Abstract
This cohort study examines the association between the use of minimally invasive surgery, capsule rupture, and survival among women with stage I epithelial ovarian cancer.
Ovarian cancer remains the deadliest gynecologic malignant neoplasm in the United States.1 Salpingo-oophorectomy with the intact removal of the ovary and fallopian tube is the standard approach for suspected ovarian malignant neoplasm apparently confined to the ovary.2 Surgery for early-stage ovarian cancer has historically been performed via laparotomy. However, in recent years, more women with ovarian cysts and masses have been treated with minimally invasive surgery (MIS), including laparoscopy.3 To date, there are limited data to support the safety and oncologic outcomes of MIS for early-stage ovarian cancer.4 The objective of this study was to examine the association between MIS use, capsule rupture, and survival of women with stage I epithelial ovarian cancer.
Methods
This is an observational study of women with stage I epithelial ovarian cancer who underwent surgery from 2010 to 2015 and were registered in the National Cancer Database. All women underwent adnexectomy. The study used deidentified data and was deemed exempt by the Columbia University Institutional Review Board.
Procedures were stratified based on the surgical approach as laparotomy vs MIS (including laparoscopic and robotic-assisted surgery). Trends in MIS use and capsule rupture over time were examined. Factors associated with capsule rupture were examined using multivariable analyses. The association between route of surgery, capsule rupture, and overall survival were examined using Cox proportional hazards regression models. All P values were from 2-sided tests and results were deemed statistically significant at P < .05.
Results
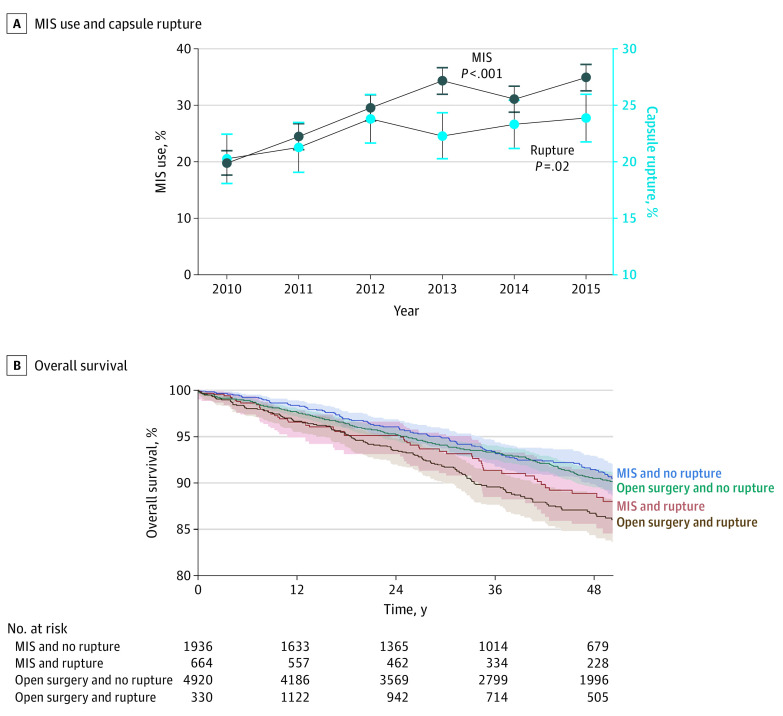
Among 8850 women (mean [SD] age, 55.6 [13.7] years) with stage I ovarian cancer, 2600 women (29.4%) underwent MIS. Use of MIS increased from 19.8% (263 of 1330) in 2010 to 34.9% (554 of 1589) in 2015 (1.8-fold increase; P < .001; Figure, A). In a multivariable model, more recent year of surgery and serous histologic characteristics were associated with use of MIS (Table).
Figure. Trends and Outcomes Associated With Capsule Rupture in Stage I Epithelial Ovarian Cancer.
A, Temporal trends of minimally invasive surgery (MIS) use and capsule rupture in stage I epithelial ovarian cancer between 2010 and 2015. Cochran-Armitage trend test for P value. Observed values and 95% CIs are shown for MIS use (dark blue) and stage I epithelial ovarian cancer with capsule rupture (light blue). B, Overall survival of women with stage I epithelial ovarian cancer stratified by surgery type and capsule rupture status. The y-axis is truncated to 80% to 100%.
Table. Multivariable Analysis for MIS Use and Capsule Rupture.
| Characteristic | No. | MIS (%/row) | aRR (95% CI) | Rupture (%/row) | aRR (95% CI) |
|---|---|---|---|---|---|
| MISa | |||||
| No | 6250 | NA | NA | 1330 (21.3) | 1 [Reference] |
| Yes | 2600 | NA | NA | 664 (25.5) | 1.17 (1.06-1.29)b |
| Age, y | |||||
| <40 | 1036 | 421 (40.6) | 1 [Reference] | 216 (20.8) | 1 [Reference] |
| 40-49 | 1713 | 528 (30.8) | 0.91 (0.71-1.17) | 374 (21.8) | 0.86 (0.61-1.21) |
| 50-59 | 2727 | 722 (26.5) | 0.80 (0.62-1.02) | 655 (24.0) | 0.92 (0.66-1.28) |
| 60-69 | 2030 | 557 (27.4) | 0.82 (0.64-1.06) | 468 (23.1) | 0.86 (0.62-1.21) |
| 70-79 | 935 | 262 (28.0) | 0.84 (0.64-1.09) | 200 (21.4) | 0.81 (0.57-1.15) |
| ≥80 | 409 | 110 (26.9) | 0.80 (0.60-1.07) | 81 (19.8) | 0.74 (0.50-1.08) |
| Race | |||||
| Non-Hispanic: white | 6929 | 2063 (29.8) | 1 [Reference] | 1557 (22.5) | 1 [Reference] |
| Non-Hispanic: black | 478 | 112 (23.4) | 0.84 (0.71-0.98)b | 90 (18.8) | 0.94 (0.76-1.15) |
| Hispanic | 574 | 167 (29.1) | 0.96 (0.83-1.10) | 120 (20.9) | 1.00 (0.83-1.20) |
| Other | 567 | 180 (31.7) | 1.02 (0.89-1.16) | 149 (26.3) | 1.13 (0.96-1.32) |
| Unknown | 302 | 78 (25.8) | 0.90 (0.73-1.10) | 78 (25.8) | 1.15 (0.93-1.40) |
| Year of diagnosis | |||||
| 2010 | 1330 | 263 (19.8) | 1 [Reference] | 269 (20.2) | 1 [Reference] |
| 2011 | 1317 | 322 (24.4) | 1.26 (1.08-1.47)b | 280 (21.3) | 1.03 (0.89-1.21) |
| 2012 | 1493 | 441 (29.5) | 1.49 (1.30-1.72)b | 355 (23.8) | 1.15 (1.00-1.33) |
| 2013 | 1584 | 543 (34.3) | 1.76 (1.53-2.03)b | 353 (22.3) | 1.08 (0.92-1.25) |
| 2014 | 1537 | 477 (31.0) | 1.64 (1.43-1.88)b | 358 (23.3) | 1.13 (0.97-1.31) |
| 2015 | 1585 | 554 (34.9) | 1.85 (1.60-2.14)b | 379 (23.9) | 1.14 (0.99-1.32) |
| Histology | |||||
| Serous | 1567 | 557 (35.5) | 1 [Reference] | 373 (23.8) | 1 [Reference] |
| Mucinous | 2211 | 541 (24.5) | 0.74 (0.67-0.82)b | 342 (15.5) | 0.75 (0.65-0.86)b |
| Endometrioid | 2389 | 742 (31.1) | 0.87 (0.80-0.96)b | 557 (23.3) | 1.06 (0.94-1.19) |
| Clear cell | 1549 | 441 (28.5) | 0.85 (0.77-0.94)b | 435 (28.1) | 1.13 (0.99-1.28) |
| Other | 1134 | 319 (28.1) | 0.84 (0.75-0.94)b | 287 (25.3) | 1.10 (0.96-1.25) |
| Tumor differentiation | |||||
| Well | 2397 | 754 (31.5) | 1 [Reference] | 437 (18.2) | 1 [Reference] |
| Moderate | 2193 | 588 (26.8) | 0.92 (0.84-1.01) | 481 (21.9) | 1.18 (1.05-1.33)b |
| Poorly | 2709 | 765 (28.2) | 0.90 (0.81-0.99)b | 754 (27.8) | 1.31 (1.15-1.50)b |
| Unknown | 1551 | 493 (31.8) | 0.95 (0.86-1.06) | 322 (20.8) | 1.07 (0.92-1.23) |
| Laterality | |||||
| Unilateral | 8353 | 2455 (29.4) | 1 [Reference] | 1869 (22.4) | 1 [Reference] |
| Bilateral | 497 | 145 (29.2) | 0.94 (0.82-1.09) | 125 (25.2) | 1.04 (0.88-1.22) |
| Tumor size, mm | |||||
| 0-20 | 1053 | 420 (39.9) | 1 [Reference] | 187 (17.8) | 1 [Reference] |
| 21-40 | 628 | 250 (39.8) | 1.05 (0.93-1.18) | 135 (21.5) | 1.17 (0.94-1.44) |
| 41-60 | 522 | 230 (44.1) | 1.15 (1.02-1.29)b | 128 (24.5) | 1.27 (1.03-1.56)b |
| 61-80 | 607 | 262 (43.2) | 1.13 (1.00-1.27)b | 176 (29.0) | 1.46 (1.21-1.75)b |
| 81-100 | 722 | 277 (38.4) | 1.01 (0.90-1.13) | 244 (33.8) | 1.74 (1.46-2.08)b |
| >100 | 4452 | 851 (19.1) | 0.53 (0.48-0.59)b | 946 (21.2) | 1.19 (1.02-1.38)b |
| Unknown | 866 | 310 (35.8) | 0.97 (0.85-1.10) | 178 (20.6) | 1.13 (0.93-1.38) |
| Lymphadenectomy | |||||
| No | 2060 | 725 (35.2) | NA | 452 (21.9) | 1 [Reference] |
| Yes | 6771 | 1868 (27.6) | NA | 1540 (22.7) | 0.92 (0.82-1.02) |
| Unknown | 19 | Suppressedc | NA | Suppressedc | 0.49 (0.12-1.99) |
Abbreviations: aRR, adjusted relative risk; MIS, minimally invasive surgery; NA, not applicable.
Data on route of surgery (MIS vs laparotomy) became available in 2010. Numbers and percentages represent row totals (factors for MIS use or risk of capsule rupture). Cases with missing information were analyzed as a discrete group. Multivariable models adjusted for age, race/ethnicity, insurance status, area average household income, area educational level, facility information (location and type), Charlson-Deyo comorbidity index, year, histologic characteristics, laterality, tumor size, lymphadenectomy, hysterectomy, and MIS use in the final model in each analysis. Surgical factors were not entered in the model for MIS use. Similar results were demonstrated when outcome measures were analyzed in purposeful selection method and backward selection method. Race was classified based on the National Cancer Database grouping.
P < .05.
Number less than 10.
A total of 1994 women (22.5%) experienced capsule rupture. The rate of rupture increased from 20.2% (269 of 1330) in 2010 to 23.9% (379 of 1589) in 2015 (18.3% relative increase; Cochran-Armitage trend test P = .02; Figure, A). In a multivariable analysis, MIS was independently associated with capsule rupture (adjusted relative risk, 1.17; 95% CI, 1.06-1.29; Table). Larger tumor size was also associated with higher risk of capsule rupture. Women with ruptured tumors were more likely than women with nonruptured tumors to receive chemotherapy (unilateral tumors: 1252 of 1869 [67.0%] vs 2500 of 6484 [38.6%]; and bilateral tumors: 100 of 125 [80.0%] vs 219 of 372 [58.9%]; P < .001).
The median follow-up was 39.4 months, and the 4-year overall survival rate decreased by 5.0% between 2010 (overall survival, 91.0%) and 2015 (overall survival, 86.0%). Women with ruptured tumors had lower overall survival compared with those with nonruptured tumors in univariable analysis: 4-year rates, 86.8% for open surgery and ruptured tumors, 88.9% for MIS and ruptured tumors, 90.5% for open surgery and nonruptured tumors, and 91.5% for MIS and nonruptured tumors (log-rank test, P = .001; Figure, B). In the adjusted model, use of MIS with capsule rupture was independently associated with an increase in all-cause mortality compared with use of MIS and nonruptured capsules (adjusted hazard ratio, 1.41; 95% CI, 1.01-1.97). A similar association was observed for laparotomy, where use of laparotomy with capsule rupture was independently associated with an increase in all-cause mortality compared with use of laparotomy and nonruptured capsules (adjusted-hazard ratio, 1.43; 95% CI, 1.18-1.73).
Discussion
There has been a significant increase in the use of MIS among women with early-stage ovarian cancer in the United States.3 In the present study, MIS was associated with an increased risk of capsule rupture, which was associated with increased mortality. An increased rate of capsular rupture among women with benign adnexal cysts undergoing MIS has been previously reported, but this association has received little attention for women with ovarian cancer.4
Previous work on the prognostic significance of capsular rupture has reported mixed findings.5 Theoretically, rupture of the ovarian capsule may result in tumor seeding of the abdominal cavity and may be associated with increased mortality.5 We recognize a number of limitations of this study, including the inability to determine the timing of capsule rupture and whether or not the rupture was contained within a tissue extraction bag at the time of occurrence. Some data, including preoperative diagnosis, tumor marker values, substage, and body mass index, were not available.
Several factors, including widespread use of MIS for other procedures, decreased perioperative morbidity, and availability of improved technology, were likely associated with the uptake of MIS adnexal surgery.6 Guidelines by the National Comprehensive Cancer Network state that MIS for early-stage ovarian cancer should be limited to selected patients and experienced surgeons.2 Until more data become available, careful preoperative patient selection and intraoperative assessment prior to endoscopic oophorectomy should be performed to minimize the risk of tumor disruption and spillage.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer. NCCN guidelines. Accessed January 5, 2020. https://www.nccn.org/
- 3.Matsuo K, Chang EJ, Matsuzaki S, et al. Minimally invasive surgery for early-stage ovarian cancer: association between hospital surgical volume and short-term perioperative outcomes. Gynecol Oncol. Published online May 10, 2020. doi: 10.1016/j.ygyno.2020.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falcetta FS, Lawrie TA, Medeiros LR, et al. Laparoscopy versus laparotomy for FIGO stage I ovarian cancer. Cochrane Database Syst Rev. 2016;10:CD005344. doi: 10.1002/14651858.CD005344.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuo K, Machida H, Yamagami W, et al. Intraoperative capsule rupture, postoperative chemotherapy, and survival of women with stage I epithelial ovarian cancer. Obstet Gynecol. 2019;134(5):1017-1026. doi: 10.1097/AOG.0000000000003507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27(32):5331-5336. doi: 10.1200/JCO.2009.22.3248 [DOI] [PMC free article] [PubMed] [Google Scholar]