Huang et al. (1) proposed a new intrauterine device: a uterine-shaped stent made with medical silicone rubber separates the anterior from posterior uterine cavity, thereby preventing intrauterine adhesion. The stent can be removed when appropriate. An experiment involving a goat showed that this stent prevented adhesion formation and there were no signs of intrauterine infection; after stent removal, the goat became pregnant. Intrauterine adhesion usually occurs by contact of the anterior/posterior endometrium, and, thus, separating them is reasonable. I wish to add two clinical suggestions from an obstetrical viewpoint.
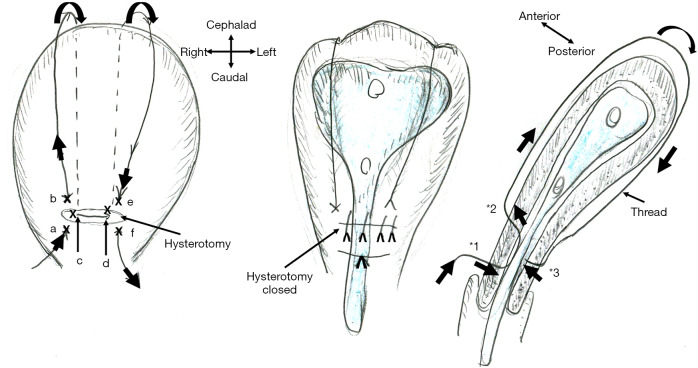
First, I believe that this stent can be applied for preventing intrauterine adhesion caused by postpartum-hemorrhage treatment, uterine compression suture (UCS), especially B-Lynch UCS. Intrauterine adhesion causes infertility, and, thus, this has long been a matter of discussion among gynecologists and reproductive medicine specialists. Huang et al.’s concept was in line with this. They bore in mind gynecologic/reproductive disorders, devising a “gynecologic” stent. For obstetricians, the year of 1997 is important, when B-Lynch UCS was first reported, which achieved hemostasis of intractable postpartum hemorrhage (2,3). A thread approximates the anterior and posterior uterine walls and thereby compresses the uterine cavity, intentionally causing “transient” intrauterine adhesion, which achieves hemostasis (Figure 1). Approximately 30 modifications of UCS have been reported. We also reported the Matsubara-Yano (MY) UCS (Figure 2) (3,4). This means that there is no single best UCS. One reason for this is that UCS causes adverse events: “permanent” intrauterine adhesion (3,5,6). UCS is a boon both for obstetricians and patients: before UCS, intractable postpartum hemorrhage required hysterectomy. UCS can preserve the uterus, and, thus, preserve fertility. Intrauterine adhesion causes a loss of fertility that could have been preserved by UCS. To overcome this adverse event, several techniques have been devised. In removable UCS, the thread is removed, thereby preventing UCS-associated adverse events including intrauterine adhesion (7,8). Laparoscopic or hysteroscopic thread removal has also been proposed (9). However, technical difficulties prevented their wide use.
Figure 1.
Schematic presentation of B-Lynch uterine compression suture and intrauterine stent placement. A thread compresses the uterine lumen in a ventral-caudal (anterior-posterior) direction, thereby achieving hemostasis of postpartum hemorrhage. The marks (x: a-f) indicate the place where the needle penetrates the anterior (a, b, e, and f) and posterior (c and d) uterine wall. As * indicates, the needle penetrates the anterior (*1, *2: a, b, e, and f) and posterior (*3: c and d) uterine walls but does not penetrate the anterior-posterior uterine wall. A new intrauterine stent can be placed intrauterine, thereby preventing intrauterine adhesion, one of the most important adverse events of B-Lynch suture.
Figure 2.
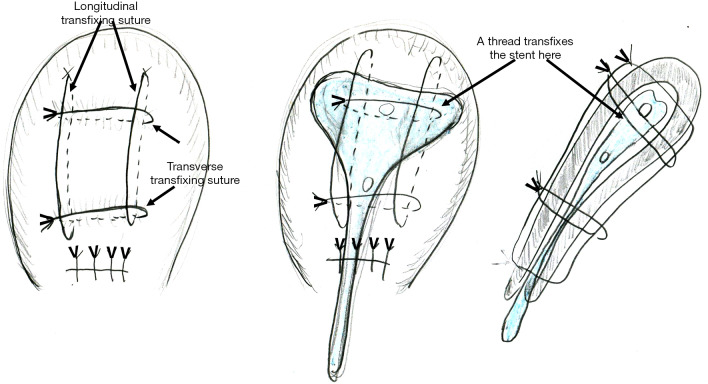
Schematic presentation of Matsubara-Yano (MY) uterine compression suture and intrauterine stent placement. A needle penetrates the anterior-posterior uterine wall eight times with two longitudinal and two transverse transfixing sutures. MY suture may be technically easier and possibly leads to tighter compression than B-Lynch suture. A needle also penetrates the intrauterine stent (arrows in middle and right figures), and, thus, an absorbable stent may be preferable.
My suggestion is: the stent should be inserted at the time of B-Lynch suture. For hemostasis of postpartum hemorrhage, “direct” contact of the anterior-posterior endometrium is not needed. Tight compression of the “anterior-endometrium/stent/posterior-endometrium as a whole” may also be effective to achieve hemostasis (Figure 1, middle and right). Stent “holes” may drain the intrauterine blood/secretion bi-directionally (anterior/posterior direction). A lower triangle tail may also drain the intrauterine blood. It may also help the cervical canal to remain open: in placenta previa, a disorder frequently requiring UCS, the cervical canal is usually closed, which prevents drainage of intrauterine blood (10). Since the postpartum uterus becomes smaller and smaller (involution), the stent size should be considered. Early stent removal may be better.
My second suggestion is: how about an absorbable stent? In some UCS, including MY UCS, the needle penetrates the anterior/posterior uterine wall. In these UCS, the thread should penetrate the stent together with uterine wall (Figure 2, middle and right). The thread is usually absorbed within one month. Also, intrauterine adhesion usually occurs within 1 month, when the uterine environment acutely changes and the endometrium is markedly stimulated. An absorbable stent can prevent intrauterine adhesion during this critical period.
I believe that Huang et al.’s idea is like “Columbus egg”: the idea looks easy once put forward, but nobody has ever noticed it. I commend Huang and colleagues for noticing this concept for the first time. The procedure is simple, straightforward, easy, and thus promising. I wish for Huang and colleagues to consider using this stent not only in gynecologic but also obstetric settings. Selection of the stent size, consideration of its removal time, and devising an absorbable stent may broaden the usage of this novel stent.
Supplementary
The article’s supplementary files as
Acknowledgments
I thank Hitoshi Yano for his help. I have obtained no financial support or grant.
Funding: None.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Translational Medicine. The article did not undergo external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.65). The author has no conflicts of interest to declare.
References
- 1.Huang H, Xu B, Cheng C, et al. A novel intrauterine stent for prevention of intrauterine adhesions. Ann Transl Med 2020;8:61. 10.21037/atm.2019.12.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.B-Lynch C , Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum haemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol 1997;104:372-5. 10.1111/j.1471-0528.1997.tb11471.x [DOI] [PubMed] [Google Scholar]
- 3.Matsubara S, Yano H, Ohkuchi A, et al. Uterine compression sutures for postpartum hemorrhage: an overview. Acta Obstet Gynecol Scand 2013;92:378-85. 10.1111/aogs.12077 [DOI] [PubMed] [Google Scholar]
- 4.Matsubara S, Yano H, Taneichi A, et al. Uterine compression suture against impending recurrence of uterine inversion immediately after laparotomy repositioning. J Obstet Gynaecol Res 2009;35:819-23. 10.1111/j.1447-0756.2008.01011.x [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim MI, Raafat TA, Ellaithy MI, et al. Risk of postpartum uterine synechiae following uterine compression suturing during postpartum haemorrhage. Aust N Z J Obstet Gynaecol 2013;53:37-45. 10.1111/ajo.12017 [DOI] [PubMed] [Google Scholar]
- 6.Rathat G, Do Trinh P, Mercier G, et al. Synechia after uterine compression sutures. Fertil Steril 2011;95:405-9. 10.1016/j.fertnstert.2010.08.055 [DOI] [PubMed] [Google Scholar]
- 7.Matsubara S. New prophylaxis methods for adverse events of uterine compression sutures: removing compression threads. Acta Obstet Gynecol Scand 2014;93:1069-70. 10.1111/aogs.12451 [DOI] [PubMed] [Google Scholar]
- 8.Zhang ZW, Liu CY, Yu N, et al. Removable uterine compression sutures for postpartum haemorrhage. BJOG 2015;122:429-33. 10.1111/1471-0528.13025 [DOI] [PubMed] [Google Scholar]
- 9.Takeda J, Kumakiri J, Makino S, et al. Laparoscopic removal of uterine vertical compression sutures. Gynecol Minim Invasive Ther 2017;6:73-5. 10.1016/j.gmit.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsubara S, Takahashi H, Baba Y, et al. Inserting the Bakri balloon during cesarean section in patients with a narrow cervix: Nelaton method (Matsubara). Acta Obstet Gynecol Scand 2015;94:1147-8. 10.1111/aogs.12693 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as