Abstract
Inherited retinal dystrophies are an assorted group of rare diseases that collectively account for the major cause of visual impairment of genetic origin worldwide. Besides clinically, these vision loss disorders present a high genetic and allelic heterogeneity. To date, over 250 genes have been associated to retinal dystrophies with reported causative variants of every nature (nonsense, missense, frameshift, splice-site, large rearrangements, and so forth). Except for a fistful of mutations, most of them are private and affect one or few families, making it a challenge to ratify the newly identified candidate genes or the pathogenicity of dubious variants in disease-associated loci. A recurrent option involves altering the gene in in vitro or in vivo systems to contrast the resulting phenotype and molecular imprint. To validate specific mutations, the process must rely on simulating the precise genetic change, which, until recently, proved to be a difficult endeavor. The rise of the CRISPR/Cas9 technology and its adaptation for genetic engineering now offers a resourceful suite of tools to alleviate the process of functional studies. Here we review the implementation of these RNA-programmable Cas9 nucleases in culture-based and animal models to elucidate the role of novel genes and variants in retinal dystrophies.
Keywords: retinal diseases, gene editing, CRISPR, cellular models, animal models, variants of unknown significance, functional studies, variant validation
1. Introduction
Inherited Retinal Dystrophies (IRDs) comprise of a diverse group of vision loss diseases of genetic origin, generally due to the progressive death of the photoreceptors. Individually, these diseases have a low incidence among the population, yet together they present a prevalence of about 1 in 3000 [1].
The clinical classification of these disorders is based on the particular type of cells that are primarily affected (cones, rods, retinal pigment epithelium or inner retinal cells) giving rise to the distinctive clinical manifestation. Typical symptoms may include a reduction of the visual field (central or peripheral), visual acuity, color perception, night vision, or photophobia. However, the presentation of these clinical traits is characteristic of each specific disorder, even though some of the phenotypes display certain overlapping of the features [2,3,4]. Some of the most common IRDs are Retinitis Pigmentosa (RP), Stargardt Disease (STGD), Leber Congenital Amaurosis (LCA), and even syndromic forms such as Usher Syndrome (USH).
IRDs are genetically very heterogeneous since there are at least 271 genes currently associated to one or more of the diseases comprised in this group (according to RetNet, accessed March 2020). Moreover, several types of inheritance patterns can be found among this set of eye disorders, including autosomal recessive, autosomal dominant, X-linked, mitochondrial, and even some digenic forms have been proposed [5]. In addition, there is a wide mutational spectrum for these diseases, being most pathogenic variants private, with only a few exceptions having a higher representation, such as the p.Glu767Serfs*21 and p.Cys759Phe in USH2A; p.Gly1961Glu in ABCA4; p.Pro347Leu in RHO, or p.Cys998* (c.2991+1655A>G) in CEP290 [6,7,8,9,10,11]. Furthermore, many of the genes (and mutations) associated with IRDs present inter- and intra-family phenotypic variability, with some genes even showing incomplete penetrance [12,13].
All these factors make both the clinical and genetic final diagnosis of IRDs cases challenging. To date, the gold-standard for the genetic characterization of patients is by means of high-throughput sequencing (HTS), usually through custom designs targeting known associated genes [14,15]. This method renders a considerable number of novel genetic variants whose clinical interpretation is initially assessed with in silico prediction software, segregation analysis, concurrence in other affected families and prevalence among the general population. However, often these mutations (particularly missense) remain officially classified as variants of unknown significance (VUS) or, at most, as possibly pathogenic. Therefore, on many occasions, functional assays are also expected to confirm the deleteriousness of a proposed variant, even more if a new candidate gene is involved.
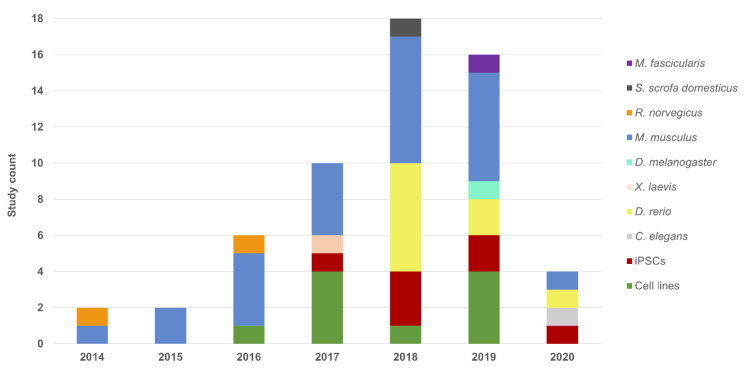
In this review, we address the CRISPR system as an asset for functional validation of VUS and for deepening into the pathogenesis role of known and novel IRD genes, given it is the increased interest in the field (Figure 1).
Figure 1.
Chart of Inherited Retinal Dystrophies (IRD) related studies using the CRISPR technology for variant interpretation and disease modeling. A growing trend in the number of works that use this editing system is observed from the year 2014 to the first quarter of 2020, as well as a diversification in terms of the models used.
2. The CRISPR Toolkit—Initial Steps
The CRISPR system has overturned research on life sciences as a whole, given its broad applicability in many areas of biology. Even though the method represents a major breakthrough for potential therapeutic-aimed gene-editing techniques, it has also transformed the basic research field just by simplifying and speeding up the process of site-directed mutagenesis.
The CRISPR system (in its simplest form) relies on two main components, namely the Cas9 protein and a guide RNA (gRNA), which together form a complex that specifically targets a desired DNA locus [16]. This happens due to the hybridization of the complementary sequence of the gRNA that steers the Cas9 endonuclease towards the precise spot.
Hence, the design of this sequence is pivotal for the edition outcome and it must adhere to certain guidelines. First, in order for the nuclease to be able to recognize and attach to the pertinent locus, a particular short nucleotide string must be adjacent to the gRNA-hybridization sequence in the host genome: the protospacer adjacent motif (PAM). The Cas9 from Streptococcus pyogenes (SpCas9) is the most commonly used and it requires a 3′ NGG motif. However, there are now many other nuclease versions either genetically engineered or coming from other organisms, such as Cpf1 or xCas9, that require different PAMs and feature other distances to the cleave point, thereby expanding the range of target possibilities [17,18,19]. Second, the choice of gRNA sequence, which should be 18–25 nt long, is determinant for the on-target efficiency and specificity, this latter translated as the potential of the fragment to mate with highly similar sequences throughout the genome (off-targets) producing unwanted DNA modifications. Fortunately, there is an increasing availability of computational tools that aid in the optimization of the design [20], as well as updated databases of these resources keeping pace with their rapid evolution [21].
Following site recognition, the nuclease cleaves the DNA and the resulting double-stranded break (DSB) is subsequently repaired mainly by two possible cellular mechanisms. The most prevalent pathway is the non-homologous end-joining repair (NHEJ), in which the cleaved ends are simply re-ligated. This is an error-prone mechanism that leaves small indels around the breaking point, due to some end resection of the strands and/or nucleotide additions [22]. Nonetheless, this somehow uncontrolled mutational outcome is of great profit when aiming for an easy and rapid generation of a knock-out or for gene disruption starting at specific points and indifferent to the following untranscribable sequence. Even more, the dual induction of DSBs using pairs of CRISPR complexes can readily replicate large structural variants, such as deletions, insertions, or translocations [23,24]. This might be the preferred strategy to confirm new candidate genes or to evaluate the specific role of its encoding protein in the molecular mechanisms of a certain cell or tissue.
The other method is known as the homology-directed repair (HDR), in which the damage is resolved through homologous recombination if a template is available; yet, even under these conditions, NHEJ still exceeds HDR. This is the method harnessed to generate precise nucleotide changes in the target DNA sequence by providing a carefully designed template, which can serve as means to either correct or introduce the underlying genetic defect of a disease. Most of the strategies used to knock-in make use of single-stranded oligonucleotides (ssODNs) or plasmids with long homologous arms to the region and the desired changes in the core as a repair template [25]. This precise method is the one usually employed to assess the impact of specific DNA changes presumed to be disease-causing.
Both the NHEJ and HDR pathways allow the directed modification of a specific DNA site while preserving the rest of the genome intact. Thus, the two CRISPR-induced strategies can be used to evaluate the pathogenicity of variants by enabling the fast generation of animal or cell/tissue-based models (as will be next discussed), spanning from days in the case of cell systems and simpler organisms like worms or flies, to weeks in vertebrate models and complex tissue-mimicking in vitro models. The strategies to address the distinct genes and mutations can vary significantly according to the appertaining form of IRDs (Table 1), just as the delivery methods differ depending on the type of targeted cells or organism (Table 2).
Table 1.
Studies using the CRISPR technology for functional validation of variants of disease modeling.
| Model | Phenotype | Gene | Genomic Target | Aim | Delivery Method | Nuclease | Reference |
|---|---|---|---|---|---|---|---|
| H HeLa | adRP | RHO | p.Pro23His | KO | Lipofection (plasmid) | SpCas9 | [34] |
| H HEK293FT | LCA | CEP290 | p.Cys998* | KI | Lipofection (plasmid) | SpCas9 | [35] |
| H HEK293 | USH2/arRP | USH2A | p.Glu767Serfs*21 | KI | Lipofection (plasmid) | SpCas9 | [36] |
| p.Cys759Phe | |||||||
| M 661W | adRP | Rp9 | Exon 5 | KO | Fugene HD (plasmid) | SpCas9 | [37] |
| p.His137Leu | KI | ||||||
| H hTERT-RPE1 | xlRP | RP2 | Exon 2 | KO | Fugene HD (plasmid) | nCas9 pairs | [38] |
| RPGR | Exons 2 and 4 | KO | Undetermined | uCas9 | [39] | ||
| sarRP | PDE6D | Exon 2 | |||||
| INPP5E | Exon 1 | ||||||
| arCORD | RPGRIP1L | Exon 3 | |||||
| M NSC | arRP | Pde6b | p.Arg560Cys | C KI | Nucleofection (plasmid) | SpCas9 | [40] |
| rd12 MEFs | LCA | Rpe65 | p.Arg44* | C KI | Electroporation (RNPs) | SpCas9 | [41] |
| PD-Fibroblasts | USH2/arRP | USH2A | p.Glu767Serfs*21 | C KI | Nucleofection (RNPs) | SpCas9 | [36] |
| PD-Keratinocytes | SHRF | EXOSC2 | Exon 1 and 4 | KO | Lentiviral transduction | SpCas9 | [42] |
| PD-iPSCs | arRP | MAK | c.1513ins353 | C KI | Nucleofection (plasmid) | SpCas9 | [43] |
| LCA | CEP290 | p.Cys998* | KO | ||||
| KO | Electroporation (plasmid) | SaCas9 | |||||
| C KI | |||||||
| adRP | RHO | p.Pro23His | KO | ||||
| C KI | Undetermined (plasmid) | SpCas9 | |||||
| PRPF31 | p.Arg372Glnfs*99 | C KI | Lipofection (plasmid) | SpCas9 | [44] | ||
| Exon 7 | KO | Nucleofection (plasmid) | SpCas9 | [45] | |||
| PRPF8 | p.Pro2301Ser | C KI | Electroporation (gRNA-plasmid and Cas9 mRNA) | Cas9-Gem | [46] | ||
| xlRP | RPGR | p.His562Argfs*20 | KI | Electroporation (plasmid) | SpCas9 | [47] | |
| ESCS | NR2E3 | p.Val41Alafs*23 | KI | Lipofection (plasmid) | SpCas9 | [48] | |
| p.Arg73Ser | KI | Electroporation (plasmid) | |||||
| USH2/arRP | USH2A | p.Glu767Serfs*21 p.Cys759Phe |
KI | Nucleofection (plasmid) | eSpCas9 | [49] | |
| XLRS | RS1 | p.Arg209Cys | KI | Nanodiamonds (linear DNA) | SpCas9 | [50] | |
|
Caenorhabditis elegans (nematode) |
adRP | prp-8 | p.Arg2310Gly | KI | Injection (RNPs) | SpCas9 | [51] |
| p.His2309del | |||||||
| snrp-200 | p.Val683Leu | ||||||
| p.Ser1087Leu | |||||||
|
Danio rerio (zebrafish) |
arRP | eys | p.Gly1163Valfs*14 | KO | Embryo injection (RNPs) | SpCas9 | [52] |
| pcare | p.Gly8Glu*19 | KO | Embryo injection (RNPs) | SpCas9 | [53] | ||
| adRP | rho | p.Cys322Argfs*116 | KO | Embryo injection (Cas9 mRNA and gRNAs) | SpCas9 | [54] | |
| LCA | cct2 | p.Leu394His-7del | KO | Embryo injection (RNPs) | SpCas9 | [55] | |
| USH2/arRP | ush2a | p.Cys780Glnfs*32 | KO | Embryo injection (Cas9 mRNA and gRNAs) | uCas9 | [56] | |
| p.Ala5174* | |||||||
| p.Lys2532Thrfs*56 | KI | Embryo injection (RNPs) | SpCas9 | [57] | |||
| ESCS | nr2e3 | p.Leu162Glnfs*30 | KO | Embryo injection (Cas9 mRNA and gRNAs) | uCas9 | [58] | |
| arCD | cacna2d4 | Undetermined | KO | Embryo injection (RNPs) | SpCas9 | [59] | |
| adFEVR | znf408 | p.His455Tyr | KI | Embryo injection (RNPs) | uCas9 | [60] | |
|
Mus musculus (mouse) |
Undetermined | Blimp1 | B108 cis-regulatory module | KO | Electroporation—subretinal injection (plasmid) | SpCas9 | [61] |
| LCA | Cep290 | p.Cys998* | KO | AAV transduction (subretinal injection) | SpCas9 | [35] | |
| Exon 3 | KO | AAV transduction (subretinal injection) | SpCas9 | [62] | |||
| Rpe65 | p.Asp477Gly | KI | Embryo injection (Cas9 mRNA and gRNAs) | SpCas9 | [63] | ||
| p.Arg44* | C KI | AAV transduction (subretinal injection) | SpCas9 | [41] | |||
| Kcnj13 | Exon 2 | KO | Zygote injection (Cas9 mRNA and gRNAs) | SpCas9 | [64] | ||
| OCA1 | Tyr | 5’ region | KO | Zygote injection (Cas9 mRNA and gRNAs) | SpCas9 | [65] | |
| Thy1-YFP | YFP | 5’ region | KO | AAV transduction (intravitreal injection) | SpCas9 | [66] | |
| arRP | Pde6b | p.Arg560Cys | KI | Electroporation—subretinal injection (plasmid) | SpCas9 | [40] | |
| p.Tyr347Ter | C KI | Embryo injection (gRNA-plasmid and Cas9 protein) | SpCas9 | [67] | |||
| Reep6 | p.Leu135Pro | KI | Embryo injection (Cas9 mRNA and gRNAs) | SpCas9 | [68] | ||
| Exon 4 | KO | Embryo injection (Cas9 mRNA and gRNAs) | SpCas9 | [69] | |||
| Arl2bp | Exon 2 | KO | Embryo injection (Cas9 mRNA and gRNAs) | SpCas9 | [70] | ||
| Hkdc1 | Exon 2 | KO | Undetermined (plasmid) | uCas9 | [71] | ||
| adRP | RHO | p.Pro23His | KO | Electroporation (plasmid) | SpCas9 | [34] | |
| Electroporation (plasmid) and AAV transduction (intravitreal injection) | SaCas9 and SaCas9-KKH | [72] | |||||
| Rho/RHO | Exon 1 | KO | AAV transduction (subretinal injection) | SpCas9 | [73] | ||
| arRP/sarRP | Cwc27 | p.Lys338Glyfs*25 | KO | Embryo injection (Cas9 mRNA and gRNAs) | SpCas9 | [74] | |
| arRP/adRP | Nrl | Undetermined | KO | AAV transduction (subretinal injection) | SpCas9 | [75] | |
| [76] | |||||||
| RD | Slc9a8 | Promoter | KO | AAV transduction (subretinal injection) | nmCas9 | [77] | |
| Usp45 | Exon 14 | KO | Embryo injection (Cas9 mRNA and gRNAs) | uCas9 | [78] | ||
| adCORD | Gucy2e | Exon 2 and 4 | KO | AAV transduction (subretinal injection) | SaCas9 | [79] | |
| Thy1-YFP | YFP | Undetermined | KO | AAV transduction (subretinal injection | SpCas9 | [80] | |
| XLRS | Rs1 | p.Arg209Cys | KI | Nanodiamonds—intravitreal injection (linear DNA) | SpCas9 | [50] | |
|
Rattus norvegicus (rat) |
OCA1 | Tyr | Exon 2 | KO | Embryo injection (Cas9 mRNA and gRNAs) | uCas9 | [81] |
| p.Arg299His | KI | ||||||
| adRP | Rho | p.Ser334Ter | KO | Electroporation—subretinal injection (plasmid) | SpCas9 | [82] | |
|
Xenopus laevis (frog) |
adRP | rho | Exon1 | KO | Embryo injection (Cas9 mRNA and gRNAs) | SpCas9 | [83] |
| Exon 5 | KI | ||||||
|
Drosophila melanogaster (fly) |
SHRF | rrp4 | Exon 1 and 4 | KO | Embryo injection (gRNA-plasmid into Cas9-expressing strain) | SpCas9 | [42] |
|
Sus scrofa domesticus (pig) |
adRP | RHO | p.Pro23His | KO | AAV transduction (subretinal injection) | SaCas9 | [43] |
|
Macaca fascicularis (macaque) |
adCORD | GUCY2D | Exon 2 and 4 | KO | AAV transduction (subretinal injection) | saCas9 | [79] |
KO: Knock-Out; KI: Knock-In; MEFs: Mouse Embryonic Fibroblasts; HeLa: Henrietta Lacks Cell Line (Uterine Cell Variety); HEK293: Human Embryonic Kidney 293 Cells; HEK293FT: Fast Growing HEK293 Line Variant; 66W: Immortalized Cone Photoreceptor Cell Line; hTERT-RPE1: Immortalized Retinal Pigment Epithelial Cell Line; NSC: Primary Cultures of Neural Stem Cells; eSpCas9: Enhanced Specificity SpCas9; nCas9: Cas9 Nickase; SpCas9: Cas9 (Streptococcus pyogenes); uCas9: Undetermined Cas9; SaCas9: Cas9 (Staphylococcus aureus); SpCas9-Gem: SpCas9 Fused to Human Geminin Protein; SaCas9-KKH: SaCas9 Recognizing NNNRRT PAMs; nmCas9: Cas9 (Neisseria meningitidis); AAV: Adeno-Associated Virus; PD: Patient-Derived; ad: Autosomal Dominant; ar: Autosomal Recessive; sar: Syndromic Autosomal Recessive; xl: X-Linked; RP: Retinitis Pigmentosa; RD: Retinal Degeneration; CD: Cone Dystrophy; USH2: Usher Syndrome Type 2; XLRS: X-Linked Juvenile Retinoschisis; SHRF: Short Stature, Hearing Loss, Retinitis Pigmentosa and Distinctive Facies Syndrome; FEVR: Familial Exudative Vitreoretinopathy; ESCS: Enhanced S-Cone Syndrome; CORD: Cone-Rod Dystrophy; OCA1: Oculocutaneous Albinism Type 1; LCA: Leber Congenital Amaurosis; Thy1-YFP: Transgenic Mice Expressing Yellow Fluorescent Protein; Symbols: H Human-origin cells; M Mouse-origin cells; C Correction-purpose.
Table 2.
Pros and cons of the CRISPR/Cas9 delivery methods described in this review.
| Method | Advantages | Disadvantages |
|---|---|---|
| Microinjection | Liberated right into the cell High efficacy |
Time-consuming Technique expertise |
| Electroporation | Normalized open-access protocols High effectiveness with plasmids |
In vitro and ex vivo cell restriction Cell cytotoxicity Not all cells are susceptible |
| Lipofection | Works in many cell types Easy manipulation Inexpensive Reduced off-targets |
Exclusive for cell culture Lysosome degradation |
| Nanodiamonds | Highly efficient delivery High biocompatibility Water solubility Fully accessible surface Inexpensive |
Genotoxicity High pressure and temperature for synthesis Tissue distribution problems |
| AAVs | Low immunogenicity and cytotoxicity Reduced off-targets High efficacy Low immune response detected Infect both dividing and non-dividing cells |
Limited cargo capacity (3.5–4 kb) High cost Technique expertise Safety obstacle Not easy to scale-up |
| Lentivirus | Expression stability Can be applied in a broad range of cell types Higher efficacy if constructs are shortened Low immune response detected Infect both dividing and non-dividing cells |
Limited cargo capacity (8–9 kb) Arbitrary integration Technique expertise Safety obstacle Not easy to scale-up |
| Microinjection delivery is based on the use of a 0.5–5.0 µm diameter needle to deliver components into a cell or intercellular space, in this case the Cas9 protein and sgRNAs in any form. The electroporation method requires high voltage currents with the purpose of opening nanopores in the cellular membrane to inlet the CRISPR components resuspended in a specific buffer. Nucleofection is a specific electroporation-based method that allows the direct entry of the components into the nucleus. Lipofection consists in the introduction of the DNA components via a liposome-based transfection, in which synthetic cationic lipids aggregate around the negatively-charged DNA molecules. Cellular uptake is based on the fusion of these liposome-like structures with the phospholipidic membrane. Nanodiamonds are carbon nanomaterials which are suspended in a colloidal solution that allow the binding with or coating of biological material for cell transfection, mainly penetrating by the clathrin-mediated endocytosis pathway. The viral transduction method leverages the natural potential of viruses to infect cells, where the vectors have been deprived of the essential pathogenic genes in their replication. The most commonly used are AAVs and lentivirus. AAVs, which consist of single-stranded DNA, present several serotype versions (allowing tissue-specific transduction) and are considered a safe option, given that they are not associated to human diseases showing low immunogenicity and entailing low cellular toxicity (as they do not integrate into the host genome). Lentiviruses are retroviruses derived from a provirus of HIV that proffer stable expression in both dividing and post-mitotic cells due to their host genome integration, and that additionally accommodate cargos up to 5–6 kb in size. | ||
Regarding the latter issue, not all means are suitable for every target type. The nuclease/gRNA can be delivered via (i) plasmids or viral carriers, which first need to be imported to the nucleus for the complex to be transcribed; (ii) as mRNA molecules, cytosolically translated; (iii) or as a pre-assembled ribonucleoprotein (RNP) enabling immediate action. Except when virally packaged, these forms can also be released using different techniques, such as electroporation or direct injection for naked delivery, lipofection, or with other coating organic and inorganic nanoparticles. RNPs and mRNA molecules are preferable, since their transient expression reduces off-target activity [26,27,28], and also because of the harmful potential of vectors, given the intrinsic cytotoxicity of plasmids and the DNA integration of some viral vehicles [29,30]. In addition, the readiness of RNPs and the fact that this pre-built method has been proven to protect the gRNA from degradation render this option as the most efficient [31]. However, even though most cultured cells may take any of these procedures in, the more complex in vivo approaches are not that tolerant and mostly conducted via embryo microinjection or are viral-mediated [32,33].
3. Cellular Models
3.1. Cell Lines
Immortalized cell lines, due to their ease of handling, are a valuable tool to study the function of selected genes in vitro and to explore the impact of certain mutations (Table 1).
The origin of some broadly used cell lines, such as HEK293 or HeLa, is very different from cells implicated in IRDs but they can be equally used to study the function of some genes involved in these blinding disorders [84,85,86,87]. Among others, HEK293 cells have been used by Fuster and collaborators to assess the efficiency of diverse RNA guides designed to target p.Glu767Serfs*21 and p.Cys759Phe mutations in USH2A by CRISPR/Cas9 before using them in patient-derived fibroblasts, widely known to be more difficult to manipulate and transfect [36]. A similar strategy is applied when the main goal is to study the validity of the CRISPR system for gene editing in a mouse model. As a first step, usually, researchers test the designed RNA guides in manageable culture cells of mouse origin, such as the Mouse Neuro 2A (N2A) cells, derived from a mouse neuroblastoma, or mouse embryonic fibroblasts [40,41].
Other cell lines seem to be more appropriate as models for the study of pathophysiological mechanisms of IRDs, since they have a similar origin to the retinal tissue affected in these diseases. The immortalized mouse cone photoreceptor-derived 661W cell line has been widely used as a model to study the processes involved in the retinal degeneration, such as oxidative stress and cell death [88,89,90,91,92]. The 661W cell line has been used by Ji-Neng and colleagues to decipher the molecular pathways associated to RP9 gene mutations, a pre-mRNA splicing factor responsible for autosomal dominant RP (adRP) [37]. By using the CRISPR/Cas9 system, these authors generated a RP9 knock-out and a knock-in model for the p.His137Leu human mutation, which allowed them to demonstrate that mutations in the RP9 gene lead to a reduction in cell proliferation and migration as well as a dysregulation in the expression of some downstream regulated genes [37].
Another common cell line derived from the female retinal pigment epithelium (RPE), hTERT-RPE1, has been used for the study of the molecular mechanisms involved in IRDs and other ciliopathies due to their origin, the presence of cilia, and the capacity to reciliate under certain conditions [93]. This cell line was chosen to investigate the role of RP2, a gene causative of X-linked retinal degeneration, because of its motility in culture. The CRISPR/Cas9 RP2 knock-out model showed a reduced motility compared to RP2 wild type cells [38]. hTERT-RPE1 was also utilized to study how mutations in RPGR, another gene causing X-linked RP, lead to photoreceptor loss [39]. To achieve this aim, knock-out cell lines for RPGR and several interactor genes (PDE6D, INPP5E and RPGRIP1L, all three are also involved in retinal degeneration) were generated with the CRISPR/Cas9 system allowing to demonstrate that PDE6D is necessary for a correct ciliary localization of prenylated proteins such as RPGR and INPP5E, but that RPGR is not essential for the localization of INPP5E in the cilia [39].
Primary cell lines harvested from patients or healthy donors represent a valid alternative to common cell lines for the study of retinal degeneration. It is important to note that these are usually difficult to handle, given their limited growth in culture conditions and reduced efficiency of transfection. However, primary cells enable the preservation of the genetic background of source patient, and fibroblasts are largely used in this sense. Fuster and collaborators were able to edit by CRISPR/Cas9 the most prevalent mutation in the USH2A gene, p.Glu767Serfs*21, in fibroblasts obtained from a patient homozygous for the mutation [36].
Recently, Yang et al., used a pool of primary human keratinocytes to study the effect of mutations in the EXOSC2, a gene encoding for one cap protein in the RNA exosome (RRP4) and associated to SHRF syndrome (short stature, hearing loss, retinitis pigmentosa and distinctives facies), which is a novel syndromic form of IRDs [42,94]. By generating an EXOSC2 knock-out model, the authors demonstrated that keratinocytes have a reduced proliferation that might explain early skin aging observed in patients with this syndrome.
3.2. Induced Pluripotent Stem Cells
Ever since the arrival of induced pluripotent stem cells (iPSCs) [95], biomedical research has taken a big leap, especially in regard to therapeutic and disease model applications. This technology allows the reprogramming of virtually any somatic human cell population to a complete pluripotent state, with the potential to subsequently generate tissue-specific progenitor cells or even a completely differentiated line [96]. This poses a great resource to explore the molecular defects of a certain pathology, including those related to differentiation mechanisms. Studies within this cellular scope consist of side-by-side assays using diseased and normal sets [97]. Rationally, those would require multiple unrelated samples to normalize the individual genetic variability of each lineage and the one resulting from the dedifferentiation process itself.
Yet to study the repercussion of a particular variant, this proceeding would turn out to be unavailing for two main reasons. First, with respect to IRDs, the low reoccurrence of disease-causing mutations would frustrate a significant statistical inference due to the poor number of independent samples in the analysis. Second, even if an adequate stock could be obtained, the prospect of any other linked VUS as the actual disease trigger would still call the results into question [98]. Indeed, even in iPSCs from the same donor, phenotypic differences have been detected and they are amplified if derived from unrelated individuals [99], encouraging the search for methods to keep this variability at a minimum.
In this matter and as previously commented, the use of CRISPR-based genome editing permits the introduction of precise genetic changes in these iPSCs without altering the rest of their genomic sequence, thereby providing isogenic cell line pairs only differing in the mutation of interest. This has a great relevance for the assessment of variant pathogenicity, since it enables the comparison of two cell populations with identical genetic backgrounds and, thus, the impact appraisal of a specific single DNA lesion in the cellular phenotype [100].
To do so, there are two essential routes [98]. The first strategy would be using patient-derived cells to repair the putative disease-causing mutation for the later monitoring of the corrected and unaltered line. However, this might be rather treacherous in some circumstances, since any detected differences would only prove the involvement of the variant yet not rule out the contribution of other changes throughout the genome. This presumption, though, might only apply for other more complex diseases in which oligogenic inheritance patterns are not uncommon, unlike with IRDs, which usually are explained by mutations in single genes. Even so, the influence of possible modifiers should not be disregarded, as evidenced by the incomplete penetrance and clinical variability of some alleles in certain retinal disorders [101,102,103,104]. Certainly, a comparative of isogenic lines should likewise serve to prove some presumed digenic or gene-modifying scenarios by testing mutation combinations. The second approach consists of the reverse procedure, in which cells obtained from healthy individuals are genetically edited to harbor the variant of study to determine if it is sufficient to produce the pathogenic phenotype regardless of the rest of genome variation.
In either case, genotype-specific disease modeling in cell cultures is much easier for dominant disorders, where only one of the two alleles need to be edited. It should be noted that the positive activity of the CRISPR complexes within a cell does not ensure their operation on both chromosomes, being the chances of producing a single vs. a dual modification greater. Likewise, the introduction of a homozygous variant under a dominant premise could bias the experiment conclusions by producing a stronger phenotype. Thus, in any way, a clone selection step is required to obtain a homogeneous cell population, even though the process might be more swift when aiming for dominant traits.
It should be recalled, that the CRISPR technique does have secondary effects in the form of potential off-targets when the designed gRNA presents a highly similar sequence to other loci of the genome, in which the complex could act by producing DSBs and, hence, further genome modifications [105]. The occurrence of these by-products is neither fixed nor abundant, yet any of these unintended genetic alterations could give rise to the anticipated differing phenotype, misattributed to the intended predesigned change. Thus, a full post-edition genome survey should be mandatory to confidently dismiss these potential changes as the actual pathogenic cause.
In order to deem a variant as a disease causative, or at least contributing, some sort of ponderable molecular or cellular trait differing from the control cell line is necessary. A good example for the use of these CRISPR editing techniques is the recent work conducted by Sanjurjo-Soriano and colleagues, where they used patient-derived iPSCs carrying the two USH2A prevalent mutations (p.Glu767Serfs*21 and p.Cys759Phe) and the corrected isogenic counterpart, and detected abnormal mRNA levels associated to the variants (see other studies in Table 1) [49].
3.3. Retinal Organoids
Despite iPSCs being such an evident profitable and manageable trial source, the method still falls short of typifying the more complex cellular networks of whole organs. Therefore, the scrutiny of merely the ultimately affected cell type might not prove to be conclusive, as the pathological mechanisms of a disease are not always attributable to its own morphology or transcriptome, but also to physiological and molecular interactions with surrounding cells. Given that the retina consists of a sophisticated stratum of neuronal cells, this is probably the case for the study of some variants in genes responsible for IRDs.
An alternative, yet more complicated, culture-based strategy to overcome this issue is the use of the recently emerged retinal organoids (ROs). These optic cups consist of the three-dimensional disposition of all retinal cells that reproduces the spatial arrangement and the development of the tissue [106]. Consequently, this rudimentary organ-like structure offers a more realistic model to study the impact of variants on a larger scale, yet still under in vitro conditions. In addition, as the organoid formation mimics the natural time-framed differentiation of the eye neuronal layers, it allows for the investigation of the consequences of a DNA change in the retinogenesis [107]. Moreover, the use of ROs might even add other read-out options for the functional appraisal, since photoreceptors in these models seem to analogously respond to light stimulus and to recapitulate the signal transmission towards the inner retinal cells [108,109].
ROs by themselves have major limitations, since they actually resemble an embryonic-staged retina. Cells are not fully developed, as evidenced in the incomplete formation of the outer and inner segments of the photoreceptors, and the 3D structure lacks vascularization and other cell types that are present in the native organ, like microglia and the RPE [110]. However, it would seem that a recently described approach is able to circumvent these shortcomings and more accurately recapitulate the in vivo scenario by co-culturing RPE sheets and ROs within a controlled microperfusion system [111]. Labelled as retina-on-a-chip (RoC), it promotes maturation of the photoreceptors by allowing the interaction of their pseudo-outer segments with the RPE and due to the constant flux of nutrients provided by the pumping platform. Hence, this evolved scaffold-like model now stands as a promising prototype recapitulating the in vivo architecture of the retina to study the inherent cellular mechanisms, effects of genome manipulations, drug treatments, and so forth.
Alike with the iPSCs, the blend of the CRISPR tools with the availability to obtain ROs would boost the cogency to validate candidate VUS. Comparatives of patient and control-derived ROs can present enough evidence supporting the deleteriousness of certain mutations [112]. However, there is also proof that 3D cups stemming from different cell lines display variation on their development [113], possibly determining their later stability and electrophysiological properties. Hence, the use of isogenic ROs should be encouraged to remove all uncontrolled variability that may bias the variant pathogenicity determination, something that can be attained using CRISPR-pre-processed iPSCs before their differentiation into optic cups.
The study of Buskin et al. is one of several examples implementing these programable nucleases in such optic cups (Table 1) [44]. The researchers produced several ROs from patient-derived cells with mutations in the PRPF31 gene, responsible for adRP. In parallel, they corrected these genetic changes in iPSCs prior to RO differentiation, providing a peer 3D-source for the appraisal of any molecular differences imputable to the variants. Indeed, results not only allowed the confirmation of PRFP31 involvement in ciliogenesis regulation, but also a consistent molecular characterization of the pathogenesis.
All these properties place the ROs as the more reliable ‘disease-in-a-dish’ model to date, and it might be more than enough to ratify the pathogenicity of a suspected mutation. Nonetheless, since these primitive organs do not engage in further physiological processes and anatomic elements of the organism, they still are lacking in replicating the whole disease picture.
4. Animal Models
4.1. Caenorhabditis elegans
Caenorhabditis elegans (C. elegans) is a nematode that plays an important role in biological research. Back in 1965, the Nobel prize-laureated Sydney Brenner chose this worm as a model to study animal development and behavior, and it has ever since been considered an excellent model to study biological processes due to several advantages: its rapid life cycle, its small size, the ease in terms of laboratory management, its transparency (which facilitates the use of microscopy in vivo), and the fact that despite its simplicity, it is still a complex multicellular organism with a variety of tissues and organs. In sum, C. elegans is a model organism that brings together the advantages of in vitro and in vivo research.
To date, mutations in 22 genes have been related with adRP (RetNet, accessed March 2020), and seven of these genes encode splicing factors. These seven genes are PRPF3, PRPF4, PRPF6, PRPF8, PRPF31, SNRNP200 and RP9. All of them, except for RP9, encode highly conserved proteins between C. elegans and humans [114]. Photoreceptor cells are characterized by an intense transcriptional activity and metabolic rate [115] and, even though they are absent in C. elegans, the animal does present other cells that have similar increased metabolic rate and high transcriptional levels during the larval phase [116,117].
The clinical variability that characterizes IRDs can be explained by the existence of genetic modifiers [118]. In this context, Kukhtar et al. generated mutant strains to mimic two pathogenic mutations reported in PRPF8 and SNRNP200 by CRISPR/Cas9 to identify potential adRP modifiers and to explore therapies that may slow the disease progression [51].
4.2. Drosophila melanogaster
Drosophila has good genetic tractability and 65% of the human genes responsible for a disease has a homolog in this fly model, including most of the genes involved in IRDs [119]. In 1995, Drosophila was used for the first time as a model to decipher the mechanisms by which mutations in RHO caused retinal degeneration, demonstrating the great potential of this model for this group of diseases [120]. Recently, Yang and collaborators generated a knock-out of EXOSC2 in the fly model assisted by the CRISPR tools and showed that some patterns, such as the autophagy, were altered in SHRF syndrome due to mutations in this gene (Table 1) [42].
4.3. Xenopus
Xenopus is an amphibian broadly used as a model to study development and cell biology because of its well-preserved development and genetic proximity to higher vertebrates. Further characteristics such as the size, external development of the embryos, type of breed, and the large number of progeny, make Xenopus a suitable animal model [121,122]. There are two main species that are used in research, namely X. laevis (allotetraploid genome) and X. tropicalis (diploid genome) [122,123], and it is the former, the one that has been used to model retinal dystrophies, with a special focus on the rhodopsin gene. Knock-out models have been generated by the microinjection of Cas9 mRNA and sgRNAs into single cell embryos [83]. Likewise, three knock-in models were produced in the same study using an external template donor. Despite the scarce research on Xenopus oriented to IRDs, the available results demonstrate that the organism can be genetically manipulated using the CRISPR/Cas9 system and be used to model eye diseases (Table 1).
4.4. Zebrafish
Another animal model widely used in research on IRDs is the zebrafish (D. rerio), a freshwater fish belonging to the family of Cyprinidae. This vertebrate model organism has been used for many years in developmental studies, toxicology, preclinical drug development, or molecular genetics, due to several beneficial characteristics like its fast life cycle, external larvae development, small size, transparency and easy maintenance and breeding [124,125,126,127,128]. However, this model has most of its genome duplicated [129], which complicates the editing of all functional gene copies. In addition, at least 70% of the human disease-related genes have their ortholog in zebrafish, which eases the study of their functions and involved molecular mechanisms, assuming an equal role in both species [130]. Moreover, compared to other vertebrate models commonly used, zebrafish has a higher number of offspring, which supposes an additional advantage [131,132].
Besides these general positive traits, it should also be noted that this organism is able to regenerate its retinal layers and some neural subtypes, thereby seeming to be a good research model for retinal diseases [133,134,135,136,137,138,139].
Additionally, following its whole genome sequencing [130], reverse genetic and other molecular tools have thrived, making it possible to perform, for example, retroviral-mediated insertional mutagenesis or CRISPR/Cas9 technology [140,141,142]. Hence, the emergence of new editing techniques has expanded the range of possibilities in the field of candidate gene or variant assessment. In fact, the zebrafish was the first model used to demonstrate the in vivo feasibility of the CRISPR/Cas9 system for genome editing, achieving up to 50% of on-target edition rate [143].
Moreover, this organism is a widely used model for functional assays in IRDs, usually based on the development of gene knock-downs or knock-outs [52,53,58], some lately generated by means of the genomic editing system here reviewed (Table 1). As an example, Minegishi and colleagues decided to confirm the involvement of the CCT2 gene in LCA [78], suspected due to identification of the compound heterozygous mutations p.Thr400Pro and p.Arg513Hisby whole exome sequencing (WES) in the affected members of a sole Chinese consanguineous family [55]. The produced cct1-L394-7del line by CRISPR/Cas9 demonstrated that the mutated cct2 gene implied serious disabilities in zebrafish, highlighting its important role in this vertebrate model. Furthermore, the homozygous cct2-L394H-7del mutant showed a phenotype rescue when injected with human wild type CCT2-coding RNA. In conclusion, this study served as a candidate gene validation, showing that mutations in the CCT2 ortholog result in a phenotype that resembles the one in human LCA patients.
A similar procedure was performed by Van De Weghe et al. in 2017 [144]. A large cohort of patients diagnosed with Joubert Syndrome (JS), were studied by WES, which led to the detection of different variants in ARMC9, including point mutations and whole exon deletions. Initial assays revealed that the gene was highly expressed in the ciliary basal body of ciliated cells, as alike with other genes involved in cilium function that are also upregulated. Leveraging the presence of an ARMC9 ortholog in zebrafish with at least 72% protein similarity, the authors introduced frameshift mutations in armc9 by the CRISPR/Cas9 technology to investigate the resulting phenotypes. Small pairs of gRNAs were co-injected to target exons 4 and 14–15 generating several mutant zebrafish strains. The resulting phenotypes confirmed that mutations in the ARMC9 gene are responsible for ciliopathy phenotypes in JS patients.
Knock-in studies to evaluate the specific IRD-causing variants have also been conducted in zebrafish. For instance, Dona and colleagues were able to reproduce two mutations responsible for USH in USH2A (p.Cys780Glnfs*32 andp.Ala5174fs*), providing finer strains modeling the disease to analyze the pathogenic molecular mechanisms underlying RP [56].
In a different study, another mutation in the same gene was introduced for two purposes, the pathogenic validation of the variant in this aquatic model and its later use for a therapeutic approach [57]. In humans, the deep intronic p.Lys2532Thrfs*56 mutation (c.7595-2144A>G) creates a potential donor splice-site in intron 40 leading to the introduction of a pseudoexon in the mRNA [145], yet Slijkerman et al. concluded that this effect was not recapitulated in zebrafish, disclosing that splice-site recognition pathways are different in the human and zebrafish organisms [57].
4.5. Rodents
Regardless of the mutant generation procedure, the mouse (Mus musculus) is by far the most commonly used organism for disease modeling. This also applies to human eye disorders, given that the mouse and human eye share many anatomic and physiologic characteristics [146]. Besides the possibility to carry out reproducible developmental and invasive studies, mice also allow relatively easy ophthalmological characterizations of the diseases through different tools, such as indirect ophthalmoscopy, fluorescein angiography, optical coherence tomography, or electroretinography. In addition, the mouse has a rather short lifespan considering its condition of mammalian organism, which is convenient in terms of model generation and later monitoring of the natural progression of the disease of study.
Despite all the progress made in the last years, the delivery of the CRISPR components remains a challenge, and mice pose a good model to investigate later human-applicable possibilities. To date, adeno-associated virus (AAV) vectors are the most effective method for delivering gene therapy compounds to retinal cells [147,148]. Following a knockdown strategy with easily detectable outcomes, Hung et al. tested the viability to target retinal cells in vivo through intravitreal administration of CRISPR AAV2 vectors inThy1-YFP mice, a reporter strain expressing a fluorescent protein in the ganglion, amacrine and bipolar cells [66].
A different study directed to photoreceptors employed a dual-AAV2/8 system for the delivery of CRISPR components to disrupt Nrl, a gene dictating rod development during development, in three different RP mice models (rd10, Rho KO and Rho P347S) [75]. Yu and colleagues established that Nrl ablation caused rods to acquire cone characteristics, mitigating the degeneration process by extending the survival of these converted-rods and preserving cone function in those cases where rod-specific mutations are accountable for the dystrophy. Other studies using this viral delivery method have gone beyond and added to the design a self-inactivating trait of the CRISPR system based on the autotargeting of the Cas9 coding sequence in the cassette once expressed, in order to limit the expression in the retina of the nuclease and, thereby, reducing undesirable off-target events, potential toxicity and immune response against Cas9 [80,149]. Another in vivo Cas9-restriction method, once again aimed to regulate the Nrl gene, was presented by Moreno et al., who devised a doxycycline-inducible nuclease expression construct [76].
CRISPR/Cas9-mediated NHEJ repair has quickly become a routine method for creating gene knock-outs in animal models, as it is a considerably simpler and more productive approach than the HDR-based knock-in process. Accordingly, many authors have demonstrated that generating knock-out mouse models by the CRISPR/Cas9 editing system is an efficient method to determine the pathogenic role of new IRD-causing candidate genes and to characterize the disease mechanisms associated with their mutations in them [62,69,70,74,78]. As an example, a recently identified homozygous missense variant (p.Trh58Met) in HKDC1 in two unrelated RP families by WES postulated the gene as candidate for the disease, and the disruption of Hkdc1 in mice using these programable nucleases resulted in a reduced scotopic electroretinogram response and thinner outer nuclear layer similar to the human patients [71]. Hence, these findings enabled the final linking of the gene to arRP.
For diseases caused by a gain of function, dominant negative variants or increased gene copy numbers, CRISPR/Cas9 can be used to selectively suppress the mutated allele that produces toxicity in the retina. In this line, an interesting proceeding was conducted in a rat model of adRP caused by the p.Ser334*stop-gain mutation in Rho, which was tackled by introducing an allele-specific gRNA/Cas9 complex by means of retina electroporation of new-born animals, preventing retinal degeneration [82].
Genome-editing nucleases can also be used to deal with regulatory elements. Seruggia et al. carried out a successful inactivation of a non-coding regulatory locus upstream the Tyr gene, employed by microinjecting mouse fertilized eggs with a combination of Cas9 and two sgRNAs targeting unique flanking sequences of the region [65]. Wang et al. also used two-sided NHEJ-mediated DSBs to produce a fundamental 108bp deletion in a cis-regulatory element controlling the Blimp1 expression, whose encoded protein is in itself a transcription factor critical for the rod versus bipolar cell differentiation, abolishing the function of the gene [61].
It must be considered that sometimes null homozygous alleles that in humans derive from autosomal recessive phenotypes can be lethal to the mouse, consequently hampering the disease emulation. This happened to Zhong and colleagues, who used the CRISPR/Cas9 system to generate mouse Kcnj13 null alleles to verify the pathogenic role of the human ortholog KCNJ13, a gene that was associated with LCA [64]. The study exposed the fatality of the complete loss of the gene, yet the retinal degeneration in animals withholding a portion of cells with the preserved wild type gene could still be used to confirm the requirement of KCNJ13 for photoreceptor survival. Moreover, these results revealed the potential use of mosaicism for in vivo gene functional validation in otherwise lethal mutations in mice.
Knock-in approaches using the CRISPR/Cas9 technology are carried out in this animal too, alike with other models, either to create more genuine disease-models or to interrogate the effect of specific genetic variants. Arno et al. exemplified this application by replicating in mice the p.Leu135Pro mutation in Reep6 in homozygosis via HDR following CRISPR/Cas9-mediated DSB, which reproduced the RP phenotype and, therefore, corroborated human REEP6 implication in the disease [68].
Almost all pathogenic mutations described in RPE65 are recessively inherited but the p.Asp477Gly variant has been reported to cause adRP in families of Irish heritage [150,151]. To prove the dominant impact of this particular mutation, Li and colleagues generated the mutation knock-in model in the mouse, again employing CRISPR/Cas9 resources [63]. They observed that heterozygous mice did not show any anomalous visual function, yet under homozygous conditions their retina did display signs of degeneration when subjected to light stress. The absence of an aberrant phenotype does not refute the dominance of the genetic change, given the similar outcome for other variants patently responsible for adRP, even though the presence genetic disease-modifiers could have been in fact neglected in the genetic screening of the patients with the p.Asp477Glymutation. Therefore, this case actually exposes the CRISPR knocking-in as a valid method to confirm but not discard proposed pathogenic effects of mutations.
4.6. Pig
Domestic pigs (Sus scrofa domesticus) share several key similarities with humans in terms of their body size, anatomy, physiology, genetics, pathophysiological responses, and diet [152]. Its use in biomedical research has some advantages like their favorable breeding characteristics. Pigs mature relatively quickly for such a large species, have a short gestational period, and produce extensive litters [153]. In general, due to the cone-rich nature of the porcine retina, the pig is considered to be an ideal model for studying IRDs, especially cone-dystrophies [146]. Burnight et al. chose a transgenic pig model that carries the human p.Pro23His mutation to perform an AAV-mediated allele-specific CRISPR correction in vivo, delivering these viral constructs by subretinal injection [43].
4.7. Macaque
Nonhuman primates have a crucial role in translational studies due to their high similarity with humans in several aspects, such as genetics and physiology. Usually, this model is used in the last preclinical phase and before starting with clinical trials in humans [154]. This step is not always necessary given the accessibility to the other model organisms explained above, which can provide enough safety and efficiency evidence for some explored treatments. Among the genus, Macaca fascicularis has been used to analyze the viability of CRISPR/Cas9 design as a therapeutic approach to treat a type of autosomal dominant cone-rod dystrophy (CORD6) due to mutations in GUCY2D, an approach in mice [79]. Subretinal injections of AAV containing the CRISPR complex sequences have been used as a delivery method to specifically knock out the GUCY2D gene in macaque photoreceptors (Table 1). The tissue-restricted model was thoroughly characterized and the results obtained served the authors as proof of concept for a potential therapeutic application of these editing tools for other retinal diseases [79].
5. Conclusions
The key contribution of the CRISPR/Cas9 technology towards variant interpretation always falls on the relatively easy and quick reproducibility of mutations in an in vitro or animal model, allowing discernment of pathogenicity if any aberrant molecular or morphological changes result from its sole conception.
This revolutionary editing technology is being increasingly used in genetics studies, keeping pace with updates of the technique that show a clear advance in its precision. This will lead to not only an improvement in the diagnosis ratio but also lead to the development of potential therapeutic options.
Taking advantage of the easy accessibility to the retina, research on non-viral delivery procedures should be fostered, aiming for hit-and-run alternatives that proffer more restrained outcomes in regard to off-target activity. This will be relevant to obtaining safer editing trials, not only with the sights set on therapeutic goals but also in terms of producing more robust disease-models and casting no doubts on their ex situ genetic integrity that, if compromised, could mask an expected phenotype.
Author Contributions
Conceptualization, C.F.-G., G.G.-G. and J.M.M.; Methodology, C.F.-G., B.G.-B. and A.R.-M.; Resources, C.F.-G., B.G.-B. and A.R.-M.; Writing—Original Draft Preparation, C.F.-G.; Writing—Review & Editing, C.F.-G., B.G.-B., A.R.-M., G.G.-G. and J.M.M.; Supervision, G.G.-G. and J.M.M.; Funding Acquisition, J.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Instituto de Salud Carlos III (ISCIII), grant numbers PI16/00539 and PI19/00303 (Co-funded by European Regional Development Fund/European Social Fund) “Investing in your future”) and Generalitat Valenciana, grant number PROMETEO/2018/135. BG-B is the recipient of a fellowship from Conselleria de Educacio, Generalitat Valenciana, number: ACIF/2019/252 and AR-M is the recipient of a contract Rio Hortega from the ISCIII (co-funded by the European Social Fund, “Investing in your future”), number: CM18/00199.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Broadgate S., Yu J., Downes S.M., Halford S. Unravelling the genetics of inherited retinal dystrophies: Past, present and future. Prog. Retin. Eye Res. 2017;59:53–96. doi: 10.1016/j.preteyeres.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Sutherland J.E., Day M.A. Advantages and disadvantages of molecular testing in ophthalmology. Expert Rev. Ophthalmol. 2011;6:221–245. doi: 10.1586/eop.11.2. [DOI] [Google Scholar]
- 3.Estrada-Cuzcano A., Roepman R., Cremers F.P.M., den Hollander A.I., Mans D.A. Non-syndromic retinal ciliopathies: Translating gene discovery into therapy. Hum. Mol. Genet. 2012;21:R111–R124. doi: 10.1093/hmg/dds298. [DOI] [PubMed] [Google Scholar]
- 4.Zeitz O. Myron Yanoff and Jay S. Duker: Ophthalmology, Fifth Edition. Graefes Arch. Clin. Exp. Ophthalmol. 2020;258:459. doi: 10.1007/s00417-019-04489-7. [DOI] [Google Scholar]
- 5.Farrar G.J., Carrigan M., Dockery A., Millington-Ward S., Palfi A., Chadderton N., Humphries M., Kiang A.S., Kenna P.F., Humphries P. Toward an elucidation of the molecular genetics of inherited retinal degenerations. Hum. Mol. Genet. 2017;26:R2–R11. doi: 10.1093/hmg/ddx185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dreyer B., Tranebjaerg L., Brox V., Rosenberg T., Möller C., Beneyto M., Weston M.D., Kimberling W.J., Cremers C.W., Liu X.Z., et al. A common ancestral origin of the frequent and widespread 2299delG USH2A mutation. Am. J. Hum. Genet. 2001;69:228–234. doi: 10.1086/321269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seyedahmadi B.J., Rivolta C., Keene J.A., Berson E.L., Dryja T.P. Comprehensive screening of the USH2A gene in Usher syndrome type II and non-syndromic recessive retinitis pigmentosa. Exp. Eye Res. 2004;79:167–173. doi: 10.1016/j.exer.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Rivera A., White K., Stöhr H., Steiner K., Hemmrich N., Grimm T., Jurklies B., Lorenz B., Scholl H.P., Apfelstedt-Sylla E., et al. A comprehensive survey of sequence variation in the ABCA4 (ABCR) gene in Stargardt disease and age-related macular degeneration. Am. J. Hum. Genet. 2000;67:800–813. doi: 10.1086/303090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziviello C., Simonelli F., Testa F., Anastasi M., Marzoli S.B., Falsini B., Ghiglione D., Macaluso C., Manitto M.P., Garrè C., et al. Molecular genetics of autosomal dominant retinitis pigmentosa (ADRP): A comprehensive study of 43 Italian families. J. Med. Genet. 2005;42:e47. doi: 10.1136/jmg.2005.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Audo I., Manes G., Mohand-Saïd S., Friedrich A., Lancelot M.-E., Antonio A., Moskova-Doumanova V., Poch O., Zanlonghi X., Hamel C.P., et al. Spectrum of rhodopsin mutations in French autosomal dominant rod-cone dystrophy patients. Investig. Ophthalmol. Vis. Sci. 2010;51:3687–3700. doi: 10.1167/iovs.09-4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valkenburg D., Van Cauwenbergh C., Lorenz B., van Genderen M.M., Bertelsen M., Pott J.W., Coppieters F., De Zaeytijd J., Thiadens A.A., Klaver C.C., et al. Clinical Characterization of 66 Patients With Congenital Retinal Disease Due to the Deep-Intronic c.2991+1655A>G Mutation in CEP290. Investig. Ophthalmol. Vis. Sci. 2018;59:4384–4391. doi: 10.1167/iovs.18-24817. [DOI] [PubMed] [Google Scholar]
- 12.Bernardis I., Chiesi L., Tenedini E., Artuso L., Percesepe A., Artusi V., Simone M.L., Manfredini R., Camparini M., Rinaldi C., et al. Unravelling the Complexity of Inherited Retinal Dystrophies Molecular Testing: Added Value of Targeted Next-Generation Sequencing. Biomed. Res. Int. 2016;2016:6341870. doi: 10.1155/2016/6341870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verbakel S.K., van Huet R.A.C., Boon C.J.F., den Hollander A.I., Collin R.W.J., Klaver C.C.W., Hoyng C.B., Roepman R., Klevering B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018;66:157–186. doi: 10.1016/j.preteyeres.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 14.De Koning T.J., Jongbloed J.D.H., Sikkema-Raddatz B., Sinke R.J. Targeted next-generation sequencing panels for monogenetic disorders in clinical diagnostics: The opportunities and challenges. Expert Rev. Mol. Diagn. 2015;15:61–70. doi: 10.1586/14737159.2015.976555. [DOI] [PubMed] [Google Scholar]
- 15.Lee H., Martinez-Agosto J.A., Rexach J., Fogel B.L. Next generation sequencing in clinical diagnosis. Lancet Neurol. 2019;18:426. doi: 10.1016/S1474-4422(19)30110-3. [DOI] [PubMed] [Google Scholar]
- 16.Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J.A., Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu J.H., Miller S.M., Geurts M.H., Tang W., Chen L., Sun N., Zeina C.M., Gao X., Rees H.A., Lin Z., et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature. 2018;556:57–63. doi: 10.1038/nature26155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Legut M., Daniloski Z., Xue X., McKenzie D., Guo X., Wessels H.-H., Sanjana N.E. High-Throughput Screens of PAM-Flexible Cas9 Variants for Gene Knockout and Transcriptional Modulation. Cell Rep. 2020;30:2859–2868. doi: 10.1016/j.celrep.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gleditzsch D., Pausch P., Müller-Esparza H., Özcan A., Guo X., Bange G., Randau L. PAM identification by CRISPR-Cas effector complexes: Diversified mechanisms and structures. RNA Biol. 2018;16:504–517. doi: 10.1080/15476286.2018.1504546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Periwal V. A comprehensive overview of computational resources to aid in precision genome editing with engineered nucleases. Brief. Bioinform. 2017;18:698–711. doi: 10.1093/bib/bbw052. [DOI] [PubMed] [Google Scholar]
- 21.Torres-Perez R., Garcia-Martin J.A., Montoliu L., Oliveros J.C., Pazos F. WeReview: CRISPR Tools-Live Repository of Computational Tools for Assisting CRISPR/Cas Experiments. Bioengineering. 2019;6:63. doi: 10.3390/bioengineering6030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickar-Oliver A., Gersbach C.A. The next generation of CRISPR–Cas technologies and applications. Nat. Rev. Mol. Cell Biol. 2019;20:490–507. doi: 10.1038/s41580-019-0131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park C.-Y., Sung J.J., Choi S.-H., Lee D.R., Park I.-H., Kim D.-W. Modeling and correction of structural variations in patient-derived iPSCs using CRISPR/Cas9. Nat. Protoc. 2016;11:2154–2169. doi: 10.1038/nprot.2016.129. [DOI] [PubMed] [Google Scholar]
- 24.Brunet E., Jasin M. Induction of Chromosomal Translocations with CRISPR-Cas9 and Other Nucleases: Understanding the Repair Mechanisms That Give Rise to Translocations. Adv. Exp. Med. Biol. 2018;1044:15–25. doi: 10.1007/978-981-13-0593-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu M., Rehman S., Tang X., Gu K., Fan Q., Chen D., Ma W. Methodologies for Improving HDR Efficiency. Front. Genet. 2019:9. doi: 10.3389/fgene.2018.00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim S., Kim D., Cho S.W., Kim J., Kim J.-S. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 2014;24:1012–1019. doi: 10.1101/gr.171322.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang X., Potter J., Kumar S., Zou Y., Quintanilla R., Sridharan M., Carte J., Chen W., Roark N., Ranganathan S., et al. Rapid and highly efficient mammalian cell engineering via Cas9 protein transfection. J. Biotechnol. 2015;208:44–53. doi: 10.1016/j.jbiotec.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 28.Horii T., Hatada I. Production of genome-edited pluripotent stem cells and mice by CRISPR/Cas. Endocr. J. 2016;63:213–219. doi: 10.1507/endocrj.EJ15-0734. [DOI] [PubMed] [Google Scholar]
- 29.Kurata M., Wolf N.K., Lahr W.S., Weg M.T., Kluesner M.G., Lee S., Hui K., Shiraiwa M., Webber B.R., Moriarity B.S. Highly multiplexed genome engineering using CRISPR/Cas9 gRNA arrays. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0198714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanlon K.S., Kleinstiver B.P., Garcia S.P., Zaborowski M.P., Volak A., Spirig S.E., Muller A., Sousa A.A., Tsai S.Q., Bengtsson N.E., et al. High levels of AAV vector integration into CRISPR-induced DNA breaks. Nat. Commun. 2019;10:4439. doi: 10.1038/s41467-019-12449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hendel A., Bak R.O., Clark J.T., Kennedy A.B., Ryan D.E., Roy S., Steinfeld I., Lunstad B.D., Kaiser R.J., Wilkens A.B., et al. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat. Biotechnol. 2015;33:985–989. doi: 10.1038/nbt.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilbie D., Walther J., Mastrobattista E. Delivery Aspects of CRISPR/Cas for in Vivo Genome Editing. Acc. Chem. Res. 2019;52:1555–1564. doi: 10.1021/acs.accounts.9b00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau C.-H., Suh Y. In vivo genome editing in animals using AAV-CRISPR system: Applications to translational research of human disease. F1000Research. 2017:6. doi: 10.12688/f1000research.11243.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Latella M.C., Salvo M.T.D., Cocchiarella F., Benati D., Grisendi G., Comitato A., Marigo V., Recchia A. In vivo Editing of the Human Mutant Rhodopsin Gene by Electroporation of Plasmid-based CRISPR/Cas9 in the Mouse Retina. Mol. Ther. Nucleic Acids. 2016:5. doi: 10.1038/mtna.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruan G.-X., Barry E., Yu D., Lukason M., Cheng S.H., Scaria A. CRISPR/Cas9-Mediated Genome Editing as a Therapeutic Approach for Leber Congenital Amaurosis 10. Mol. Ther. 2017;25:331–341. doi: 10.1016/j.ymthe.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuster-García C., García-García G., González-Romero E., Jaijo T., Sequedo M.D., Ayuso C., Vázquez-Manrique R.P., Millán J.M., Aller E. USH2A Gene Editing Using the CRISPR System. Mol. Ther. Nucleic Acids. 2017;8:529–541. doi: 10.1016/j.omtn.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lv J.-N., Zhou G.-H., Chen X., Chen H., Wu K.-C., Xiang L., Lei X.-L., Zhang X., Wu R.-H., Jin Z.-B. Targeted RP9 ablation and mutagenesis in mouse photoreceptor cells by CRISPR-Cas9. Sci. Rep. 2017;7:43062. doi: 10.1038/srep43062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyraki R., Lokaj M., Soares D.C., Little A., Vermeren M., Marsh J.A., Wittinghofer A., Hurd T. Characterization of a novel RP2-OSTF1 interaction and its implication for actin remodelling. J. Cell Sci. 2018:131. doi: 10.1242/jcs.211748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Q., Giacalone J.C., Searby C., Stone E.M., Tucker B.A., Sheffield V.C. Disruption of RPGR protein interaction network is the common feature of RPGR missense variations that cause XLRP. Proc. Natl. Acad. Sci. USA. 2019;116:1353–1360. doi: 10.1073/pnas.1817639116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vagni P., Perlini L.E., Chenais N.A.L., Marchetti T., Parrini M., Contestabile A., Cancedda L., Ghezzi D. Gene Editing Preserves Visual Functions in a Mouse Model of Retinal Degeneration. Front. Neurosci. 2019;13:1–18. doi: 10.3389/fnins.2019.00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jo D.H., Song D.W., Cho C.S., Kim U.G., Lee K.J., Lee K., Park S.W., Kim D., Kim J.H., Kim J.S., et al. CRISPR-Cas9–mediated therapeutic editing of Rpe65 ameliorates the disease phenotypes in a mouse model of Leber congenital amaurosis. Sci. Adv. 2019;5:1–9. doi: 10.1126/sciadv.aax1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X., Bayat V., DiDonato N., Zhao Y., Zarnegar B., Siprashvili Z., Lopez-Pajares V., Sun T., Tao S., Li C., et al. Genetic and genomic studies of pathogenic EXOSC2 mutations in the newly described disease SHRF implicate the autophagy pathway in disease pathogenesis. Hum. Mol. Genet. 2019;29:541–553. doi: 10.1093/hmg/ddz251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burnight E.R., Gupta M., Wiley L.A., Anfinson K.R., Tran A., Triboulet R., Hoffmann J.M., Klaahsen D.L., Andorf J.L., Jiao C., et al. Using CRISPR-Cas9 to Generate Gene-Corrected Autologous iPSCs for the Treatment of Inherited Retinal Degeneration. Mol. Ther. 2017;25:1999–2013. doi: 10.1016/j.ymthe.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buskin A., Zhu L., Chichagova V., Basu B., Mozaffari-Jovin S., Dolan D., Droop A., Collin J., Bronstein R., Mehrotra S., et al. Disrupted alternative splicing for genes implicated in splicing and ciliogenesis causes PRPF31 retinitis pigmentosa. Nat. Commun. 2018:9. doi: 10.1038/s41467-018-06448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brydon E.M., Bronstein R., Buskin A., Lako M., Pierce E.A., Fernandez-Godino R. AAV-Mediated Gene Augmentation Therapy Restores Critical Functions in Mutant PRPF31+/− iPSC-Derived RPE Cells. Mol. Ther. Methods Clin. Dev. 2019;15:392–402. doi: 10.1016/j.omtm.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foltz L.P., Howden S.E., Thomson J.A., Clegg D.O. Functional Assessment of Patient-Derived Retinal Pigment Epithelial Cells Edited by CRISPR/Cas9. Int. J. Mol. Sci. 2018;19:4127. doi: 10.3390/ijms19124127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deng W.-L., Gao M.-L., Lei X.-L., Lv J.-N., Zhao H., He K.-W., Xia X.-X., Li L.-Y., Chen Y.-C., Li Y.-P., et al. Gene Correction Reverses Ciliopathy and Photoreceptor Loss in iPSC-Derived Retinal Organoids from Retinitis Pigmentosa Patients. Stem Cell Rep. 2018;10:1267–1281. doi: 10.1016/j.stemcr.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bohrer L.R., Wiley L.A., Burnight E.R., Cooke J.A., Giacalone J.C., Anfinson K.R., Andorf J.L., Mullins R.F., Stone E.M., Tucker B.A. Correction of NR2E3 Associated Enhanced S-cone Syndrome Patient-specific iPSCs using CRISPR-Cas9. Genes. 2019;10:278. doi: 10.3390/genes10040278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanjurjo-Soriano C., Erkilic N., Baux D., Mamaeva D., Hamel C.P., Meunier I., Roux A.-F., Kalatzis V. Genome Editing in Patient iPSCs Corrects the Most Prevalent USH2A Mutations and Reveals Intriguing Mutant mRNA Expression Profiles. Mol. Ther. Methods Clin. Dev. 2020;17:156–173. doi: 10.1016/j.omtm.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang T.-C., Chang C.-Y., Yarmishyn A.A., Mao Y.-S., Yang Y.-P., Wang M.-L., Hsu C.-C., Yang H.-Y., Hwang D.-K., Chen S.-J., et al. Carboxylated nanodiamond-mediated CRISPR-Cas9 delivery of human retinoschisis mutation into human iPSCs and mouse retina. Acta Biomater. 2020;101:484–494. doi: 10.1016/j.actbio.2019.10.037. [DOI] [PubMed] [Google Scholar]
- 51.Kukhtar D., Rubio-Peña K., Serrat X., Cerón J. Mimicking of splicing-related retinitis pigmentosa mutations in C. elegans allow drug screens and identification of disease modifiers. Hum. Mol. Genet. 2020;29:756–765. doi: 10.1093/hmg/ddz315. [DOI] [PubMed] [Google Scholar]
- 52.Messchaert M., Dona M., Broekman S., Peters T.A., Corral-Serrano J.C., Slijkerman R.W.N., van Wijk E., Collin R.W.J. Eyes shut homolog is important for the maintenance of photoreceptor morphology and visual function in zebrafish. PLoS ONE. 2018;13:e0200789. doi: 10.1371/journal.pone.0200789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corral-Serrano J.C., Messchaert M., Dona M., Peters T.A., Kamminga L.M., van Wijk E., Collin R.W.J. C2orf71a/pcare1 is important for photoreceptor outer segment morphogenesis and visual function in zebrafish. Sci. Rep. 2018;8:1–10. doi: 10.1038/s41598-018-27928-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Unal Eroglu A., Mulligan T.S., Zhang L., White D.T., Sengupta S., Nie C., Lu N.Y., Qian J., Xu L., Pei W., et al. Multiplexed CRISPR/Cas9 Targeting of Genes Implicated in Retinal Regeneration and Degeneration. Front. Cell Dev. Biol. 2018:6. doi: 10.3389/fcell.2018.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Minegishi Y., Nakaya N., Tomarev S.I. Mutation in the Zebrafish cct2 Gene Leads to Abnormalities of Cell Cycle and Cell Death in the Retina: A Model of CCT2-Related Leber Congenital Amaurosis. Investig. Ophthalmol. Vis. Sci. 2018;59:995–1004. doi: 10.1167/iovs.17-22919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dona M., Slijkerman R., Lerner K., Broekman S., Wegner J., Howat T., Peters T., Hetterschijt L., Boon N., de Vrieze E., et al. Usherin defects lead to early-onset retinal dysfunction in zebrafish. Exp. Eye Res. 2018;173:148–159. doi: 10.1016/j.exer.2018.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slijkerman R., Goloborodko A., Broekman S., de Vrieze E., Hetterschijt L., Peters T., Gerits M., Kremer H., van Wijk E. Poor Splice-Site Recognition in a Humanized Zebrafish Knockin Model for the Recurrent Deep-Intronic c.7595-2144A>G Mutation in USH2A. Zebrafish. 2018;15:597–609. doi: 10.1089/zeb.2018.1613. [DOI] [PubMed] [Google Scholar]
- 58.Xie S., Han S., Qu Z., Liu F., Li J., Yu S., Reilly J., Tu J., Liu X., Lu Z., et al. Knockout of Nr2e3 prevents rod photoreceptor differentiation and leads to selective L-/M-cone photoreceptor degeneration in zebrafish. Biochim. Biophys. Acta Mol. Basis Dis. 2019;1865:1273–1283. doi: 10.1016/j.bbadis.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 59.Schlegel D.K., Glasauer S.M.K., Mateos J.M., Barmettler G., Ziegler U., Neuhauss S.C.F. A New Zebrafish Model for CACNA2D4-Dysfunction. Investig. Ophthalmol. Vis. Sci. 2019;60:5124–5135. doi: 10.1167/iovs.19-26759. [DOI] [PubMed] [Google Scholar]
- 60.Karjosukarso D.W., Ali Z., Peters T.A., Zhang J.Q.C., Hoogendoorn A.D.M., Garanto A., van Wijk E., Jensen L.D., Collin R.W.J. Modeling ZNF408-Associated FEVR in Zebrafish Results in Abnormal Retinal Vasculature. Investig. Ophthalmol. Vis. Sci. 2020;61:39. doi: 10.1167/iovs.61.2.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang S., Sengel C., Emerson M.M., Cepko C.L. A gene regulatory network controls the binary fate decision of rod and bipolar cells in the vertebrate retina. Dev. Cell. 2014;30:513–527. doi: 10.1016/j.devcel.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mookherjee S., Chen H.Y., Isgrig K., Yu W., Hiriyanna S., Levron R., Li T., Colosi P., Chien W., Swaroop A., et al. A CEP290 C-terminal Domain Complements the Mutant CEP290 of Rd16 Mice in Trans and Rescues Retinal Degeneration. Cell Rep. 2018;25:611–623. doi: 10.1016/j.celrep.2018.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y., Furhang R., Ray A., Duncan T., Soucy J., Mahdi R., Chaitankar V., Gieser L., Poliakov E., Qian H., et al. Aberrant RNA splicing is the major pathogenic effect in a knock-in mouse model of the dominantly inherited c.1430A>G human RPE65 mutation. Hum. Mutat. 2019;40:426–443. doi: 10.1002/humu.23706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhong H., Chen Y., Li Y., Chen R., Mardon G. CRISPR-engineered mosaicism rapidly reveals that loss of Kcnj13 function in mice mimics human disease phenotypes. Sci. Rep. 2015;5:1–8. doi: 10.1038/srep08366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seruggia D., Fernández A., Cantero M., Pelczar P., Montoliu L. Functional validation of mouse tyrosinase non-coding regulatory DNA elements by CRISPR-Cas9-mediated mutagenesis. Nucleic Acids Res. 2015;43:4855–4867. doi: 10.1093/nar/gkv375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hung S.S.C., Chrysostomou V., Li F., Lim J.K.H., Wang J.-H., Powell J.E., Tu L., Daniszewski M., Lo C., Wong R.C., et al. AAV-Mediated CRISPR/Cas Gene Editing of Retinal Cells In Vivo. Investig. Ophthalmol. Vis. Sci. 2016;57:3470–3476. doi: 10.1167/iovs.16-19316. [DOI] [PubMed] [Google Scholar]
- 67.Wu W.-H., Tsai Y.-T., Justus S., Lee T.-T., Zhang L., Lin C.-S., Bassuk A.G., Mahajan V.B., Tsang S.H. CRISPR Repair Reveals Causative Mutation in a Preclinical Model of Retinitis Pigmentosa. Mol. Ther. 2016;24:1388–1394. doi: 10.1038/mt.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arno G., Agrawal S.A., Eblimit A., Bellingham J., Xu M., Wang F., Chakarova C., Parfitt D.A., Lane A., Burgoyne T., et al. Mutations in REEP6 Cause Autosomal-Recessive Retinitis Pigmentosa. Am. J. Hum. Genet. 2016;99:1305–1315. doi: 10.1016/j.ajhg.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agrawal S.A., Burgoyne T., Eblimit A., Bellingham J., Parfitt D.A., Lane A., Nichols R., Asomugha C., Hayes M.J., Munro P.M., et al. REEP6 deficiency leads to retinal degeneration through disruption of ER homeostasis and protein trafficking. Hum. Mol. Genet. 2017;26:2667–2677. doi: 10.1093/hmg/ddx149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moye A.R., Singh R., Kimler V.A., Dilan T.L., Munezero D., Saravanan T., Goldberg A.F.X., Ramamurthy V. ARL2BP, a protein linked to retinitis pigmentosa, is needed for normal photoreceptor cilia doublets and outer segment structure. Mol. Biol. Cell. 2018;29:1590–1598. doi: 10.1091/mbc.E18-01-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang L., Sun Z., Zhao P., Huang L., Xu M., Yang Y., Chen X., Lu F., Zhang X., Wang H., et al. Whole-exome sequencing revealed HKDC1 as a candidate gene associated with autosomal-recessive retinitis pigmentosa. Hum. Mol. Genet. 2018;27:4157–4168. doi: 10.1093/hmg/ddy281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giannelli S.G., Luoni M., Castoldi V., Massimino L., Cabassi T., Angeloni D., Demontis G.C., Leocani L., Andreazzoli M., Broccoli V. Cas9/sgRNA selective targeting of the P23H Rhodopsin mutant allele for treating retinitis pigmentosa by intravitreal AAV9.PHP.B-based delivery. Hum. Mol. Genet. 2018;27:761–779. doi: 10.1093/hmg/ddx438. [DOI] [PubMed] [Google Scholar]
- 73.Tsai Y.-T., Wu W.-H., Lee T.-T., Wu W.-P., Xu C.L., Park K.S., Cui X., Justus S., Lin C.-S., Jauregui R., et al. CRISPR-based genome surgery for the treatment of autosomal dominant retinitis pigmentosa. Ophthalmology. 2018;125:1421–1430. doi: 10.1016/j.ophtha.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu M., Xie Y., Abouzeid H., Gordon C.T., Fiorentino A., Sun Z., Lehman A., Osman I.S., Dharmat R., Riveiro-Alvarez R., et al. Mutations in the Spliceosome Component CWC27 Cause Retinal Degeneration with or without Additional Developmental Anomalies. Am. J. Hum. Genet. 2017;100:592–604. doi: 10.1016/j.ajhg.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu W., Mookherjee S., Chaitankar V., Hiriyanna S., Kim J.-W., Brooks M., Ataeijannati Y., Sun X., Dong L., Li T., et al. Nrl knockdown by AAV-delivered CRISPR/Cas9 prevents retinal degeneration in mice. Nat. Commun. 2017;8:14716. doi: 10.1038/ncomms14716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moreno A.M., Fu X., Zhu J., Katrekar D., Shih Y.R.V., Marlett J., Cabotaje J., Tat J., Naughton J., Lisowski L., et al. In Situ Gene Therapy via AAV-CRISPR-Cas9-Mediated Targeted Gene Regulation. Mol. Ther. 2018;26:1818–1827. doi: 10.1016/j.ymthe.2018.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xia C.-H., Ferguson I., Li M., Kim A., Onishi A., Li L., Su B., Gong X. Essential function of NHE8 in mouse retina demonstrated by AAV-mediated CRISPR/Cas9 knockdown. Exp. Eye Res. 2018;176:29–39. doi: 10.1016/j.exer.2018.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yi Z., Ouyang J., Sun W., Xiao X., Li S., Jia X., Wang P., Zhang Q. Biallelic mutations in USP45, encoding a deubiquitinating enzyme, are associated with Leber congenital amaurosis. J. Med. Genet. 2019;56:325–331. doi: 10.1136/jmedgenet-2018-105709. [DOI] [PubMed] [Google Scholar]
- 79.McCullough K.T., Boye S.L., Fajardo D., Calabro K., Peterson J.J., Strang C.E., Chakraborty D., Gloskowski S., Haskett S., Samuelsson S., et al. Somatic Gene Editing of GUCY2D by AAV-CRISPR/Cas9 Alters Retinal Structure and Function in Mouse and Macaque. Hum. Gene Ther. 2019;30:571–589. doi: 10.1089/hum.2018.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li F., Hung S.S.C., Mohd Khalid M.K.N., Wang J.-H., Chrysostomou V., Wong V.H.Y., Singh V., Wing K., Tu L., Bender J.A., et al. Utility of Self-Destructing CRISPR/Cas Constructs for Targeted Gene Editing in the Retina. Hum. Gene Ther. 2019;30:1349–1360. doi: 10.1089/hum.2019.021. [DOI] [PubMed] [Google Scholar]
- 81.Yoshimi K., Kaneko T., Voigt B., Mashimo T. Allele-specific genome editing and correction of disease-associated phenotypes in rats using the CRISPR–Cas platform. Nat. Commun. 2014;5:1–9. doi: 10.1038/ncomms5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bakondi B., Lv W., Lu B., Jones M.K., Tsai Y., Kim K.J., Levy R., Akhtar A.A., Breunig J.J., Svendsen C.N., et al. In Vivo CRISPR/Cas9 Gene Editing Corrects Retinal Dystrophy in the S334ter-3 Rat Model of Autosomal Dominant Retinitis Pigmentosa. Mol. Ther. J. Am. Soc. Gene Ther. 2016;24:556–563. doi: 10.1038/mt.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feehan J.M., Chiu C.N., Stanar P., Tam B.M., Ahmed S.N., Moritz O.L. Modeling Dominant and Recessive Forms of Retinitis Pigmentosa by Editing Three Rhodopsin-Encoding Genes in Xenopus Laevis Using Crispr/Cas9. Sci. Rep. 2017;7:6920. doi: 10.1038/s41598-017-07153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mitchell J., Balem F., Tirupula K., Man D., Dhiman H.K., Yanamala N., Ollesch J., Planas-Iglesias J., Jennings B.J., Gerwert K., et al. Comparison of the molecular properties of retinitis pigmentosa P23H and N15S amino acid replacements in rhodopsin. PLoS ONE. 2019;14:1–14. doi: 10.1371/journal.pone.0214639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wimberg H., Lev D., Yosovich K., Namburi P., Banin E., Sharon D., Koch K.W. Photoreceptor Guanylate Cyclase (GUCY2D) Mutations Cause Retinal Dystrophies by Severe Malfunction of Ca2+-Dependent Cyclic GMP Synthesis. Front. Mol. Neurosci. 2018;11:1–12. doi: 10.3389/fnmol.2018.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Street V.A., Li J., Robbins C.A., Kallman J.C. A DNA variant within the MYO7A promoter regulates YY1 transcription factor binding and gene expression serving as a potential dominant DFNA11 auditory genetic modifier. J. Biol. Chem. 2011;286:15278–15286. doi: 10.1074/jbc.M111.228304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rodríguez-Pascau L., Coll M.J., Vilageliu L., Grinberg D. Antisense oligonucleotide treatment for a pseudoexon-generating mutation in the NPC1 gene causing Niemann-Pick type C disease. Hum. Mutat. 2009;30:E993–E1001. doi: 10.1002/humu.21119. [DOI] [PubMed] [Google Scholar]
- 88.Tan E., Ding X.Q., Saadi A., Agarwal N., Naash M.I., Al-Ubaidi M.R. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Investig. Ophthalmol. Vis. Sci. 2004;45:764–768. doi: 10.1167/iovs.03-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Imamura T., Tsuruma K., Inoue Y., Otsuka T., Ohno Y., Ogami S., Yamane S., Shimazawa M., Hara H. Involvement of cannabinoid receptor type 2 in light-induced degeneration of cells from mouse retinal cell line in vitro and mouse photoreceptors in vivo. Exp. Eye Res. 2018;167:44–50. doi: 10.1016/j.exer.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 90.Wang Y., Yin Z., Gao L., Sun D., Hu X., Xue L., Dai J., Zeng Y.X., Chen S., Pan B., et al. Curcumin Delays Retinal Degeneration by Regulating Microglia Activation in the Retina of rd1 Mice. Cell. Physiol. Biochem. 2017;44:479–493. doi: 10.1159/000485085. [DOI] [PubMed] [Google Scholar]
- 91.Balmer J., Zulliger R., Roberti S., Enzmann V. Retinal cell death caused by sodium iodate involves multiple caspase-dependent and caspase-independent cell-death pathways. Int. J. Mol. Sci. 2015;16:15086–15103. doi: 10.3390/ijms160715086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wheway G., Nazlamova L., Turner D., Cross S. 661W photoreceptor cell line as a cell model for studying retinal ciliopathies. Front. Genet. 2019;10:1–14. doi: 10.3389/fgene.2019.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spalluto C., Wilson D.I., Hearn T. Evidence for reciliation of RPE1 cells in late G1 phase, and ciliary localisation of cyclin B1. FEBS Open Bio. 2013;3:334–340. doi: 10.1016/j.fob.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Di Donato N., Neuhann T., Kahlert A.K., Klink B., Hackmann K., Neuhann I., Novotna B., Schallner J., Krause C., Glass I.A., et al. Mutations in EXOSC2 are associated with a novel syndrome characterised by retinitis pigmentosa, progressive hearing loss, premature ageing, short stature, mild intellectual disability and distinctive gestalt. J. Med. Genet. 2016;53:419–425. doi: 10.1136/jmedgenet-2015-103511. [DOI] [PubMed] [Google Scholar]
- 95.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 96.Avior Y., Sagi I., Benvenisty N. Pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Mol. Cell Biol. 2016;17:170–182. doi: 10.1038/nrm.2015.27. [DOI] [PubMed] [Google Scholar]
- 97.Foltz L.P., Clegg D.O. Patient-derived induced pluripotent stem cells for modelling genetic retinal dystrophies. Prog. Retin. Eye Res. 2019;68:54–66. doi: 10.1016/j.preteyeres.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 98.Bassett A.R. Editing the genome of hiPSC with CRISPR/Cas9: Disease models. Mamm. Genome. 2017;28:348–364. doi: 10.1007/s00335-017-9684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ovando-Roche P., Georgiadis A., Smith A.J., Pearson R.A., Ali R.R. Harnessing the Potential of Human Pluripotent Stem Cells and Gene Editing for the Treatment of Retinal Degeneration. Curr. Stem Cell Rep. 2017;3:112–123. doi: 10.1007/s40778-017-0078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ben Jehuda R., Shemer Y., Binah O. Genome Editing in Induced Pluripotent Stem Cells using CRISPR/Cas9. Stem Cell Rev. Rep. 2018;14:323–336. doi: 10.1007/s12015-018-9811-3. [DOI] [PubMed] [Google Scholar]
- 101.Michaelides M., Holder G.E., Bradshaw K., Hunt D.M., Moore A.T. Cone-rod dystrophy, intrafamilial variability, and incomplete penetrance associated with the R172W mutation in the peripherin/RDS gene. Ophthalmology. 2005;112:1592–1598. doi: 10.1016/j.ophtha.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 102.Maubaret C.G., Vaclavik V., Mukhopadhyay R., Waseem N.H., Churchill A., Holder G.E., Moore A.T., Bhattacharya S.S., Webster A.R. Autosomal Dominant Retinitis Pigmentosa with Intrafamilial Variability and Incomplete Penetrance in Two Families Carrying Mutations in PRPF8. Investig. Ophthalmol. Vis. Sci. 2011;52:9304–9309. doi: 10.1167/iovs.11-8372. [DOI] [PubMed] [Google Scholar]
- 103.Rose A.M., Bhattacharya S.S. Variant haploinsufficiency and phenotypic non-penetrance in PRPF31-associated retinitis pigmentosa. Clin. Genet. 2016;90:118–126. doi: 10.1111/cge.12758. [DOI] [PubMed] [Google Scholar]
- 104.Chapi M., Sabbaghi H., Suri F., Alehabib E., Rahimi-Aliabadi S., Jamali F., Jamshidi J., Emamalizadeh B., Darvish H., Mirrahimi M., et al. Incomplete penetrance of CRX gene for autosomal dominant form of cone-rod dystrophy. Ophthalmic Genet. 2019;40:259–266. doi: 10.1080/13816810.2019.1622023. [DOI] [PubMed] [Google Scholar]
- 105.Zhang X.-H., Tee L.Y., Wang X.-G., Huang Q.-S., Yang S.-H. Off-target Effects in CRISPR/Cas9-mediated Genome Engineering. Mol. Ther. Nucleic Acids. 2015;4:e264. doi: 10.1038/mtna.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eiraku M., Takata N., Ishibashi H., Kawada M., Sakakura E., Okuda S., Sekiguchi K., Adachi T., Sasai Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 107.Fligor C.M., Langer K.B., Sridhar A., Ren Y., Shields P.K., Edler M.C., Ohlemacher S.K., Sluch V.M., Zack D.J., Zhang C., et al. Three-Dimensional Retinal Organoids Facilitate the Investigation of Retinal Ganglion Cell Development, Organization and Neurite Outgrowth from Human Pluripotent Stem Cells. Sci. Rep. 2018;8:1–14. doi: 10.1038/s41598-018-32871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhong X., Gutierrez C., Xue T., Hampton C., Vergara M.N., Cao L.-H., Peters A., Park T.S., Zambidis E.T., Meyer J.S., et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat. Commun. 2014;5:4047. doi: 10.1038/ncomms5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wahlin K.J., Maruotti J.A., Sripathi S.R., Ball J., Angueyra J.M., Kim C., Grebe R., Li W., Jones B.W., Zack D.J. Photoreceptor Outer Segment-like Structures in Long-Term 3D Retinas from Human Pluripotent Stem Cells. Sci. Rep. 2017;7:766. doi: 10.1038/s41598-017-00774-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Achberger K., Haderspeck J.C., Kleger A., Liebau S. Stem cell-based retina models. Adv. Drug Deliv. Rev. 2019;140:33–50. doi: 10.1016/j.addr.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 111.Achberger K., Probst C., Haderspeck J., Bolz S., Rogal J., Chuchuy J., Nikolova M., Cora V., Antkowiak L., Haq W., et al. Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform. Elife. 2019:8. doi: 10.7554/eLife.46188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guo Y., Wang P., Ma J.H., Cui Z., Yu Q., Liu S., Xue Y., Zhu D., Cao J., Li Z., et al. Modeling Retinitis Pigmentosa: Retinal Organoids Generated From the iPSCs of a Patient With the USH2A Mutation Show Early Developmental Abnormalities. Front. Cell. Neurosci. 2019:13. doi: 10.3389/fncel.2019.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mellough C.B., Collin J., Queen R., Hilgen G., Dorgau B., Zerti D., Felemban M., White K., Sernagor E., Lako M. Systematic Comparison of Retinal Organoid Differentiation from Human Pluripotent Stem Cells Reveals Stage Specific, Cell Line, and Methodological Differences. Stem Cells Transl. Med. 2019;8:694–706. doi: 10.1002/sctm.18-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rubio-Peña K., Fontrodona L., Aristizábal-Corrales D., Torres S., Cornes E., García-Rodríguez F.J., Serrat X., González-Knowles D., Foissac S., Porta-De-La-Riva M., et al. Modeling of autosomal-dominant retinitis pigmentosa in Caenorhabditis elegans uncovers a nexus between global impaired functioning of certain splicing factors and cell type-specific apoptosis. RNA. 2015;21:2119–2131. doi: 10.1261/RNA.053397.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bramall A.N., Wright A.F., Jacobson S.G., McInnes R.R. The Genomic, Biochemical, and Cellular Responses of the Retina in Inherited Photoreceptor Degenerations and Prospects for the Treatment of These Disorders. Annu. Rev. Neurosci. 2010;33:441–472. doi: 10.1146/annurev-neuro-060909-153227. [DOI] [PubMed] [Google Scholar]
- 116.Houthoofd K., Braeckman B.P., Lenaerts I., Brys K., De Vreese A., Van Eygen S., Vanfleteren J.R. Ageing is reversed, and metabolism is reset to young levels in recovering dauer larvae of C. elegans. Exp. Gerontol. 2002;37:1015–1021. doi: 10.1016/S0531-5565(02)00063-3. [DOI] [PubMed] [Google Scholar]
- 117.Grün D., Kirchner M., Thierfelder N., Stoeckius M., Selbach M., Rajewsky N. Conservation of mRNA and protein expression during development of C.elegans. Cell Rep. 2014;6:565–577. doi: 10.1016/j.celrep.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 118.Venturini G., Rose A.M., Shah A.Z., Bhattacharya S.S., Rivolta C. CNOT3 is a modifier of PRPF31 mutations in retinitis pigmentosa with incomplete penetrance. PLoS Genet. 2012;8:e1003040. doi: 10.1371/journal.pgen.1003040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ugur B., Chen K., Bellen H.J. Drosophila tools and assays for the study of human diseases. DMM Dis. Models Mech. 2016;9:235–244. doi: 10.1242/dmm.023762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Colley N.J., Cassill J.A., Baker E.K., Zuker C.S. Defective intracellular transport is the molecular basis of rhodopsin- dependent dominant retinal degeneration. Proc. Natl. Acad. Sci. USA. 1995;92:3070–3074. doi: 10.1073/pnas.92.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang F., Shi Z., Cui Y., Guo X., Shi Y.B., Chen Y. Targeted gene disruption in Xenopus laevis using CRISPR/Cas9. Cell Biosci. 2015;5:1–5. doi: 10.1186/s13578-015-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tandon P., Conlon F., Furlow J.D., Horb M.E. Expanding the genetic toolkit in Xenopus: Approaches and opportunities for human disease modeling. Dev. Biol. 2017;426:325–335. doi: 10.1016/j.ydbio.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feehan J.M., Stanar P., Tam B.M., Chiu C., Moritz O.L. Generation and Analysis of Xenopus Laevis Models of Retinal Degeneration Using CRISPR/Cas9. Methods Mol. Biol. 2019;1834:193–207. doi: 10.1007/978-1-4939-8669-9_14. [DOI] [PubMed] [Google Scholar]
- 124.Streisinger G., Walker C., Dower N., Knauber D., Singer F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio) Nature. 1981;291:293–296. doi: 10.1038/291293a0. [DOI] [PubMed] [Google Scholar]
- 125.Kimmel C.B. Genetics and early development of zebrafish. Trends Genet. 1989;5:283–288. doi: 10.1016/0168-9525(89)90103-0. [DOI] [PubMed] [Google Scholar]
- 126.Driever W., Stemple D., Schier A., Solnica-Krezel L. Zebrafish: Genetic tools for studying vertebrate development. Trends Genet. 1994;10:152–159. doi: 10.1016/0168-9525(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 127.Haffter P., Nüsslein-Volhard C. Large scale genetics in a small vertebrate, the zebrafish. Int. J. Dev. Biol. 1996;40:221–227. [PubMed] [Google Scholar]
- 128.Lawson N.D., Wolfe S.A. Forward and reverse genetic approaches for the analysis of vertebrate development in the zebrafish. Dev. Cell. 2011;21:48–64. doi: 10.1016/j.devcel.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 129.Meyer A., Schartl M. Gene and genome duplications in vertebrates: The one-to-four (-to-eight in fish) rule and the evolution of novel gene functions. Curr. Opin. Cell Biol. 1999;11:699–704. doi: 10.1016/s0955-0674(99)00039-3. [DOI] [PubMed] [Google Scholar]
- 130.Howe K., Clark M.D., Torroja C.F., Torrance J., Berthelot C., Muffato M., Collins J.E., Humphray S., McLaren K., Matthews L., et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kettleborough R.N.W., Busch-Nentwich E.M., Harvey S.A., Dooley C.M., de Bruijn E., van Eeden F., Sealy I., White R.J., Herd C., Nijman I.J., et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature. 2013;496:494–497. doi: 10.1038/nature11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Varshney G.K., Burgess S.M. Mutagenesis and phenotyping resources in zebrafish for studying development and human disease. Brief. Funct. Genom. 2014;13:82–94. doi: 10.1093/bfgp/elt042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vihtelic T.S., Hyde D.R. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J. Neurobiol. 2000;44:289–307. doi: 10.1002/1097-4695(20000905)44:3<289::aid-neu1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 134.Fimbel S.M., Montgomery J.E., Burket C.T., Hyde D.R. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J. Neurosci. 2007;27:1712–1724. doi: 10.1523/JNEUROSCI.5317-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ariga J., Walker S.L., Mumm J.S. Multicolor Time-lapse Imaging of Transgenic Zebrafish: Visualizing Retinal Stem Cells Activated by Targeted Neuronal Cell Ablation. J. Vis. Exp. 2010;43:2093. doi: 10.3791/2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Montgomery J.E., Parsons M.J., Hyde D.R. A Novel Model of Retinal Ablation Demonstrates That the Extent of Rod Cell Death Regulates the Origin of the Regenerated Zebrafish Rod Photoreceptors. J. Comp. Neurol. 2010;518:800–814. doi: 10.1002/cne.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fraser B., DuVal M.G., Wang H., Allison W.T. Regeneration of cone photoreceptors when cell ablation is primarily restricted to a particular cone subtype. PLoS ONE. 2013;8:e55410. doi: 10.1371/journal.pone.0055410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tappeiner C., Balmer J., Iglicki M., Schuerch K., Jazwinska A., Enzmann V., Tschopp M. Characteristics of rod regeneration in a novel zebrafish retinal degeneration model using N-methyl-N-nitrosourea (MNU) PLoS ONE. 2013;8:e71064. doi: 10.1371/journal.pone.0071064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Powell C., Cornblath E., Elsaeidi F., Wan J., Goldman D. Zebrafish Müller glia-derived progenitors are multipotent, exhibit proliferative biases and regenerate excess neurons. Sci. Rep. 2016;6:24851. doi: 10.1038/srep24851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Amsterdam A., Varshney G.K., Burgess S.M. Retroviral-mediated Insertional Mutagenesis in Zebrafish. Methods Cell Biol. 2011;104:59–82. doi: 10.1016/B978-0-12-374814-0.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mali P., Yang L., Esvelt K.M., Aach J., Guell M., DiCarlo J.E., Norville J.E., Church G.M. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hsu P.D., Lander E.S., Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hwang W.Y., Fu Y., Reyon D., Maeder M.L., Tsai S.Q., Sander J.D., Peterson R.T., Yeh J.-R.J., Joung J.K. Efficient In Vivo Genome Editing Using RNA-Guided Nucleases. Nat. Biotechnol. 2013;31:227–229. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Van De Weghe J.C., Rusterholz T.D.S., Latour B., Grout M.E., Aldinger K.A., Shaheen R., Dempsey J.C., Maddirevula S., Cheng Y.-H.H., Phelps I.G., et al. Mutations in ARMC9, which Encodes a Basal Body Protein, Cause Joubert Syndrome in Humans and Ciliopathy Phenotypes in Zebrafish. Am. J. Hum. Genet. 2017;101:23–36. doi: 10.1016/j.ajhg.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vaché C., Besnard T., le Berre P., García-García G., Baux D., Larrieu L., Abadie C., Blanchet C., Bolz H.J., Millan J., et al. Usher syndrome type 2 caused by activation of an USH2A pseudoexon: Implications for diagnosis and therapy. Hum. Mutat. 2012;33:104–108. doi: 10.1002/humu.21634. [DOI] [PubMed] [Google Scholar]
- 146.Slijkerman R.W.N., Song F., Astuti G.D.N., Huynen M.A., van Wijk E., Stieger K., Collin R.W.J. The pros and cons of vertebrate animal models for functional and therapeutic research on inherited retinal dystrophies. Prog. Retin. Eye Res. 2015;48:137–159. doi: 10.1016/j.preteyeres.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 147.Kotterman M.A., Chalberg T.W., Schaffer D.V. Viral Vectors for Gene Therapy: Translational and Clinical Outlook. Annu. Rev. Biomed. Eng. 2015;17:63–89. doi: 10.1146/annurev-bioeng-071813-104938. [DOI] [PubMed] [Google Scholar]
- 148.Ramlogan-Steel C.A., Murali A., Andrzejewski S., Dhungel B., Steel J.C., Layton C.J. Gene therapy and the adeno-associated virus in the treatment of genetic and acquired ophthalmic diseases in humans: Trials, future directions and safety considerations. Clin. Exp. Ophthalmol. 2019;47:521–536. doi: 10.1111/ceo.13416. [DOI] [PubMed] [Google Scholar]
- 149.Wang D., Mou H., Li S., Li Y., Hough S., Tran K., Li J., Yin H., Anderson D.G., Sontheimer E.J., et al. Adenovirus-Mediated Somatic Genome Editing of Pten by CRISPR/Cas9 in Mouse Liver in Spite of Cas9-Specific Immune Responses. Hum. Gene Ther. 2015;26:432–442. doi: 10.1089/hum.2015.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bowne S.J., Humphries M.M., Sullivan L.S., Kenna P.F., Tam L.C.S., Kiang A.S., Campbell M., Weinstock G.M., Koboldt D.C., Ding L., et al. A dominant mutation in RPE65 identified by whole-exome sequencing causes retinitis pigmentosa with choroidal involvement. Eur. J. Hum. Genet. 2011;19:1074–1081. doi: 10.1038/ejhg.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hull S., Mukherjee R., Holder G.E., Moore A.T., Webster A.R. The clinical features of retinal disease due to a dominant mutation in RPE65. Mol. Vis. 2016;22:626–635. [PMC free article] [PubMed] [Google Scholar]
- 152.Perleberg C., Kind A., Schnieke A. Genetically engineered pigs as models for human disease. Dis. Models Mech. 2018:11. doi: 10.1242/dmm.030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sachs D.H. The pig as a potential xenograft donor. Vet. Immunol. Immunopathol. 1994;43:185–191. doi: 10.1016/0165-2427(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 154.Harding J.D. Nonhuman primates and translational research: Progress, opportunities, and challenges. ILAR J. 2017;58:141–150. doi: 10.1093/ilar/ilx033. [DOI] [PMC free article] [PubMed] [Google Scholar]