This cohort study evaluates the host factors associated with prognosis in patients with oral cavity squamous cell carcinoma and their interactions for development of a numerical index that quantifies their prognostic capacity.
Key Points
Questions
What host factors are associated with prognosis in patients with oral cavity cancer, and can these factors be used to develop an index that quantifies their prognostic capacity?
Findings
In this cohort study of 1309 patients with oral cavity squamous cell carcinomas, an index (H-index) was developed that combined all the host factors that were independently associated with overall survival. The index, which included the pretreatment neutrophil count, monocyte count, lymphocyte count, albumin level, and hemoglobin level, accurately stratified patients into groups with differences in overall and disease-specific survival.
Meaning
The findings suggest that the H-index can be used to quantify the prognostic capacity of host characteristics associated with oral cavity squamous cell carcinomas.
Abstract
Importance
The association and interaction of host characteristics with prognosis in patients with oral cavity squamous cell carcinoma (OSCC) are poorly understood. There is increasing evidence that host characteristics are associated with treatment outcomes of many cancers.
Objectives
To examine the host factors associated with prognosis in patients with OSCC and their interactions to create a numerical index that quantifies the prognostic capacity of these host characteristics.
Design, Setting, and Participants
This retrospective cohort study included patients with OSCC treated surgically at a tertiary care center from January 1, 1998, to December 31, 2015. From a departmental OSCC database of 1377 previously untreated patients, 68 patients with missing data on any host variable of interest within a month before the start of treatment were excluded, leaving 1309 patients. Data analysis was performed from October 21, 2019, to December 10, 2019.
Exposure
Primary surgery for OSCC.
Main Outcomes and Measures
Overall survival (OS) was the primary end point, and disease-specific survival (DSS) was the secondary end point. Optimal cutoffs for each variable were identified using recursive-partitioning analysis with the classification and regression tree method using OS as the dependent variable. Body mass index (BMI) and pretreatment peripheral blood leukocyte count, platelet count, hemoglobin level, and albumin level were analyzed. A host index (H-index) was developed using independent factors associated with OS.
Results
A total of 1309 patients (731 [55.8%] male; mean [SD] age, 62 [14.3] years) participated in the study. When including all the host-related factors in a multivariable analysis, all except BMI (hazard ratio [HR], 1.14; 95% CI, 0.80-1.63) were independently associated with outcomes. For example, compared with a hemoglobin level of 14.1 g/dL or greater, the HR for a level of 12.9 to 14.0 g/dL was 1.42 (95% CI, 1.13-1.77) and for a level of 12.8 g/dL or less was 1.51 (95% CI, 1.18-1.94), and compared with an albumin level of 4.3 g/dL or greater, the HR for a level of 3.7 to 4.2 g/dL was 1.18 (95% CI, 0.95-1.45) and for a level of 3.6 g/dL or less was 3.64 (95% CI, 2.37-5.58). An H-index of 1.4 or less was associated with a 74% 5-year OS, an H-index of 1.5 to 3.5 with a 65% 5-year OS, and an H-index of 3.6 or higher with a 38% 5-year OS; for DSS, the 5-year survival was 84%, 80%, and 64%, respectively. Compared with patients with an H-index score of 1.4 or less, patients with H-index scores of 1.5 to 3.5 (hazard ratio, 1.474; 95% CI, 1.208-1.798) and 3.6 or higher (hazard ratio, 3.221; 95% CI, 2.557-4.058) had a higher risk of death.
Conclusions and Relevance
The findings suggest that pretreatment values of neutrophils, monocytes, lymphocytes, hemoglobin, and albumin are independently associated with prognosis in patients with OSCC. The interactions between these host factors were incorporated into a novel H-index that quantified the prognostic capacity of host characteristics associated with OSCC.
Introduction
The American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) staging system generally estimates prognosis of patients based on only tumor factors.1 However, there is increasing evidence of several host characteristics being associated with oncologic outcomes.2,3,4,5,6 To our knowledge, the only example of a staging system for head and neck cancer that takes into consideration a host factor is the staging system for differentiated thyroid cancers, in which age is incorporated in the prognostic stratification of patients.
Besides well-known host characteristics, such as age, tobacco and alcohol consumption, and medical comorbidities, the most widely investigated host prognostic factors are pretreatment peripheral blood leukocytes.7,8,9,10 Additional host factors, such as platelet count, hemoglobin level, albumin level, and body mass index (BMI) (calculated as weight in kilograms divided by height in meters squared), have also been studied as potential prognostic factors in several cancer types.3,11,12,13
It is logical to assume that these and other host factors are not associated with the host-tumor interaction in isolation. Many of these factors are interdependent and interact in a complex manner to influence prognosis not only directly but also indirectly by affecting the treatment decisions for patients. Therefore, attempts have been made to combine various significant host factors into a comprehensive index for assessing this interaction in association with prognosis.14,15 For example, Jafri et al14 described the advanced lung cancer inflammation index (ALI), an index that combines host factors, such as albumin level, BMI, and the neutrophil-lymphocyte ratio.
A previous study16 reported the association of pretreatment peripheral blood leukocyte levels with outcomes of oral cavity squamous cell carcinomas (OSCCs). Higher neutrophil and monocyte counts and lower lymphocyte counts were associated with poorer outcomes. The aims of this study were to assess the association of host factors with prognosis and to examine the interaction between all the analyzed factors to create a numerical index to quantify the prognostic capacity of host characteristics in OSCCs.
Methods
This cohort study used our departmental database of 1377 patients with a biopsy-proven invasive OSCC treated with primary surgery at Memorial Sloan Kettering Cancer Center from January 1, 1998, to December 31, 2015. Data analysis was performed from October 21, 2019, to December 10, 2019. The study design was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board, which determined that no informed consent was required from participants because of the retrospective, deidentified nature of the study.
Exclusion criteria were synchronous head and neck squamous cell carcinomas, prior treatment of the reference carcinoma, distant metastasis at presentation, and history of nonendocrine head and neck cancer. In addition to tumor variables and pretreatment peripheral blood leukocyte levels (neutrophil, monocyte, and lymphocyte counts), the host-related variables of interest were BMI and the pretreatment peripheral blood absolute counts of eosinophils, basophils, platelets, hemoglobin, and albumin. We used the blood test closest to surgery, only considering those blood tests performed within a month before surgery. We excluded 68 patients with missing data on any variable of interest, leaving 1309 patients.
Optimal cutoffs for each variable were identified using a recursive-partitioning analysis with the classification and regression tree method using overall survival (OS) as the primary outcome of interest.17 The established cutoffs were also used to analyze differences in disease-specific survival (DSS) as the secondary outcome of interest. In addition to the aforementioned variables, we analyzed other previously published ratios and indexes, such as the platelet-lymphocyte ratio and the ALI, calculated as: (BMI × albumin)/neutrophil-lymphocyte ratio.14 Parameters for which the recursive-partitioning analysis did not find a feasible cutoff or for which the cutoff stratified patients in 2 groups with 1 of them representing less than 5% of the cohort were excluded from the analyses.
We analyzed the prognostic capacity of each host variable (for the variables of interest from this study and a previous study16) using univariable analysis for both OS and DSS and then we conducted a multivariable analysis that included as independent variables the clinicopathologic characteristics and the host variables that were significant in the univariable analyses (eTable 1 in the Supplement). The host variables that were independently associated with outcomes in the multivariable analysis were then combined to create the host index (H-index), which is calculated using the following formula: ([neutrophils × monocytes]/[lymphocytes × hemoglobin × albumin]) × 100.
Statistical Analysis
We evaluated the association between host variables and patient characteristics using the t test or 1-way analysis of variance. Survival curves were calculated according to the Kaplan-Meier method, and differences in survival were compared using the log-rank test. Hazard ratios (HRs) were calculated according to the Cox proportional hazards regression model, which was also used to perform the multivariable analyses. All statistical analyses were conducted using SPSS software, version 25.0 (IBM Corp) and Stata software, version 15 (StataCorp).
Results
A total of 1309 patients (731 [55.8%] male; mean [SD] age, 62 [14.3] years) participated in the study. The clinicopathologic characteristics of the study cohort are given in Table 1. History of tobacco and alcohol use was reported by 821 (62.7%) and 917 (70.1%), respectively. We considered patients as smokers (ever) if they reported a history of actively smoking or having smoked tobacco at any point in their life. Comorbidities were recorded according to the Washington University Head and Neck Comorbidity Index (WUHNCI) (scores range from 0 to 15, with higher scores indicating worse prognosis because of comorbidities), with 376 patients (28.7%) having a WUHNCI score of 1 or higher at the time of diagnosis.18 The most common primary tumor subsite was the tongue (696 [53.2%]). A total of 630 patients (48.2%) had an advanced pathologic stage (stages III–IV) according to AJCC TNM classification.1 Median follow-up time from the date of surgery was 39 months (range, 1-221 months). Five-year OS was 64.2%, and 5-year DSS was 79.7%.
Table 1. Clinicopathologic Characteristics of the Study Cohort .
| Characteristic | Finding (N = 1309)a |
|---|---|
| Age, mean (SD) [range], y | 62.1 (14.3) [18.3-100.4] |
| Sex | |
| Male | 731 (55.8) |
| Female | 578 (44.2) |
| Tobacco use | |
| Never | 488 (37.3) |
| Ever | 821 (62.7) |
| Alcohol use | |
| Never | 392 (29.9) |
| Ever | 917 (70.1) |
| WUHNCI scoreb | |
| 0 | 933 (71.3) |
| ≥1 | 376 (28.7) |
| Subsite | |
| Oral tongue | 696 (53.2) |
| Lower gum | 174 (13.3) |
| Floor of the mouth | 155 (11.8) |
| Buccal mucosa | 104 (7.9) |
| Upper gum | 88 (6.7) |
| Retromolar trigone | 67 (5.1) |
| Hard palate | 25 (1.9) |
| pTc | |
| pT1 | 420 (32.1) |
| pT2 | 327 (25.0) |
| pT3 | 246 (18.8) |
| pT4 | 252 (19.3) |
| Not recorded | 64 (4.9) |
| pNc | |
| pN0 | 891 (68.1) |
| pN1 | 118 (9.0) |
| pN2 | 129 (9.9) |
| pN3 | 152 (11.6) |
| Not recorded | 19 (1.5) |
| pStagec | |
| I | 381 (29.1) |
| II | 230 (17.6) |
| III | 201 (15.4) |
| IV | 429 (32.8) |
| Not recorded | 68 (5.2) |
| Grade | |
| Differentiated | |
| Well | 219 (16.7) |
| Moderately | 830 (63.4) |
| Poorly | 199 (15.2) |
| Not recorded | 61 (4.7) |
| Perineural invasion | |
| Absent | 819 (62.6) |
| Present | 402 (30.7) |
| Not recorded | 88 (6.7) |
| Lymphovascular invasion | |
| Absent | 1044 (79.8) |
| Present | 177 (13.5) |
| Not recorded | 88 (6.7) |
| Margins | |
| Negative | 385 (29.4) |
| Close | 781 (59.7) |
| Positive | 137 (10.5) |
| Not recorded | 6 (0.5) |
| Treatment | |
| Surgery | 807 (61.7) |
| Surgery and adjuvant radiotherapy | 379 (29.0) |
| Surgery and adjuvant chemoradiotherapy | 123 (9.4) |
Abbreviation: WUHNCI, Washington University Head and Neck Comorbidity Index.
Data are presented as number (percentage) of patients unless otherwise indicated.
The WUHNCI scores range from 0 to 15, with higher scores indicating worse prognosis because of comorbidities.18
Based on the American Joint Committee on Cancer, 8th edition.1
When conducting the recursive-partitioning analysis for each of the host variables of interest and following the previously mentioned exclusion criteria (no cutoff found or cutoff creating only 2 groups with 1 of them representing <5% of the cohort), we excluded eosinophil count, basophil count, platelet count, and platelet-lymphocyte ratio from further analyses. The recursive-partitioning analysis for these variables is shown in eFigure 1 in the Supplement. The remaining host variables analyzed had a feasible cutoff and were used for further analyses.
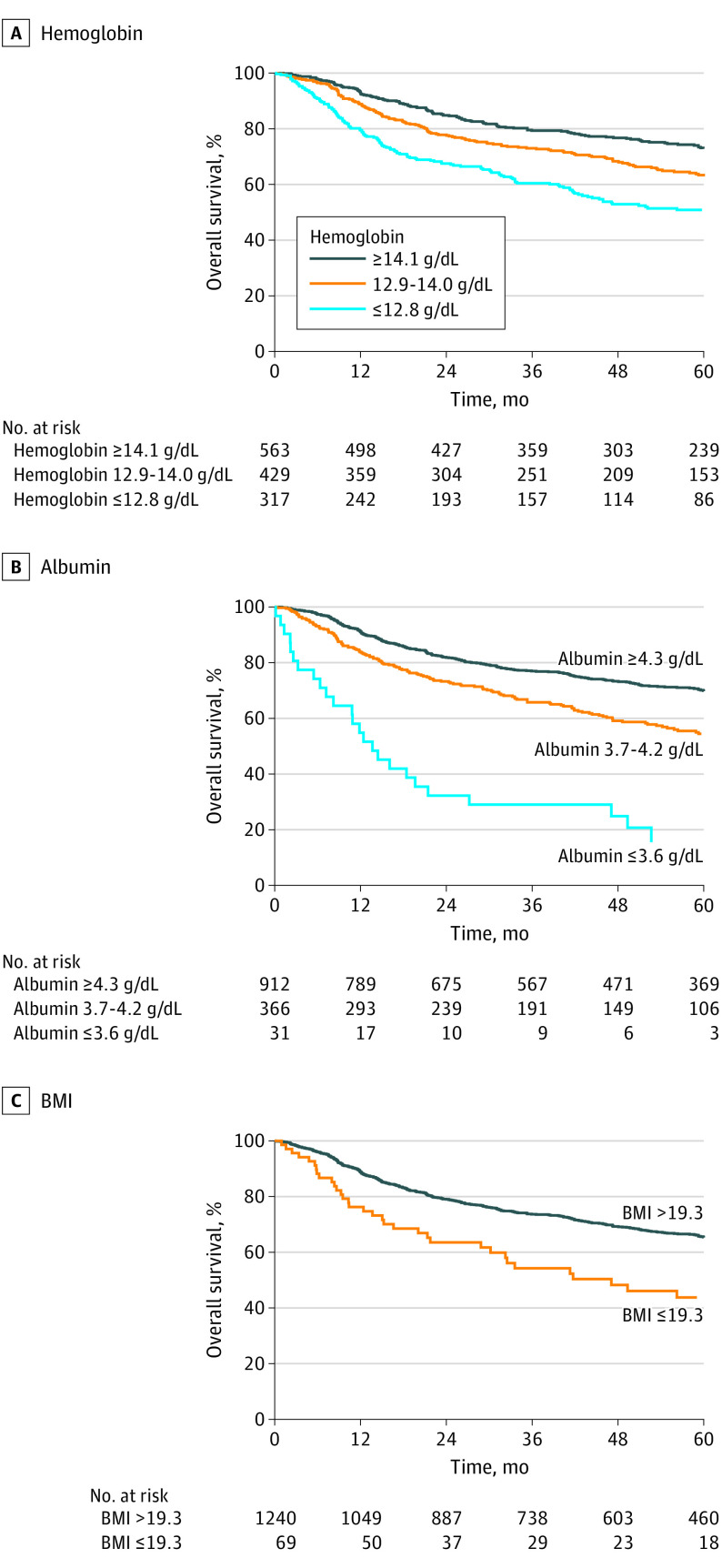
We first analyzed the 5-year OS and DSS for each host variable according to the categories defined by the recursive-partitioning analysis for the variables of interest from this study and a previous study (Table 2).16 All variables were associated with OS and DSS. An ordered decrease in OS was associated with decreases in hemoglobin level (≥14.1 vs 12.9-14.0 g/dL: HR, 1.372 [95% CI, 1.121-1.680]; ≥14.1 vs ≤12.8 g/dL: HR, 2.071 [95% CI, 1.682-2.549]), albumin level (≥4.3 vs 3.7-4.2 g/dL: HR, 1.726 [95% CI, 1.438-2.071]; ≥4.3 vs ≤3.6 g/dL: HR, 5.754 [95% CI, 3.898-8.492]), BMI (>19.3 vs ≤19.3: HR, 2.078 [95% CI, 1.526-2.831]), and lymphocyte count (>0.8 vs ≤0.8/μL: HR, 2.358 [95% CI, 1.731-3.213]). An ordered decrease in DSS was also associated with decreases in these measures. An ordered decrease in OS was associated with increases in neutrophil count (≤4.8 vs 4.7-9.0/μL: HR, 1.504 [95% CI, 1.264-1.791]; ≤4.8 vs ≥9.1/μL: HR, 3.060 [95% CI, 2.157-4.341]) and monocyte count (≤0.3 vs >0.3: HR, 1.418 [95% CI, 1.191-1.689]). An ordered decrease in DSS was also associated with increases in neutrophil and monocyte counts. The OS and DSS curves according to hemoglobin levels, albumin levels, and BMI are shown in Figure 1 and eFigure 2 in the Supplement, respectively. When analyzing ALI, patients with the lowest score had a decrease in OS compared with the rest of the cohort, and patients with the highest score had the best OS. However, the 2 intermediate groups did not follow the expected order. For DSS, the categories did not stratify survival in an ordered manner, with the 2 intermediate categories being the ones with the best and worst DSS. The OS and DSS curves according to ALI categories are shown in eFigure 3 in the Supplement.
Table 2. Survival Outcomes According to Host Variables Using the Cutoffs Found in a Previous Study16 and in This Study.
| Variable | No. of patients | Overall survival | Disease-specific survival | ||
|---|---|---|---|---|---|
| 5-y Survival, % | HR (95% CI) | 5-y Survival, % | HR (95% CI) | ||
| Neutrophil count, /μL | |||||
| ≤4.8 | 735 | 69.5 | 1 [Reference] | 83.1 | 1 [Reference] |
| 4.7-9.0 | 524 | 60.0 | 1.504 (1.264-1.791) | 76.7 | 1.517 (1.162-1.982) |
| ≥9.1 | 50 | 31.5 | 3.060 (2.157-4.341) | 60.0 | 3.045 (1.800-5.153) |
| Monocyte count, /μL | |||||
| ≤0.3 | 552 | 70.9 | 1 [Reference] | 83.1 | 1 [Reference] |
| >0.3 | 757 | 59.1 | 1.418 (1.191-1.689) | 77.0 | 1.368 (1.048-1.785) |
| Lymphocyte count, /μL | |||||
| >0.8 | 1242 | 65.3 | 1 [Reference] | 80.0 | 1 [Reference] |
| ≤0.8 | 67 | 42.7 | 2.358 (1.731-3.213) | 74.9 | 1.453 (0.817-2.600) |
| Hemoglobin level, g/dL | |||||
| ≥14.1 | 563 | 72.8 | 1 [Reference] | 85.2 | 1 [Reference] |
| 12.9-14.0 | 429 | 62.9 | 1.372 (1.121-1.680) | 78.1 | 1.516 (1.111-2.067) |
| ≤12.8 | 317 | 50.8 | 2.071 (1.682-2.549) | 71.6 | 2.117 (1.534-2.921) |
| Albumin level, g/dL | |||||
| ≥4.3 | 912 | 69.8 | 1 [Reference] | 80.7 | 1 [Reference] |
| 3.7-4.2 | 366 | 54.5 | 1.726 (1.438-2.071) | 79.1 | 1.255 (0.941-1.673) |
| ≤3.6 | 31 | 15.6 | 5.754 (3.898-8.492) | 61.8 | 3.360 (1.712-6.593) |
| BMI | |||||
| >19.3 | 1240 | 65.3 | 1 [Reference] | 80.4 | 1 [Reference] |
| ≤19.3 | 69 | 43.8 | 2.078 (1.526-2.831) | 65.0 | 2.107 (1.302-3.410) |
| ALI14 | |||||
| ≥30.0 | 969 | 70.6 | 1 [Reference] | 82.7 | 1 [Reference] |
| 27.5-29.9 | 60 | 44.8 | 2.455 (1.754-3.436) | 58.2 | 2.902 (1.798-4.684) |
| 21.8-27.4 | 112 | 65.3 | 1.155 (0.846-1.576) | 86.5 | 0.911 (0.536-1.550) |
| ≤21.9 | 168 | 34.8 | 2.932 (2.379-3.614) | 62.7 | 2.654 (1.916-3.677) |
| H-index | |||||
| ≤1.4 | 505 | 73.8 | 1 [Reference] | 84.4 | 1 [Reference] |
| 1.5-3.5 | 614 | 64.6 | 1.474 (1.208-1.798) | 80.0 | 1.372 (1.019-1.847) |
| ≥3.6 | 190 | 37.7 | 3.221 (2.557-4.058) | 64.1 | 2.704 (1.772-3.873) |
Abbreviations: ALI, advanced lung cancer inflammation index; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); H-index, host index; HR, hazard ratio.
SI conversion factors: To convert neutrophils, monocytes, and lymphocytes to ×109/L, multiply by 0.001; to convert hemoglobin and albumin to grams per liter, multiply by 10.
Figure 1. Overall Survival Curves.
The group stratification was based on the categories obtained with the recursive-partitioning analysis. BMI is calculated as weight in kilograms divided by height in meters squared. To convert hemoglobin and albumin to grams per liter, multiply by 10.
When including all the host-related factors in a multivariable analysis, all except BMI (hazard ratio [HR], 1.14; 95% CI, 0.80-1.63) were independently associated with outcomes. For example, compared with a hemoglobin level of 14.1 g/dL or greater, the HR for a level of 12.9 to 14.0 g/dL was 1.42 (95% CI, 1.13-1.77) and for a level of 12.8 g/dL or less was 1.51 (95% CI, 1.18-1.94), and compared with an albumin level of 4.3 g/dL or greater, the HR for a level of 3.7 to 4.2 g/dL was 1.18 (95% CI, 0.95-1.45) and for a level of 3.6 g/dL or less was 3.64 (95% CI, 2.37-5.58) (eTable 1 in the Supplement). On the basis of these results, we created the H-index, an index that accounts for the prognostic capacity and interactions of all the host-related factors that were independently associated with outcomes. An H-index of 1.4 or less was associated with a 74% 5-year OS, an H-index of 1.5 to 3.5 with a 65% 5-year OS, and an H-index of 3.6 or higher with a 38% 5-year OS; for DSS, the 5-year survival was 84%, 80%, and 64%, respectively. Compared with patients with an H-index score of 1.4 or less, patients with an H-index score of 1.5 to 3.5 (HR, 1.474; 95% CI, 1.208-1.798) and those with an H-index of 3.6 or higher (HR, 3.221; 95% CI, 2.557-4.058) had a greater risk of death.
Survival curves according to H-index categories defined by the recursive-partitioning analysis cutoffs are shown in Figure 2. In multivariable analysis using the association of H-index with OS (Table 3), the H-index showed a good balance in the number of patients included in each category. After controlling for clinicopathologic characteristics in the multivariable analysis, the H-index showed a 2 times greater risk of death for patients in the highest category (H-index ≥3.6: HR, 1.988; 95% CI, 1.521-2.598) compared with the patients in the lowest category (H-index ≤1.4 [reference category]).
Figure 2. Survival Curves for the Host Index (H-Index).

The group stratification was based on the categories obtained with the recursive-partitioning analysis.
Table 3. Multivariable Analysis Including the H-Index as an Independent Variable Associated With Overall Survival.
| Variable | HR (95% CI) | |
|---|---|---|
| Univariable analysis | Multivariable analysis | |
| Age, y | ||
| ≤60 | 1 [Reference] | 1 [Reference] |
| >60 | 1.955 (1.631-2.343) | 1.807 (1.481-2.203) |
| Tobacco use | ||
| Never | 1 [Reference] | 1 [Reference] |
| Ever | 1.305 (1.091-1.561) | 1.033 (0.850-1.255) |
| WUHNCIa | ||
| 0 | 1 [Reference] | 1 [Reference] |
| ≥1 | 1.624 (1.363-1.936) | 1.273 (1.050-1.544) |
| Lymphovascular invasion | ||
| Absent | 1 [Reference] | 1 [Reference] |
| Present | 2.100 (1.690-2.611) | 1.181 (0.922-1.512) |
| Perineural invasion | ||
| Absent | 1 [Reference] | 1 [Reference] |
| Present | 2.209 (1.855-2.630) | 1.374 (1.111-1.699) |
| Margin status | ||
| Negative | 1 [Reference] | 1 [Reference] |
| Close | 1.544 (1.256-1.900) | 1.262 (1.000-1.591) |
| Positive | 3.051 (2.343-3.973) | 1.949 (1.426-2.663) |
| Histologic grade | ||
| Differentiated | ||
| Well | 1 [Reference] | 1 [Reference] |
| Moderately | 1.548 (1.204-1.990) | 1.084 (0.808-1.454) |
| Poorly | 2.179 (1.619-2.931) | 1.035 (0.721-1.486) |
| pTb | ||
| pT1 | 1 [Reference] | 1 [Reference] |
| pT2 | 1.657 (1.274-2.153) | 1.197 (0.897-1.596) |
| pT3 | 2.624 (2.024-3.401) | 1.309 (0.958-1.789) |
| pT4 | 4.136 (3.239-5.281) | 1.582 (1.161-2.157) |
| pNb | ||
| pN0 | 1 [Reference] | 1 [Reference] |
| pN1 | 1.487 (1.109-1.993) | 1.264 (0.921-1.736) |
| pN2 | 2.200 (1.699-2.850) | 1.594 (1.200-2.118) |
| pN3 | 5.681 (4.565-7.069) | 2.966 (2.255-3.901) |
| H-index | ||
| ≤1.4 | 1 [Reference] | 1 [Reference] |
| 1.5-3.5 | 1.474 (1.208-1.798) | 1.262 (1.016-1.567) |
| ≥3.6 | 3.221 (2.557-4.058) | 1.988 (1.521-2.598) |
Discussion
The current AJCC/UICC staging system for most head and neck cancers, including OSCC, considers only tumor-related factors to stratify patients into prognostic groups. Despite the current system’s ability to estimate prognosis, heterogeneity within staging groups still exists. We hypothesized that inclusion of both tumor and host-related factors in the staging system would increase its prognostic fidelity by decreasing heterogeneity within each group.
Screening of the possible significant host prognostic factors is the natural first step toward including host factors in the staging system. The most commonly investigated host factors besides age, tobacco and alcohol consumption, and medical comorbidities are pretreatment peripheral blood leukocyte counts. A previous study16 found that elevated neutrophil count, monocyte count, neutrophil-lymphocyte ratio, or systemic inflammatory response index and a decreased lymphocyte count are correlated with worse oncologic outcomes. Other host factors, such as pretreatment peripheral blood eosinophil count, basophil count, platelet count, hemoglobin level, albumin level, C-reactive protein (CRP) level, and a wide range of ratios and indexes combining these parameters, have been analyzed.
Only a few studies19,20,21 have analyzed the prognostic capacity of eosinophil and basophil counts by evaluating their pretreatment values, and the results were consistent with our findings, in which neither eosinophil nor basophil count were associated with outcomes. However, Wei et al22 found that lower eosinophil and basophil counts were associated with worse outcomes in patients with stage I to III colorectal cancer, and Holub and Biete4 found the same results for eosinophil counts in patients with cervical cancer.
Several studies11,23,24,25,26,27,28 have analyzed the association of platelet count with outcomes, and, similarly to neutrophil-lymphocyte ratio, the platelet-to-lymphocyte ratio has also been used. Li et al23 found differences in survival associated with platelet or platelet-lymphocyte ratio counts, revealing that higher counts were associated with worse survival, with platelet count being a surrogate marker of inflammation and lymphocyte count with immune status. On the other hand, in several studies,11,24,25,26,27,28 including the present study, platelet count and platelet-lymphocyte ratio were not associated with outcomes. It is possible that platelet values would act as a prognostic factor only at extreme values; low values are likely associated with poor performance status, whereas high values may be associated with a proinflammatory status, both of which are associated with worse outcomes.
C-reactive protein level and various indexes that include CRP level, such as the Glasgow prognostic score, have been shown to be associated with outcomes based on CRP level being a proinflammatory marker.29,30,31 We did not include this factor in our analyses because CRP level is not routinely included in the preoperative assessment of patients with OSCC and therefore is not a good candidate to be used on a routine basis in clinical practice.
Studies23,32,33,34,35,36 that analyzed hemoglobin level as a prognostic factor found that lower levels of hemoglobin are correlated with worse oncologic outcomes. Hemoglobin can be considered a surrogate marker for a patient’s general nutritional and performance status, with patients with lower hemoglobin levels having worse outcomes.32 It has also been proposed that decreased levels of hemoglobin lead to reduced oxygen concentrations that will facilitate a hypoxic environment that promotes tumor progression by increasing tumor cell resistance to therapy and promoting distant metastases.23,33,34,35,36 Many studies3,32,33,37,38 that have analyzed hemoglobin level in different tumor models have found that hemoglobin level is independently associated with outcomes. Only a few studies23,27 did not corroborate these results.
Besides inflammation and the immune system status, nutritional status is associated with patient outcomes. Malnutrition can influence the intensity of treatment that a patient is able to receive as well as the host’s immune response to the tumor. Nutritional status is partially mirrored by albumin level, and some studies12,39 have found that lower albumin levels, corresponding to malnutrition, are correlated with worse outcomes. Moreover, a low BMI can also act as a surrogate marker of malnutrition. In our study, patients with the lowest BMI had worse outcomes. The cutoff point in our study (≤19.3) was close to the World Health Organization BMI underweight range (<18.5); our analysis revealed that only severe malnourishment was associated with prognosis.
Bi et al37 have shown that underweight patients had the worst prognosis, and Peter et al3 have shown that low BMI is not significantly associated with prognosis. Another study13 reported that the other extreme of BMI measure, obesity, is also correlated with worse outcomes. These contradictory results may be seen because the analyses using BMI generally compare obesity with healthy weight only, not including the subset of patients with cachexia, and are mostly restricted to early-stage tumors. We analyzed BMI as a potential variable associated with outcomes in our study cohort comparing healthy weight (BMI, 18.5-24.9) with overweight (BMI, 25.0-29.9) and obese (BMI, ≥30.0), following the universal World Health Organization BMI ranges. No differences were found in OS, but when analyzing DSS, patients with obesity had worse outcomes. The differences were even larger when only analyzing T1/T2 tumors. Therefore, excluding malnourished patients, BMI analysis showed that patients with obesity had worse outcomes than patients with healthy weight. It is possible that patients with obesity have a worse disease-specific outcome because a higher amount of fat tissue can act as a reservoir of inflammatory cells, such as macrophages, which when recruited to the tumor transform in protumoral cells, facilitating tumor progression, angiogenesis, and metastases.40,41,42
The BMI correlation with survival is not linear; lower BMI is associated with worse outcomes because it is a surrogate of a poor performance status, and higher BMI is also associated with worse outcomes not only because of a higher proinflammatory status based on a higher volume of fat tissue but also because of associated comorbidities in patients with high BMI. We believe that both extremes of BMI harbor an adverse host environment that allows tumors to progress, but this simple measure is probably not strong enough to emerge as an independent prognostic factor in all patients.
The ALI was one of the first attempts to combine different host factors in 1 index by analyzing the combination of albumin level, BMI, and neutrophil-lymphocyte ratio.14 Albumin level and BMI represent the nutritional status of the patient. The neutrophil-lymphocyte ratio represents the balance between inflammation and immune system response. In our study, ALI showed only significant and ordered differences in OS when comparing the highest and lowest categories obtained in this study but showed a contrary to expected trend in survival within the 2 intermediate categories. We also tested the cutoff reported in the article published by Jafri et al14 and observed that it stratified patients better than the cutoffs found in our study (eFigure 4 in the Supplement). However, the balance in the number of patients included in each group was limited in the study by Jafri et al,14 with only 8% of patients in the lower category.
Other attempts have been made to combine host nutritional and immune status and inflammation into 1 index. Chen et al15 created an index combining hemoglobin level, albumin level, lymphocyte count, and platelet count (HALP). We believe that this index can be improved by the incorporation of neutrophil and monocyte counts, which had strong associations with outcomes in our study, and by excluding platelet count, which was not associated with outcomes in our study.
By creating the H-index, we were able to include all the independent host-related prognostic factors identified in our studies. Because the 3 individual factors analyzed in the previous study (neutrophil, monocyte, and lymphocyte count) were independently associated with prognosis, we included all 3 as components of the H-index jointly with the independent host factors found in this study (hemoglobin and albumin levels). To create an index that inversely correlates with outcomes, we included in the numerator the variables with a negative correlation with survival (neutrophil and monocyte counts) and in the denominator the variables with a positive correlation (lymphocyte count, hemoglobin level, and albumin level). The H-index stratified patients better than the ALI, with an improved balance in the number of patients included in each category.
Some variables included in the H-index, such as leukocyte count, may be associated with other clinicopathologic characteristics.2,16 To analyze whether the remaining variables of the H-index are also correlated with patients’ clinicopathologic characteristics, we reported the mean number of each of the host variables included in this study according to patients’ clinicopathologic characteristics (eTable 2 in the Supplement). We observed a correlation between different variables, such as age, smoking status, or cancer stage, and the different host factors analyzed. Nevertheless, these host factors maintained independent associations with outcomes when controlling for clinicopathologic characteristics in the multivariable analyses.
Furthermore, to elucidate whether the H-index goes beyond tracking comorbidities, subanalyses based on the presence or absence of comorbidities are shown in eFigure 5 in the Supplement. We categorized patients based on the WUHNCI. Patients with a score of 0 were considered as the group without comorbidities, and patients with a score of 1 or higher were considered as the group with comorbidities. The H-index accurately stratified patients in terms of OS and DSS both for the subgroup of patients without comorbidities and for the subgroup of patients with comorbidities. This observation suggests that the H-index accounts not only for comorbidities and performance status but also for other interactions of the host against the tumor. We believe that the H-index is a more comprehensive evaluation of the patient status, improving previous classifications that did not account for all the independent host-related prognostic factors. Even though statistically the prognostic capacity of the H-index might be slightly better than when analyzing leukocyte count alone, by introducing nutritional status based on albumin levels and hypoxic status based on hemoglobin levels, clinically meaningful factors were added in the holistic assessment of patients with OSCC.
Several studies43,44,45,46 that supported the inclusion of host factors in the assessment of patients with cancer have been published. A systematic effort is needed to identify relevant host factors that should be included in prediction models for OSCC. In addition, there is the issue of geographic heterogeneity because it has been previously proposed that host-related variables, such as CRP level and neutrophil-lymphocyte ratio, differ among populations.5,45,47 A previous study16 reported a cohort from Europe that had different median values in terms of leukocyte counts compared with the cohort in the present study. A multi-institutional study that incorporated cohorts from different continents would be the next step to account for geographic variations. This study would allow the development of a more reproducible and generalizable model for prediction of outcomes in OSCC. Every patient undergoing surgery for OSCC in any setting around the world will have a presurgical blood test that measures leukocyte count, hemoglobin level, and albumin level, and BMI measurement is also universally available. Therefore, we identified a set of powerful and universally available host-related factors associated with outcomes that can be incorporated into a statistical tool, such as a nomogram, to assess risk among individual patients but also allow worldwide use in future iterations of the staging system for OSCC.46
Limitations
This study has inherent limitations because of its retrospective nature. Moreover, values were only analyzed at a single time point before initial treatment. The association of non–cancer-related conditions (eg, infection and the treatment and its complications) with host factors, such as peripheral blood leukocyte count, hemoglobin level, albumin level, and body weight, could not be analyzed and accounted for in our study.
Conclusions
The findings suggest that pretreatment values of neutrophils, monocytes, lymphocytes, hemoglobin, and albumin are independently associated with prognosis in patients with OSCC. The interactions between these host factors were incorporated into a novel H-index that quantified the prognostic capacity of host characteristics associated with OSCC.
eTable 1. Multivariable Analysis Including All the Significant Host Factors as Independent Variables for Predicting Overall Survival
eTable 2. Average Count of Each Host Variable According to the Clinicopathologic Characteristics
eFigure 1. Recursive-Partitioning Analyses for the Host Variables That Did Not Reach Our Inclusion Criteria
eFigure 2. Disease-Specific Survival Curves for A) Hemoglobin (HGB), B) Albumin and C) Body Mass Index (BMI) According of the Categories Obtained With the Recursive-Partitioning Analysis
eFigure 3. A) Overall Survival and B) Disease-Specific Survival Curves for Advanced Lung Cancer Inflammation Index (ALI) According of the Categories Obtained With the Recursive-Partitioning Analysis
eFigure 4. A) Overall Survival and B) Disease-Specific Survival Curves for Advanced Lung Cancer Inflammation Index (ALI) According of the Categories Obtained With the Cutoff From Jafri et al
eFigure 5. Overall Survival and Disease-Specific Survival Curves According to H-Index in the Subgroup of Patients Without Comorbidities (A, B) and in the Subgroup of Patients With Comorbidities (C, D)
References
- 1.Amin MB, Edge SB, Greene FL, et al. , eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017. doi: 10.1007/978-3-319-40618-3 [DOI] [Google Scholar]
- 2.Valero C, Pardo L, López M, et al. Pretreatment count of peripheral neutrophils, monocytes, and lymphocytes as independent prognostic factor in patients with head and neck cancer. Head Neck. 2017;39(2):219-226. doi: 10.1002/hed.24561 [DOI] [PubMed] [Google Scholar]
- 3.Peter F, Wittekindt C, Finkensieper M, Kiehntopf M, Guntinas-Lichius O. Prognostic impact of pretherapeutic laboratory values in head and neck cancer patients. J Cancer Res Clin Oncol. 2013;139(1):171-178. doi: 10.1007/s00432-012-1320-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holub K, Biete A. Impact of systemic inflammation biomarkers on the survival outcomes of cervical cancer patients. Clin Transl Oncol. 2019;21(7):836-844. doi: 10.1007/s12094-018-1991-4 [DOI] [PubMed] [Google Scholar]
- 5.Dolan RD, Lim J, McSorley ST, Horgan PG, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: systematic review and meta-analysis. Sci Rep. 2017;7(1):16717. doi: 10.1038/s41598-017-16955-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luvián-Morales J, González-Trejo S, Carrillo JF, et al. Association of the prognostic nutritional index and overall survival in patients with colorectal cancer: a STROBE compliant retrospective cohort study. Cancer Med. 2019;8(7):3379-3388. doi: 10.1002/cam4.2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu W, Dou Y, Wang K, et al. Preoperative neutrophil lymphocyte ratio but not platelet lymphocyte ratio predicts survival and early relapse in patients with oral, pharyngeal, and lip cancer. Head Neck. 2019;41(5):1468-1474. doi: 10.1002/hed.25580 [DOI] [PubMed] [Google Scholar]
- 8.Tham T, Bardash Y, Herman SW, Costantino PD. Neutrophil-to-lymphocyte ratio as a prognostic indicator in head and neck cancer: a systematic review and meta-analysis. Head Neck. 2018;40(11):2546-2557. doi: 10.1002/hed.25324 [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Jiang W, Xi D, et al. Development and validation of nomogram based on SIRI for predicting the clinical outcome in patients with nasopharyngeal carcinomas. J Investig Med. 2019;67(3):691-698. doi: 10.1136/jim-2018-000801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 11.Pardo L, Valero C, López M, et al. The prognostic value of pretreatment platelet count in patients with head and neck squamous cell carcinoma. Auris Nasus Larynx. 2017;44(3):313-318. doi: 10.1016/j.anl.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 12.Ayhan A, Günakan E, Alyazıcı İ, Haberal N, Altundağ Ö, Dursun P. The preoperative albumin level is an independent prognostic factor for optimally debulked epithelial ovarian cancer. Arch Gynecol Obstet. 2017;296(5):989-995. doi: 10.1007/s00404-017-4511-9 [DOI] [PubMed] [Google Scholar]
- 13.Iyengar NM, Kochhar A, Morris PG, et al. Impact of obesity on the survival of patients with early-stage squamous cell carcinoma of the oral tongue. Cancer. 2014;120(7):983-991. doi: 10.1002/cncr.28532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer. 2013;13:158. doi: 10.1186/1471-2407-13-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen XL, Xue L, Wang W, et al. Prognostic significance of the combination of preoperative hemoglobin, albumin, lymphocyte and platelet in patients with gastric carcinoma: a retrospective cohort study. Oncotarget. 2015;6(38):41370-41382. doi: 10.18632/oncotarget.5629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valero C, Zanoni DK, McGill MR, et al. Pretreatment peripheral blood leukocytes are independent predictors of survival in oral cavity cancer. Cancer. 2020;126(5):994-1003. doi: 10.1002/cncr.32591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Puten W. CART: Stata module to perform classification and regression tree analysis. Stat Softw Components. November 2006;S456776.
- 18.Piccirillo JF, Lacy PD, Basu A, Spitznagel EL. Development of a new head and neck cancer-specific comorbidity index. Arch Otolaryngol Head Neck Surg. 2002;128(10):1172-1179. doi: 10.1001/archotol.128.10.1172 [DOI] [PubMed] [Google Scholar]
- 19.Cihan YB, Arslan A, Cetindag MF, Mutlu H. Lack of prognostic value of blood parameters in patients receiving adjuvant radiotherapy for breast cancer. Asian Pac J Cancer Prev. 2014;15(10):4225-4231. doi: 10.7314/APJCP.2014.15.10.4225 [DOI] [PubMed] [Google Scholar]
- 20.Holub K, Biete A. New pre-treatment eosinophil-related ratios as prognostic biomarkers for survival outcomes in endometrial cancer. BMC Cancer. 2018;18(1):1280. doi: 10.1186/s12885-018-5131-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimm M, Rieth J, Hoefert S, et al. Standardized pretreatment inflammatory laboratory markers and calculated ratios in patients with oral squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2016;273(10):3371-3384. doi: 10.1007/s00405-016-3950-4 [DOI] [PubMed] [Google Scholar]
- 22.Wei Y, Zhang X, Wang G, et al. The impacts of pretreatment circulating eosinophils and basophils on prognosis of stage I-III colorectal cancer. Asia Pac J Clin Oncol. 2018;14(5):e243-e251. doi: 10.1111/ajco.12871 [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Xu Z, Huang Y, et al. Prognostic values of preoperative platelet-to-lymphocyte ratio, albumin and hemoglobin in patients with non-metastatic colon cancer. Cancer Manag Res. 2019;11:3265-3274. doi: 10.2147/CMAR.S191432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tangthongkum M, Tiyanuchit S, Kirtsreesakul V, Supanimitjaroenporn P, Sinkitjaroenchai W. Platelet to lymphocyte ratio and red cell distribution width as prognostic factors for survival and recurrence in patients with oral cancer. Eur Arch Otorhinolaryngol. 2017;274(11):3985-3992. doi: 10.1007/s00405-017-4734-1 [DOI] [PubMed] [Google Scholar]
- 25.Chen S, Guo J, Feng C, Ke Z, Chen L, Pan Y. The preoperative platelet-lymphocyte ratio versus neutrophil-lymphocyte ratio: which is better as a prognostic factor in oral squamous cell carcinoma? Ther Adv Med Oncol. 2016;8(3):160-167. doi: 10.1177/1758834016638019 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Zhang Y, Zheng L, Quan L, Du L. Prognostic role of platelet-to-lymphocyte ratio in oral cancer: a meta-analysis. J Oral Pathol Med. Published online January 25, 2019. doi: 10.1111/jop.12832 [DOI] [PubMed] [Google Scholar]
- 27.Sakin A, Sahin S, Yasar N, et al. The relation between hemogram parameters and survival in extensive-stage small cell lung cancer. Oncol Res Treat. 2019;42(10):506-515. doi: 10.1159/000501595 [DOI] [PubMed] [Google Scholar]
- 28.Solak Mekić M, Pedišić I, Šobat H, et al. The role of complete blood count parameters in patients with colorectal cancer. Acta Clin Croat. 2018;57(4):624-629. doi: 10.20471/acc.2018.57.04.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Q, Song X, Zhao Y, et al. Preoperative high c-reactive protein/albumin ratio is a poor prognostic factor of oral squamous cell carcinoma. Future Oncol. 2019;15(19):2277-2286. doi: 10.2217/fon-2019-0063 [DOI] [PubMed] [Google Scholar]
- 30.DE Paz D, Young CK, Chien HT, et al. Prognostic roles of SCC antigen, CRP and CYFRA 21-1 in oral cavity squamous cell carcinoma. Anticancer Res. 2019;39(4):2025-2033. doi: 10.21873/anticanres.13313 [DOI] [PubMed] [Google Scholar]
- 31.Hanai N, Sawabe M, Kimura T, et al. The high-sensitivity modified Glasgow prognostic score is superior to the modified Glasgow prognostic score as a prognostic predictor for head and neck cancer. Oncotarget. 2018;9(97):37008-37016. doi: 10.18632/oncotarget.26438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jazieh AR, Hussain M, Howington JA, et al. Prognostic factors in patients with surgically resected stages I and II non-small cell lung cancer. Ann Thorac Surg. 2000;70(4):1168-1171. doi: 10.1016/S0003-4975(00)01529-0 [DOI] [PubMed] [Google Scholar]
- 33.Oblak I, Strojan P, Zakotnik B, Budihna M, Šmid L. Hemoglobin as a factor influencing the outcome in inoperable oropharyngeal carcinoma treated by concomitant radiochemotherapy. Neoplasma. 2003;50(6):452-458. [PubMed] [Google Scholar]
- 34.Vaupel P, Mayer A, Höckel M. Impact of hemoglobin levels on tumor oxygenation: the higher, the better? Strahlenther Onkol. 2006;182(2):63-71. doi: 10.1007/s00066-006-1543-7 [DOI] [PubMed] [Google Scholar]
- 35.Franco P, Montagnani F, Arcadipane F, et al. The prognostic role of hemoglobin levels in patients undergoing concurrent chemo-radiation for anal cancer. Radiat Oncol. 2018;13(1):83. doi: 10.1186/s13014-018-1035-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nordsmark M, Bentzen SM, Rudat V, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy: an international multi-center study. Radiother Oncol. 2005;77(1):18-24. doi: 10.1016/j.radonc.2005.06.038 [DOI] [PubMed] [Google Scholar]
- 37.Bi H, Huang Y, Wang G, Ma L, Lu M. Impact of body mass index and pretreatment hemoglobin level on prognosis following radical cystectomy for bladder cancer in males and females. Urol Int. 2020;104(1-2):28-35. doi: 10.1159/000500561 [DOI] [PubMed] [Google Scholar]
- 38.Schäfer U, Micke O, Müller SB, Schüller P, Willich N. Hemoglobin as an independent prognostic factor in the radiotherapy of head and neck tumors. Strahlenther Onkol. 2003;179(8):527-534. doi: 10.1007/s00066-003-1117-x [DOI] [PubMed] [Google Scholar]
- 39.Toiyama Y, Yasuda H, Ohi M, et al. Clinical impact of preoperative albumin to globulin ratio in gastric cancer patients with curative intent. Am J Surg. 2017;213(1):120-126. doi: 10.1016/j.amjsurg.2016.05.012 [DOI] [PubMed] [Google Scholar]
- 40.Wu P, Wu D, Zhao L, et al. Inverse role of distinct subsets and distribution of macrophage in lung cancer prognosis: a meta-analysis. Oncotarget. 2016;7(26):40451-40460. doi: 10.18632/oncotarget.9625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin S, Huang J, Li Z, et al. The prognostic and clinicopathological significance of tumor-associated macrophages in patients with gastric cancer: a meta-analysis. PLoS One. 2017;12(1):e0170042. doi: 10.1371/journal.pone.0170042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao X, Qu J, Sun Y, et al. Prognostic significance of tumor-associated macrophages in breast cancer: a meta-analysis of the literature. Oncotarget. 2017;8(18):30576-30586. doi: 10.18632/oncotarget.15736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kao HK, Löfstrand J, Loh CYY, et al. Nomogram based on albumin and neutrophil-to-lymphocyte ratio for predicting the prognosis of patients with oral cavity squamous cell carcinoma. Sci Rep. 2018;8(1):13081. doi: 10.1038/s41598-018-31498-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mattavelli D, Lombardi D, Missale F, et al. Prognostic nomograms in oral squamous cell carcinoma: the negative impact of low neutrophil to lymphocyte ratio. Front Oncol. 2019;9:339. doi: 10.3389/fonc.2019.00339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park JH, Ishizuka M, McSorley ST, et al. Staging the tumor and staging the host: a two centre, two country comparison of systemic inflammatory responses of patients undergoing resection of primary operable colorectal cancer. Am J Surg. 2018;216(3):458-464. doi: 10.1016/j.amjsurg.2017.08.044 [DOI] [PubMed] [Google Scholar]
- 46.Patel SG, Lydiatt WM. Staging of head and neck cancers: is it time to change the balance between the ideal and the practical? J Surg Oncol. 2008;97(8):653-657. doi: 10.1002/jso.21021 [DOI] [PubMed] [Google Scholar]
- 47.Azab B, Camacho-Rivera M, Taioli E. Average values and racial differences of neutrophil lymphocyte ratio among a nationally representative sample of United States subjects. PLoS One. 2014;9(11):e112361. doi: 10.1371/journal.pone.0112361 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Multivariable Analysis Including All the Significant Host Factors as Independent Variables for Predicting Overall Survival
eTable 2. Average Count of Each Host Variable According to the Clinicopathologic Characteristics
eFigure 1. Recursive-Partitioning Analyses for the Host Variables That Did Not Reach Our Inclusion Criteria
eFigure 2. Disease-Specific Survival Curves for A) Hemoglobin (HGB), B) Albumin and C) Body Mass Index (BMI) According of the Categories Obtained With the Recursive-Partitioning Analysis
eFigure 3. A) Overall Survival and B) Disease-Specific Survival Curves for Advanced Lung Cancer Inflammation Index (ALI) According of the Categories Obtained With the Recursive-Partitioning Analysis
eFigure 4. A) Overall Survival and B) Disease-Specific Survival Curves for Advanced Lung Cancer Inflammation Index (ALI) According of the Categories Obtained With the Cutoff From Jafri et al
eFigure 5. Overall Survival and Disease-Specific Survival Curves According to H-Index in the Subgroup of Patients Without Comorbidities (A, B) and in the Subgroup of Patients With Comorbidities (C, D)