detect NAFLD. For this, optimal cutoff values were selected by maximizing the sum of sensitivity and specificity, the sensitivity, specificity, and areas under the ROC curves (AUCs) of GGT/HDL-C ratio were obtained by comparing participants with and without NAFLD (NAFLD vs. non-NAFLD), and the differences between the AUCs were evaluated by the Z statistical test (19). Values of P<0.05 were considered statistically significant.
Results
Baseline characteristics of the participants
The baseline characteristics of the participants are shown in Table 1. For the NAFLD group, BMI, WC, SBP, DBP, ALT, AST, ALP, GGT, TG, TC, HDL-C, GGT/HDL-C ratio, LDL-C, FPG, Cr, UA, HOMA-IR, and smoking differed significantly compared with the non-NAFLD group (P<0.05 for all). Participants with NAFLD were older and had higher BMI, WC, GGT/HDL-C ratio, TG, TC, LDL-C, INS, HOMA-IR, and FPG than participants without NAFLD. The prevalence of MS was 58.3% in NAFLD participants, compared with only 14.5% in non-NAFLD participants.
Table 1. Baseline characteristics of the study population (n=6,326).
| Characteristics | Overall (n=6,326) | NO-NAFLD (n=4,513) | NAFLD (n=1,813) | P |
|---|---|---|---|---|
| Age (year) | 47.81±10.18 | 47.08±10.32 | 49.63±9.58 | <0.001* |
| Female (%) | 3,077 (48.6) | 2,551 (56.5) | 526 (29.0) | <0.001$ |
| WC (cm) | 85.12±9.37 | 82.01±8.18 | 92.9±7.48 | <0.001* |
| BMI (kg/m2) | 24.09±3.21 | 23.03±2.72 | 26.73±2.77 | <0.001* |
| Obesity (%) | 693 (11.0) | 178 (3.9) | 515 (28.4) | <0.001$ |
| SBP (mmHg) | 128±18 | 125±18 | 136±18 | <0.001* |
| DBP (mmHg) | 77±12 | 75±11 | 83±11 | <0.001* |
| ALP (U/L) | 66 [54–79] | 64 [53–78] | 70 [59–82] | <0.001# |
| ALT (U/L) | 18 [13–28] | 16 [12–23] | 27 [19–40] | <0.001# |
| AST (U/L) | 20 [17–24] | 19 [16–23] | 22 [19–28] | <0.001# |
| GGT (U/L) | 21 [14–34] | 18 [12–27] | 33 [23–50] | <0.001# |
| Cr (μmol/L) | 70 [59–81] | 67 [58–80] | 75 [63–85] | <0.001# |
| Glu (mmol/L) | 4.82 (4.51–5.22) | 4.73 (4.46–5.07) | 5.11 (4.69–5.78) | <0.001# |
| TC (mmol/L) | 4.65 (4.11–5.25) | 4.58 (4.07–5.16) | 4.84 (4.28–5.46) | <0.001# |
| TG (mmol/L) | 1.23 (0.87–1.82) | 1.05 (0.78–1.49) | 1.8 (1.34–2.56) | <0.001# |
| HDL-C (mmol/L) | 1.23 (1.02–1.48) | 1.31 (1.10–1.55) | 1.06 (0.91–1.23) | <0.001# |
| LDL-C (mmol/L) | 2.69 (2.23–3.20) | 2.63 (2.20–3.13) | 2.83 (2.36–3.34) | <0.001# |
| UA (μmol/L) | 313 [256–378] | 292 [242–351] | 368 [312–426] | <0.001# |
| INS (μU/mL) | 8.5 (5.9–12) | 7.4 (5.3–10.2) | 12 (9.1–16) | <0.001# |
| HOMA-IR | 1.86 (1.24–2.71) | 1.57 (1.10–2.22) | 2.82 (2.07–3.95) | <0.001# |
| GGT/HDL-C | 17.65 (10.16–31.83) | 13.60 (8.71–23.08) | 32.00 (20.89–50.99) | <0.001# |
| Diabetes (%) | 631 (10.0) | 245 (5.4) | 386 (21.3) | <0.001$ |
| MS (%) | 1710 (27.0) | 653 (14.5) | 1057 (58.3) | <0.001$ |
| Smoke (%) | 966 (15.3) | 577 (12.8) | 389 (21.5) | <0.001$ |
| Physical exercise (%) | 1,933 (30.6) | 1,397 (31.0) | 536 (29.6) | 0.278$ |
NAFLD, nonalcoholic fatty liver disease; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; WC, waist circumstance; MS, metabolic syndrome; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; Cr, creatinine; UA, uric acid; INS, insulin; Glu, glucose; HOMA-IR, homeostasis model assessment of insulin resistance; GGT, γ-glutamyl transpeptidase; HDL-C, high-density lipoprotein cholesterol; GGT/HDL-C, GGT/HDL-C ratio. Continuous variables were presented as mean ± SD or median (interquartile range). The statistical significance of differences between the non-NAFLD group and NAFLD group were analyzed by Student’s t-test (*), the Mann-Whitney U test (#), or the chi-square test ($).
Characteristics of NAFLD participants according to quartiles of GGT/HDL-C ratio
We divided the NAFLD participants into four groups: Q1 (lowest), Q2, Q3, and Q4 (highest) according to the quartiles of GGT/HDL-C ratio. As the GGT/HDL-C ratio quartiles increased, prevalence of MS and NAFLD gradually increased. Each quartile was further divided into three body weight groups: normal-weight, overweight, and obesity groups. From the first to fourth quartiles: normal-weight participants, the prevalences of NAFLD (0.6–3.2%; P<0.001) and MS (0.4–2.8%; P<0.001) increased; for overweight participants, the prevalences of NAFLD (0.9–21.1%; P<0.001) and MS (1.0–21.0%; P<0.001) increased; and for obese participants, the prevalences of NAFLD (1.4–43.4%; P<0.001) and MS (1.2–40.1%; P<0.001) increased. Therefore, the higher the GGT/HDL-C ratio quartile was and the heavier the participants were, the higher the prevalence of NAFLD and MS was.
Correlation analyses between GGT/HDL-C ratio and other variables
The correlation analyses revealed that GGT/HDL-C ratio in NAFLD participants was correlated with age (P<0.001), WC (P<0.001), BMI (P<0.001), SBP (P=0.001), DBP (P<0.001), ALT (P<0.001), AST (P<0.001), ALP (P<0.001), TG (P<0.001), TC (P<0.001), UA (P<0.001), HOMA-IR (P<0.001), prevalence of MS (P<0.001), and smoking (P<0.001). Therefore, GGT/HDL-C ratio was associated with liver enzymology markers (ALT, AST, and ALP), abdominal obesity (BMI and WC), IR (HOMA-IR), and prevalence of MS. These findings suggest that the indicated variables may act as cofactors for the link between GGT/HDL-C ratio and NAFLD.
Association between GGT/HDL-C ratio and prevalence of NAFLD/MS
Univariate regression analyses were performed to analyze the associations between GGT/HDL-C ratio and prevalence of NAFLD/MS in the 6,326 participants (Table 2) with forward selection. Data in Table 2 shows that age, sex, WC, BMI, SBP, DBP, ALT, AST, TG, TC, LDL-C, UA, HOMA-IR, and smoking (all P<0.05) could impact the prevalence of NAFLD/MS. On the basis of the univariate analyses, these indicators were included in the multivariate regression analysis for NAFLD/MS. The results of the adjusted binary logistic regression analysis models are shown in Table 3. In the multivariable adjusted models, the odds ratio (OR) and 95% confidence interval (CI) for NAFLD was 1.003 (1.001–1.006) and that for MS was 1.003 (1.000–1.007) in all 6326 participants (Table 3). Thus, each 1 unit increase in GGT/HDL-C ratio will increase the prevalence of NAFLD by 0.3% in all participants, and by 0.4% in overweight participants (Table 4). Furthermore, each 1 unit increase in GGT/HDL-C ratio will increase the prevalence of MS by 0.5% in overweight participants (Table 4).
Table 2. Associations of GGT/HDL-C ratio with prevalence of NAFLD/MS by univariate regression analyses.
| Characteristics | NAFLD | MS | |||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Sex (male) | 3.181 (2.829–3.577) | <0.001 | 2.165 (1.929–2.428) | <0.001 | |
| Age | 1.025 (1.020–1.031) | <0.001 | 1.042 (1.036–1.048) | <0.001 | |
| WC | 1.195 (1.183–1.207) | <0.001 | 1.177 (1.166–1.189) | <0.001 | |
| BMI | 1.633 (1.589–1.679) | <0.001 | 1.505 (1.468–1.543) | <0.001 | |
| SBP | 1.034 (1.031–1.037) | <0.001 | 1.050 (1.046–1.053) | <0.001 | |
| DBP | 1.061 (1.056–1.067) | <0.001 | 1.080 (1.074–1.086) | <0.001 | |
| ALP | 1.013 (1.011–1.016) | <0.001 | 1.018 (1.015–1.021) | <0.001 | |
| ALT | 1.047 (1.043–1.051) | <0.001 | 1.025 (1.022–1.029) | <0.001 | |
| AST | 1.042 (1.036–1.049) | <0.001 | 1.024 (1.018–1.030) | <0.001 | |
| Cr | 1.026 (1.022–1.030) | <0.001 | 1.018 (1.014–1.022) | <0.001 | |
| TC | 1.381 (1.299–1.469) | <0.001 | 1.233 (1.160–1.312) | <0.001 | |
| TG | 2.333 (2.177–2.500) | <0.001 | 5.970 (5.379–6.625) | <0.001 | |
| LDL-C | 1.389 (1.293–1.492) | <0.001 | 1.069 (0.994–1.149) | 0.072 | |
| UA | 1.010 (1.009–1.011) | <0.001 | 1.007 (1.006–1.008) | <0.001 | |
| HOMA-IR | 2.386 (2.255–2.526) | <0.001 | 2.259 (2.138–2.387) | <0.001 | |
| Smoke (%) | 1.864 (1.617–2.149) | <0.001 | 1.816 (1.572–2.097) | <0.001 | |
| GGT/HDL-C | 1.036 (1.033–1.039) | <0.001 | 1.035 (1.032–1.038) | <0.001 | |
NAFLD, nonalcoholic fatty liver disease; MS, metabolic syndrome; OR, odds ratio; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; WC, waist circumstance; MS, metabolic syndrome; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; Cr, creatinine; UA, uric acid; HOMA-IR, homeostasis model assessment of insulin resistance; GGT/HDL-C, GGT/HDL-C ratio.
Table 3. Associations of GGT/HDL-C ratio with prevalence of NAFLD/MS by multivariate regression analyses.
| Model | NAFLD | MS | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| 1 | 1.017 (1.014–1.020) | <0.001 | 1.021 (1.018–1.024) | <0.001 | |
| 2 | 1.013 (1.010–1.016) | <0.001 | 1.023 (1.019–1.026) | <0.001 | |
| 3 | 1.011 (1.008–1.014) | <0.001 | 1.022 (1.018–1.028) | <0.001 | |
| 4 | 1.004 (1.001–1.007) | 0.006 | 1.004 (1.001–1.008) | 0.026 | |
| 5 | 1.003 (1.001–1.006) | 0.037 | 1.003 (1.000–1.007) | 0.084 | |
Adjusted model 1: Age, WC, BMI, SBP, DBP, sex, and smoking (baseline indexes); model 2: model 1 + ALP, AST, and ALT (liver markers); model 3: model 2 + Cr and UA (kidney markers); model 4: model 3 + TC, TG, and LDL-C (lipid markers); model 5: model 4 + HOMA-IR. NAFLD, nonalcoholic fatty liver disease; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; WC, waist circumstance; MS, metabolic syndrome; GGT, γ-glutamyl transpeptidase; HDL-C, high-density lipoprotein cholesterol; GGT/HDL-C, GGT/HDL-C ratio.
Table 4. ORs of GGT/HDL-C ratio in normal-weight and overweight participants.
| Groups | OR (95% CI) | P value |
|---|---|---|
| NAFLD | ||
| Normal weight | 1.007 (1.000–1.014) | 0.062 |
| Overweight | 1.004 (1.001–1.008) | 0.014 |
| MS | ||
| Normal weight | 1.002 (0.993–1.010) | 0.704 |
| Overweight | 1.005 (1.001–1.009) | 0.035 |
Adjusted model 5 (shown in Table 3).
To increase the ability of the models to identify risks for the prevalence of NAFLD/MS, we conducted binary regression analyses to analyze the associations between GGT/HDL-C ratio quartiles and prevalence of NAFLD/MS (Table 5). In the multivariable adjusted models, the ORs of GGT/HDL-C ratio quartiles comparing the highest quartile (Q4) versus the lowest quartile (Q1) were 6.362 (4.420–9.156) for NAFLD and 3.968 (2.641–5.962) for MS in all participants. Therefore, the prevalence of NAFLD/MS in the Q4 group will be 6.362/3.968 times higher than that in the Q1 group. In overweight participants, the ORs (Q4 vs. Q1) were 4.934 (2.879–8.457; P<0.001) for NAFLD and 2.580 (1.422–4.680; P<0.001) for MS, indicating that the prevalence of NAFLD/MS in the Q4 group will be 4.934/2.580 times higher than that in the Q1 group. In normal-weight participants, the ORs (Q4 vs. Q1) were 4.437 (2.306–8.537; P<0.001) for NAFLD and 3.117 (1.462–6.644; P<0.001) for MS, meaning that the prevalence of NAFLD/MS in the Q4 group will be 4.437/3.117 times higher than that in the Q1 group.
Table 5. ORs of GGT/HDL-C ratio quartiles by multivariate regression analyses.
| Quartiles | NAFLD | MS | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value for trend | OR (95% CI) | P value for trend | ||
| All | <0.001 | <0.001 | |||
| Q4 (highest) | 6.362 (4.420–9.156) | 3.968 (2.641–5.962) | |||
| Q3 | 5.213 (3.704–7.339) | 3.511 (2.397–5.142) | |||
| Q2 | 3.077 (2.203–4.300) | 2.303 (1.588–3.340) | |||
| Q1 (lowest) | 1 | 1 | |||
| Normal weight | <0.001 | <0.001 | |||
| Q4 (highest) | 4.437 (2.306–8.537) | 3.117 (1.462–6.644) | |||
| Q3 | 4.149 (2.272–7.577) | 5.061 (2.591–9.884) | |||
| Q2 | 1.699 (0.931–3.102) | 1.514 (2.142–5.394) | |||
| Q1 (lowest) | 1 | 1 | |||
| Overweight | <0.001 | <0.001 | |||
| Q4 (highest) | 4.934 (2.879–8.457) | 2.580 (1.422–4.680) | |||
| Q3 | 4.362 (2.612–7.285) | 2.182 (1.242–3.835) | |||
| Q2 | 3.300 (2.002–5.440) | 1.627 (0.939–2.809) | |||
| Q1 (lowest) | 1 | 1 | |||
Adjusted model 5 (showed in Table 3); According to the quartiles of GGT/HDL-C ratio, Q1: lowest quartiles; Q2: lower quartiles; Q3: higher quartiles; Q4: highest quartiles.
Predictive value of GGT/HDL-C ratio for prevalence of NAFLD
To compare the predictive value of GGT/HDL-C ratio for NAFLD, we performed ROC curve analyses (Figure 1). For prediction of NAFLD, the AUCs for GGT/HDL-C ratio, GGT, and HDL-C were 0.799 (0.788–0.810), 0.773 (0.761–0.785), and 0.726 (0.712–0.739), respectively (all P<0.001). The AUCs for GGT/HDL-C ratio were significantly higher than those for GGT or HDL-C alone (both P<0.001, by Z-statistics). The optimal cut-off value for GGT/HDL-C ratio was 21.3, with sensitivity of 74.5% and specificity of 71.7%. Briefly, GGT/HDL-C ratio was an independent predictive factor for prevalence of NAFLD.
Figure 1.
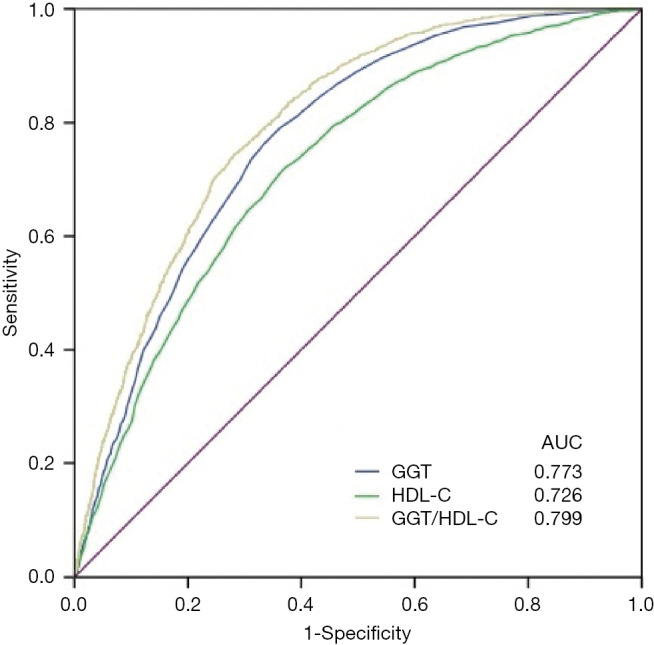
ROC curves of GGT/HDL-C ratio for prevalence of NAFLD. ROC curves, receiver-operating characteristic curves; AUCs, areas under the ROC curves; GGT, γ-glutamyl transpeptidase; HDL-C, high-density lipoprotein cholesterol; GGT/HDL-C ratio, GGT/HDL-C ratio; NAFLD, nonalcoholic fatty liver disease.
Conclusions
Participants with NAFLD had higher GGT/HDL-C ratio, BMI, WC, TG, TC, GGT, and HOMA-IR than participants without NAFLD, but lower HDL-C. GGT/HDL-C ratio was closely associated with liver enzymology markers (ALT and AST), abdominal obesity (BMI and WC), and IR (INS and HOMA-IR) by correlation analyses. The AUCs (95% confidence interval) for GGT/HDL-C ratio [0.799 (0.788–0.810)] were significantly higher than those for GGT or HDL-C alone. GGT/HDL-C ratio was significantly associated with prevalence of NAFLD: for each 1 unit increase in GGT/HDL-C ratio, the prevalence of NAFLD will increase by 0.3%. To increase the ability to identify risks for the prevalence of NAFLD/MS, we divided the NAFLD participants into four groups: Q1 (lowest), Q2, Q3, and Q4 (highest) according to the quartiles of GGT/HDL-C ratio, and found that the prevalence of NAFLD/MS in the Q4 group will be 6.362/3.968 times higher than that in the Q1 group. As the GGT/HDL-C ratio quartiles increased, the prevalence of MS and NAFLD gradually increased.
GGT is a surface enzyme that cleaves extracellular glutathione (GSH), maintains GSH homeostasis, and plays a key role in mitigating the effects of oxidative stress (20). Elevated GGT activity is associated with MS, cardiovascular risk factors, systemic inflammation, and oxidative stress, and serum GGT activity is widely used as a sensitive indicator of fatty liver disease, alcohol ingestion, hepatic inflammation, and hepatitis (20,21). Oxidative stress upregulates intracellular GGT level, and thus intracellular GGT level can be considered a biomarker for oxidative stress associated with GSH metabolism (22,23). Alam and colleagues advised NASH patients to undertake moderate-intensity exercise and dietary restriction for 1 year, and found that both lean and non-lean NASH patients exhibited weight loss and reduced GGT, which was more obvious in non-lean than lean NASH patients (12,24). Thus, GGT is closely related to NASH in both lean and non-lean patients, but more closely associated with NASH in non-lean patients (24,25). These findings are similar to those in the present study, wherein the ORs for GGT/HDL-C ratio quartile with prevalence of NAFLD in normal-weight and overweight people were 4.437 and 4.934, respectively, indicating that a higher quartile was associated with a higher risk of NAFLD in obese people. These findings probably arise because NAFLD is a manifestation of MS in the liver, and thus liver damage in NAFLD patients was more obvious than that in MS patients. Saoi et al. (26) found that high-risk NASH patients were indicative for lower circulating γ-glutamyl dipeptide concentrations and higher serum GGT activity. Therefore, the γ-glutamyl cycle has an important role for intracellular GSH reserves during oxidative stress, which is relevant to the pathophysiology of advanced NAFLD stages with severe liver inflammation and fibrosis (26). Hossain et al. (21) found that Bangladeshi adults with elevated levels of GGT and IR were more likely to develop NAFLD and demonstrated a significant positive association between HOMA-IR and GGT after adjusting for the effects of WC in Bangladeshi NAFLD subjects.
Dyslipidemia is a risk factor for NAFLD. Hypertriglyceridemia leads to decreased HDL-C through enhanced clearance of hepatic lipase (27,28). Studies by Klisic and colleagues showed that higher HDL-C was an independent predictor for advanced liver fibrosis (29,30). Wu et al. (31) demonstrated that NAFLD patients had reduced HDL-C, and especially severe NAFLD. In newly diagnosed type 2 diabetes mellitus patients, Ren et al. (32) found that low HDL-C was associated with IR, and that IR may be an underlying mechanism leading to dyslipidemia. Alkassabany et al. (33) further showed that NAFLD was significantly associated with low HDL-C, high TG, HOMA-IR, and number of MS components in schoolchildren. After adjustment for adiposity and IR, DeFilippis et al. (34) found that NAFLD was associated with low serum HDL-C in the normal-weight population. Other studies indicated that low HDL-C was associated with increased risk of cardiovascular events, IR, energy intake, carbohydrate intake, and weight gain, and that both energy and carbohydrate restriction should be considered in overweight and obese subjects with low HDL-C (35,36). In our study, NAFLD patients had low HDL-C and IR compared with non-NAFLD participants.
Single high GGT can be used as an indicator of steatosis in liver cells, while single low HDL-C is associated with IR and dyslipidemia, suggesting that GGT/HDL-C ratio can combine the functions of GGT and HDL-C alone. Thus, higher GGT/HDL-C ratio may be correlated with IR, dyslipidemia, and steatosis in liver cells in the pathomechanism of NAFLD. Therefore, we performed ROC curve analyses to predict the diagnostic value of GGT/HDL-C ratio for NAFLD, and found that the AUC for GGT/HDL-C ratio was significantly higher than those for GGT or HDL-C alone, and had high diagnostic value (AUC: 0.799) (19). GGT/HDL-C ratio can be considered to play a role in predicting the prevalence of NAFLD. Furthermore, because GGT and HDL are routine test indicators in clinical laboratories, the tests are easy to conduct and the cost performance index is high, indicating good application prospects of GGT/HDL-C ratio in NAFLD patients.
The present study has several potential limitations. First, it was a single-center cross-sectional study. Therefore, cause-and-effect relationships could not be inferred between changes in GGT/HDL-C ratio and NAFLD. Second, the diagnosis of NAFLD was based on ultrasonographic examination, which may lead to incorrect diagnosis of NAFLD in 10–30% of cases (37). Third, our samples were limited to Chinese adults, and thus the results of our study may not be applicable to other ethnic groups and children. Finally, there were significant differences in baseline characteristics between the NAFLD and non-NAFLD groups, and we did not match the two groups. Despite these limitations, the strengths of our study were the large sample size, the use of multiple regression models to adjust for many confounding factors, and the fact that GGT/HDL-C ratio is an easy and inexpensive marker with wide application value.
In conclusion, our results demonstrated a significant correlation between GGT/HDL-C ratio and NAFLD. Thus, GGT/HDL-C ratio can be considered to predict the prevalence of NAFLD after adjustment for confounding variables in both normal-weight and overweight Chinese populations.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors thank Alison Sherwin, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac) for editing the English text of a draft of this manuscript.
Funding: This work was supported by grants from the Science and Technology Project (LGF19H040017), Natural Science Foundation (LY20H200005), and Education Department (Y201636519) of Zhejiang Province.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This work was approved by the Ethics Committee of the First Affiliated Hospital of Medical College at Zhejiang University (Ethics Approval Ref: 2019-1486).
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-19-4516
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-19-4516
Peer Review File: Available at http://dx.doi.org/10.21037/atm-19-4516
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-19-4516). YZ serves as an unpaid Section Editor of Annals of Translational Medicine from Oct 2019 to Sep 2020. The other authors have no conflicts of interest to declare.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73-84. 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 2.Fang YL, Chen H, Wang CL, et al. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: From "two hit theory" to "multiple hit model". World J Gastroenterol 2018;24:2974-83. 10.3748/wjg.v24.i27.2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrell GC, Wong VW, Chitturi S. NAFLD in Asia--as common and important as in the West. Nat Rev Gastroenterol Hepatol 2013;10:307-18. 10.1038/nrgastro.2013.34 [DOI] [PubMed] [Google Scholar]
- 4.Preuss HG, Kaats GR, Mrvichin N, et al. Examining the Relationship Between Nonalcoholic Fatty Liver Disease and the Metabolic Syndrome in Nondiabetic Subjects. J Am Coll Nutr 2018;37:457-65. 10.1080/07315724.2018.1443292 [DOI] [PubMed] [Google Scholar]
- 5.Fan N, Zhang L, Xia Z, et al. Sex-Specific Association between Serum Uric Acid and Nonalcoholic Fatty Liver Disease in Type 2 Diabetic Patients. J Diabetes Res 2016;2016:3805372. 10.1155/2016/3805372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albracht-Schulte K, Rosairo S, Ramalingam L, et al. Obesity, adipocyte hypertrophy, fasting glucose, and resistin are potential contributors to nonalcoholic fatty liver disease in South Asian women. Diabetes Metab Syndr Obes 2019;12:863-72. 10.2147/DMSO.S203937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borrelli A, Bonelli P, Tuccillo FM, et al. Role of gut microbiota and oxidative stress in the progression of non-alcoholic fatty liver disease to hepatocarcinoma: Current and innovative therapeutic approaches. Redox Biol 2018;15:467-79. 10.1016/j.redox.2018.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen SH, He F, Zhou HL, et al. Relationship between nonalcoholic fatty liver disease and metabolic syndrome. J Dig Dis 2011;12:125-30. 10.1111/j.1751-2980.2011.00487.x [DOI] [PubMed] [Google Scholar]
- 9.Cruz MA, Cruz JF, Macena LB, et al. Association of the Nonalcoholic Hepatic Steatosis and Its Degrees With the Values of Liver Enzymes and Homeostasis Model Assessment-Insulin Resistance Index. Gastroenterology Res 2015;8:260-4. 10.14740/gr685w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansour-Ghanaei R, Mansour-Ghanaei F, Naghipour M, et al. Biochemical markers and lipid profile in nonalcoholic fatty liver disease patients in the PERSIAN Guilan cohort study (PGCS), Iran. J Family Med Prim Care 2019;8:923-8. 10.4103/jfmpc.jfmpc_243_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novakovic T, Mekic M, Smilic L, et al. Anthropometric and biochemical characteristics of patients with nonalcoholic fatty liver diagnosed by non-invasive diagnostic methods. Med Arch 2014;68:22-6. 10.5455/medarh.2014.68.22-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alam S, Noor EASM, Chowdhury ZR, et al. Nonalcoholic steatohepatitis in nonalcoholic fatty liver disease patients of Bangladesh. World J Hepatol 2013;5:281-7. 10.4254/wjh.v5.i5.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbalho SM, Tofano RJ, de Oliveira MB, et al. HDL-C and non-HDL-C levels are associated with anthropometric and biochemical parameters. J Vasc Bras 2019;18:e20180109. 10.1590/1677-5449.180109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fadaei R, Meshkani R, Poustchi H, et al. Association of carotid intima media thickness with atherogenic index of plasma, apo B/apo A-I ratio and paraoxonase activity in patients with non-alcoholic fatty liver disease. Arch Physiol Biochem 2019;125:19-24. 10.1080/13813455.2018.1429475 [DOI] [PubMed] [Google Scholar]
- 15.Cho J, Hong H, Park S, et al. Insulin Resistance and Its Association with Metabolic Syndrome in Korean Children. Biomed Res Int 2017;2017:8728017. 10.1155/2017/8728017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H, Xiong C, Shao X, et al. Lymphocyte To High-Density Lipoprotein Ratio As A New Indicator Of Inflammation And Metabolic Syndrome. Diabetes Metab Syndr Obes 2019;12:2117-23. 10.2147/DMSO.S219363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Workshop on Fatty Liver and Alcoholic Liver Disease CSoH, Chinese Medical Association; Fatty Liver Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: a 2018 update. Zhonghua Gan Zang Bing Za Zhi 2018;26:195-203. [DOI] [PubMed]
- 18.Weng J, Ji L, Jia W, et al. Standards of care for type 2 diabetes in China. Diabetes Metab Res Rev 2016;32:442-58. 10.1002/dmrr.2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin J, Tian L. Joint confidence region estimation for area under ROC curve and Youden index. Stat Med 2014;33:985-1000. 10.1002/sim.5992 [DOI] [PubMed] [Google Scholar]
- 20.Ndrepepa G, Colleran R, Kastrati A. Gamma-glutamyl transferase and the risk of atherosclerosis and coronary heart disease. Clin Chim Acta 2018;476:130-8. 10.1016/j.cca.2017.11.026 [DOI] [PubMed] [Google Scholar]
- 21.Hossain IA, Rahman Shah MM, Rahman MK, et al. Gamma glutamyl transferase is an independent determinant for the association of insulin resistance with nonalcoholic fatty liver disease in Bangladeshi adults: Association of GGT and HOMA-IR with NAFLD. Diabetes Metab Syndr 2016;10:S25-9. 10.1016/j.dsx.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 22.Wang P, Zhang J, Liu HW, et al. An efficient two-photon fluorescent probe for measuring gamma-glutamyltranspeptidase activity during the oxidative stress process in tumor cells and tissues. Analyst 2017;142:1813-20. 10.1039/C7AN00229G [DOI] [PubMed] [Google Scholar]
- 23.Karp DR, Shimooku K, Lipsky PE. Expression of gamma-glutamyl transpeptidase protects ramos B cells from oxidation-induced cell death. J Biol Chem 2001;276:3798-804. 10.1074/jbc.M008484200 [DOI] [PubMed] [Google Scholar]
- 24.Alam S, Jahid Hasan M, Khan MAS, et al. Effect of Weight Reduction on Histological Activity and Fibrosis of Lean Nonalcoholic Steatohepatitis Patient. J Transl Int Med 2019;7:106-14. 10.2478/jtim-2019-0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasapoglu B, Turkay C, Yalcin KS, et al. Role of gamma-glutamyl transferase levels in prediction of high cardiovascular risk among patients with non-alcoholic fatty liver disease. Indian J Med Res 2016;143:30-6. 10.4103/0971-5916.178585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saoi M, Sasaki K, Sagawa H, et al. High Throughput Screening of Serum gamma-Glutamyl Dipeptides for Risk Assessment of Nonalcoholic Steatohepatitis with Impaired Glutathione Salvage Pathway. J Proteome Res 2019. [Epub ahead of print]. 10.1021/acs.jproteome.9b00405 [DOI] [PubMed] [Google Scholar]
- 27.Corey KE, Vuppalanchi R, Vos M, et al. Improvement in liver histology is associated with reduction in dyslipidemia in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr 2015;60:360-7. 10.1097/MPG.0000000000000584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corey KE, Vuppalanchi R, Wilson LA, et al. NASH resolution is associated with improvements in HDL and triglyceride levels but not improvement in LDL or non-HDL-C levels. Aliment Pharmacol Ther 2015;41:301-9. 10.1111/apt.13035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klisic A, Abenavoli L, Fagoonee S, et al. Older age and HDL-cholesterol as independent predictors of liver fibrosis assessed by BARD score. Minerva Med 2019;110:191-8. 10.23736/S0026-4806.19.05978-0 [DOI] [PubMed] [Google Scholar]
- 30.Klisic A, Isakovic A, Kocic G, et al. Relationship between Oxidative Stress, Inflammation and Dyslipidemia with Fatty Liver Index in Patients with Type 2 Diabetes Mellitus. Exp Clin Endocrinol Diabetes 2018;126:371-8. 10.1055/s-0043-118667 [DOI] [PubMed] [Google Scholar]
- 31.Wu KT, Kuo PL, Su SB, et al. Nonalcoholic fatty liver disease severity is associated with the ratios of total cholesterol and triglycerides to high-density lipoprotein cholesterol. J Clin Lipidol 2016;10:420-5.e1. 10.1016/j.jacl.2015.12.026 [DOI] [PubMed] [Google Scholar]
- 32.Ren X, Chen ZA, Zheng S, et al. Association between Triglyceride to HDL-C Ratio (TG/HDL-C) and Insulin Resistance in Chinese Patients with Newly Diagnosed Type 2 Diabetes Mellitus. PLoS One 2016;11:e0154345. 10.1371/journal.pone.0154345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alkassabany YM, Farghaly AG, El-Ghitany EM. Prevalence, risk factors, and predictors of nonalcoholic fatty liver disease among schoolchildren: a hospital-based study in Alexandria, Egypt. Arab J Gastroenterol 2014;15:76-81. 10.1016/j.ajg.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 34.DeFilippis AP, Blaha MJ, Martin SS, et al. Nonalcoholic fatty liver disease and serum lipoproteins: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2013;227:429-36. 10.1016/j.atherosclerosis.2013.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Can AS, Uysal C, Palaoglu KE. Short term effects of a low-carbohydrate diet in overweight and obese subjects with low HDL-C levels. BMC Endocr Disord 2010;10:18. 10.1186/1472-6823-10-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dowla S, Aslibekyan S, Goss A, et al. Dyslipidemia is associated with pediatric nonalcoholic fatty liver disease. J Clin Lipidol 2018;12:981-7. 10.1016/j.jacl.2018.03.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang MH, Sung J, Gwak GY. The associations between apolipoprotein B, A1, and the B/A1 ratio and nonalcoholic fatty liver disease in both normal-weight and overweight Korean population. J Clin Lipidol 2016;10:289-98. 10.1016/j.jacl.2015.11.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


