Abstract
Neurotoxicity linked to excessive brain manganese levels can occur as a result of high level Mn exposures and/or metabolic aberrations (liver disease and decreased biliary excretion). Increased brain manganese levels have been reported to induce oxidative stress, as well as alterations in neurotransmitter metabolism with concurrent neurobehavioral and motor deficits. Two putative mechanisms in which manganese can produce oxidative stress in the brain are: (1) via its oxidation of dopamine, and (2) interference with normal mitochondrial respiration. Measurements of antioxidant species (e.g., glutathione and metallothionein), and the abundance of proteins (enzymes) exquisitely sensitive to oxidation (e.g., glutamine synthetase) have been commonly used as biomarkers of oxidative stress, particularly in rat brain tissue. This paper examines the link between manganese neurotoxicity in the rat brain and common pathways to oxidative stress.
Keywords: Brain, Manganese, Neurotoxicity, Glutathione, Metallothionein, Glutamine synthetase, MMT
1. Introduction
1.1. Manganese neurotoxicity
Manganese is an essential nutrient and it is important for optimal cellular function. However, exceedingly high brain manganese concentrations are known to cause neurotoxicity (Cotzias et al., 1968). Manganese has been implicated in oxidative stress (Stokes et al., 2000; DeSole et al., 1997), as well as disturbance of neurotransmitter metabolism (Miele et al., 2000; Montes et al., 2001). Few reports exist on brain manganese concentrations upon manganese intoxication. The concentrations of manganese in human striatum and globus pallidus (primary target areas) are unknown. Cerebellar concentrations of manganese are available from Japanese accident victims, and at the time of autopsy they ranged between 9 and 10 μM (Sumino et al., 1975). However, these values are likely lower than those in the striatum and globus pallidus, both of which are known to accumulate more manganese than cerebellum. A study performed almost three decades ago examined manganese concentration in striatum and globus pallidus (two regions in the nonhuman primate known to accumulate manganese) of monkeys dosed for 3 months with manganese dioxide (Suzuki et al., 1975). Striatal manganese concentration reached 264 μM, while manganese concentration in globus pallidus peaked at 334 μM. In rats, manganese concentrations can reach up to 200 μM depending upon the brain region and dosing regiment (Ingersoll et al., 1999; Lai et al., 1999; Roels et al., 1997). Thus, during manganese toxicity, it is possible for brain levels to exceed 350 μM. Manganese concentrations in various parts of the rat striatum have been reported to range from 4.4 to 18 μM, and in exposed rats, the levels of manganese in the striatum increase to 23–70 μM (Ingersoll et al., 1999; Lai et al., 1999; Roels et al., 1997). Notably, these studies suggest that both in primates and rodents, homeostatic control of brain manganese concentrations is tight, because even in conditions of high exposures, levels of brain manganese increase only by several fold (3–4).
The manganese from MMT is in the form of several aerosolized salts when it is combusted. The most abundant species represented by manganese phosphate and manganese sulfate (Lynam et al., 1999; Zayed et al., 1999). Pharmacokinetic studies have shown that the salt characteristics will determine the rate of transport into the brain with the following rank order: MnCl2>MnSO4>MnPO4 (Drown et al., 1986; Dorman et al., 2001). Nonetheless, it is important to note that regardless of exposure, once absorbed and within biological media (e.g., blood, cerebrospinal fluid), manganese would be expected to bind to the same ligands and behave in an analogous pharmacokinetic fashion. Thus, the physical and chemical properties of these aerosolized salts will only govern their absorption and elimination properties, but overall the tissue distribution and their mechanisms of toxicity would be expected to be similar, yet on a differential temporal scale (e.g., attainment of toxic concentrations of manganese in target tissue would be reached faster for the more soluble manganese salts).
Manganese neurotoxicity (manganism) shares neurological symptoms with several clinical disorders commonly described as “extrapyramidal motor system dysfunction”, and in particular, Parkinson’s disease (PD) (Calne et al., 1994). Evidence for the involvement of oxidative stress in the etiology of PD exists, supported by the following observations: (1) Monoamine oxidase (MAO) activity, which catabolizes intraneuronal dopamine and yields hydrogen peroxide (H2O2), increases with age (Fahn and Cohen, 1992). (2) Pharmacological manipulations that enhance dopamine turnover cause an increase in oxidized glutathione (GSH), which can be suppressed by simultaneous treatment with the MAO inhibitors clorgyline and deprenyl, indicating that MAO activity is a source of oxidative stress (Cohen and Spina, 1989). (3) In postmortem studies of PD brains, the activity of glutathione peroxidase and the amounts of glutathione (GSH) in the substantia nigra are reduced (Riederer et al., 1989). (4) Oxidative stress is one of the proximate causes of 1-methyl-4-phenyl-1,2,3,6-tetrahyrdopyridine (MPTP)-induced dopaminergic neuronal degeneration, and transgenic mice overexpressing copper/zinc superoxide dismutase are resistant to the neurotoxic action of MPTP (Przedborski et al., 1992).
1.2. Oxidative stress
Oxidative stress has been implicated as a contributing mechanism by which manganese can be toxic to cells (Galvani et al., 1995; Aschner, 1997). A potential mechanism for manganese-induced oxidative stress is via the oxidation of dopamine and other catecholamines (Sloot et al., 1996). This is likely because in primates, manganese accumulates in dopamine-rich regions, especially in the basal ganglia (Newland, 1999). Another possibility is that sequestration of manganese in mitochondria interferes with proper respiration, thereby leading to excessive production of reactive oxygen species (ROS). Small initial amounts of oxygen can be self-propagating via damage to the electron transport chain in mitochondria. When proteins or quinones that participate in transfer of electrons are damaged, the chain begins to donate electrons directly to molecular oxygen, thereby creating the highly reactive superoxide radical. This leak of electrons to form superoxide is most commonly reported at complex I (Blake et al., 1997; Bautista et al., 2000).
Galvani et al. (1995) reported inhibition of complex I of the electron transport chain after treatment of PC12 cell cultures with MnCl2. Divalent manganese treatment in HeLa cells was shown to increase in the production of ROS (peroxides), and to induce manganese superoxide dismutase and catalase activities without an effect on the Cu,Zn-superoxide dismutase level (Oubrahim et al., 2001). Gavin et al. (1999) showed evidence suggesting that the ATPase complex is inhibited at very low levels of mitochondrial manganese, and that complex I is inhibited only at higher manganese concentrations. In agreement with the earlier suggestion of Tyree and Archibald and Tyree (1987) and the findings of Ali et al. (1995), Chen et al. (2001) demonstrated that trivalent manganese is more potent at inhibiting complex I, but the divalent form is the predominant species within cells and is largely bound to ATP. Nevertheless, manganese in any oxidation state will likely spontaneously give rise to infinitesimal amounts of trivalent manganese, and HaMai et al. (2001) demonstrated that even at trace amounts, trivalent manganese can cause formation of ROS. They also showed evidence that divalent manganese fails to induce oxidative effects. Finally, a recent report showed that exposure of dopaminergic cells to MMT resulted in rapid increases in ROS followed by mitochondrial-induced apoptosis (Anantharam et al., 2002). However, because combustion of MMT in cars yields various manganese salts, direct exposure of cells to MMT does not seem to represent a toxicologically relevant experimental model, and it is not germane to a discussion on the neurotoxicity of inorganic manganese.
Notably, other studies have failed to support ROS generation and oxidative stress as a result of exposure to manganese. Brenneman et al. (1999) examined the effect of orally [0, 25, or 50 mg/kg/day of MnCl2 from postnatal day (PND) 1 to 49] administered manganese on brain function in developing CD rats. The high-dose manganese exposure was associated with increased spontaneous motor activity on PND 21 and ROS levels (8hydroxy-2′ -deoxyguanosine) were elevated in cerebellum but not striatum, the latter representing the primary target site in primates. Additional studies showed that iron sulfate, copper sulfate, vanadyl sulfate, cobalt sulfate, nickel sulfate, and zinc sulfate generate ROS (formation of malondialdehyde) in aqueous extracts of National Institute of Standards and Technology (NIST) ambient particulate matter and diesel engine particles, but that manganese failed to do so (oral communication, Lynam). Furthermore, it has been shown that low concentrations of divalent manganese protect cells against oxidative stress and that this protective effect derives from the fact that manganese can catalyze the dismutation of superoxide radical anions and H2O2 under physiological conditions (Oubrahim et al., 2001).
Astrocytes take up glutamate by a Na+-dependent mechanism (Hertz, 1979). In the presence of ammonia, glutamate is metabolized to glutamine by the astrocyte-specific enzyme glutamine synthetase (GS; Martinez-Hernandez et al., 1977), maintaining [glutamate]O at 0.3 μM (Waniewski and Martin, 1986). This represents a 10,000-fold gradient vs. [glutamate]i (3 mM). This glutamate-glutamine pathway constitutes the source of a glutamate pool in brain (Berl et al., 1961). GS contains eight manganese ions per octamer, and accounts for approximately 80% of total manganese in brain. Unlike neurons, astrocytes have the ability to concentrate manganese at levels 50-fold higher than the culture media (Wedler et al., 1989; Aschner et al., 1992), providing a mechanism by which manganese concentrations in astrocytic cytosol could attain the range required for activation of GS. Recent studies in our laboratory (Erikson and Aschner, 2002; Erikson et al., 2002) and others (Hazell and Norenberg, 1997) have demonstrated that glutamate uptake is significantly attenuated in primary astrocyte cultures upon addition of manganese to the culture medium. Lafon-Cazal et al. (1993) showed that glutamate receptor (NMDA) stimulation produces significant elevations in both superoxide and hydroxyl radicals in cultured cerebellar granule cells. Interference with glutamate transporter function will lead to increased extracellular glutamate concentrations. Potential ROS generation as a consequence of manganese exposure will further oppose the removal of extracellular glutamate by inhibiting the high affinity glutamate transporters (Trotti et al., 1998). The cumulative sum of these events will trigger an amplifying cycle, potentially contributing to the dysfunction of astrocytes and their inability to maintain optimal control over the extracellular milieu, thereby indirectly leading to neuronal demise via NMDA receptor activation.
1.3. Biomarkers of oxidative stress
Oxidative stress can be measured by detection of different chemical species. Dichlorofluroescein (DCF) is useful for detecting oxygen radicals via a fluorescence-producing reaction. Ali et al. (1995) demonstrated that manganese injected in vivo and exposure of brain regions to manganese in vitro both resulted in detection of RO5 by DCF fluorescence. A limitation of this method is that living cells are required for analysis. Measurement of products directly related to RO5 is also a practical method for assessing oxidative stress in tissue. Examples include the measurement of oxidative damage in DNA (comet assay), lipid [thiobarbituric acid reactive species (TBAR5)] and protein (glutathione peroxidase activity). However, inconsistency between methods has been problematic. Another approach for detection of oxidants is to measure the levels of antioxidants [glutathione (G5H) or metallothionein (MT)], as well as the abundance of proteins [glutamine synthetase (G5)] that are exquisitely sensitive to oxidative stress.
The tripeptide G5H (γ-glutamylcysteinylglycine) is a major antioxidant in mammalian cells constituting nearly 90% of the intracellular non-protein thiols (Anderson and Meister, 1983). It is important in maintaining the intracellular redox status of protein thiols, for protection against endogenous and exogenous sources of oxidative stress, and for the conjugation and excretion of toxic molecules (Meister, 1988,1991).
The MTs, a class of cysteine-containing intracellular proteins, are highly conserved and are widely distributed throughout all cells in an organism. They are an important metal binding protein, with zinc serving as the primary regulator of MT metabolism in cells (Andrews, 1990; Dunn et al., 1987; Hamer, 1986). Evidence suggests that MT acts as an antioxidant by neutralizing RO5 both systemically and in the brain. In situ hybridization studies showed that bacterial endotoxin induces MT gene expression (Itano et al., 1991). Oxidative stress, kainic acid, and 6-hydroxydopamine, a known dopaminergic toxin and RO5 generator, induced MT-I gene expression in the brain (Shiraga et al., 1993). Likewise, compounds (e.g., diquat) that generate free oxygen species via the redox cycling increase MT in tissue, along with compounds (e.g., 3-methylindole) that cause lipid peroxidation, and compounds (e.g., diamide and di-methyl maleate) that deplete cellular defense mechanisms (Bauman et al., 1991).
Within the CN5, G5 is exclusively expressed in astrocytes (Martinez-Hernandez et al., 1977). A manganese-dependent enzyme, G5 catalyzes the formation of glutamine from glutamate. This glutamine is taken up by neighboring glutamatergic or γ-aminobutyric acid (GABA-ergic) neurons and deamination back to glutamate occurs. This process is considered the primary glutamate-recycling pathway (Van den Berg and Garfinkel, 1971; Westergaard et al., 1995; Ottersen et al., 1992). Inhibition of G5 activity can have serious consequences on neuronal functioning (e.g., decreased glutamate and GABA levels and the inability to detoxify ammonia). Given its high susceptibility to oxidation, and subsequent rapid degradation, G5 serves as an excellent marker for the presence of RO5 in the brain (Stadtman, 1992).
2. Results and discussion
Given the above, two recent studies in our laboratory have examined the relationship between manganese exposure and oxidative stress in rat brain tissues. The first study employed oral administration of manganese chloride (MnCl2) (0, 25, and 50 mg/kg) to rat neonates throughout the lactational period (postnatal days, PND 0–21) (Dorman et al., 2000; Weber et al., 2002), and the second study investigated the effects of inhaled manganese sulfate (MnSO4) (0, 0.03, 0.3, 3.0 mg Mn/m3) for 6 h/day for 14 days on adult rats (PND 70) (Dorman et al., 2001; Dobson et al., 2003).
Due to differences in study protocols and design, and limitations in tissue availability the neonatal study was restricted to investigating oxidative stress biomarkers in the cortex and cerebellum (Weber et al., 2002). The inhalation study (adult rat) was broader and included the striatum, olfactory bulb, hypothalamus, hippocampus, and cerebellum (Dobson et al., 2003). Accordingly, direct comparisons between the two studies are limited, and the discussion of the results is focused on the effects of manganese exposure on oxidative stress biomarkers only within the cerebellum.
A 50 mg/kg oral manganese caused a significant increase in cerebellar manganese concentrations in neonatal rats (Dorman et al., 2000), and there was a slight, albeit not statistically significant, increase in cerebellar manganese concentration upon inhalation of 3.0 mg Mn/m3 in adult rats (Dorman et al., 2001) (Fig. 1). Given differences in exposure paradigms (oral vs. inhaled) and differences in total administered doses, the two studies cannot be directly compared in terms of net brain manganese accumulation. Nevertheless, the magnitude of increased manganese brain concentrations is greater in the neonatal cohort, corroborating earlier observation that the developing brain takes up a significant proportion of the manganese retained by the body during the early neonatal period.
Fig. 1.
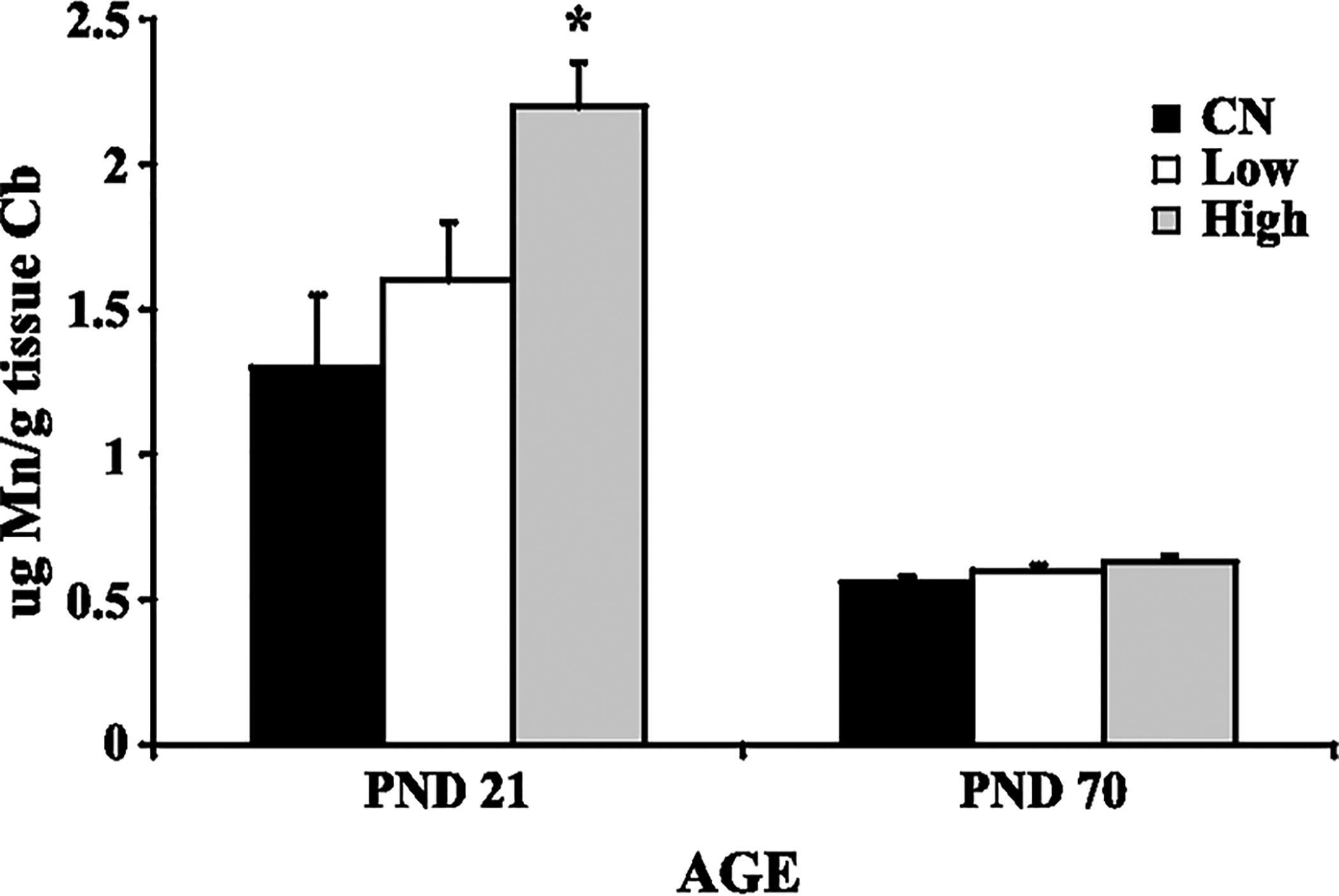
Manganese (Mn) concentration in the cerebellum of developing (PND 21) and adult (PND 70) rats: control (CN), low dose (Low), high dose (High). Data represent means ± S.E.M. High dose of manganese administered orally caused a significant increase (p < 0.05) in cerebellar manganese concentration. Statistical analysis was carried out by one-way analysis of variance followed by Newman-Keuls.
For example, Keen et al. (1986) have reported that approximately 8% of the total oral manganese dose is retained in the neonatal rat brain, exceeding the retention in the adult CNS. Known pharmacokinetic processes that may contribute to the increased tissue concentrations and susceptibility of neonatal animals to manganese-induced neurotoxicity include increased manganese absorption from the gastrointestinal tract, an incompletely formed blood-brain barrier, and the virtual absence of biliary manganese excretory mechanisms until weaning (Ballatori et al., 1987; Rehnberg et al., 1982; Miller et al., 1975). The relative higher net increase in brain manganese concentrations in the neonatal vs. adult brain (Fig. 1) should not necessarily be construed as evidence for heightened neurotoxicity in developing animals, given the known requirement for manganese for optimal CNS development in the neonate. Manganese concentration in the brain of developing rats is highest of all age groups, suggesting that manganese is required in a high amount during infancy, and that a sufficient manganese supply is critical for normal brain development (Takeda et al., 1999). The high uptake of manganese into the brain of neonatal rats reflects blood concentrations of manganese that are threefold higher than in adults (Dupont and Tanaka, 1985; Spencer, 1999).
Increased GS mRNA expression in the cerebellum of both PND 21 rats (high dose exposure) and PND 70 rats (low and high dose exposures) exposed to manganese was observed (Fig. 2) (Dobson et al., 2003; Weber et al., 2002). This increased GS mRNA expression at the high dose in the PND 21 rats correlated with increased cerebellar manganese concentration (Fig. 1). There was no effect of manganese on cerebellar GS protein levels in either study (Fig. 3), nor was there any significant effect on total GSH levels in the cerebellum of manganese exposed rats (Fig. 4). Finally, manganese exposure in both studies did not significantly affect MT mRNA expression in the cerebellum (Fig. 5).
Fig. 2.
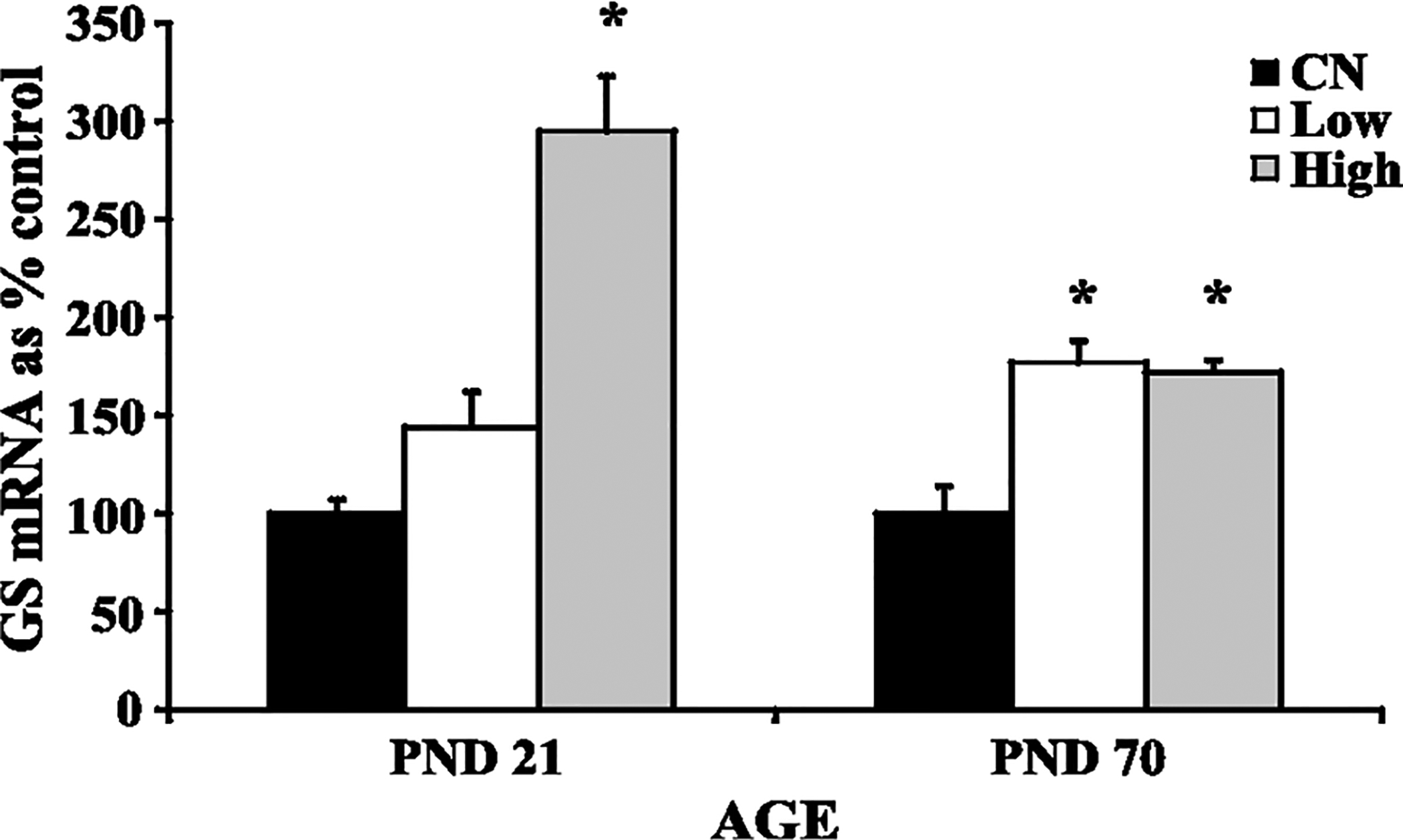
Glutamine synthetase (GS) mRNA levels in cerebellum of developing (PND 21) and adult (PND 70) rats: control (CN), low dose (Low), high dose (High). Data represent means ± S.E.M. expressed as percentage of control. There was a significant (p < 0.05) increase in GS mRNA levels in the cerebellum of rats exposed to manganese, both via the oral and inhaled routes. Statistical analysis was carried out by one-way analysis of variance followed by Newman-Keuls.
Fig. 3.
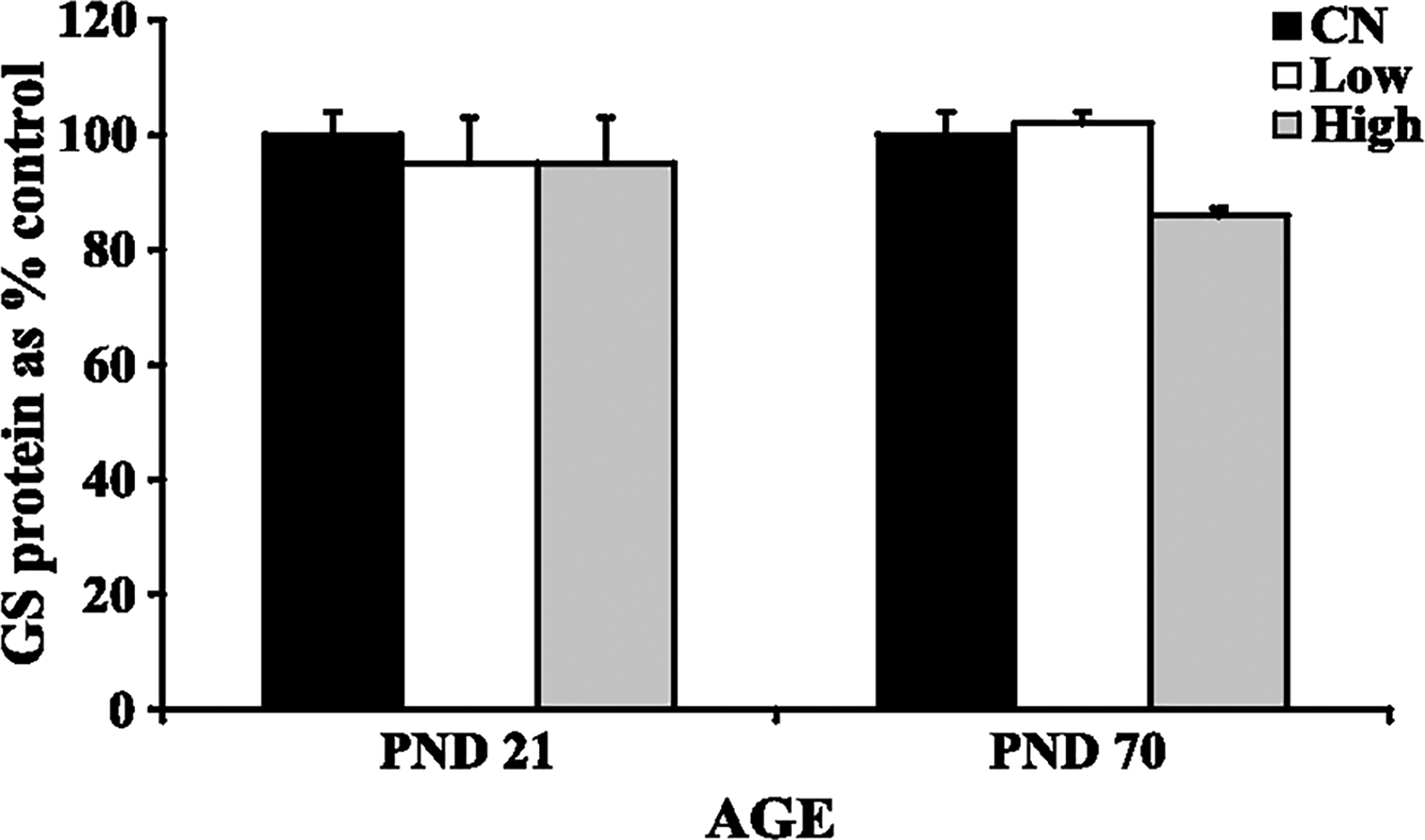
Glutamine synthetase (GS) protein levels in cerebellum of developing (PND 21) and adult (PND 70) rats: control (CN), low dose (Low), high dose (High). Data represent means ± S.E.M. expressed as percentage of control. There was no effect of manganese exposure on GS levels in the cerebellum of developing or adult rats. Statistical analysis was carried out by one-way analysis of variance followed by Newman-Keuls.
Fig. 4.
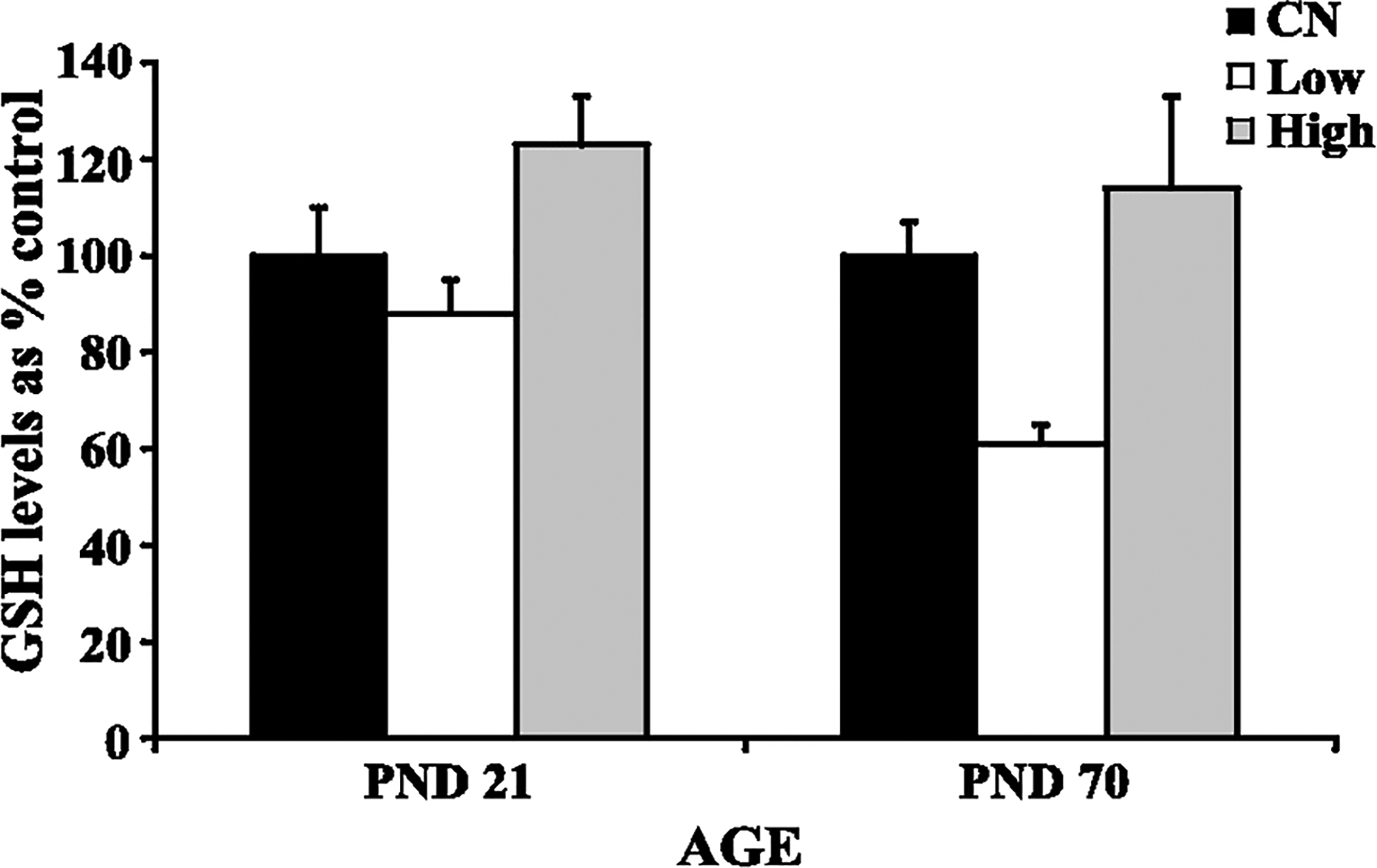
Glutathione (GSH) levels in cerebellum of developing (PND 21) and adult (PND 70) rats: control (CN), low dose (Low), high dose (High). Data represent means ± S.E.M. expressed as percentage of control. Although GSH levels increased in rats exposed to high manganese levels, it was not statistically significant. Statistical analysis was carried out by one-way analysis of variance followed by Newman-Keuls.
Fig. 5.
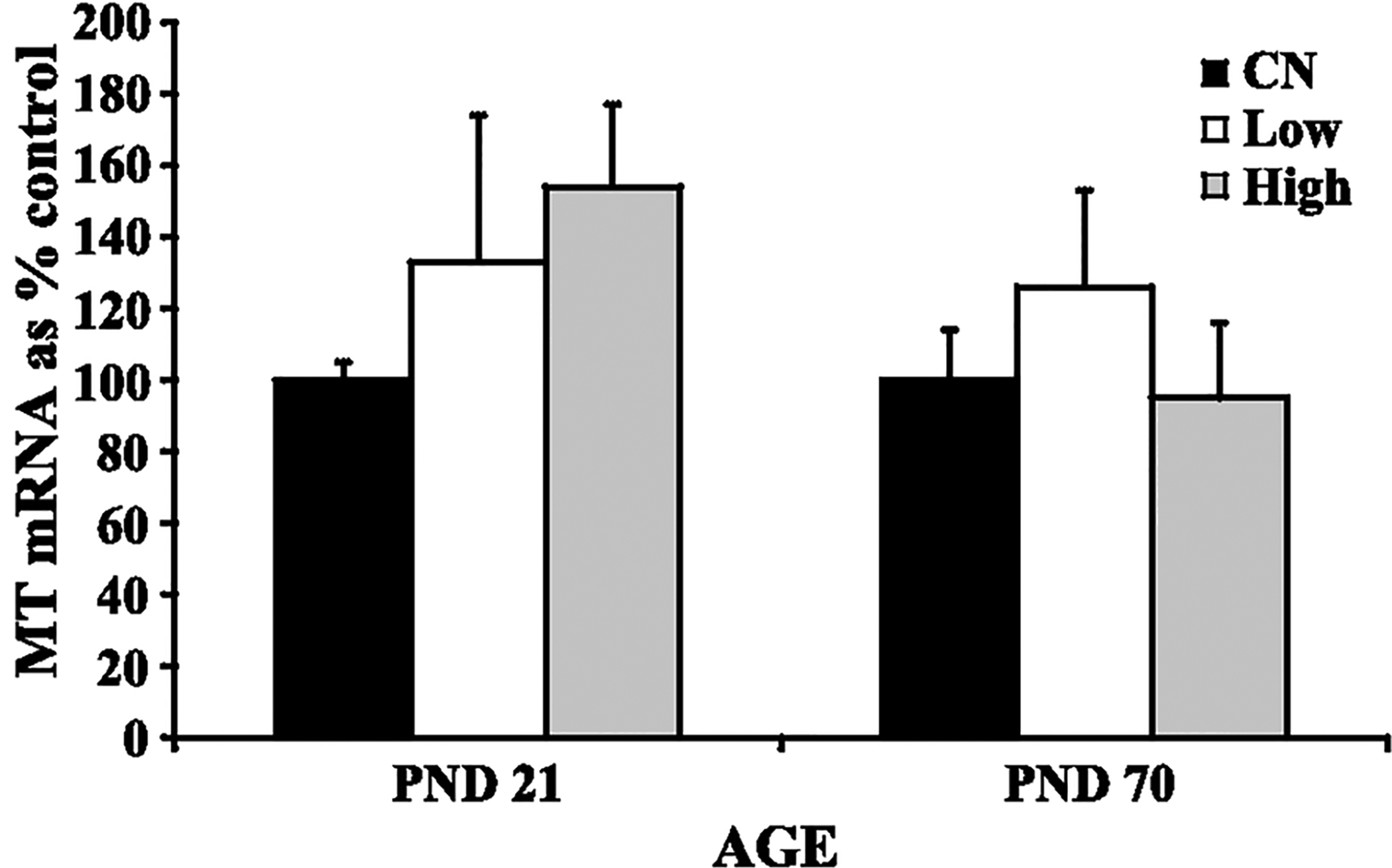
Metallothionein (MT) mRNA levels in the cerebellum of developing (PND 21) and adult (PND 70) rats: control (CN), low dose (Low), high dose (High). Data represent means + S.E.M. expressed as percentage of control. There was no effect of manganese exposure on MT mRNA expression in the cerebellum of developing or adult rats. Statistical analysis was carried out by one-way analysis of variance followed by Newman-Keuls.
3. Conclusion
These studies demonstrate that both of these routes of exposure, oral and inhaled (neonatal and adult rats, respectively), led to minimal biochemical changes indicative of manganese-induced oxidative stress in cerebellar tissues. The only ensuing change in response to manganese exposures was an increase GS mRNA (in both cohorts), but notably, GS protein levels were unchanged, suggesting that posttranslational process maintain adequate GS protein expression. Accordingly, in the cerebellum, manganese treatment does not appear to inflict functional damage that is correlated with the generation of oxidative stress.
References:
- Ali SF, Duhart HM, Newport GD, Lipe GW, Slikker W. Manganese-induced reactive oxygen species: comparison between Mn+2 and Mn+3. Neurodegeneration 1995;4:329–34. [DOI] [PubMed] [Google Scholar]
- Anantharam V, Kitazawa M, Wagner J, Kaul S, Kanthasamy AG. Caspase-3-dependent proteolytic cleavage of protein kinase C delta is essential for oxidative stress-mediated dopaminergic cell death after exposure to methylcyclopentadienyl manganese tricarbonyl. J Neurosci 2002;22:1738–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ME, Meister A. Transport and direct utilization of gamma-glutamylcyst(e)ine for glutathione synthesis. Proc Natl Acad Sci 1983;80:707–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews GK. Regulation of metallothioneins gene expression. Prog Food Nutr Sci 1990;14:193–258. [PubMed] [Google Scholar]
- Archibald FS, Tyree C. Manganese poisoning and the attack of trivalent manganese upon catecholamines. Arch Biochem Biophys 1987;256:638–50. [DOI] [PubMed] [Google Scholar]
- Aschner M Manganese neurotoxicity and oxidative damage Metals and Oxidative Damage in Neurological Disorders. New York: Plenum Press; 1997. p. 77–93. [Google Scholar]
- Aschner M, Gannon M, Kimelberg HK. Manganese (Mn) uptake and efflux in cultured rat astrocytes. J Neurochem 1992;58:730–5. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Miles E, Clarkson TW. Homeostatic control of manganese excretion in the neonatal rat. Am J Physiol 1987;252:R842–7. [DOI] [PubMed] [Google Scholar]
- Bauman JW, Liu J, Liu YP, Klaassen CD. Increase in metallothionein produced by chemicals that induced oxidative stress. Toxicol Appl Pharmacol 1991;110:347–54. [DOI] [PubMed] [Google Scholar]
- Bautista J, Corpas R, Ramos R, Cremades O, Gutierrez JF, Alegre S. Brain mitochondrial complex I inactivation by oxidative modification. Biochem Biophys Res Commun 2000;275: 890–4. [DOI] [PubMed] [Google Scholar]
- Berl S, Lajtha A, Waelsch A. Amino acid and protein metabolism: VI. Cerebral compartments of glutamic acid metabolism. J Neurochem 1961;7:186–97. [DOI] [PubMed] [Google Scholar]
- Blake CI, Spitz E, Leehey M, Hoffer BJ, Boyson SJ. Platelet mitochondrial respiratory chain function in Parkinson’s disease. Mov Disord 1997;12:3–8. [DOI] [PubMed] [Google Scholar]
- Brenneman KA, Cattley RC, Ali SF, Dorman DC. Manganese-induced developmental neurotoxicity in the CD rat: is oxidative damage a mechanism of action? Neurotoxicology 1999;20: 477–87. [PubMed] [Google Scholar]
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic Parkinsonism: similarities and difference. Neurology 1994;44:1583–6. [DOI] [PubMed] [Google Scholar]
- Chen JY, Tsao GC, Zhao Q, Zheng W. Differential cytotoxicity of Mn(II) and Mn(III): special reference to mitochondrial [Fe–S] containing enzymes. Toxicol Appl Pharmacol 2001;175: 160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G, Spina MB. Deprenyl suppresses the oxidant stress associated with dopamine turnover. Ann Neurol 1989;26: 689–90. [DOI] [PubMed] [Google Scholar]
- DeSole MS, Esposito G, Migheli R, Sircana S, Delogu MR, Fresu L, et al. Glutathione deficiency potentiates manganese toxicity in rat striatum and brainstem and in PC 12 cells. Pharmacol Res 1997;36:285–92. [DOI] [PubMed] [Google Scholar]
- Dobson AW, Weber S, Dorman DC, Lash LK, Erikson KM, Aschner M. Inhaled manganese sulfate and measures of oxidative stress in rat brain. Biol Trace Elem Res 2003;93:113–26. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, Vitarella D, Byerly FL, Goetz J, Miller R. Neurotoxicity of manganese chloride in neonatal and adult CD rats following subchronic (21-day) high-dose oral exposure. J Appl Toxicol 2000;20:179–87. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, James RA, Marshall MW, Parkinson CU, Wong BA. Influence of particle solubility on the delivery of inhaled manganese to the rat brain: manganese sulfate and manganese tetroxide pharmacokinetics following repeated (14-day) exposure. Toxicol Appl Pharmacol 2001;170:79–87. [DOI] [PubMed] [Google Scholar]
- Drown DB, Oberg SG, Sharma SP. Pulmonary clearance of soluble and insoluble forms of manganese. J Toxicol Environ Health 1986;17:201–12. [DOI] [PubMed] [Google Scholar]
- Dunn MT, Blalock TL, Cousins RJ. Metallothionein. Proc Soc Exp Biol Med 1987;185:107–19. [DOI] [PubMed] [Google Scholar]
- Dupont CL, Tanaka Y. Blood manganese levels in children with convulsive disorder. Biochem Med 1985;33:246–55. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Aschner M. Manganese causes differential regulation of glutamate transporter, taurine transporter and metallothionein in cultured rat astrocytes. Neurotoxicology 2002;23:595–602. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Suber RL, Aschner M. Glutamate/aspartate (GLAST), taurine transporter, and metallothionein mRNA levels are differentially altered in astrocytes exposed to manganese chloride, manganese phosphate, and manganese sulfate. Neurotoxicology 2002;23:281–8. [DOI] [PubMed] [Google Scholar]
- Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson’s disease: evidence supporting it. Ann Neurol 1992;32:804–12. [DOI] [PubMed] [Google Scholar]
- Galvani P, Fumagalli P, Santagostino A. Vulnerability of mitochondrial complex I in PC12 cells exposed to manganese. Eur J Pharmacol 1995;293:377–83. [DOI] [PubMed] [Google Scholar]
- Gavin CE, Gunter KK, Gunter TE. Manganese and calcium transport in mitochondria: implications for manganese toxicity. Neurotoxicology 1999;20:445–53. [PubMed] [Google Scholar]
- HaMai D, Campbell A, Bondy SC. Modulation of oxidative events by multivalent manganese complexes in brain tissue. Free Radic Biol Med 2001;31:763–8. [DOI] [PubMed] [Google Scholar]
- Hamer DH. Metallothioneins. Annu Rev Biochem 1986;55:913–51. [DOI] [PubMed] [Google Scholar]
- Hazell AS, Norenberg MD. Manganese decreases glutamate uptake in cultured astrocytes. Neurochem Res 1997;22:1443–7. [DOI] [PubMed] [Google Scholar]
- Hertz L Functional interactions between neurons and astrocytes I. Turnover and metabolism of putative amino acid transmitters. Prog Neurobiol 1979;13:277–323. [DOI] [PubMed] [Google Scholar]
- Ingersoll RT, Montgomery EB, Aposhian HV. Central nervous system toxicity of manganese: II. Cocaine or reserpine inhibits manganese concentration in the rat brain. Neurotoxicology 1999;20:467–76. [PubMed] [Google Scholar]
- Itano Y, Noji S, Koyama E, Taniguchi S, Taga N, Takahashi T, et al. Bacterial endotoxin-induced expression of metallothionein genes in rat brain, as revealed by in situ hybridization. Neurosci Lett 1991;124:13–6. [DOI] [PubMed] [Google Scholar]
- Keen CL, Bell JG, Lonnerdal B. The effect of age on manganese uptake and retention from milk and infant formulas in rats. J Nutr 1986;116:395–402. [DOI] [PubMed] [Google Scholar]
- Lafon-Cazal M, Culcasi M, Gaven F, Pietri S, Bockaert J. Nitric oxide, superoxide, and peroxynitrite: putative mediators of NMDA-induced cell death in cerebellar granule cells. Neuropharmacology 1993;32:1259–66. [DOI] [PubMed] [Google Scholar]
- Lai JC, Minski MJ, Chan AW, Leung TK, Lim L. Manganese mineral interactions in brain. Neurotoxicology 1999;20:433–44. [PubMed] [Google Scholar]
- Lynam DR, Roos JW, Pfeifer GD, Fort BF, Pullin TG. Environmental effects and exposures to manganese from use of methylcyclopentadienyl manganese tricarbonyl (MMT) in gasoline. Neurotoxicology 1999;20:145–50. [PubMed] [Google Scholar]
- Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science 1977;195:1356–8. [DOI] [PubMed] [Google Scholar]
- Meister A Glutathione metabolism and its selective modification. J Biol Chem 1988;263:17205–8. [PubMed] [Google Scholar]
- Meister A Glutathione deficiency produced by inhibition of its synthesis, and reversal; applications in research and therapy. Pharmacol Ther 1991;51:155–94. [DOI] [PubMed] [Google Scholar]
- Miele M, Serra PA, Esposito G, Delogu MR, Migheli R, Rocchitta G, et al. Glutamate and catabolites of high energy phosphates in the striatum and brainstem of young and aged rats subchronically exposed to manganese. Aging 2000;12:393–7. [DOI] [PubMed] [Google Scholar]
- Miller ST, Cotzias GC, Evert HA. Control of tissue manganese: initial absence and sudden emergence of excretion in the neonatal mouse. Am J Physiol 1975;229:1080–4. [DOI] [PubMed] [Google Scholar]
- Montes S, Alcaraz-Zubeldia M, Muriel P, Rios C. Striatal manganese accumulation induces changes in dopamine metabolism in the cirrhotic rat. Brain Res 2001;891:123–9. [DOI] [PubMed] [Google Scholar]
- Newland MC. Animal models of manganese’s neurotoxicity. Neurotoxicology 1999;20:415–32. [PubMed] [Google Scholar]
- Ottersen OP, Zhang N, Walberg F. Metabolic compartmentation of glutamate and glutamine: morphological evidence obtained by quantitative immunocytochemistry in rat cerebellum. Neuroscience 1992;46:519–34. [DOI] [PubMed] [Google Scholar]
- Oubrahim H, Stadtman ER, Chock PB. Mitochondria play no roles in Mn(II)-induced apoptosis in HeLa cells. Proc Natl Acad Sci U S A 2001;98:9505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S, Kostic V, Jackson-Lewis V, Naini AB, Simonetti S, Fahn S, et al. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J Neurosci 1992;12: 1658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehnberg GL, Hein JF, Carter SD, Linko RS, Laskey JW. Chronic manganese oxide administration to preweaning rats: manganese accumulation and distribution. J Toxicol Environ Health 1982;6: 217–26. [DOI] [PubMed] [Google Scholar]
- Riederer P, Sofic E, Rausch WD, Schmidt B, Reynolds GP, Jellinger K, et al. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem 1989;52:515–20. [DOI] [PubMed] [Google Scholar]
- Roels H, Meiers G, Delos M, Ortega I, Lauwerys R, Buchet JP, et al. Influence of The route of administration and the chemical form (MnCl2, MnO2) on the absorption and cerebral distribution of manganese in rats. Arch Toxicol 1997;71:223–30. [DOI] [PubMed] [Google Scholar]
- Shiraga H, Pfeiffer RF, Ebadi M. The effects of 6-hydroxydopamine and oxidative stress on the level of brain metallothionein. Neurochem Int 1993;23:561–6. [DOI] [PubMed] [Google Scholar]
- Sloot WN, Korf J, Koster JF, DeWit LEA, Gramsbergen JBP. Manganese-induced hydroxyl radical formation in rat striatum is not attenuated by dopamine depletion or iron chelation in vivo. Exp Neurol 1996;138:236–45. [DOI] [PubMed] [Google Scholar]
- Spencer A Whole blood manganese levels in pregnancy and the neonate. Nutrition 1999;15:731–4. [DOI] [PubMed] [Google Scholar]
- Stadtman ER. Protein oxidation and aging. Science 1992;257: 1220–4. [DOI] [PubMed] [Google Scholar]
- Stokes AH, Lewis DY, Lash LH, Jerome WG, Grant KW, Aschner M, et al. Dopamine toxicity in neuroblastoma cells: role of glutathione depletion by L-BSO and apoptosis. Brain Res 2000;858:1–8. [DOI] [PubMed] [Google Scholar]
- Sumino K, Hayakawa K, Shibata T, Kitamura S. Heavy metals in normal Japanese tissues. Arch Environ Health 1975;30: 487–94. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Mouri T, Suzuki Y, Nishiyama K, Fuju N, Yano H. Study of substance toxicity of manganese dioxide in monkeys. Tokushima J Exp Med 1975;22:5–10. [PubMed] [Google Scholar]
- Takeda A, Ishiwatari S, Okada S. Manganese uptake into rat brain during development and aging. J Neurosci Res 1999;56: 93–8. [DOI] [PubMed] [Google Scholar]
- Trotti D, Danbolt NC, Volterra A. Glutamate transporters are oxidant-vulnerable: a molecular link between oxidative and excitotoxic neurodegeneration? Trends Pharmacol Sci 1998; 19:328–34. [DOI] [PubMed] [Google Scholar]
- Van den Berg CJ, Garfinkel D. A simulation study of brain compartments—metabolism of glutamate and related substances in mouse brain. Biochem J 1971;123:211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waniewski RA, Martin DL. Exogenous glutamate is metabolized to glutamine and exported by primary astrocyte cultures. J Neurochem 1986;47:304–13. [DOI] [PubMed] [Google Scholar]
- Weber S, Dorman DC, Lash LH, Erikson K, Vrana KE, Aschner M. Effects of Manganese (Mn) on the developing rat brain: oxidative-stress related endpoints. Neurotoxicology 2002;23: 169–75. [DOI] [PubMed] [Google Scholar]
- Wedler FC, Ley BW, Grippo AA. Manganese (II) dynamics and distribution in glial cells cultured from chick cerebral cortex. Biochem Res 1989;14:1129–35. [DOI] [PubMed] [Google Scholar]
- Westergaard N, Sonnewald U, Schousboe A. Metabolic trafficking between neurons and astrocytes: the glutamate glutamine cycle revisited. Dev Neurosci 1995;17:203–11. [DOI] [PubMed] [Google Scholar]
- Zayed J, Hong B, L’Esperance G. Characterization of manganese-containing particles collected from the exhaust emissions of automobiles running with MMT additive. Environ Sci Technol 1999;33:3341–6. [Google Scholar]


