Key Points
Question
Is the selection of primary treatment modality associated with survival among patients with sarcopenia and localized oropharyngeal squamous cell carcinoma?
Findings
In this cohort study, a matched analysis of patients with sarcopenia and localized oropharyngeal squamous cell carcinoma demonstrated an association between patients treated by primary surgical resection and improved overall and disease-specific survival compared with patients treated by definitive radiotherapy.
Meaning
Up-front surgical resection may be associated with improved survival for sarcopenic patients with localized oropharyngeal squamous cell carcinoma.
Abstract
Importance
The negative association of low lean muscle mass (sarcopenia) with survival outcomes in head and neck cancers, including oropharyngeal carcinoma, is established. However, it is not known whether the choice of primary treatment modality (surgery or radiotherapy) is associated with oncologic outcomes of patients with sarcopenia and oropharyngeal squamous cell carcinoma (OPSCC).
Objective
To examine whether primary surgical resection or definitive radiotherapy is associated with improved survival for patients with sarcopenia and localized OPSCC.
Design, Setting, and Participants
A cohort study was conducted of patients with clinically staged T1 to T2, N0 to N2 OPSCC with cross-sectional abdominal imaging within 60 days prior to treatment and treated between January 1, 2005, and December 31, 2017. Skeletal muscle mass was measured at the third lumbar vertebra using previously defined techniques and sarcopenia was defined as less than 52.4 cm2/m2 of muscle for men and less than 38.5 cm2/m2 for women. In addition, associated patient demographic characteristics, cancer data, treatment information, and survival outcomes were assessed. Statistical analysis was performed from December 3, 2018, to August 28, 2019.
Main Outcomes and Measures
Primary outcomes were overall survival and disease-specific survival.
Results
Among the 245 patients who met study inclusion criteria, 209 were men (85.3%) and the mean (SD) age was 62.3 (7.8) years. Sarcopenia was detected in 135 patients (55.1%), while normal skeletal muscle mass was detected in 110 patients (44.9%). For the 110 patients without sarcopenia, primary treatment modality was not associated with improved survival. For patients with sarcopenia at diagnosis, primary surgical resection was associated with improved overall survival (hazard ratio [HR], 0.37; 95% CI, 0.17-0.82) and disease-specific survival (HR, 0.22; 95% CI, 0.07-0.68). This association persisted after propensity score matching, as up-front surgery was associated with improved overall survival (HR, 0.33; 95% CI, 0.12-0.91) and disease-specific survival (HR, 0.17; 95% CI, 0.04-0.75) survival.
Conclusions and Relevance
This study suggests that sarcopenia has a negative association with survival for patients with OPSCC. Primary surgery and radiotherapy confer similar survival associations for patients with normal skeletal muscle mass and localized OPSCC. However, up-front surgical resection may be associated with improved survival outcomes for patients with sarcopenia.
This cohort study examines whether primary surgical resection or definitive radiotherapy is associated with improved survival for patients with sarcopenia and localized oropharyngeal squamous cell carcinoma (SCC).
Introduction
Patients with head and neck squamous cell carcinoma frequently experience changes in body weight prior to treatment, and many studies demonstrate that increased body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) is associated with increased survival in patients with solid tumors.1,2,3,4 However, research demonstrates that BMI incompletely describes the contributions of fat, muscle, and water compartments to the overall composition of the body.5,6 Sarcopenia, defined as a degenerative loss of skeletal muscle, was recently identified as a factor associated with morbidity and mortality in patients with solid tumors, including patients with head and neck squamous cell carcinoma.6,7,8,9,10 The prognostic utility of sarcopenia is independent of BMI,6 which is exemplified by the subset of oncologic patients who have both sarcopenia and obesity; these patients have worse outcomes than BMI-matched patients without sarcopenia. With the recent identification of sarcopenia as an independent factor associated with outcomes for oncology patients, significant efforts are being made to improve detection methods of sarcopenia, understand its biological implications, and determine best clinical practices for treating patients with cancer and excessive muscle catabolism.
For patients with localized oropharyngeal squamous cell carcinoma (OPSCC), primary surgery and definitive radiotherapy (RT) treatments historically demonstrate similar survival for both human papillomavirus (HPV)–positive and HPV-negative disease.11,12,13 Thus, the decision between primary surgical treatment and definitive RT for localized OPSCC can be difficult, and is further complicated by uncertainties concerning whether adjuvant therapy is needed. As a result, the identification of comorbidities and risk factors that allow patients to differentially benefit from either primary surgical resection or definitive RT is an area of active research. To our knowledge, there are no published reports investigating the oncologic outcomes for localized OPSCC in a sarcopenic cohort receiving either primary surgery or definitive RT. Therefore, we sought to examine whether selection of primary treatment modality for sarcopenic patients with localized OPSCC is associated with clinically meaningful changes in survival. Because the decision to add adjuvant therapy is typically made after pathologic staging and tumor features are made available after resection, we structured this analysis around the clinical decision-making involved in selecting a primary treatment modality before the need for adjuvant therapy is known. Accordingly, the primary aim of this study was to characterize the association between sarcopenia and OPSCC survival for patients treated by either primary surgery or definitive RT.
Methods
Population Cohort and End Points
A retrospective review of the medical records for patients with OPSCC was performed between January 1, 2005, and December 31, 2017, at Oregon Health & Science University in Portland, Oregon. Study inclusion was restricted to patients treated with primary surgical resection or definitive RT with OPSCC clinically staged as T1 to T2, N0 to N2, M0 by American Joint Committee on Cancer, Eighth edition,14 criteria. Furthermore, patients were required to have undergone whole-body positron emission tomographic–computed tomographic (PET-CT) imaging or abdominal CT scans within 60 days prior to treatment initiation. Electronic health records were reviewed for data collection, including: patient demographics, body mass and height, comorbidities, tumor staging and subsite, HPV or p16 status (defined by >75% of tumor cells displaying strong nuclear and cytoplasmic staining), smoking status, primary treatment modality details, evidence for recurrent disease, date and cause of death, and date of last follow-up. After patient data were abstracted and correlated with imaging, data were deidentified for subsequent analysis. This study was approved by the institutional review board of Oregon Health & Science University, and requirement for informed consent was deemed unnecessary given the retrospective nature of the study. Overall survival was defined as the time from clinical diagnosis to the date of death as a result of any cause. Disease-specific survival was defined as the time from diagnosis to the date of death due to OPSCC. Morphologic spread of disease was staged according to the American Joint Committee on Cancer using the eighth edition TNM system.
CT Body Composition Analysis
Body composition analysis of skeletal muscle was conducted using previously described and widely accepted methods.15 In brief, the cross-sectional area of skeletal muscle at the center of the third lumbar vertebra was calculated for each patient by analyzing axial CT images of the abdomen. These CT images were often taken along with PET-CT images during the patient’s clinical workup for neoplastic disease. Muscle tissue was defined as −29 to 150 Hounsfield units (Slice-o-Matic software, version 5.0; Tomovision), as described previously,16 and include the rectus abdominus, abdominal wall, psoas, and paraspinal muscles. After automated segmentation, each patient scan was examined for precise skeletal muscle labeling, and manual corrections of mislabeled muscle were performed if necessary. The resulting cross-sectional muscle area was normalized to the patient’s square of height in meters and used to calculate skeletal muscle index.15 Sarcopenia was defined a priori as a skeletal muscle index of less than 52.4 for men and 38.5 for women; these cutoffs are consistent with previous reports in patients with head and neck cancer.8,9
Statistical Analysis
Data were analyzed between December 3, 2018, and August 28, 2019. Descriptive statistics were performed and differences between groups were assessed by Pearson χ2 tests for categorical variables or 2-tailed t test for continuous variables; P < .05 was considered significant. Odds ratios and 95% CIs are reported for all appropriate calculations. Survival curves were generated using the Kaplan-Meier technique. Log-rank tests and hazard ratios (HRs) were calculated to compare both overall survival and disease-specific survival among groups. Univariable and multivariable analyses for overall survival were performed using the Cox proportional hazards regression model, and variables with P < .20 by univariable analysis were included in multivariable analysis. Hazard ratios and corresponding 95% CIs are reported. Propensity score matching (PSM) was performed among the groups undergoing primary surgery and definitive RT to identify matched cohorts. In brief, propensity scores were estimated using logistic regression of select covariates and matched using nearest neighbor matching with a caliper of 0.2 (defined by units of SDs of the logit of the estimated propensity scores). The PSM was performed using the following covariates: age (<65 or ≥65 years), sex, clinical T stage (1 or 2), clinical N stage (0 or 1-2), smoking status (dichotomized by a 10 pack-year history cutoff), p16 reactivity, Charlson Comorbidity Index (CCI) score (<5 or ≥5), and BMI (<18.5, 18.5-24.9, and ≥25.0). All statistical analyses and graph construction were performed using SPSS statistical software, version 25 (IBM Corp).
Results
Baseline Patient Characteristics
A total of 245 patients met inclusion criteria for this study. Of these 245 patients, 209 (85.3%) identified as men and 36 (14.7%) identified as women, with a mean (SD) age of 62.3 (7.8) years (Table 1). All 142 patients who underwent surgery and were included in the study had transoral resections with neck dissection, with 127 of those operations being robot assisted. Patients’ smoking status was dichotomized as less than or greater than or equal to a 10 pack-year history, with 158 patients (64.5%) reporting less than a 10 pack-year history and 87 patients (35.5%) reporting greater than or equal to a 10 pack-year history. Using p16 reactivity as a surrogate for HPV-positive disease, 28 of 225 patients (12.4%) had p16-negative disease and 197 of 225 patients (87.6%) had p16-positive disease. For the entire study, 135 patients (55.1%) were sarcopenic and 110 (44.9%) were not. A CCI score was calculated for each patient as previously described,17 and patients were stratified as either low risk (CCI, <5; 211 [86.1%]) and high risk (CCI, ≥5; 34 [13.9%]). Most patients in both groups (172 [70.2%] for the entire study) were overweight, with a BMI greater than or equal to 25. Baseline characteristics for the entire study are shown in Table 1. Of the 142 patients treated by primary surgical resection, 43 went on to receive adjuvant RT and 48 received adjuvant chemoradiotherapy. Of the 103 patients treated by primary RT, 83 also received concurrent chemotherapy.
Table 1. Demographic Characteristics of Patients.
| Characteristic | Patients, No. (%) | OR (95% CI) | ||
|---|---|---|---|---|
| Surgery (n = 142) | Radiotherapy (n = 103) | Total (N = 245) | ||
| Age, mean (SD), y | 62.1 (7.5) | 62.5 (8.2) | 62.3 (7.8) | |
| <65 | 107 (75.4) | 60 (58.3) | 167 (68.2) | 2.2 (1.2-3.7) |
| ≥65 | 35 (24.6) | 43 (41.7) | 78 (31.8) | 1 [Reference] |
| Sex | ||||
| Male | 111 (78.2) | 98 (95.1) | 209 (85.3) | 0.2 (0.07-0.5) |
| Female | 31 (21.8) | 5 (4.9) | 36 (14.7) | 1 [Reference] |
| Clinical tumor stage | ||||
| 1 | 62 (43.7) | 33 (32.0) | 95 (38.8) | 1.6 (1.0-2.8) |
| 2 | 80 (56.3) | 70 (68.0) | 150 (61.2) | 1 [Reference] |
| Clinical nodal stage | ||||
| 0 | 27 (19.0) | 7 (6.8) | 34 (13.9) | 3.2 (1.3-7.7) |
| 1-2 | 115 (81.0) | 96 (93.2) | 211 (86.1) | 1 [Reference] |
| Smoking, pack-years | ||||
| <10 | 108 (76.1) | 50 (48.5) | 158 (64.5) | 3.4 (2.0-5.8) |
| ≥10 | 34 (23.9) | 53 (51.5) | 87 (35.5) | 1 [Reference] |
| HPV status | ||||
| Negative | 18/137 (13.1) | 10/88 (11.4) | 28/225 (12.4) | 1.2 (0.5-2.7) |
| Positive | 119/137 (86.9) | 78/88 (88.6) | 197/225 (87.6) | 1 [Reference] |
| Sarcopenic | ||||
| No | 71 (50.0) | 39 (37.9) | 110 (44.9) | 1.7 (1.0-2.8) |
| Yes | 71 (50.0) | 64 (62.1) | 135 (55.1) | 1 [Reference] |
| CCI score | ||||
| <5 | 124 (87.3) | 87 (84.5) | 211 (86.1) | 1.3 (0.6-2.6) |
| ≥5 | 18 (12.7) | 16 (15.5) | 34 (13.9) | 1 [Reference] |
| Body mass indexa | ||||
| <18.5 | 4 (2.8) | 5 (4.9) | 9 (3.7) | 1.7 (0.4-6.4) |
| 18.5-24.9 | 40 (28.2) | 24 (23.3) | 64 (26.1) | 0.8 (0.4-1.4) |
| ≥25 | 98 (69.0) | 74 (71.8) | 172 (70.2) | 1 [Reference] |
Abbreviations: CCI, Charlson Comorbidity Index; HPV, human papilloma virus p16 variant; OR, odds ratio.
Calculated as weight in kilograms divided by height in meters squared.
We then analyzed the sarcopenic subset of patients before and after PSM. Table 2 details the baseline characteristics of sarcopenic patients treated by either primary surgery or definitive RT before and after PSM to control for important survival covariates. Prior to PSM, the primary surgery and definitive RT groups were clinically comparable in the following variables: age, N stage, HPV status, CCI score, and BMI. After PSM, a comparison of the primary surgery and definitive RT groups did not show clinically meaningful differences with respect to age, sex, tumor stage, nodal stage, smoking status, HPV status, CCI score, and BMI.
Table 2. Demographic Characteristics of Patients With Sarcopenia Before and After Propensity Score Matching.
| Characteristic | Patients before propensity score matching, No. (%) | OR (95% CI) | Patients after propensity score matching, No. (%) | OR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Surgery (n = 71) | Radiotherapy (n = 64) | Total (N = 135) | Surgery (n = 42) | Radiotherapy (n = 42) | Total (N = 84) | |||
| Age, mean (SD), y | 62.4 (8.6) | 61.9 (8.1) | 62.2 (8.4) | NA | 62.1 (7.5) | 62.5 (8.2) | 62.3 (7.8) | NA |
| <65 | 44 (62.0) | 48 (75.0) | 92 (68.1) | 0.5 (0.3-1.1) | 29 (69.0) | 32 (76.2) | 61 (72.6) | 0.7 (0.3-1.8) |
| ≥65 | 27 (38.0) | 16 (25.0) | 43 (31.9) | 1 [Reference] | 13 (31.0) | 10 (23.8) | 23 (27.4) | 1 [Reference] |
| Sex | ||||||||
| Male | 59 (83.1) | 61 (95.3) | 120 (88.9) | 0.2 (0.1-0.9) | 34 (81.0) | 40 (95.2) | 74 (88.1) | 0.2 (0.04-1.1) |
| Female | 12 (16.9) | 3 (4.7) | 15 (11.1) | 1 [Reference] | 8 (19.0) | 2 (4.8) | 10 (11.9) | 1 [Reference] |
| Clinical tumor stage | ||||||||
| 1 | 34 (47.9) | 19 (29.7) | 53 (39.3) | 2.2 (1.1-4.4) | 16 (38.1) | 15 (35.7) | 31 (36.9) | 1.1 (0.5-2.7) |
| 2 | 37 (52.1) | 45 (70.3) | 82 (60.7) | 1 [Reference] | 26 (61.9) | 27 (64.3) | 53 (63.1) | 1 [Reference] |
| Clinical nodal stage | ||||||||
| 0 | 12 (16.9) | 4 (6.3) | 16 (11.9) | 3.1 (0.9-10.0) | 7 (16.7) | 4 (9.5) | 11 (13.1) | 1.9 (0.5-7.1) |
| 1-2 | 59 (83.1) | 60 (93.8) | 110 (88.1) | 1 [Reference] | 35 (83.3) | 38 (90.5) | 73 (86.9) | 1 [Reference] |
| Smoking status, pack-years | ||||||||
| <10 | 49 (69.0) | 25 (39.1) | 74 (54.8) | 3.5 (1.7-7.1) | 24 (57.1) | 25 (59.5) | 49 (58.3) | 0.9 (0.4-2.2) |
| ≥10 | 22 (31.0) | 39 (60.9) | 61 (45.2) | 1 [Reference] | 18 (42.9) | 17 (40.5) | 35 (41.7) | 1 [Reference] |
| HPV status | ||||||||
| Negative | 8/68 (11.8) | 10/57 (17.5) | 18/125 (14.4) | 0.62 (0.3-1.7) | 8 (19.0) | 6 (14.3) | 14 (16.7) | 1.6 (0.5-5.1) |
| Positive | 60/68 (88.2) | 47/57 (82.5) | 107/125 (85.6) | 1 [Reference] | 34 (81.0) | 36 (85.7) | 70 (83.3) | 1 [Reference] |
| CCI score | ||||||||
| <5 | 59 (83.1) | 58 (90.6) | 117 (86.7) | 0.5 (0.2-1.4) | 37 (88.1) | 37 (88.1) | 74 (88.1) | 1.0 (0.3-3.7) |
| ≥5 | 12 (16.9) | 6 (9.4) | 18 (13.3) | 1 [Reference] | 5 (11.9) | 5 (11.9) | 10 (11.9) | 1 [Reference] |
| Body mass indexa | ||||||||
| <18.5 | 3 (4.2) | 4 (6.3) | 7 (5.2) | 1.3 (0.3-6.0) | 1 (2.4) | 1 (2.4) | 2 (2.4) | 0.9 (0.1-14.4) |
| 18.5-24.9 | 31 (43.7) | 21 (32.8) | 52 (38.5) | 0.6 (0.3-1.3) | 18 (42.9) | 14 (33.3) | 32 (38.1) | 0.7 (0.3-1.6) |
| ≥25 | 37 (52.1) | 39 (60.9) | 76 (56.3) | 1 [Reference] | 23 (54.8) | 27 (64.3) | 50 (59.5) | 1 [Reference] |
Abbreviations: CCI, Charlson Comorbidity Index; HPV, human papilloma virus p16 variant; NA, not applicable; OR, odds ratio.
Calculated as weight in kilograms divided by height in meters squared.
Sarcopenia and Survival by Primary Treatment Modality
Kaplan-Meier analysis of overall survival and disease-specific survival was performed for patients treated by either primary surgery or definitive RT and stratified by the presence or absence of sarcopenia (Figure 1). For nonsarcopenic patients, both overall survival and disease-specific survival were not statistically different between groups for patients treated by either primary surgical resection or definitive RT (Figure 1A and B). Similarly, there was no overall survival benefit observed for nonsarcopenic patients with HPV-positive disease treated by either surgical resection or definitive RT (Figure 1C). Prior to PSM, sarcopenic patients treated with up-front surgical resection demonstrated increased overall survival (HR, 0.37; 95% CI, 0.17-0.82) and disease-specific survival (HR, 0.22; 95% CI, 0.07-0.68). This survival benefit was also observed when controlling for HPV status, as sarcopenic patients with HPV-positive disease demonstrated increased overall survival when treated by primary surgical resection. After PSM, these survival trends persisted for patients with sarcopenia, as patients treated with primary surgical resection demonstrated an increase in overall survival (HR, 0.33; 95% CI, 0.12-0.91) and disease-specific survival (HR, 0.17; 95% CI, 0.04-0.75) (Figure 2). On univariable analysis of the matched sarcopenic patients, less than 10 pack-year smoking history (HR, 0.16; 95% CI, 0.05-0.50), negative HPV status (HR, 3.55; 95% CI, 1.30-10.25), BMI less than 18.5 (HR, 18.04; 95% CI, 3.62-89.96), and primary surgical resection (HR, 0.33; 95% CI, 0.12-0.91) were factors associated with overall survival (Table 3). Multivariable analysis was then performed using covariates of smoking status, HPV status, CCI score, BMI, and primary treatment modality. This analysis demonstrated that less than 10 pack-year history smoking status (HR, 0.26; 95% CI, 0.08-0.86) and surgical resection (HR, 0.25; 95% CI, 0.08-0.81) were associated with improved overall survival, while negative HPV status (HR, 1.29; 95% CI, 1.09-3.15) and a BMI of less than 18.5 (HR, 19.59; 95% CI, 2.64-145.55) were negatively associated with overall survival.
Figure 1. Survival Among Patients With or Without Sarcopenia.
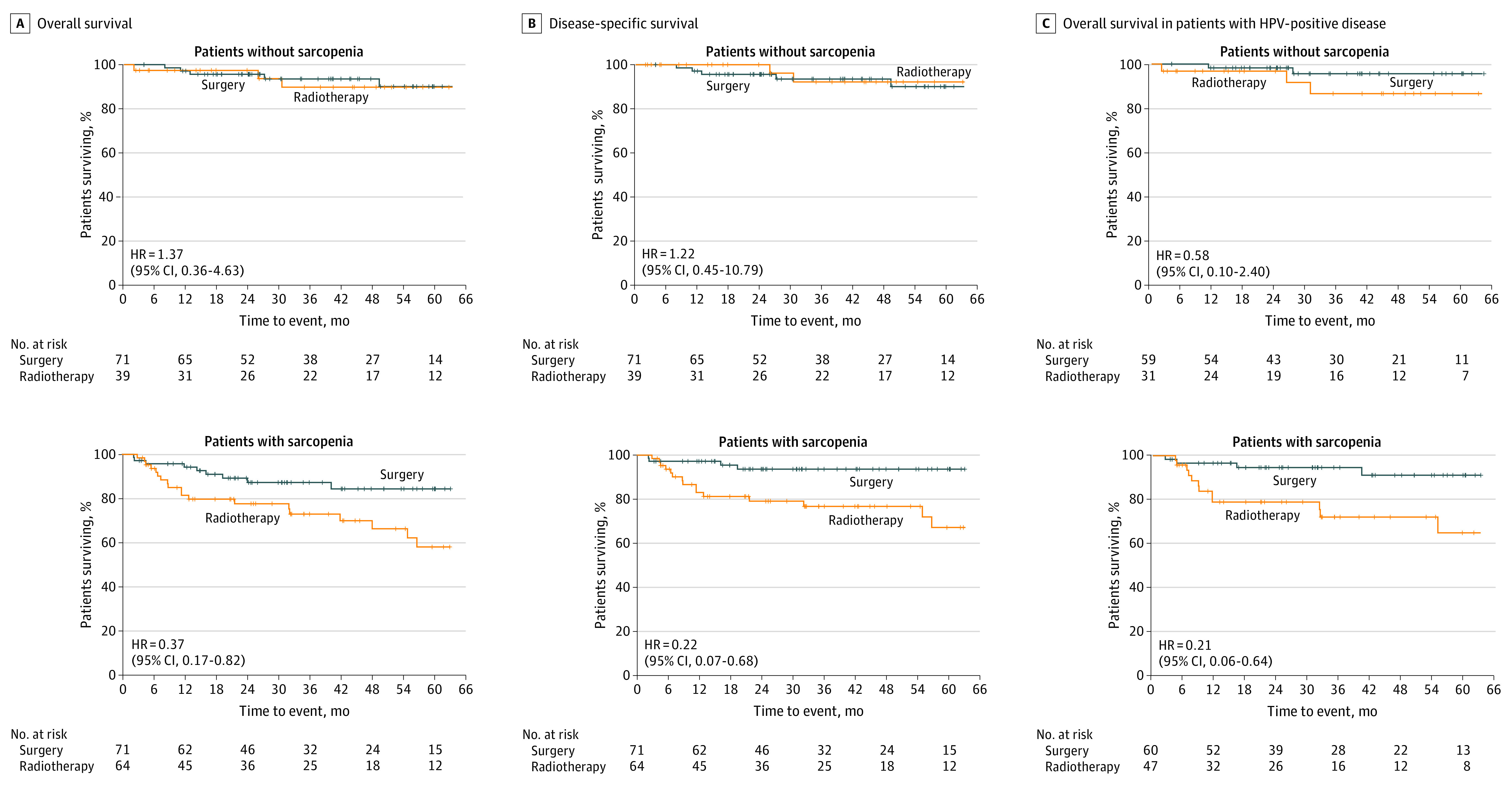
A, Overall survival for sarcopenic and nonsarcopenic patients treated by primary surgery or definitive radiotherapy. B, Disease-specific survival for sarcopenic and nonsarcopenic patients treated by primary surgery or definitive radiotherapy. C, Overall survival among patients with human papillomavirus (HPV)–positive disease with or without sarcopenia.
Figure 2. Survival Analysis in a Propensity Score–Matched Cohort of Patients With Sarcopenia.
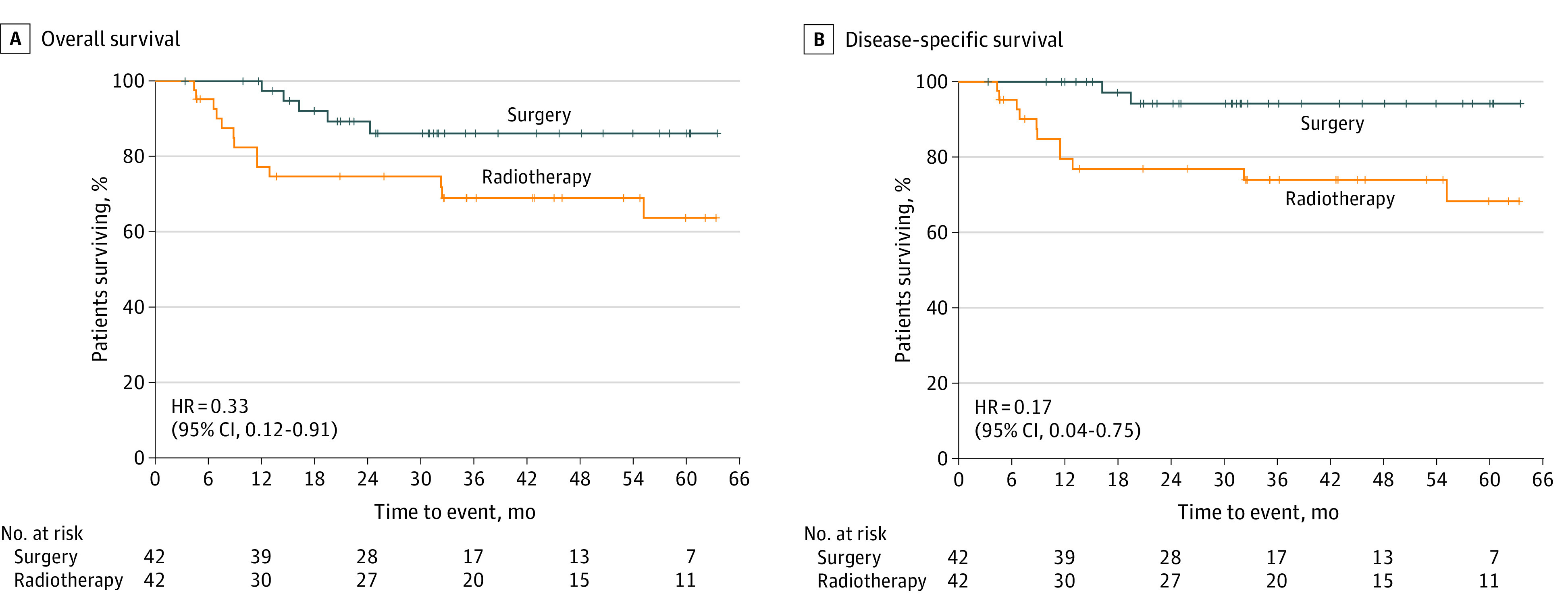
A, Overall survival for patients with sarcopenia treated by either primary surgery or definitive radiotherapy. B, Disease-specific survival for patients with sarcopenia treated by either primary surgery or definitive radiotherapy.
Table 3. Univariable and Multivariable Analyses.
| Analysis | Hazard ratio (95% CI) |
|---|---|
| Univariable analysis | |
| Age, y | |
| <65 | 0.15 (0.02-1.99) |
| ≥65 | 1 [Reference] |
| Sex | |
| Male | 0.39 (0.05-2.90) |
| Female | 1 [Reference] |
| Clinical tumor stage | |
| 1 | 0.92 (0.36-2.35) |
| 2 | 1 [Reference] |
| Clinical nodal stage | |
| 0 | 0.62 (0.14-2.71) |
| 1-2 | 1 [Reference] |
| Smoking status, pack-years | |
| <10 | 0.16 (0.05-0.50) |
| ≥10 | 1 [Reference] |
| HPV status | |
| Negative | 3.55 (1.30-10.25) |
| Positive | 1 [Reference] |
| CCI score | |
| <5 | 0.49 (0.19-1.21) |
| ≥5 | 1 [Reference] |
| Body mass indexa | |
| <18.5 | 18.04 (3.62-89.96) |
| 18.5-24.9 | 1.83 (0.71-4.75) |
| ≥25 | 1 [Reference] |
| Primary treatment | |
| Surgery | 0.33 (0.12-0.91) |
| RT | 1 [Reference] |
| Multivariable analysis | |
| Smoking status, pack-years | |
| <10 | 0.26 (0.08-0.86) |
| ≥10 | 1 [Reference] |
| HPV status | |
| Negative | 1.29 (1.09-3.15) |
| Positive | 1 [Reference] |
| CCI score | |
| <5 | 0.84 (0.09-7.54) |
| ≥5 | 1 [Reference] |
| Body mass indexa | |
| <18.5 | 19.59 (2.64-145.55) |
| 18.5-24.9 | 1.76 (0.65-4.73) |
| ≥25 | 1 [Reference] |
| Primary treatment | |
| Surgery | 0.25 (0.08-0.81) |
| RT | 1 [Reference] |
Abbreviations: CCI, Charlson Comorbidity Index; HPV, human papilloma virus p16 variant; RT, radiotherapy.
Calculated as weight in kilograms divided by height in meters squared.
Discussion
It is well established that skeletal muscle depletion is a significant indicator of surgical and oncologic outcomes across numerous cancer types,18,19,20,21 with a more recent identification of its prognostic utility in head and neck cancer treated by either primary surgical resection or definitive RT.8,9,10 However, to our knowledge, there are no published reports evaluating survival for sarcopenic patients treated by either up-front surgical resection or definitive RT in any oncologic setting. Thus, this study sought to evaluate if pretreatment sarcopenia is associated with a differential survival benefit for patients with localized OPSCC treated by either primary surgical resection or definitive RT, as this disease is currently treated by either primary treatment modality, with similar overall survival and recurrence-free survival.11,12 To address this question, we identified 245 patients with localized OPSCC at clinical diagnosis and performed CT imaging analysis to identify patients with and without sarcopenia. We found that patients with normal skeletal musculature at diagnosis demonstrated no difference in overall survival and disease-specific survival when treated by either primary surgery or definitive RT; however, when analyzing patients with sarcopenia at diagnosis, up-front surgical resection was associated with improved overall survival and disease-specific survival in this study.
In the literature, the estimates of sarcopenia in patients with head and neck cancer fluctuates significantly, with reports ranging from 35% to nearly 80%.8,10 However, these reports vary in inclusion criteria and disease stage. In our study, inclusion criteria were limited to patients with clinically staged T1 to T2, N0 to N2 disease, and 55.1% of the population had sarcopenia at diagnosis. This value is consistent with previous reports demonstrating that skeletal muscle wasting could be present in nearly half of diagnosed cases of head and neck cancer.9,10,22 Despite these large fluctuations in the prevalence of sarcopenia in patients with head and neck cancer, it is clear that a large portion of patients with head and neck cancer present with sarcopenia at diagnosis. As current guidelines for patients with clinically staged T1 to T2, N0 to N2 OPSCC recommend a range of treatment options, including primary surgery with pathologically directed adjuvant therapy, definitive RT, concurrent chemoradiotherapy, induction chemotherapy and RT, or clinical trials, clinicians are continually in search of risk-stratification methods to best determine which patients are most likely to benefit from these varied treatment modalities. Thus, identification of novel risk factors that extend beyond tumor features have gained increased attention during the past decade, and include pretreatment measures of body composition, dietary patterns, and neurocognitive impairment.23,24,25,26
It is possible that sarcopenia is a consequence of other pathophysiological processes associated with cancer, including the unique biology of the primary tumor and the chronic disease–associated syndrome cachexia. In the case of the former, recent studies demonstrate the ability of HPV-positive cancers to secrete extracellular vesicles that increase tumor innervation and aggressiveness.27,28 Furthermore, solid tumors may be directly associated with skeletal muscle wasting through release of heat shock proteins and extracellular vesicles that activate catabolic signaling pathways.29,30 In this case, in which the primary tumor biology is directly associated with muscle catabolism, it is conceivable that sarcopenic patients would glean benefit from rapid surgical excision of the primary tumor. As mentioned previously, sarcopenia is also a sign of cachexia, a disease-associated metabolic syndrome that significantly reduces patients’ quality of life and ultimate survival. Cachexia is a multiorgan wasting syndrome in which patients experience decreased appetite, yet have a paradoxical increase in basal metabolic rate.31,32 Discriminating between simple nutritional deficiencies and cachexia in the clinic is especially challenging in patients with head and neck cancer because the primary tumor location is often directly positioned in the aerodigestive tract. As a result, determining the causative mechanism of sarcopenia (eg, cachexia, malnutrition, dysphagia, or old age) may lead to clearer risk-stratification methods for sarcopenic patients. Collectively, these data and more suggest that nutritional interventions for patients with sarcopenia would be beneficial, independent of the underlying cause of muscle wasting.
Taken together, these results highlight the unique biological state of the sarcopenic patient, and suggest that patients with excessive muscle loss at diagnosis may differentially respond to treatment modalities compared with their nonsarcopenic counterparts. There are many plausible explanations for this differential effectiveness of primary surgery vs RT in sarcopenic patients. Baseline sarcopenia may limit the ability of patients with head and neck cancer to tolerate RT and chemoradiotherapy, as sarcopenic patients are more likely than nonsarcopenic patients to require treatment holidays.33,34 Similarly, the long-term outcomes of RT, such as dysphagia, xerostomia, and other issues, may be more significant for sarcopenic patients than nonsarcopenic patients, making surgery and subsequent de-escalation of RT and/or chemotherapy a more viable treatment paradigm in these patients. Although the recently published ORATOR (Radiotherapy Versus Transoral Robotic Surgery and Neck Dissection for Oropharyngeal Squamous Cell Carcinoma) trial demonstrated that patients receiving definitive RT had improved quality of life swallowing scores compared with patients who underwent transoral robotic surgery, the 1-year end point of this study may be too short to capture some of the long-term morbidities associated with RT, including dysphagia and aspiration.35 Alternatively, sarcopenia may be correlated with other host factors, such as reduced immune surveillance, that may render RT less effective. Further study into this observation will be required to better understand these factors and to test the utility of the clinical assessment of sarcopenia in clinical practice.
Limitations
As with any retrospective review, this study has important limitations to consider when interpreting the presented data. Retrospective studies are routinely subject to missing data and lack of patient follow-up data. Because abdominal CT imaging is not always performed during workup for head and neck cancer or outside imaging may not have been available for analysis, several patients were excluded from analysis owing to a lack of abdominal imaging. Thus, this selection of patients with abdominal imaging limits the overall power of the study and may introduce a selection bias. Additional treatment bias may be introduced as this was not a controlled study, and the choice of treatment modality was entirely up to the patients and treatment team. Similarly, precise clinical rationale on how or why a patient was selected for a given treatment modality was not easily captured in the health records. Finally, since this study was performed at a single tertiary care institution, care should be taken in interpreting these results, as they may not generalize to the broader head and neck oncology community.
Conclusions
Patients with OPSCC and depleted skeletal muscle mass, or sarcopenia, exhibit decreased overall survival and disease-specific survival, and patients with localized disease are routinely treated by primary surgical resection or definitive RT. To our knowledge, no published reports have investigated the association between primary treatment modality and oncologic outcomes in patients with baseline skeletal muscle wasting. In this single-institution study, we report that patients with normal skeletal musculature at diagnosis benefit equally from primary surgical resection or definitive RT. However, up-front ablative surgery for sarcopenic patients was associated with an increase in overall survival and disease-specific survival compared with their counterparts who received definitive RT. As such, these data suggest that sarcopenia may be a clinically significant variable when considering treatment modalities for patients with localized OPSCC.
References
- 1.Jager-Wittenaar H, Dijkstra PU, Vissink A, et al. Changes in nutritional status and dietary intake during and after head and neck cancer treatment. Head Neck. 2011;33(6):863-870. doi: 10.1002/hed.21546 [DOI] [PubMed] [Google Scholar]
- 2.Nejatinamini S, Debenham BJ, Clugston RD, et al. Poor vitamin status is associated with skeletal muscle loss and mucositis in head and neck cancer patients. Nutrients. 2018;10(9):E1236. doi: 10.3390/nu10091236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson W, Alexander N, Schipper M, Fig L, Feng F, Jolly S. Characterization of changes in total body composition for patients with head and neck cancer undergoing chemoradiotherapy using dual-energy x-ray absorptiometry. Head Neck. 2014;36(9):1356-1362. [DOI] [PubMed] [Google Scholar]
- 4.Ghadjar P, Hayoz S, Zimmermann F, et al. ; Swiss Group for Clinical Cancer Research (SAKK) . Impact of weight loss on survival after chemoradiation for locally advanced head and neck cancer: secondary results of a randomized phase III trial (SAKK 10/94). Radiat Oncol. 2015;10:21. doi: 10.1186/s13014-014-0319-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;22(12):1164-1171. doi: 10.1038/sj.ijo.0800741 [DOI] [PubMed] [Google Scholar]
- 6.Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539-1547. doi: 10.1200/JCO.2012.45.2722 [DOI] [PubMed] [Google Scholar]
- 7.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629-635. doi: 10.1016/S1470-2045(08)70153-0 [DOI] [PubMed] [Google Scholar]
- 8.Grossberg AJ, Chamchod S, Fuller CD, et al. Association of body composition with survival and locoregional control of radiotherapy-treated head and neck squamous cell carcinoma. JAMA Oncol. 2016;2(6):782-789. doi: 10.1001/jamaoncol.2015.6339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone L, Olson B, Mowery A, et al. Association between sarcopenia and mortality in patients undergoing surgical excision of head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2019;145(7):647-654. doi: 10.1001/jamaoto.2019.1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Achim V, Bash J, Mowery A, et al. Prognostic indication of sarcopenia for wound complication after total laryngectomy. JAMA Otolaryngol Head Neck Surg. 2017;143(12):1159-1165. doi: 10.1001/jamaoto.2017.0547 [DOI] [PubMed] [Google Scholar]
- 11.Pedro C, Mira B, Silva P, et al. Surgery vs. primary radiotherapy in early-stage oropharyngeal cancer. Clin Transl Radiat Oncol. 2017;9:18-22. doi: 10.1016/j.ctro.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly JR, Park HS, An Y, et al. Comparison of survival outcomes among human papillomavirus-negative cT1-2 N1-2b patients with oropharyngeal squamous cell cancer treated with upfront surgery vs definitive chemoradiation therapy: an observational study. JAMA Oncol. 2017;3(8):1107-1111. doi: 10.1001/jamaoncol.2016.5769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly JR, Park HS, An Y, et al. Upfront surgery versus definitive chemoradiotherapy in patients with human papillomavirus–associated oropharyngeal squamous cell cancer. Oral Oncol. 2018;79:64-70. doi: 10.1016/j.oraloncology.2018.02.017 [DOI] [PubMed] [Google Scholar]
- 14.Amin MB, Edge S, Greene F, et al, eds. AJCC Cancer Staging Manual. 8th ed Springer International Publishing; 2017. [Google Scholar]
- 15.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(5):997-1006. doi: 10.1139/H08-075 [DOI] [PubMed] [Google Scholar]
- 16.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985). 1998;85(1):115-122. doi: 10.1152/jappl.1998.85.1.115 [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 18.da Cunha LP, Silveira MN, Mendes MCS, et al. Sarcopenia as an independent prognostic factor in patients with metastatic colorectal cancer: a retrospective evaluation. Clin Nutr ESPEN. 2019;32:107-112. doi: 10.1016/j.clnesp.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 19.Ninomiya G, Fujii T, Yamada S, et al. Clinical impact of sarcopenia on prognosis in pancreatic ductal adenocarcinoma: A retrospective cohort study. Int J Surg. 2017;39:45-51. doi: 10.1016/j.ijsu.2017.01.075 [DOI] [PubMed] [Google Scholar]
- 20.Begini P, Gigante E, Antonelli G, et al. Sarcopenia predicts reduced survival in patients with hepatocellular carcinoma at first diagnosis. Ann Hepatol. 2017;16(1):107-114. doi: 10.5604/16652681.1226821 [DOI] [PubMed] [Google Scholar]
- 21.Iritani S, Imai K, Takai K, et al. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J Gastroenterol. 2015;50(3):323-332. doi: 10.1007/s00535-014-0964-9 [DOI] [PubMed] [Google Scholar]
- 22.Wendrich AW, Swartz JE, Bril SI, et al. Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol. 2017;71:26-33. doi: 10.1016/j.oraloncology.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 23.Olson B, Marks DL. Pretreatment cancer-related cognitive impairment-mechanisms and outlook. Cancers (Basel). 2019;11(5):687. doi: 10.3390/cancers11050687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hshieh TT, Jung WF, Grande LJ, et al. Prevalence of cognitive impairment and association with survival among older patients with hematologic cancers. JAMA Oncol. 2018;4(5):686-693. doi: 10.1001/jamaoncol.2017.5674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown JC, Cespedes Feliciano EM, Caan BJ. The evolution of body composition in oncology-epidemiology, clinical trials, and the future of patient care: facts and numbers. J Cachexia Sarcopenia Muscle. 2018;9(7):1200-1208. doi: 10.1002/jcsm.12379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crowder SL, Sarma KP, Mondul AM, et al. Pretreatment dietary patterns are associated with the presence of nutrition impact symptoms 1 year after diagnosis in patients with head and neck cancer. Cancer Epidemiol Biomarkers Prev. 2019;28(10):1652-1659. doi: 10.1158/1055-9965.EPI-19-0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madeo M, Colbert PL, Vermeer DW, et al. Cancer exosomes induce tumor innervation. Nat Commun. 2018;9(1):4284. doi: 10.1038/s41467-018-06640-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucido CT, Wynja E, Madeo M, et al. Innervation of cervical carcinoma is mediated by cancer-derived exosomes. Gynecol Oncol. 2019;154(1):228-235. doi: 10.1016/j.ygyno.2019.04.651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang G, Liu Z, Ding H, et al. Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat Commun. 2017;8(1):589. doi: 10.1038/s41467-017-00726-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Zhang Z, Zhang Y, et al. ZIP4 promotes muscle wasting and cachexia in mice with orthotopic pancreatic tumors by stimulating RAB27B-regulated release of extracellular vesicles from cancer cells. Gastroenterology. 2019;156(3):722-734. doi: 10.1053/j.gastro.2018.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grossberg AJ, Scarlett JM, Marks DL. Hypothalamic mechanisms in cachexia. Physiol Behav. 2010;100(5):478-489. doi: 10.1016/j.physbeh.2010.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4:17105. doi: 10.1038/nrdp.2017.105 [DOI] [PubMed] [Google Scholar]
- 33.Ganju RG, Morse R, Hoover A, TenNapel M, Lominska CE. The impact of sarcopenia on tolerance of radiation and outcome in patients with head and neck cancer receiving chemoradiation. Radiother Oncol. 2019;137:117-124. doi: 10.1016/j.radonc.2019.04.023 [DOI] [PubMed] [Google Scholar]
- 34.Cho Y, Kim JW, Keum KC, Lee CG, Jeung HC, Lee IJ. Prognostic significance of sarcopenia with inflammation in patients with head and neck cancer who underwent definitive chemoradiotherapy. Front Oncol. 2018;8:457-457. doi: 10.3389/fonc.2018.00457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nichols AC, Theurer J, Prisman E, et al. Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): an open-label, phase 2, randomised trial. Lancet Oncol. 2019;20(10):1349-1359. doi: 10.1016/S1470-2045(19)30410-3 [DOI] [PubMed] [Google Scholar]


