This multicenter cohort study assesses the accuracy of each definition of hyperprogressive disease to identify the frequency and associations with poorer outcomes of immune-checkpoint inhibitor therapy in patients with advanced non–small cell lung cancer and offers an optimized and homogenized definition based on all previous criteria for identifying hyperprogressive disease.
Key Points
Question
Are the different definitions used to assess hyperprogressive disease during immunotherapy for non–small cell lung cancer representative of the same tumoral behavior?
Findings
For this multicenter cohort study of 406 patients with advanced non–small cell lung cancer treated with programmed cell death 1/programmed cell death 1 ligand inhibitors, the 5 disease definitions assessed resulted in diverse incidences, different patient characteristics, and different associations with survival outcomes. A new criterion of difference in tumor growth rate of greater than 100 showed more accuracy in assessing hyperprogressive disease.
Meaning
These findings suggest that the 5 definitions assessed are not representative of the same tumoral behavior.
Abstract
Importance
Hyperprogressive disease (HPD) is an aggressive pattern of progression reported for patients treated with programmed cell death 1 (PD-1)/programmed cell death 1 ligand (PD-L1) inhibitors as a single agent in several studies. However, the use of different definitions of HPD introduces the risk of describing different tumoral behaviors.
Objective
To assess the accuracy of each HPD definition to identify the frequency of HPD and the association with poorer outcomes of immune-checkpoint inhibitor (ICI) treatment in patients with advanced non–small cell lung cancer (NSCLC) and to provide an optimized and homogenized definition based on all previous criteria for identifying HPD.
Design, Setting, and Participants
This retrospective cohort study included 406 patients with advanced NSCLC treated with PD-1/PD-L1 inhibitors from November 1, 2012, to April 5, 2017, in 8 French institutions. Measurable lesions were defined using the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria on at least 2 computed tomographic scans before the initiation of ICI therapy and 1 computed tomographic scan during treatment. Data were analyzed from November 1, 2012, to August 1, 2019.
Exposures
Advanced NSCLC and treatment with PD-1/PD-L1 inhibitors.
Main Outcomes and Measures
Association of the definition with the related incidence and the HPD subset constitution and the association between each HPD definition and overall survival. All dynamic indexes used in the previous proposed definitions, such as the tumor growth rate (TGR) or tumor growth kinetics (TGK), were calculated before and during treatment.
Results
Among the 406 patients with NSCLC included in the analysis (259 male [63.8%]; median age at start of ICI treatment, 64 [range, 30-91] years), the different definitions resulted in incidences of the HPD phenomenon varying from 5.4% (n = 22; definition based on a progression pace >2-fold and a time to treatment failure of <2 months) to 18.5% (n = 75; definition based on the TGR ratio). The concordance between these different definitions (using the Jaccard similarity index) varied from 33.3% to 69.3%. For every definition, HPD was associated with poorer survival (range of median overall survival, 3.4 [95% CI, 1.9-8.4] to 6.0 [95% CI, 3.7-9.4] months). The difference between TGR before and during therapy (ΔTGR) was the most correlated with poor overall survival with an initial plateau for a larger number of patients and a slower increase, and it had the highest ability to distinguish patients with HPD from those with progressive disease not classified as HPD. In addition, an optimal threshold of ΔTGR of greater than 100 was identified for this distinction.
Conclusions and Relevance
The findings of this retrospective cohort study of patients with NSCLC suggest that the previous 5 definitions of HPD were not associated with the same tumor behavior. A new definition, based on ΔTGR of greater than 100, appeared to be associated with the characteristics expected with HPD (increase of the tumor kinetics and poor survival). Additional studies on larger groups of patients are necessary to confirm the accuracy and validate this proposed definition.
Introduction
Immune-checkpoint inhibitors (ICIs) have been one of the major developments in cancer therapy in the past decade and are approved for various tumor types, such as melanoma, non–small cell lung cancer (NSCLC), renal cell carcinoma, or head and neck squamous cell carcinoma.1,2,3,4 Their approval has caused a revolution in the therapeutic approach, because they differ from conventional cytotoxic treatments and molecularly targeted agents in their mechanism and in the response patterns with which they are associated.
Immune-checkpoint inhibitors have indeed demonstrated survival benefits, including complete and partial responses as well as long-term remission, and have been associated with novel patterns of responses such as pseudoprogression, defined as an initial increase in the tumor burden followed by a later or a dissociated response.5 Of greater concern, several studies have reported a possible deleterious effect of ICI in a subpopulation of patients, with dramatic tumor growth following the initiation of the therapy, termed hyperprogressive disease (HPD).6 This phenomenon is clinically defined as an unexpected acceleration of the tumor kinetics that can be measured on imaging with dynamic parameters.
To define and quantify the incidence of this phenomenon, parameters have been used that include tumor growth rate (TGR),7 tumor growth kinetics (TGK),8 and time to treatment failure.9 However, to date, no consensual HPD definition has been proposed, and the risk of describing different tumoral behaviors exists. The objectives of the present study are to achieve an advanced comprehensive comparison of the different definitions of HPD in a cohort of patients with NSCLC to evaluate the association of the definition with the related incidence and the HPD subset constitution and the association between each HPD definition and overall survival.
Methods
Patients
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. This study was approved by the institutional review board of Gustave Roussy, which did not require informed consent from participants because of the retrospective nature. Data from patients with NSCLC who were treated with programmed cell death 1 (PD-1)/programmed cell death 1 ligand (PD-L1) inhibitors from November 1, 2012, to April 5, 2017, in 8 French institutions were retrospectively collected and analyzed. Patients with confirmed stage III or IV NSCLC and for whom computed tomographic (CT) scans were available for radiological evaluation were included. Most patients received prior treatments (eTable in the Supplement).
At least 2 CT scans before the beginning of the ICI therapy and 1 CT scan during the treatment were required for assessing the dynamic indexes. The baseline CT scan was performed in the 6 weeks preceding the initiation of ICI treatment, and a minimum of 2 weeks between different CT evaluations was expected, resulting in the inclusion of 406 patients in the final cohort (eFigure 1 in the Supplement). The median interval from prebaseline to baseline CT scans was 60 days (range, 14-120 days); from baseline to follow-up CT scans, 62 days (range, 14-90 days).
Definitions of Tumor Dynamics
In the recent literature, HPD has been defined in different studies using 5 different criteria as already emphasized in the study by Fuentes-Antrás et al.10 A summary of existing definitions is available in Table 1. For a better understanding of our analysis, a distinction between the terms definition and index has been established, with index referring to the mathematical parameters, such as TGR or TGK, combined and used with thresholds to define HPD.
Table 1. Main Different Definitions of HPD According to Previous Studies.
| Component | Source | ||||
|---|---|---|---|---|---|
| Champiat et al,6 November 2016 | Kato et al,9 March 2017 | Saâda-Bouzid et al,11 April 2017 | Singavi et al,12 September 2017 | Ferrara et al,13 November 2018 | |
| Letter | A | B | C | D | E |
| Definitiona | RECIST progression and TGR-exp/TGR-ref >2 | TTF <2 mo, RECIST>50%, and progression pace >2-fold | TGK-exp/TGK-ref >2 | RECIST progression, RECIST >50%, and TGR-exp/TGR-ref >2 | RECIST progression and TGR-exp − TGR-ref >50 |
| Reported HPD incidence, No. (%) | 12/131 (9.2) | 6/155 (3.9) | 10/34 (29.4) | 5 patients | 56/406 (13.8) |
| Histologic types | Various: melanoma, 34%; lung, 10% | Various: melanoma, 33%; NSCLC, 25% | Head and neck squamous cell carcinoma | Various | NSCLC |
Abbreviations: HPD, hyperprogressive disease; NSCLC, non–small cell lung carcinoma; RECIST, Response Evaluation Criteria in Solid Tumours; TGK, tumor growth kinetics; TGR, tumor growth rate; TTF, time to treatment failure.
Indexes are calculated using TGR and TGK values before (ref) and during (exp) therapy.
Tumor growth rate is defined by Gomez-Roca et al7 as the percentage increase in tumor volume per month, following TGR = 100 [exp(TG) – 1], where TG is 3-log (St/S0) and where St and S0 are the tumor sizes at times t and 0, defined as the sum of the longest diameters of the target lesions as per the Response Evaluation Criteria in Solid Tumours (RECIST) 1.1 criteria.14 Tumor growth kinetics are defined as the change in the tumor size per unit of time (in mm/d) as reported by Le Tourneau et al8: TGK = (St – S0)/(t – t0). Both indexes were calculated before and during treatment to evaluate any change in the tumor kinetics. For both indexes and for every patient, the RECIST sum was computed with the target lesions defined at baseline of ICI. For an easier reading, each of these definitions will be named using letters from A to E as in Table 1. Of these 5 definitions, 3 (A, D, and E) rest on the hypothesis of a natural exponential growth of the tumor volume with time. Assuming that at time t, the volume Vt can be expressed as Vt = V0 exp (TG × t), this hypothesis leads directly to the use of TGR as the HPD index. Nevertheless, these 3 definitions are not strictly equivalent.
Champiat et al6 (definition A) defined HPD as at least a 2-fold increase of the TGR from before (ref) to during (exp) therapy (TGR-exp:TGR-ref ≥2). In other words, patients with HPD are characterized by a twice higher percentage increase in volume per month during immunotherapy than before. A later study by Singavi et al12 (definition D) takes the same definition as A and adds a condition on the RECIST percentage increase during ICI treatment of more than 50%. The study by Ferrara et al13 (definition E) assumes that HPD is characterized by a difference (and not a ratio) superior to 50% between TGR-exp and TGR-ref (ΔTGR), suggesting that the increase in volume per month during ICI therapy must be 50% higher than that expected with the increase before treatment.
Saâda-Bouzid et al11 (definition C) relied on the use of TGK, which does not account for the hypothesis of the natural exponential growth of the tumor and uses the diameters rather than the volume. Finally, Kato et al9 (definition B) defined HPD using 3 conditions: tumor kinetics (progression pace >2-fold), the RECIST percentage (increase in the tumor burden during ICI >50%), and ongoing treatment (time to treatment failure, <2 months). In the analysis, tumor burden at prebaseline, baseline, and postbaseline evaluations will be termed S-prebaseline, S-baseline, and S-postbaseline, respectively.
Statistical Analysis
Data were analyzed from November 1, 2012, to August 1, 2019. Concordance between the constitutions of the HPD groups for the different definitions was evaluated using the similarity Jaccard index. The influence of each definition on the designation of HPD was then theoretically analyzed as a function of the RECIST percentage before immunotherapy and the RECIST percentage during immunotherapy. To do so, we represented the mathematical criteria (TGK-exp:TGK-ref >2, TGR-exp:TGR-ref >2, and ΔTGR>50) under the form of isolines and compared the respective positions of the curves. For an identical RECIST percentage before immunotherapy, the curve corresponds to the definition that requires a higher RECIST percentage during therapy to assess HPD and that is therefore more restrictive.
To investigate the association between HPD status and survival outcomes, patients with initial PD as defined per RECIST 1.1 were divided into those with HPD and those with PD without HPD. Overall survival was defined as the time from the initiation of the ICI therapy to the death of the patient due to any cause; time to treatment failure, as the duration from the beginning to the discontinuation of the treatment for any reason, including toxicity, progression, patient’s choice, or death. Landmark survival analyses (eFigure 2 and 3 in the Supplement) were performed using the Kaplan-Meier method,15 and the log-rank test was used for comparison. Two-sided P < .05 was considered statistically significant.
To evaluate the prognostic value of each index, we divided the patients with PD into 2 groups: those with the highest values of the index (N) and those with other PD. We then computed the median overall survival as a function of the number N. Studying the influence of N on the landmark analysis, our objective was to determine which indexes showed a clear ability to distinguish patients with both acceleration of the tumor growth and poor survival and thereby determine which index and threshold led to the most significant distinction between the groups. All the statistical and mathematical analyses were performed using Python software, version 3.0 (Python Software Foundation, Python Language Reference).
Results
Of 406 patients included in the analyses, 259 (63.8%) were male and 147 (36.2%) were female. The median age at the beginning of ICI was 64 (range, 30-91) years. Of these, 207 were assessed as having PD while receiving ICI therapy at first evaluation, owing to an increase in target lesions of greater than 20% in 143 and an increase in nontarget lesions or appearance in 64. Nineteen patients with PD were retrospectively assessed as having pseudoprogression and were excluded from the analysis. The complete characteristics of the patients have previously been described by Ferrara et al.13
Influence of the Definition on Incidence
When we applied the different definitions of HPD to the 406 patients in the cohort analysis, HPD incidence varied from 22 (5.4%) to 75 (18.5%) patients. Definition B of HPD appeared to be the most restrictive definition, with the smallest incidence (5.4%) (Table 2).
Table 2. Incidence and Overall Survival of Patients With HPD According to the Different Definitions.
| Variable | Definition A | Definition B | Definition C | Definition D | Definition E |
|---|---|---|---|---|---|
| Incidence, No./total No. (%) | 52/406 (12.8) | 22/406 (5.4) | 75/406 (18.5) | 25/406 (6.2) | 56/406 (13.8) |
| Median OS (95% CI), mo | |||||
| HPD group | 5.1 (3.3-9.4) | 3.4 (1.9-8.4) | 6.0 (3.7-9.4) | 5.1 (3.2-10.3) | 4.0 (2.9-7.5) |
| PD non-HPD group | 6.3 (5.0-7.5) | 6.4 (5.6-8.4) | 6.2 (5.0-7.5) | 6.2 (5.3-7.5) | 6.4 (5.5-8.4) |
| P valuea | .45 | <.001 | .62 | .59 | .14 |
Abbreviations: HPD, hyperprogressive disease; OS, overall survival; PD, progressive disease. For HPD definitions A through E, see Table 1.
Log-rank tests were computed to test the significance of this distinction.
HPD Subsets With the Different HPD Definitions
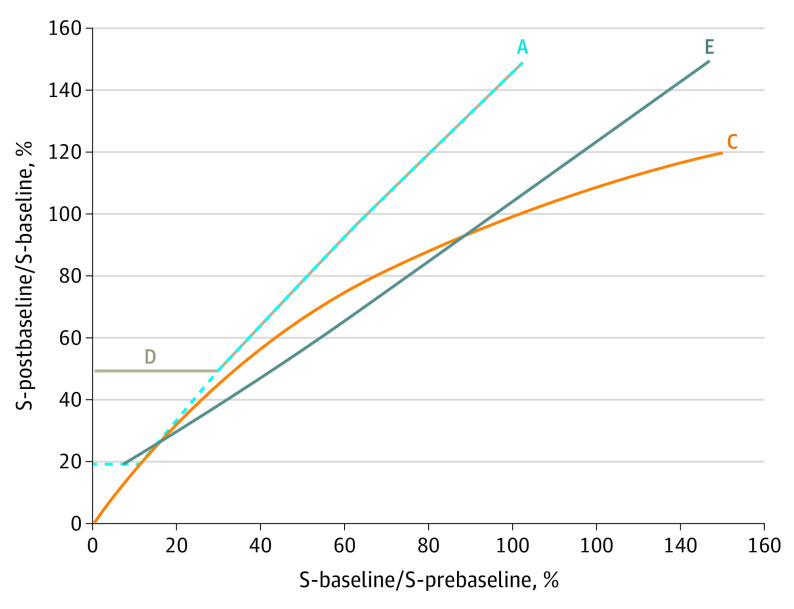
Nineteen patients underwent assessment of HPD by all 5 definitions (A, B, C, D, and E). The maximum value of the similarity index was 69.3%, reached for definitions A and C; and the minimum value was 33.3%, reached for the definitions of C and D (Table 3), demonstrating different HPD subpopulations with each definition. To understand which patients are classified as having HPD by each definition, the characteristics of patients for definitions A, C, D, and E as regarding the RECIST percentages before and during ICI therapy have been modeled in Figure 1.
Table 3. Influence of the Definition on the HPD Subset Constitution.
| Definition | Jaccard index value, No. (%)a | ||||
|---|---|---|---|---|---|
| Definition A (n = 52) | Definition B (n = 22) | Definition C (n = 75) | Definition D (n = 25) | Definition E (n = 56) | |
| A | NA | NA | NA | NA | NA |
| B | 19 (34.5) | NA | NA | NA | NA |
| C | 52 (69.3) | 21 (27.6) | NA | NA | NA |
| D | 25 (48.1) | 22 (67.9) | 25 (33.3) | NA | NA |
| E | 44 (68.8) | 23 (34.8) | 49 (59.8) | 24 (47.4) | NA |
Abbreviations: HPD, hyperprogressive disease; NA, not applicable. For HPD definitions A through E, see Table 1.
The Jaccard index value corresponds to the proportion of patients with HPD in common among all patients assessed as having HPD by one definition or the other. For instance, definitions A and E assessed 44 patients with HPD in common but another 20 patients were assessed as having HPD by only 1 of the 2 definitions (52 – 44 = 8 in A and 56 – 44 = 12 in E), leading to a similarity index of 44 of 64 = 68.8%.
Figure 1. Areas of Hyperprogressive Disease (HPD) Incidence According to Response Evaluation Criteria in Solid Tumours (RECIST) .
Data are shown before (x-axis) and during (y-axis) programmed cell death 1/programmed cell death 1 ligand therapy for S-baseline/S-prebaseline of greater than 1, where S indicates tumor size. Lines representing the definitions mark the frontier between patients with progressive disease and those with HPD. For each definition, patients in the area above are assessed as having HPD, whereas patients in the area below are not. These lines were drawn in the ideal case of a period of 1 month between 2 computed tomographic scans. Definition B does not only rely on considerations of the tumor size and kinetics and therefore could not be included in this comparison. See Table 1 for definitions A, C, D, and E of HPD.
Two cases were distinguished. In S-baseline/S-prebaseline of greater than 1, for a pretherapy increase of the target lesions’ size, definitions based on TGR or TGK tended to associate HPD with high values of the RECIST percentage during ICI therapy (above the line in Figure 1). However, the corresponding curves did not overlap, and different situations must be distinguished. Definition C is mathematically more likely to diagnose HPD among patients with a pretreatment increase from 1% to 15% (stable disease according to RECIST criteria) and among patients with a high pretreatment progression of greater than 90%.
Definition A requires the highest RECIST percentage during therapy to define patients with HPD. Definition D adds to definition A the condition RECIST percentage of greater than 50% and is even more restrictive until a pretreatment increase of 40%, when both curves overlap. Finally, definition E tends to diagnose more HPD among patients with a pretreatment progression with a RECIST percentage from 15% to 90% than with other definitions.
For a pretherapy decrease of the RECIST sum, the difference between the definitions is even more substantial. Using mathematical ratios, such as definitions A, B, and D, no patient with S-baseline/S-prebaseline of less than 1 can ever be considered to have HPD; the 3 conditions S-baseline/S-prebaseline of less than 1, S-postbaseline/S-baseline of greater than 1.2, and TGR ratio of greater than 2 (or TGK ratio >2) cannot be satisfied simultaneously. However, using a subtraction, such as definitions C and E, allows patients with a small S-baseline/S-prebaseline and a high S-postbaseline/S-baseline to be assessed as having HPD. These patients were nonetheless declared to have PD during pretreatment because of the appearance of new lesions only, which are accounted for in none of the definitions.
Association Between HPD Definitions and OS
For each of the 5 definitions, the landmark survival analysis emphasized a worse outcome for the patients with HPD compared with the patients with PD. The median overall survival for patients with HPD, which ranged from 3.4 (95% CI, 1.9-8.4) to 6.0 (95% CI, 3.7-9.4) months, was smaller than that for patients without HPD, which ranged from 6.2 (95% CI, 5.0-7.5) to 6.4 (95% CI, 5.6-8.4) months. The gap between the median overall survival of the 2 groups varied from 0.2 months (definition C) to 3.0 months (definition B), thus highlighting a disparity in the correlation of the different HPD definitions with outcomes (Table 2).
Log-rank tests were computed to test the statistical significance of the differentiation between PD with and without HPD. Only definition B demonstrated a significant distinction (3.0 months; P < .001) (Table 2).
Prognostic Value of the Different Indexes
To study the prognostic values of the different indexes (TGK-exp:TGK-ref, TGR-exp:TGR-ref, and ΔTGR) on the 2 groups gathering the highest values on the index (N) and the 207 – N other values on the index (207 patients had PD at first evaluation), we obtained the curves shown in Figure 2. For all 3 indexes, the highest values (ie, the highest increases of TGK during therapy) tended to be associated with the poorest survival outcomes. However, the curves of Figure 2 showed differences in the size of the population associated with these characteristics and in the amplitude and the stability of the gap between the median overall survival of both groups.
Figure 2. Evaluation of the Prognostic Value of Each Hyperprogressive Disease (HDP) Index.
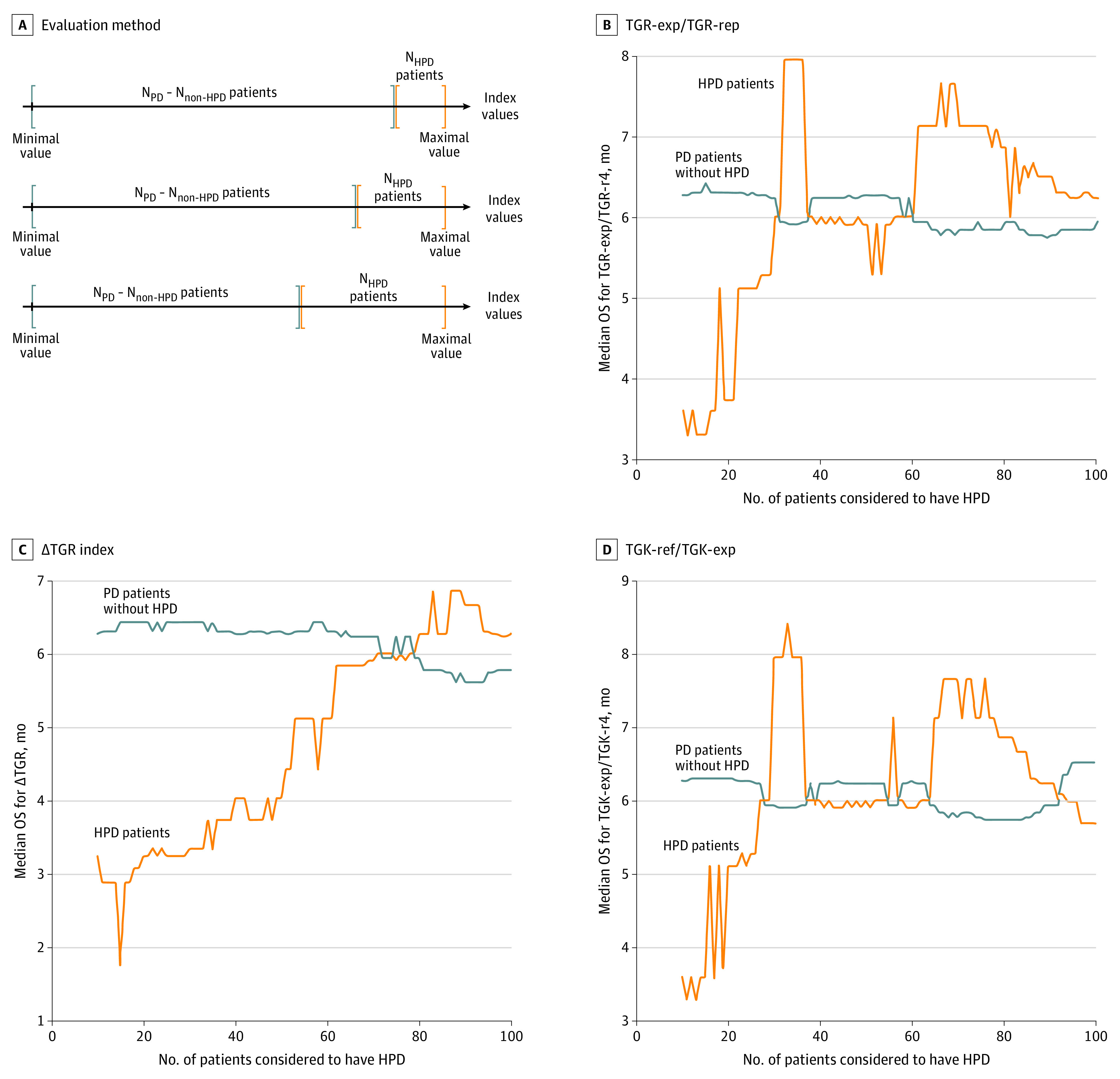
A, Evaluation method of the prognostic value of each index. For each index, patients were categorized into 2 groups according to their index value: those with the highest value (N) and those with progressive disease (NPD) minus N patients, to study the difference between the N with worst progression and the NPD – N other patients. We first set N = 10 and then increased its value until it reached half the number of patients with PD. This determines for which N the difference becomes negligible, which is the limit between patients with HPD and PD. A comparative landmark analysis was performed on these 2 groups for each value of N. B, Median overall survival (OS) curves for patients with HPD compared with PD without HPD according to the threshold ratio of tumor growth rate before (ref) and during (exp) therapy (TGR-exp:TGR-ref index) as a function of the number N of patients considered to have HPD. C, Median OS curves for HPD compared with PD without HPD according to the threshold TGR-exp − TGR-ref (ΔTGR) index as a function of the number N of patients considered to have HPD. D, Median OS curves for HPD compared with PD without HPD according to the threshold tumor growth kinetics before (ref) and during (exp) therapy (TGK-exp:TGK-ref) index as a function of the number N of patients considered to have HPD.
Figure 2B-D describes the testing of each specific index. Each time, the objective was to determine how efficiently each index discriminated between patients with HPD and those with PD without HPD. In other words, how different are the overall survival curves for both groups? Each time, N = 1 is equivalent to the patient with the most HPD according to the chosen index and N = 2, the 2 patients with the most HPD, etc. When the number N is small, the few patients with the most HPD are assessed as having HPD, and the median overall survival of the HPD group should be much smaller than that of the non-HPD group. When the number N is larger, many patients are assessed as having HPD, which means that the overall survival of the HPD group should be closer to that of the non-HPD group. Therefore, a good index is associated with a large gap between the curves for small N values. The wider and the longer the gap between curves for both groups, the better the index is able to discriminate between patients with HPD and those with PD without HPD. The curves of TGR-exp:TGR-ref and TGK-exp:TGK-ref (Figure 2B and D) appeared to be similar, highlighting a similar distribution of the values among patients (ie, ranking patients according to their values of TGR-exp:TGR-ref or TGK-exp:TGK-ref leads to a close result). Both curves showed an initial plateau until 20 patients but emphasized an important instability followed by a sharp increase of the median overall survival that even overtakes that of patients with PD for a larger number N.
The curve of ΔTGR (Figure 2C) also revealed an initial plateau for a larger number of 40 to 50 patients and a slower increase, demonstrating a greater correlation with overall survival. To confirm the relevance of these distinctions between the 2 groups, we further investigated whether a log-rank test was significant for the different indexes and the different thresholds N. All 3 indexes locally reached a significant P < .05 for a small N. However, only ΔTGR remained at P < .05 for a larger range of N, with P values for both other indexes oscillating between significant and nonsignificant values while N increased. The P value for ΔTGR remained significant until a maximum N of 34 patients in the first group corresponding to a threshold ΔTGR of greater than 102.
Discussion
Previous studies6,9,10,11,12,13 on HPD during immunotherapy reported different incidences of the phenomenon, varying from 4% to 29%. The causes for such a disparity might include the diversity in cancer histology as well as the size and source of the study cohort constitution (Table 1). However, as already emphasized by Kim et al,16 the metrics used for HPD assessment could also be a major explanation for this inconsistency. To our knowledge, our study is the first one to offer a detailed analytical comparison of all the definitions that have been used so far to assess this phenomenon.
In this study, the rates of HPD with the different definitions applied to the same NSCLC cohort appeared to be concordant with the previously reported studies, with the exception of definition C from Saâda-Bouzid11 that showed a smaller incidence of patients with HPD in our cohort (18.5% vs 29%). No reasoned explanation can be given for such a gap at this stage, but the effect of the patients’ characteristics and the histologic findings (NSCLC vs previously irradiated squamous cell carcinoma of the head and neck) on such a result should be further analyzed.
The results of the present study first and foremost point out the high disparity in HPD incidences due to the definitions themselves, with a number of patients with HPD that can vary from 1 to 4 in the same cohort (22 patients for definition B compared with 75 patients for definition C). The choice of the definition seems therefore to be a major reason for the diversity observed among previous studies. Beyond the question of incidences, these results also highlight the fact that the groups of patients with HPD appear to differ from one definition to the other, with only 19 patients being common to all definitions. More precisely, the similarity measures show that the so-called HPD for different definitions is not representative of the same tumoral behavior.
Consequently, the definitions do not correlate in the same way with overall survival. Most of them proved no ability to establish a clear distinction between the overall survival of patients with HPD compared with PD without HPD. Indeed, only 2 definitions appeared to be (statistically) significantly correlated with a worse overall survival for patients with HPD. This result should be moderated by the fact that the small overall survival is itself a criterion taken into account in both these definitions.
Trying to extract thresholds to align the patients with the worst progression and the worst survival prognosis, we showed that only the index ΔTGR appeared to be likely to distinguish subsets of patients with the characteristics expected with HPD status: acceleration of the tumor growth combined with a poor overall survival. For this index, the significance of the distinction between patients with HPD and those with PD without HPD in terms of median overall survival was reached for 34 patients (8.4%) less than in the previous studies using ΔTGR and corresponding to an approximate threshold ΔTGR of greater than 100. Therefore, to be in accordance with the concept of HPD that assumes both a high increase of the tumor kinetics and a poor survival outcome, the following definition based on our cohort could appear more relevant: RECIST percentage during therapy of greater than 20% (S-postbaseline/S-baseline >1.2) and ΔTGR of greater than 100.
Limitations
Some limitations to our study should be noted. First, whereas HPD behavior was initially evaluated in a mixed oncologic population6,9 or in patients with head and neck squamous cell carcinoma,11 our model only includes patients with NSCLC. Studies with larger groups of patients with different characteristics would be necessary to confirm the accuracy of our definition of HPD that was empirically determined.
Second, the characterization of HPD remains difficult on a routine basis, because pretreatment imaging is required. Moreover, all definitions of HPD are based on the measurement of target lesions following RECIST 1.1 criteria, thus not accounting for the unequivocal progression of nontarget lesions or the appearance of a new lesion. This bias, as well as the artificial exclusion of patients who died before having the requisite posttreatment imaging, making it impossible to associate the death with HPD, may lead to an underestimation of the phenomenon.
Patients with 2-month intervals between CT scans were selected when possible. A more precise time frame would be ideal for improved precision of HPD assessment, and a larger prospective database would be necessary in further studies.
Conclusions
In this study, we observed the existence of an increase of kinetics of some patients with NSCLC receiving ICI, which we define as HPD. We demonstrated highly variable HPD rates, based on the 5 previous definitions of HPD, and thus suggest an optimized definition: greater than 20% RECIST and ΔTGR of greater than 100, correlated with poor OS outcomes. A biological explanation and surrogate are urgently needed to identify as soon as possible patients with HPD receiving ICI.
eTable. Patient Prior Treatments for Immunotherapy-Treated Patients With NSCLC
eFigure 1. Case Study of a Patient With Hyperprogressive Disease
eFigure 2. Overall Survival for Hyperprogressive Disease Compared With Progressive Disease Without Hyperprogression Under Immunotherapy (Landmark at the Beginning of Immunotherapy)
eFigure 3. Overall Survival for Hyperprogressive Disease Compared With Progressive Disease Without Hyperprogression Under Immunotherapy (6-wk Landmark Analysis)
References
- 1.Robert C, Schachter J, Long GV, et al. ; KEYNOTE-006 investigators . Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521-2532. doi: 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 2.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small cell lung cancer. N Engl J Med. 2015;373(2):123-135. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motzer RJ, Escudier B, McDermott DF, et al. ; CheckMate 025 Investigators . Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803-1813. doi: 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856-1867. doi: 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412-7420. doi: 10.1158/1078-0432.CCR-09-1624 [DOI] [PubMed] [Google Scholar]
- 6.Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920-1928. doi: 10.1158/1078-0432.CCR-16-1741 [DOI] [PubMed] [Google Scholar]
- 7.Gomez-Roca C, Koscielny S, Ribrag V, et al. Tumour growth rates and RECIST criteria in early drug development. Eur J Cancer. 2011;47(17):2512-2516. doi: 10.1016/j.ejca.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 8.Le Tourneau C, Servois V, Diéras V, Ollivier L, Tresca P, Paoletti X. Tumour growth kinetics assessment: added value to RECIST in cancer patients treated with molecularly targeted agents. Br J Cancer. 2012;106(5):854-857. doi: 10.1038/bjc.2012.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23(15):4242-4250. doi: 10.1158/1078-0432.CCR-16-3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuentes-Antrás J, Provencio M, Díaz-Rubio E. Hyperprogression as a distinct outcome after immunotherapy. Cancer Treat Rev. 2018;70:16-21. doi: 10.1016/j.ctrv.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 11.Saâda-Bouzid E, Defaucheux C, Karabajakian A, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017;28(7):1605-1611. doi: 10.1093/annonc/mdx178 [DOI] [PubMed] [Google Scholar]
- 12.Singavi AK, Menon S, Kilari D, et al. 1140PD Predictive biomarkers for hyper-progression (HP) in response to immune checkpoint inhibitors (ICI): analysis of somatic alterations (SAs). Ann Oncol. 2017;28(5). doi: 10.1093/annonc/mdx376.006 [DOI] [Google Scholar]
- 13.Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4(11):1543-1552. doi: 10.1001/jamaoncol.2018.3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1-update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132-137. doi: 10.1016/j.ejca.2016.03.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1(11):710-719. doi: 10.1200/JCO.1983.1.11.710 [DOI] [PubMed] [Google Scholar]
- 16.Kim CG, Kim KH, Pyo K-H, et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol. 2019;30(7):1104-1113. doi: 10.1093/annonc/mdz123 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Patient Prior Treatments for Immunotherapy-Treated Patients With NSCLC
eFigure 1. Case Study of a Patient With Hyperprogressive Disease
eFigure 2. Overall Survival for Hyperprogressive Disease Compared With Progressive Disease Without Hyperprogression Under Immunotherapy (Landmark at the Beginning of Immunotherapy)
eFigure 3. Overall Survival for Hyperprogressive Disease Compared With Progressive Disease Without Hyperprogression Under Immunotherapy (6-wk Landmark Analysis)