Abstract
To investigate pufferfish accumulation, elimination, and distribution of tetrodotoxin (TTX), Takifugu obscurus was fed with wild TTX-containing gastropod Nassarius semiplicata to simulate the natural food chain. Three-month-old non-poisonous T. obscurus was fed with wild toxic N. semiplicata at three exposure dose for 28 days, and later, with toxin-free food until day 67. Three fish individuals from each treatment were sampled, and the distribution of TTX in different tissues was measured. The results showed that the accumulation ratio of TTX in the three exposure dose groups ranged from 35.76% to 40.20%. The accumulation ratio in the skin and liver was the highest amongst all tissues, accounting for more than 85% of the total TTX, whereas that in the kidney and gallbladder was the lowest (0.11–0.78%). Studies on the kinetic of TTX accumulation and elimination revealed that the skin was the tissue with the highest accumulation speed constant (8.06), while the liver, kidney, and intestinal tract showed the highest speed of TTX elimination. The time required for TTX reduction to reach the safety limit could be predicted by using standard elimination equations. Qualitative analysis by UPLC-MS/MS revealed the occurrence of seven TTX derivatives in T. obscurus; of these TTX, 5-deoxy TTX, 11-deoxy TTX, 4,9-anhydro TTX were found in all tested tissues.
Keywords: tetrodotoxin, Takifugu obscurus, accumulation, elimination, Nassarius semiplicata
1. Introduction
Tetrodotoxin (TTX) is a heterocyclic organic perhydroquinazoline compound that was initially isolated from wild Tetraodontidae by Tahara in 1909 [1,2,3,4]. TTX has the chemical formula of C11H17O8N3 and a low molecular weight of 319 [5]. TTX, mainly found in pufferfish, is a chemical with high stability. As a potent non-protein neurotoxin, TTX can selectively block sodium channels to inhibit nerve and muscle conductions, leading to death from nerve paralysis and respiratory failure [6,7,8]. The median lethal dose (LD50) for TTX found after intraperitoneal injection in mice was 10.7 μg/kg [9,10]. In the case of human intoxication, the onset of symptoms could occur within 10 min after ingestion, with a delay of up to 4–6 h. No effective antidote for TTX has been reported yet. Pufferfish is a delicacy appreciated in East Asia. In recent years, there have been cases of poisoning and even death caused by eating poisonous pufferfish [10,11,12,13,14,15,16]. To maintain seafood safety and a healthy development of the pufferfish industry, health authorities in Japan have set an acceptable limit of TTX of < 10 MU/g in edible parts of pufferfish [16] and a regulatory limit in seafood of 2000 μg/kg [9,10]. There are no food regulations concerning TTX in the United States of America [17].
Previous studies have suggested that TTX may be initially synthesized by marine bacteria and transferred to pufferfish via the food chain [2,18]. Nevertheless, the mechanisms of TTX accumulation in the pufferfish through a toxic food chain, and its elimination remains to be elucidated [19]. It has been shown that cultured non-toxic pufferfish when fed on a TTX-containing diet become toxic, and TTX mainly accumulated into the liver and skin [20], while pufferfish should become non-toxic when fed TTX-free diets after hatching in an environment in which the invasion of TTX-bearing organisms has been eliminated [21]. Furthermore, TTX accumulation and transfer kinetics in Takifugu rubripes [22], Takifugu porphyreus [23], Takifugu poecilonotus [24], Takifugu niphobles (current name: Takifugu alboplumbeus) [20] and other pufferfish [25] indicated that the distribution, accumulation, and elimination of TTX varied among different species.
In 2016, China allowed certified companies to cultivate pufferfish T. rubripes and T. obscurus. The accumulation and elimination patterns of TTX in T. rubripes have been extensively studied [19]. TTX mainly accumulated in the skin, liver, and ovary [20], but there were important differences according to the age group: TTX accumulated mainly in the liver in adult fish, whereas in juvenile fish the maximum was found in the skin [19,26,27]. Study on the accumulation, elimination, and distribution of TTX in T. obscurus has not been reported, yet the related information is essential to prevent and control the occurrence of TTX poisoning.
In this study, T. obscurus was used as a model organism to simulate the accumulation and elimination of TTX in the natural food chain by feeding it with wild TTX-containing marine snail Nassarius semiplicata. The study aims to describe the temporal–spatial patterns of TTX distribution in different tissues by analyzing time–tissue data, thereby understanding the accumulation and elimination patterns of TTX in T. obscurus under natural feeding conditions.
2. Results
2.1. Anatomical Distribution of TTX
The accumulation of TTX by T. obscurus was evaluated by comparing the actual intake and feeding amount at the end of the accumulation period. Total TTX content in samples from the three exposure dose groups was determined on day 28, and the actual intake, actual accumulation, and accumulation ratio of TTX were calculated and recorded (Table 1). TTX intake ranged from 1032 to 5420 ng, and the TTX accumulation ratio from 35.76% to 40.20%. The actual feed intake of different dose groups was similar, and the actual TTX accumulation gradually increased with the dose concentration. Unlike the total accumulation pattern, the TTX accumulation rate did not increase with the increase in intake but showed a unique pattern. The accumulation ratio of the low-dose group was the highest, reaching a value of 40.20%, whereas that of the medium-dose group (35.76%) was the lowest. The above results showed that TTX could be accumulated more efficiently in T. obscurus under low-dose exposure, but there was no linear relationship between TTX accumulation and the exposure dose.
Table 1.
Tetrodotoxin (TTX) accumulation ratio of whole T. obscurus in exposure dose groups n = 6.
| Exposure Dose Group | TTX in Feed (ng/g) | Feed Intake (g) | Total TTX (ng) | Actual Accumulation of TTX (ng) | Accumulation Ratio (%) |
|---|---|---|---|---|---|
| Low-dose group | 100 | 25.67 ± 2.1 | 2567 ± 210 | 1032 ± 104 | 40.20 ± 5.2 |
| Medium-dose group | 210 | 26.03 ± 3.2 | 5466 ± 320 | 1955 ± 110 | 35.76 ± 4.9 |
| High-dose group | 600 | 23.75 ± 2.4 | 14250 ± 240 | 5420 ± 173 | 38.03 ± 4.1 |
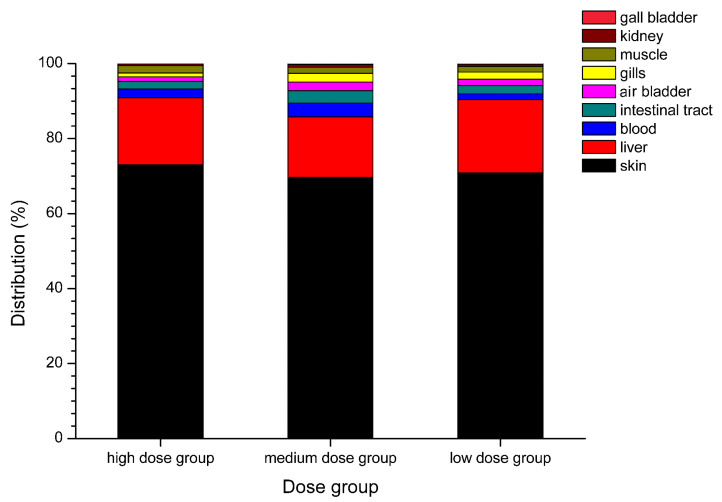
Various tissues of T. obscurus were collected on day 28 at the end of the accumulation stage to measure TTX concentration by UPLC-MS/MS. TTX distribution was subsequently analyzed by combining data of TTX concentration with tissue weight. The proportion of the accumulated amount of TTX in each tissue with respect to content is shown in Figure 1. Overall, the TTX amount in the skin accounts for the largest proportion (69.70–73.00%), followed by the liver (16.05–19.58%). These two tissues account for more than 85% of the total amount of TTX. The TTX amount in the blood, intestinal tract, air bladder, gills, and muscle was relatively low, proportion values ranging from 3.72% to 0.99%. The TTX proportion in the kidney and gallbladder was the lowest, accounting for only 0.11–0.78%. Differences in TTX distribution were also observed among different dose groups. The TTX proportion in the blood from the medium- and high-dose groups was higher than that in the intestine, air bladder, gills, and muscle, while the TTX proportion in the blood from the low-dose group was significantly lower.
Figure 1.
Anatomic distribution of tetrodotoxin (TTX) in Takifugu obscurus under low-dose (100 ng TTX/g), medium-dose (210 ng TTX/g), and high-dose (600 ng TTX/g) feeding conditions.
2.2. Patterns in TTX Accumulation and Elimination
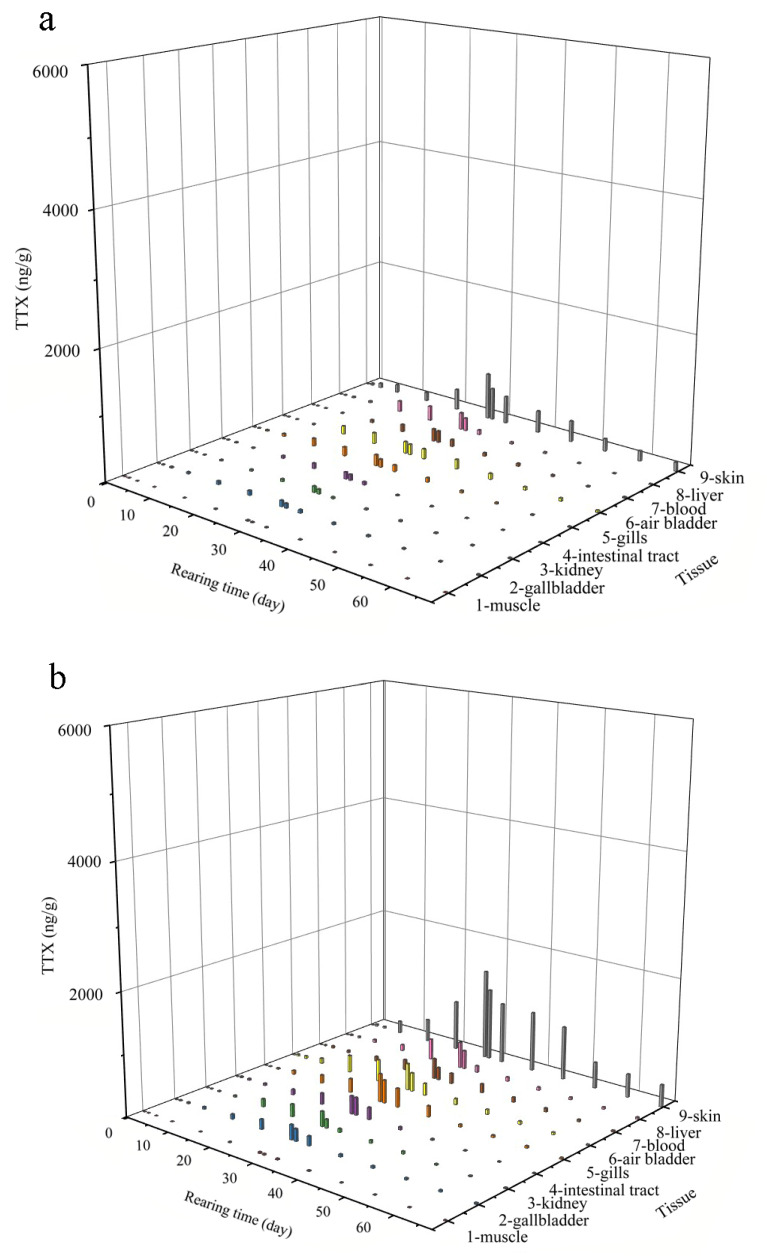
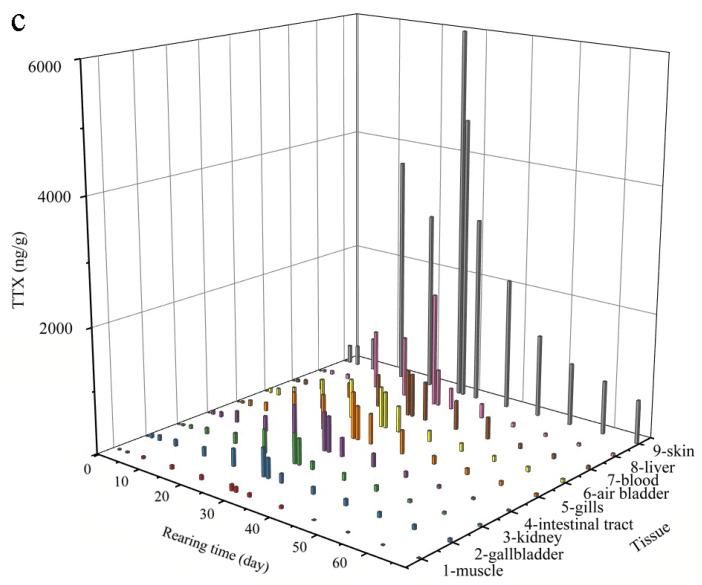
To systematically study the accumulation and elimination pattern in T. obscurus, various tissues were collected at different time points during the accumulation and elimination period to determine the TTX concentrations, and the data were analyzed to obtain a 3D change diagram of time-tissue-concentration (Figure 2). The TTX concentration first increased and then decreased with time throughout the accumulation and elimination process, showing a Gaussian distribution. The accumulation and elimination of TTX in different tissues were significantly different.
Figure 2.
Patterns of TTX accumulation and elimination in different tissues of T. obscurus under low-dose (100 ng TTX/g), medium-dose (210 ng TTX/g), and high-dose (600 ng TTX/g) feeding conditions. (a) low-dose. (b) medium-dose. (c) high-dose.
During the 28-day accumulation period, TTX levels in of the three exposure dose groups of T. obscurus increased with the number of toxic feeding days. In all tissues, the initial accumulation rates were relatively slow (day 1–7), ranging from 0.17 ng/g/d to 39.20 ng/g/d. The accumulation rates were the fastest in the middle period (day 7–21), rising from 0.86 ng/g/d to 338.09 ng/g/d. In the later stage (day 21–28), the accumulation rates gradually slowed down, dropping from 0.33 ng/g/d to 328.57 ng/g/d. At the end of the accumulation stage (day 28), the TTX concentration in the skin reached the highest values (Figure 2), with 717.4, 1484, and 6000 ng/g in the low-, medium- and high-dose groups, respectively. For the liver tissue, values were 262.2, 427.6, and 1840 ng/g, respectively, for muscle tissue, the values were 10.68, 20.51, and 99.37 ng/g. To evaluate the accumulation in T. obscurus, the TTX accumulation rate constant was calculated by using the relationship between the accumulation concentration and feeding concentration. The variance analysis showed that there was no significant difference (p > 0.05) in the accumulation rate constant of the same tissue among three exposure doses. Different tissues were divided into four groups according to the value of their accumulation rate constant: (i) rapid-constant group (skin tissue) with a maximum of 8.06; (ii) sub-rapid-constant group, including the liver (2.58), air bladder (1.74), gills (1.62), and blood (1.58); (iii) medium-rapid-constant group, including the intestine (1.15), kidney (0.96), and gallbladder (0.93), and (iv) low-constant group, including muscle tissue, with a minimum value of 0.12.
During the elimination period, the TTX content in the tissues showed a downward pattern, with the fastest decline from the 29th to the 39th day, and a much slower in the later period (day 39–67). To gain a better understanding of the characteristics of TTX elimination, the kinetics of toxin elimination in different tissues was studied (Table 2). The elimination rate constant K could directly reflect the elimination rate of the toxin. Variance analysis revealed there were no significant differences in the elimination rate constant K (p < 0.05) among different dose groups using variance analysis, which indicated that different dosages had little effect on the elimination rate, leading to a very similar elimination process. This pattern can also be visualized in Figure 2. In terms of different tissues, the elimination rates were obviously different. The tissues with the highest clearance rates were the liver, kidney, and intestinal tract, and their K values were 0.952, 0.496, and 0.236, respectively. The tissues with lower elimination rates included the gallbladder, air bladder, and gills, with K values of 0.132, 0.138, and 0.100, respectively. The tissues with the lowest rate of elimination were the gallbladder, muscle, and skin, with the K values of 0.059, 0.040, and 0.035, respectively. There were also obvious differences in the total elimination of TTX in different tissues. After 39 days of elimination experiment, the residual TTX content in the muscle from the low-, medium-, and high-dose feeding groups were all found to be the lowest, reaching 2.32 ng/g, 2.79 ng/g, and 7.4 ng/g, respectively. Skin tissues of the three groups with values of 135.66, 358.79, and 699.35 ng/g, respectively, contained the highest content of residual TTX. TTX in the skin reached a maximum concentration of 6000 ng/g, which is three times the regulatory limit at the end of accumulation (Table 2). By substituting the concentration into the elimination model, it is estimated that TTX in the skin could be below the safety limit after 12.3 days.
Table 2.
Parameters of the TTX elimination kinetics in Takifugu obscurus.
| Dose Group | Tissues | TTX-C0 Day 28 (ng/g) | TTX-Ct Day 67 (ng/g) | Elimination Rate K (d−1) | Half-Life Period B1/2 (d) |
|---|---|---|---|---|---|
| Low-dose group | gallbladder | 87.96 | 9.53 | 0.084 | 8.25 |
| kidney | 91.53 | 8.55 | 0.391 | 1.77 | |
| air bladder | 178.1 | 29.04 | 0.042 | 16.50 | |
| gills | 170.29 | 6.72 | 0.088 | 7.88 | |
| intestinal tract | 101.23 | 3.33 | 0.236 | 2.94 | |
| muscle | 10.68 | 2.32 | 0.040 | 9.16 | |
| liver | 262.18 | 4.42 | 0.387 | 1.79 | |
| skin | 717.34 | 135.66 | 0.042 | 16.50 | |
| blood | 189.35 | 14.42 | 0.105 | 6.60 | |
| Medium-dose group | gallbladder | 238.84 | 19.79 | 0.132 | 5.25 |
| kidney | 244.47 | 14.03 | 0.496 | 1.40 | |
| air bladder | 458.22 | 22.909 | 0.138 | 5.02 | |
| gills | 435.86 | 10.49 | 0.100 | 6.93 | |
| intestinal tract | 291.46 | 8.14 | 0.156 | 4.44 | |
| muscle | 20.51 | 2.79 | 0.056 | 12.38 | |
| liver | 427.62 | 27.88 | 0.399 | 1.74 | |
| skin | 1484.20 | 358.79 | 0.035 | 19.80 | |
| blood | 333.96 | 22.25 | 0.068 | 10.19 | |
| High-dose group | gallbladder | 460 | 50.25 | 0.100 | 6.93 |
| kidney | 486.47 | 29.07 | 0.320 | 2.17 | |
| air bladder | 757 | 43.86 | 0.098 | 7.07 | |
| gills | 650.12 | 54.82 | 0.075 | 9.24 | |
| intestinal tract | 622.93 | 18.1 | 0.12 | 5.78 | |
| muscle | 99.36 | 7.4 | 0.092 | 7.53 | |
| liver | 1840.3 | 41.18 | 0.952 | 0.73 | |
| skin | 6000 | 699.35 | 0.076 | 9.12 | |
| blood | 750.13 | 50.1 | 0.059 | 11.75 |
2.3. TTX Derivatives in Takifugu obscurus
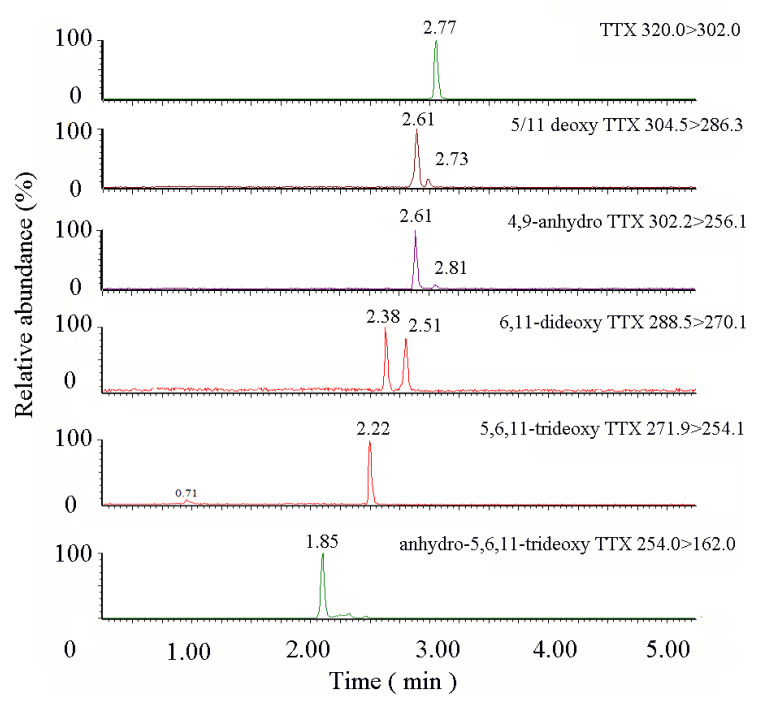
To know whether TTX is transformed or degraded in T. obscurus during the accumulation period, TTX derivatives in the feed and the pufferfish after the enrichment experiment were analyzed. The results showed that only TTX was detected in Nassarius semiplicata, while a variety of TTX derivatives were found in different pufferfish tissues, as shown in Table 3. TTX, 5-deoxy TTX, 11-deoxy TTX, 4,9-anhydro TTX were found in all tissues, indicating the common existence of the above substances. While anhydro-5,6,11-trideoxy TTX was only detected in the skin. The UPLC-MS/MS chromatogram of TTX derivatives in T. obscurus is shown in Figure 3.
Table 3.
TTX and its derivatives found in Takifugu obscurus.
| TTX Analogues | Takifugu obscurus Tissues | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gallbladder | Kidney | Air Bladder | Gills | Intestinal Tract | Muscle | Liver | Skin | Blood | |
| TTX | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 5-deoxy TTX | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 11-deoxy TTX | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 4,9-anhydro TTX | √ | √ | √ | √ | √ | / | √ | √ | √ |
| 6,11-dideoxy TTX | / | √ | √ | √ | √ | √ | √ | √ | √ |
| 5,6,11-trideoxy TTX | √ | / | √ | √ | √ | / | √ | √ | √ |
| anhydro-5,6,11-trideoxy TTX | / | / | / | / | / | / | / | √ | / |
√ means present, / means not detected.
Figure 3.
UPLC-MS/MS Chromatogram of TTX derivatives in Takifugu obscurus.
3. Discussion
A TTX exposure model of T. obscurus cultured in brackish water was established. The pufferfish was fed with wild Nassarius semiplicata to simulate the natural food chain environment. Differences in the anatomical distribution of TTX have been observed between marine and freshwater pufferfish [10,25], but also between juvenile and mature individuals [22]. Here we studied detection and anatomical distribution of TTX in 3-month-old T. obscurus, which is the least studied freshwater species. There is a variety of TTX administration modes. In most studies, TTX administration mode is in the form of intramuscular administration (IMA) [26], in which a one-time injection cannot reflect the real accumulation process. Oral gavage administration (OGA) was used in other studies [22,23], but the passive acceptance process cannot reflect the real natural accumulation. In this study, TTX-containing N. semiplicata was chosen as the 28-day feeding source to get TTX-containing T. obscurus, followed by 36-day of observations on the elimination process. These conditions simulated well the natural food chain.
The pufferfish T. obscurus could accumulate TTX efficiently with an accumulation ratio of 35.76–40.20%. The ability to accumulate toxins varies among different organisms. Pufferfish, mussel, starfish, and salamander have proven to be capable of accumulating biotoxins. Among them, pufferfishes are the most common TTX-accumulating species [2]. The capacity to accumulate TTX in different pufferfish species is obviously different. Wang et al. [23] performed an accumulation experiment with the artificial hybrid of Takifugu rubripes and Takifugu porphyreus using gastric gavage and intramuscular injection and found that the accumulated amount of TTX in the above groups ranged from 31% to 45% and from 42% to 74%, respectively. Tatsuno et al. [22] fed a TTX-containing homogenate to non-toxic cultured T. rubripes by gastric gavage and the total amount of TTX accumulated per fish was 31% of the given dose. In this study, T. obscurus was fed with poisonous wild N. semiplicata to mimic the predator’s behavior in the field. The average amount of TTX accumulated in T. obscurus was 38.03–40.20%, which is similar to that obtained from the gastric gavage method [23], but significantly lower than that obtained from the intramuscular injection method. Gao et al. [25] compared the selective accumulation of TTX between freshwater and marine pufferfish and found that freshwater P. suvatti could only accumulate a very small amount of TTX (0.03–0.5%). As brackish-water pufferfish, T. obscurus showed much higher TTX accumulation capacity in this study.
We also found that the distribution of TTX in T. obscurus was tissue-specific. Noguchi et al. [2] demonstrated that in adult fish, the liver and ovaries usually contained the highest toxin content, followed by the intestine and skin. Tatsuno et al. [22] compared TTX accumulation in the juvenile T. rubripes (6 and 15 months old). The 6-month-old pufferfish had the highest content of TTX accumulated in the skin (71%), followed by the liver (21%). For the 15-month-old pufferfish, the amount of TTX was highest in the liver (83%), and that in the skin was 14%. T. obscurus specimens selected for the current study were 3-month old, so their ovary development was still incomplete. UPLC-MS/MS analysis of the juvenile T. obscurus revealed that the TTX content in the skin (69.35–73.00%) was significantly higher than that in the liver (16.05–19.16%), which was similar to the reported values in 6-month-old T. rubripes. Itoi et al. [28,29] observed that non-toxic fish would rapidly spit out juvenile pufferfish (T. rubripes and T. niphobles) after swallowing them. Therefore, it can be assumed that the high accumulation of TTX in the skin of T. obscurus is also a self-protection adaption to repel predators in nature.
For the high-dose group, the TTX concentration in the skin decreased on the 21st day (Figure 2c), while in the medium- and low-concentration groups, the TTX concentration increased gradually with time during the whole accumulation period (Figure 2a,b). This phenomenon underlines the fact that the TTX accumulation in the skin had changed due to unknown reasons. Tatsuno et al. [19] found that the molecular mechanisms of TTX transfer and accumulation in the skin and liver are different, and that the accumulation rate of TTX in the skin will decrease with the increase in the dosage. Yosu-Yamhasita et al. [30] reported that a toxin binding protein (PSTBP) was involved in TTX transfer from the blood to the skin. When a large amount of TTX circulates in the blood, PSTBP could be saturated with TTX, leading to a decrease in the TTX accumulation rate in the skin. Therefore, it was speculated that when the PSTBP and TTX binding reached saturation, the TTX in the blood was no longer transported to the skin, resulting in the decrease in TTX concentration in the skin but the increase of TTX concentration in the blood. At the same time, TTX had been transferred to other tissues, including the gallbladder and kidney by other transporters, leading to the corresponding increase in toxin concentration.
In the present study, a dynamic model of TTX in T. obscurus was established to reflect the long-term elimination pattern. TTX content in the tissues showed a downward pattern, with elimination rate constant K from 0.12 to 8.06. Earlier TTX elimination experiments [20,23,26] were mostly carried out within short periods of time(60–168 h), mainly for observing TTX transfer, accumulation, and anatomic distribution. Ikeda et al. [26] studied TTX elimination in T. rubripes for 168 h after the IMA, and found that the toxin content showed a decreasing pattern of 8–12 h, then gradually increased from 24–168 h, and finally, about 89% accumulated in the skin. Wang et al. [23] used OGA for administration and found that TTX absorbed by the digestive tract was first transferred to the liver, and then to the skin, resulting in a pattern of decreasing and then increasing toxin content in the skin during the elimination stage. To observe the elimination stage after a long-term accumulation, the experiment was carried out for as long as 39 days (936 h). It was found that TTX content decreased gradually in all tissues during the whole elimination period, and there was not a notable increase in TTX in the skin tissue. This may be explained by the fact that the elimination stage was relatively long and short-term patterns could not be reflected. Unlike previous reports, a dynamic model was established in the present study to reflect the long-term elimination pattern. Analysis of the elimination process in nine tissues of T. obscurus showed that the process in vivo fit to a first-order elimination kinetic equation, similar to the Dyble kinetic equation for the elimination of microcystins [31]. It was possible to calculate the elimination rate constant and half-life. It was possible to predict that TTX in the skin of the high-dose group of the exposure group would be below the regulatory limit after elimination for 12.3 days. The kinetic model could be applied to a TTX level reduction control in pufferfish cultivation.
Seven kinds of TTX derivatives were found in different tissues of T. obscurus, but one of these, the anhydro-5,6,11-trideoxy TTX was only found in the skin. So far, more than 20 TTX structural analogs with different toxic potential have been found in pufferfish [3]. Jang et al. [32] reported that TTX and 5,6,11-trideoxyTTX were the major toxin components, whereas 4-epiTTX 4,9-anhydroTTX, 5-deoxyTTX, and 11-deoxyTTX were minor TTX analogs in the toxin profile of Fugu niphobles, Tetraodon nigroviridis, and Tetradon biocellatus. Recently, Tonol et al. [33] discovered new TTX analogs in Brazilian pufferfish tissues and microbiome through UPLC qTOF-MS/MS analysis in combination with molecular network techniques. Our study is the first to report the distribution of TTX analogs among skin, liver, blood, and another six tissues in T. obscurus, and the existence of five kinds of TTX derivatives. It should be noted that due to the lack of TTX derivative standards, only qualitative analyzes by UPLC-MS/MS of these derivatives were possible. The above findings indicate that TTX in the feed had indeed undergone biotransformation or degradation in T. obscurus being converted into a series of derivatives. Nevertheless, the mechanism of transformation needs to be further studied. Yang et al. [34] isolated a TTX-producing Aeromonas strain from the ovary of the pufferfish Takifugu obscurus. The possible effects of the strain on the TTX accumulation in the adult pufferfish or the existence of other toxin-producing strains in the target tissues should be considered. TTX and five other derivatives were also found in T. niphobles (current name: T. alboplumbeus) [32], but anhydro-5,6,11-trideoxy TTX was not detected. This toxin was previously detected in Fugu poecilonotus [35]. Nagashima et al. [36] found that non-toxic pufferfish species contained TTX derivatives with a lower toxic potential, and suggested that TTX could be biotransformed into other less toxic forms. Further studies on TTX derivatives in T. obscurus will help to identify the toxic potential of these derivatives.
4. Conclusions
This paper reports the first accumulation and elimination study of TTX in the pufferfish T. obscurus. A TTX exposure model of T. obscurus was established by feeding with wild N. semiplicata to simulate the natural food chain environment. The juvenile T. obscurus could accumulate TTX efficiently, and the distribution of TTX was tissue-specific. TTX content in the tissues showed a downward pattern, with an elimination rate constant K values ranging from 0.12 to 8.06. A Kinetic model to reflect the long-term elimination pattern of TTX in T. obscurus was established. This model could be applied to the control of TTX reduction during pufferfish cultivation in the future. The identification of seven TTX derivatives in different tissues of T. obscurus will be helpful to investigate the toxic potential of these derivatives.
5. Materials and Methods
5.1. Materials
5.1.1. Instruments and Reagents
Analytical instruments included an ACQUITY ultra-performance liquid chromatograph (UPLC, Waters, Miford, MA, USA), and a Xevo TQS quadrupole mass spectrometer (Waters, Manchester, UK), with an electrospray ion source. Other instruments included an MS2 Vortex Mixer (IKA, Wilmington, NC, USA); an Avanti JXN-30 refrigerated high-speed centrifuge (Beckman Coulter, Brea, CA, USA); an N-EVAP112 nitrogen evaporator (Organomation Associates, Berlin, MA, USA); a Visiprep SPE Vacuum Manifold (Supelco, Bellefonte, PA, USA); and an SK8200GT ultrasonic cleaner (Shanghai Kudos Ultrasonic Instrument Co., Ltd., Shanghai, China).
The TTX standard was purchased from Dr. Ehrenstorfer GmbH, Germany. Immuno-affinity columns (3 mL, 60 mg) were purchased from Jiangsu Meizheng Bio-Tech Inc., China. Milli-Q ultrapure water was used. Methanol, acetonitrile, acetic acid, and ammonium acetate were HPLC grade.
5.1.2. Biological Materials
Two hundred three-month-old T. obscurus (20 ± 1 g, 4–5 cm) were purchased from the Shanghai Fisheries Research Institute. The protocol was approved by the Ethics Committee of the Marine Fishery Research Institute of Zhejiang on 5 May 2018 (Project identification code: 2017YFC1600701).
Fish formula feed contained 45% of crude protein, 4% of fat, and 15% of lysine. It was stored in a dry environment at room temperature.
Three batches of the sea snail N. semiplicata were collected from coastal areas of Xiangshan, Ningbo city in Zhejiang, from July to September 2018. and used as toxic feed. The soft tissue was taken out, cut into small pieces, thoroughly homogenized, packed in bags and stored at −18 °C until subsequent use. The TTX concentration in the aforementioned homogenates was determined by UPLC-MS/MS as 600 ng/g, 210 ng/g, and 100 ng/g, respectively. No TTX derivatives were detected in any of the N. semiplicata samples.
5.2. Culture Environment and Diet
T. obscurus was acclimated in 120 L tank, at a density of 30 fish per tank, and kept without feeding for 1 week in brackish water with salinity 8–10 and temperature of 22–25 °C, under a 12 h light:12 h dark photoperiod. Dissolved oxygen (>6 mg/L), ammonia nitrogen (<1 mg/L), and pH (7.5–8.0) conditions were maintained. After the acclimation period, T. obscurus was fed daily with fish feed (3% fish weight).
T. obscurus specimens were randomly divided into four groups: control group and low-, medium- and high-dose exposure groups, in which juvenile fish were fed with formula feed mixed with non-toxic shrimp (control), 100 ng TTX/g (low), 210 ng TTX/g (medium), and 600 ng TTX/g (high) dose exposure of TTX-containing N. semiplicata homogenate (N. semiplicata meat: formula feed = 1:2 wt).
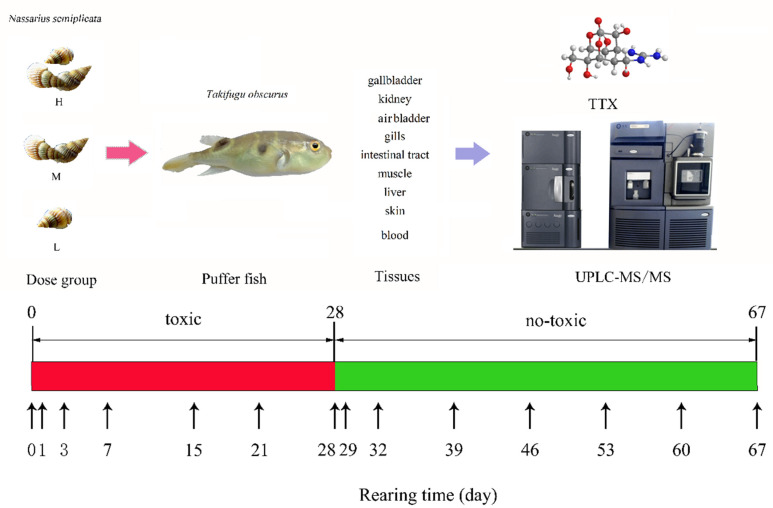
During the 28-d accumulation period, juveniles were fed the mixed feed daily (3% of their body weight). The unconsumed feed was removed after 0.5 h and weighed to calculate the actual daily intake. One-third of the tank water was renewed daily, and it was continuously filtered to maintain water quality. On day 28, the juveniles were transferred to new brackish water, and the feed for T. obscurus in the dose exposure groups was changed to the regular formula feed during the whole elimination period. The accumulation and elimination experiment schedule is shown in Figure 4. Three T. obscurus were randomly collected on days 0, 1, 3, 7, 15, 21, 28, 29, 32, 39, 46, 53, 60, and 67. Tissues including the gallbladder, kidney, air bladder, gills, intestinal tract, muscle, liver, skin, and blood were sampled and stored at −18 °C until analysis.
Figure 4.
Diagram of the TTX accumulation and elimination experiment. The fish was fed with toxic feed until the 28th day, then fed with non-toxic feed until 67th day.
5.3. Analysis of TTX and Its Analogues
Quantitative detection of TTX was performed using the previously reported UPLC-MS/MS method [37]. Briefly, 10 mL of 1% acetic acid in methanol was added to an appropriate amount (0.5–2 g) of homogenized samples (skin, liver, muscle, etc.). The samples were vortexed for 3 min, sonicated at 60 °C for 15 min, cooled to room temperature, and centrifuged at 8000 rpm for 7 min. Five milliliters of the supernatant was diluted with 20 mL of PBS buffer, and the solution pH adjusted to 6.9–7.1 with 1 mol/L NaOH. Subsequently, the sample solution was loaded onto an immuno-affinity column, rinsed with 8 mL of 20% aqueous methanol solution, and finally eluted with 4 mL of 2% acetic acid in methanol. The obtained solution was concentrated with nitrogen at 60 °C and then dissolved in 5 mmol/L ammonium acetate/acetonitrile (V/V = 1/9) solution containing 0.1% formic acid to a final volume of 1 mL. Finally, the samples were filtered through a 0.22-μm filter prior to the analysis.
The chromatographic parameters were as follows: column, ACQUITY UPLC BEH amide (50 mm × 2.1 mm, 1.7 μm); sample chamber temperature, 4 °C; column temperature, 40 °C; injection volume, 10 μL; mobile phase A, 5 mmol/L ammonium acetate solution containing 0.1% formic acid, mobile phase B, acetonitrile; and flow rate, 0.3 mL/min. The gradient elution conditions were set as follows: 0–1.5 min, 10% A; 1.5–4 min, 60% A; 4–4.1 min, 60%A; and 4.1–5 min, 10% A.
The mass spectrometry parameters were as follows: electrospray ion source, positive ion (ESI+); mode, multiple reactions monitoring (MRM); capillary voltage, 3.0 kV; ion source temperature, 110 °C; desolventizing gas temperature, 350 °C; cone gas flow rate, 50 L/h; and desolventizing gas flow rate, 600 L/h. Data acquisition was conducted in MRM mode, and related parameters for TTX and its analogs are shown in Table 4.
Table 4.
Parameters of the UPLC-MS/MS system to measure TTX and analogs.
| Analyte | Retention Time(min) | Precursor Ion(m/z) | Product Ion(m/z) | Cone Voltage (V) |
Collision Energy(eV) |
|---|---|---|---|---|---|
| TTX | 2.77 | 320.0 | 302.0 * 161.8 |
45 | 25 35 |
| 5-deoxy TTX/ 11-deoxy TTX |
2.61 | 304.5 | 286.29 162.10 |
40 | 25 20 |
| 4,9-anhydro TTX | 2.61 | 302.20 | 256.13 161.83 |
40 | 35 25 |
| 6,11-dideoxy TTX | 2.51 | 288.54 | 270.11 224.69 |
40 | 25 25 |
| 5,6,11-trideoxy TTX | 2.22 | 271.90 | 254.11 162.07 |
40 | 25 20 |
| anhydro-5,6,11-trideoxy TTX | 1.85 | 254.02 | 161.98 | 40 | 25 |
*: Quantitative ion.
5.4. Kinetic TTX Elimination Model
The elimination stage of TTX in T. obscurus was fit to a first-order loss kinetic model. The TTX concentration and elimination time were calculated by using the equation:
| (1) |
where C0 and Ct are the TTX concentration in the tissue (ng g−1) at time 0 (beginning) and time t (end of the elimination process), respectively, t is time (day), and K is the elimination rate constant (d−1) obtained from the equation:
| (2) |
The half-life (B1/2) of TTX in each tissue was calculated based on the elimination rate constant K, as shown in Equation (3) [38]:
| (3) |
5.5. Statistical Analysis
The accumulation ratio of TTX was evaluated by comparing the actual intake and feeding amount at the end of the accumulation period. The accumulation rate was calculated by dividing the TTX concentration by accumulation days. The accumulation constant was calculated by dividing the TTX concentration in the tissue by feeding concentration. All experimental measurements were performed with at least three replicates for each data point. Elimination model regression analyses were performed according to the CurveExpert Professional software (version 2.6.5, Hyams Development, Chattanooga, TN, USA). p-values less than 0.05 were considered statistically significant. OriginPro software (version 9.6, OriginLab, Northampton, MA, USA) was performed for statistical analyses and scientific mapping, respectively.
Acknowledgments
The authors would like to thank the colleagues for their valuable technical assistance.
Author Contributions
The project was conceived and designed by X.Z. and H.X.; organized and performed the experiments, J.Z., R.W.; analyzed and interpreted the data, S.C. and Y.L. The manuscript was written by X.Z.; funding acquisition, M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China (2017YFC1600701).
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
This paper first reports the accumulation and elimination of TTX in the pufferfish T. obscurus. A kinetic model of TTX in T. obscurus was established to reflect the long-term elimination pattern.
References
- 1.Moczydlowski E.G. The molecular mystique of tetrodotoxin. Toxicon. 2013;63:165–183. doi: 10.1016/j.toxicon.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 2.Noguchi T., Arakawa O. Tetrodotoxin--distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar. Drugs. 2008;6:220–242. doi: 10.3390/md20080011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bane V., Lehane M., Dikshit M., O’Riordan A., Furey A. Tetrodotoxin: Chemistry, toxicity, source, distribution and detection. Toxins. 2014;6:693–755. doi: 10.3390/toxins6020693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X., Han C., Chen S., Li L., Zong J., Zeng J., Mei G. Response Surface Methodology for the Optimization of Ultrasound-Assisted Extraction of Tetrodotoxin from the Liver of Takifugu pseudommus. Toxins. 2018;10:529. doi: 10.3390/toxins10120529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorentz M.N., Stokes A.N., Rossler D.C., Lotters S. Tetrodotoxin. Curr. Biol. 2016;26:R870–R872. doi: 10.1016/j.cub.2016.05.067. [DOI] [PubMed] [Google Scholar]
- 6.Colquhoun D., Henderson R., Ritchie J.M. The binding of labelled tetrodotoxin to non-myelinated nerve fibres. J. Physiol. 1972;227:95–126. doi: 10.1113/jphysiol.1972.sp010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narahashi T. PHARMACOLOGY OF TETRODOTOXIN. J. Toxicol. Toxin Rev. 2001;20:67–84. doi: 10.1081/TXR-100102537. [DOI] [Google Scholar]
- 8.Noguchi T., Arakawa O., Takatani T. TTX accumulation in pufferfish. Comp. Biochem. Physiol. Part D Genom. Proteom. 2006;1:145–152. doi: 10.1016/j.cbd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Noguchi T., Ebesu J.S.M. PUFFER POISONING: EPIDEMIOLOGY AND TREATMENT. J. Toxicol. 2001;20:1–10. doi: 10.1081/TXR-100103080. [DOI] [Google Scholar]
- 10.Lago J., Rodriguez L.P., Blanco L., Vieites J.M., Cabado A.G. Tetrodotoxin, an Extremely Potent Marine Neurotoxin: Distribution, Toxicity, Origin and Therapeutical Uses. Mar. Drugs. 2015;13:6384–6406. doi: 10.3390/md13106384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen T.Y., Hsieh C.H., Hwang D.F. Development of standardized methodology for identifying toxins in clinical samples and fish species associated with tetrodotoxin-borne poisoning incidents. J. Food Drug Anal. 2016;24:9–14. doi: 10.1016/j.jfda.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravaonindrina N., Andriamaso T.H., Rasolofonirina N. Puffer fish poisoning in Madagascar: Four case reports. Arch. Inst. Pasteur Madag. 2001;67:61–64. [PubMed] [Google Scholar]
- 13.Chowdhury F.R., Ahasan H.A., Al Mamun A., Rashid A.K., Al Mahboob A. Puffer fish (Tetrodotoxin) poisoning: An analysis and outcome of six cases. Trop. Doct. 2007;37:263–264. doi: 10.1258/004947507782333017. [DOI] [PubMed] [Google Scholar]
- 14.Islam Q.T., Razzak M.A., Islam M.A., Bari M.I., Basher A., Chowdhury F.R., Sayeduzzaman A.B., Ahasan H.A., Faiz M.A., Arakawa O., et al. Puffer fish poisoning in Bangladesh: Clinical and toxicological results from large outbreaks in 2008. Trans. R. Soc. Trop. Med. Hyg. 2011;105:74–80. doi: 10.1016/j.trstmh.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Ortega J.F., Morales-de los Santos J.M., Herrera-Gutierrez M.E., Fernandez-Sanchez V., Rodriguez Loureo P., Rancano A.A., Tellez-Andrade A. Seafood intoxication by tetrodotoxin: First case in Europe. J. Emerg. Med. 2010;39:612–617. doi: 10.1016/j.jemermed.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Katikou P., Georgantelis D., Sinouris N., Petsi A., Fotaras T. First report on toxicity assessment of the Lessepsian migrant pufferfish Lagocephalus sceleratus (Gmelin, 1789) from European waters (Aegean Sea, Greece) Toxicon. 2009;54:50–55. doi: 10.1016/j.toxicon.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Yakes B.J., Deeds J., White K., Degrasse S.L. Evaluation of surface plasmon resonance biosensors for detection of tetrodotoxin in food matrices and comparison to analytical methods. J. Agric. Food Chem. 2011;59:839–846. doi: 10.1021/jf103779k. [DOI] [PubMed] [Google Scholar]
- 18.Arakawa O., Takatani T., Taniyama S., Tatsuno R. Toxins of Pufferfish—Distribution, Accumulation Mechanism, and Physiologic Functions. Aqua-Biosci. Monogr. 2017;10:41–80. doi: 10.5047/absm.2017.01003.0041. [DOI] [Google Scholar]
- 19.Tatsuno R., Gao W., Ibi K., Mine T., Okita K., Nishihara G.N., Takatani T., Arakawa O. Profile differences in tetrodotoxin transfer to skin and liver in the pufferfish Takifugu rubripes. Toxicon. 2017;130:73–78. doi: 10.1016/j.toxicon.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Kono M., Matsui T., Furukawa K., Yotsu-Yamashita M., Yamamori K. Accumulation of tetrodotoxin and 4,9-anhydrotetrodotoxin in cultured juvenile kusafugu Fugu niphobles by dietary administration of natural toxic komonfugu Fugu poecilonotus liver. Toxicon. 2008;51:1269–1273. doi: 10.1016/j.toxicon.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Noguchi T., Arakawa O., Takatani T. Toxicity of pufferfish Takifugu rubripes cultured in netcages at sea or aquaria on land. Comp. Biochem. Physiol. Part D Genom. Proteom. 2006;1:153–157. doi: 10.1016/j.cbd.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Tatsuno R., Shikina M., Shirai Y., Wang J., Soyano K., Nishihara G.N., Takatani T., Arakawa O. Change in the transfer profile of orally administered tetrodotoxin to non-toxic cultured pufferfish Takifugu rubripes depending of its development stage. Toxicon. 2013;65:76–80. doi: 10.1016/j.toxicon.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Wang J., Araki T., Tatsuno R., Nina S., Ikeda K., Takatani T., Arakawa O. Transfer profile of orally and intramuscularly administered tetrodotoxin to artificial hybrid specimens of the pufferfish Takifugu rubripes and Takifugu porphyreus. Shokuhin Eiseigaku Zasshi. 2012;53:33–38. doi: 10.3358/shokueishi.53.33. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda K., Emoto Y., Tatsuno R., Wang J.J., Ngy L., Taniyama S., Takatani T., Arakawa O. Maturation-associated changes in toxicity of the pufferfish Takifugu poecilonotus. Toxicon. 2010;55:289–297. doi: 10.1016/j.toxicon.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Gao W., Kanahara Y., Yamada M., Tatsuno R., Yoshikawa H., Doi H., Takatani T., Arakawa O. Contrasting Toxin Selectivity between the Marine Pufferfish Takifugu pardalis and the Freshwater Pufferfish Pao suvattii. Toxins. 2019;11:470. doi: 10.3390/toxins11080470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda K., Murakami Y., Emoto Y., Ngy L., Taniyama S., Yagi M., Takatani T., Arakawa O. Transfer profile of intramuscularly administered tetrodotoxin to non-toxic cultured specimens of the pufferfish Takifugu rubripes. Toxicon. 2009;53:99–103. doi: 10.1016/j.toxicon.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Itoi S., Kozaki A., Komori K., Tsunashima T., Noguchi S., Kawane M., Sugita H. Toxic Takifugu pardalis eggs found in Takifugu niphobles gut: Implications for TTX accumulation in the pufferfish. Toxicon. 2015;108:141–146. doi: 10.1016/j.toxicon.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Itoi S., Yoshikawa S., Asahina K., Suzuki M., Ishizuka K., Takimoto N., Mitsuoka R., Yokoyama N., Detake A., Takayanagi C., et al. Larval pufferfish protected by maternal tetrodotoxin. Toxicon. 2014;78:35–40. doi: 10.1016/j.toxicon.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Itoi S., Suzuki M., Asahina K., Sawayama E., Nishikubo J., Oyama H., Takei M., Shiibashi N., Takatani T., Arakawa O., et al. Role of maternal tetrodotoxin in survival of larval pufferfish. Toxicon. 2018;148:95–100. doi: 10.1016/j.toxicon.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Yotsu-Yamashita M., Okoshi N., Watanabe K., Araki N., Yamaki H., Shoji Y., Terakawa T. Localization of pufferfish saxitoxin and tetrodotoxin binding protein (PSTBP) in the tissues of the pufferfish, Takifugu pardalis, analyzed by immunohistochemical staining. Toxicon. 2013;72:23–28. doi: 10.1016/j.toxicon.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Dyble J., Gossiaux D., Landrum P., Kashian D.R., Pothoven S. A kinetic study of accumulation and elimination of microcystin-LR in yellow perch (Perca flavescens) tissue and implications for human fish consumption. Mar. Drugs. 2011;9:2553–2571. doi: 10.3390/md9122553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jang J.H., Lee J.S., Yotsu-Yamashita M. LC/MS analysis of tetrodotoxin and its deoxy analogs in the marine puffer fish Fugu niphobles from the southern coast of Korea, and in the brackishwater puffer fishes Tetraodon nigroviridis and Tetraodon biocellatus from Southeast Asia. Mar. Drugs. 2010;8:1049–1058. doi: 10.3390/md8041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tonon L.A.C., de Azevedo G.P.R., Monteiro A.F., Bernardi D.I., Gubiani J.R., Ioca L.P., Mattsson H.K., Moreira A.P.B., Gomes A.F., Pires Junior O.R., et al. New tetrodotoxin analogs in Brazilian pufferfishes tissues and microbiome. Chemosphere. 2020;242:125211. doi: 10.1016/j.chemosphere.2019.125211. [DOI] [PubMed] [Google Scholar]
- 34.Yang G., Xu J., Liang S., Ren D., Yan X., Bao B. A novel TTX-producing Aeromonas isolated from the ovary of Takifugu obscurus. Toxicon. 2010;56:324–329. doi: 10.1016/j.toxicon.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Kudo Y., Yasumoto T., Konoki K., Cho Y., Yotsu-Yamashita M. Isolation and structural determination of the first 8-epi-type tetrodotoxin analogs from the newt, Cynops ensicauda popei, and comparison of tetrodotoxin analogs profiles of this newt and the puffer fish, Fugu poecilonotus. Mar. Drugs. 2012;10:655–667. doi: 10.3390/md10030655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagashima Y., Tanaka N., Shimakura K., Shiomi K., Shida Y. Occurrence of tetrodotoxin-related substances in the nontoxic puffer Takifugu xanthopterus. Toxicon. 2001;39:415–418. doi: 10.1016/S0041-0101(00)00136-7. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X., Yan Z., Wang Y., Jiang T., Wang J., Sun X., Guo Y. Immunoaffinity chromatography purification and ultrahigh performance liquid chromatography tandem mass spectrometry determination of tetrodotoxin in marine organisms. J. Agric. Food Chem. 2015;63:3129–3134. doi: 10.1021/acs.jafc.5b00045. [DOI] [PubMed] [Google Scholar]
- 38.Gall B.G., Stokes A.N., French S.S., Brodie E.D., 3rd, Brodie E.D., Jr. Female newts (Taricha granulosa) produce tetrodotoxin laden eggs after long term captivity. Toxicon. 2012;60:1057–1062. doi: 10.1016/j.toxicon.2012.07.017. [DOI] [PubMed] [Google Scholar]