Abstract
West Nile virus (WNV) is a widespread zoonotic arbovirus and a threat to public health in Germany since its first emergence in 2018. It has become of particular relevance in Germany in 2019 due to its rapid geographical spread and the detection of the first human clinical cases. The susceptibility of indigenous Culex pipiens (biotypes pipiens and molestus) for a German WNV lineage 2 strain was experimentally compared to that of Serbian Cx. pipiens biotype molestus and invasive German Aedes albopictus. All tested populations proved to be competent laboratory vectors of WNV. Culex pipiens biotype pipiens displayed the highest transmission efficiencies (40.0%–52.9%) at 25 °C. This biotype was also able to transmit WNV at 18 °C (transmission efficiencies of 4.4%–8.3%), proving that temperate climates in Central and Northern Europe may support WNV circulation. Furthermore, due to their feeding behaviors, Cx. pipiens biotype molestus and Ae. albopictus can act as “bridge vectors”, leading to human WNV infections.
Keywords: West Nile virus, vector competence, transmission, arbovirus, Culex pipiens, Aedes albopictus
1. Introduction
West Nile virus (WNV; Flaviviridae; Flavivirus) is the most dispersed zoonotic arbovirus worldwide and the causative agent of viral neurological diseases in susceptible animals and humans. The virus is maintained in an enzootic transmission cycle between ornithophilic mosquitoes as vectors and susceptible avian species as amplifying hosts [1]. Raptors (such as hawks and owls) and passeriform bird species (such as corvids) are highly susceptible, usually developing neuroinvasive diseases with high morbidity and mortality rates [2]. Rapid onset of disease and severe clinical symptoms are often associated with high viremia levels, sufficient to infect feeding mosquitoes and perpetuate the transmission cycle. However, amplifying hosts such as the American robin (Turdus migratorius) and the house sparrow (Passer domesticus) can show low mortality rates and still produce viremia levels high enough to infect mosquitoes [2,3]. Non-avian vertebrates, so-called “dead-end” hosts that are inadequate to pass on the virus to mosquitoes, can be infected with WNV via “bridge vectors” (mosquito species that feed on both birds and mammals) [1].
WNV was initially isolated in the West Nile district in Uganda in 1937 [4]. After extensive geographical dispersion mainly due to migratory birds [5], WNV is today endemic to every continent apart from Antarctica [1]. WNV lineage 1 was introduced into the United States in 1999 [6]. Through extensive infection of immunologically naïve avian hosts [7], it rapidly spread from the East to the West Coast within three years [1]. Unlike in the United States, WNV lineage 1 has been present in Europe and the Mediterranean at least since the late 1950s and was primarily associated with sporadic infections of low pathogenicity [1]. Only with the appearance of WNV lineage 2 in 2004 in several southern and southeastern European countries was there an increase in the severity and incidence of clinical cases in humans and equines [8,9,10]. In 2018, autochthonous WNV cases (12 in total) in resident wild and captive birds were recorded for the first time in Germany. The detected cases extended from the north to the south of eastern Germany (i.e., Munich to Rostock) [11,12]. In 2019, WNV incidence increased with numerous cases in birds and horses, including severe and fatal infections, primarily in Central and Eastern Germany [12]. In the same year, the first human infections were confirmed, with a total of five cases, which included WNV neuroinvasive disease [13].
Numerous studies indicated Culex pipiens, Cx. quinquefasciatus, and Cx. tarsalis to be principal vector species in the United States [14,15,16]. Similarly, Cx. pipiens plays a primary role in the transmission of WNV in Europe [17]. Vector competence studies with French [18], Italian [19], Spanish [20], Dutch [21,22,23,24,25], and German [26,27] Cx. pipiens populations proved their susceptibility to several WNV strains. Yet it remains unclear how efficient German mosquito populations are at transmitting WNV, especially regarding the two different Cx. pipiens biotypes (pipiens and molestus). These biotypes are known to differ in their seasonal activity and feeding-behavior (ornithophilic vs. mammalophilic) [28,29]. Furthermore, to better comprehend the spread of WNV in Germany and throughout Europe it is important to assess whether German mosquito species can transmit WNV under temperate climate conditions as found, for example, in Northern Europe. Finally, little is known about whether invasive mosquito species such as Aedes albopictus [30] play a role in WNV transmission in Central Europe.
The aim of this study was to investigate the vector competence of Cx. pipiens populations (biotypes pipiens and molestus) indigenous to Germany for the first German isolate of WNV lineage 2 [11] and to compare it to a Serbian (i.e., southern European) Cx. pipiens biotype molestus colony as well as an invasive German Ae. albopictus strain. Special focus was also placed on the influence of temperature on the vector competence of Cx. pipiens biotype pipiens.
2. Materials and Methods
2.1. Mosquito Origin and Rearing
Vector competence studies were performed with three mosquito taxa from Germany (Cx. pipiens biotypes pipiens and molestus and Ae. albopictus) and one colony (Cx. pipiens biotype molestus) from the Republic of Serbia (Table 1). Culex pipiens biotype pipiens egg rafts were collected in Brandenburg, Germany (“Schöneiche” and “Rehfelde” (S)), in 2018 and propagated in the laboratory, as well as in 2019 (“Groß Kreutz” (G)), where the F0 generation was directly used in the experiments. The Cx. pipiens biotype molestus colony was established in 2012 (“Wendland” (W), Lower Saxony, Germany) [26]. The Ae. albopictus colony was initiated from eggs acquired from an overwintering population in Jena (J), Thuringia, Germany, in 2016 [31]. For comparative purposes, a Cx. pipiens biotype molestus colony from Novi Sad (N), Republic of Serbia, established in 2012, was also tested.
Table 1.
Mosquito taxa and blood sources used in the infection experiments.
| Mosquito Taxon | Collection Site | Year of Collection | Laboratory Colony | Field-Collected | Blood Source |
|---|---|---|---|---|---|
| Culex pipiens biotype pipiens | “Schöneiche“ and “Rehfelde” (S), Brandenburg, Germany |
2018 | X | Chicken | |
| “Groß Kreutz” (G), Brandenburg, Germany |
2019 | X | Chicken | ||
| Cx. pipiens biotype molestus | “Wendland” (W), Lower Saxony, Germany |
2012 | X | Bovine | |
| Novi Sad (N), Republic of Serbia |
2012 | X | Bovine | ||
| Aedes albopictus | Jena (J), Thuringia, Germany |
2016 | X | Bovine |
For the identification of field-collected Cx. pipiens to species and biotype level, larvae hatching from each egg raft were tested by a real-time polymerase chain reaction (PCR) [32]. After pupation, the pupae were transferred into mosquito breeding cages (BugDorm; MegaView Science Co., Ltd., Taichung, Taiwan), and emerging adult mosquitoes were offered a 5% sugar solution ad libitum. They were kept at 24 °C ± 1 °C with a relative humidity of 60%–70% and a 16 h light/8 h dark photocycle. For the maintenance of established colonies, mosquitoes were artificially fed with chicken or bovine blood using the Hemotek Membrane Feeding System (Hemotek Ltd., Blackburn, United Kingdom). To assess whether mosquito populations were free from flaviviruses prior to the experiments, a minimum of two non-engorged females per population were examined via a WNV-specific reverse transcription quantitative real-time PCR (RT-qPCR) [33] and individual females were also tested in a SYBR® Green-based quantitative real-time pan-flavivirus assay [34].
The WNV lineage 2 strain (GenBank accession no. MH924836) had been isolated from the brain of the first confirmed WNV infected bird, a great grey owl (Strix nebulosa), in Germany [11]. The virus was passaged three times alternately on Vero and Ae. albopictus C6/36 cell monolayers (the Collection of Cell Lines in Veterinary Medicine (CCLV), Friedrich-Loeffler-Institut (FLI), Greifswald-Insel Riems, Germany) and maintained in modified minimum essential medium (MEM) supplemented with 2% fetal calf serum (FCS). The last passage (P3) was performed on a Vero cell monolayer with a multiplicity of infection of 0.001. The virus stock was harvested four days post infection (dpi). The virus titer was quantified by means of an endpoint dilution assay on Vero cells and calculated with the Spearman–Karber algorithm [35]. The used stock had a titer of 9.3 log10 50% tissue culture infective dose (TCID50) per mL. The virus stock was stored at −70 °C in 500 µL aliquots.
2.2. Mosquito Infection
Twenty-four hours prior to infection, female mosquitoes (5–14 days old) were sorted into transparent tubes and deprived of sugar solution and water. The infectious blood meal was composed of 90% heparinized chicken or bovine blood, based on the mosquito taxa’s feeding preference (Table 1), and 10% virus stock (i.e., 1:10 dilution of the virus stock with a titer of 9.3 log10 TCID50/mL). The blood was obtained from quarantine animals kept at the FLI, Greifswald-Insel Riems, Germany. As WNV-specific antibodies have not been detected in sentinel birds (mallards, Anas platyrhynchos) kept on the Isle of Riems (personal communication [36]), the chicken blood source was considered negative prior to the experiments. Bovine serum samples were tested to be free from WNV-specific antibodies using the ID Screen® WN competition enzyme-linked immunosorbent assay (ELISA) (IDVet, Grabels, France). ATP (Merck, St. Louis, MO, USA) was added as a phagostimulant at a final concentration of 0.5 mM. In each tube, the mosquitoes were offered two cotton stick ends fully soaked in the infectious blood meal for three hours. The blood meal was titrated both before feeding and afterward from the cotton sticks on Vero cells to confirm the exact virus titer offered to the mosquitoes. Engorged females were transferred to new modified tubes under CO2-sedation. Two freshly engorged females per species were stored at −70 °C, to later confirm virus ingestion and construct a baseline for virus development in the mosquitoes via the WNV-specific RT-qPCR [33]. The remaining engorged mosquitoes were incubated under controlled conditions (18 °C, 25 °C, or 28 °C ± 1 °C, relative humidity of 80%–85%, 16 h light/8 h dark photocycle) in an incubator (MLR-352H-PE; Panasonic Corporation, Osaka, Japan) for 14/15 or 20/21 days, respectively. During this period, the mosquitoes were offered cotton pads soaked with 5% sugar solution ad libitum.
2.3. Mosquito Processing
After 14/15 or 20/21 days, respectively (i.e., salivation assay was performed on two consecutive days), mosquitoes were examined for virus presence in their bodies (thorax and abdomen), legs plus wings, and saliva. First, the mosquitoes were immobilized by detaching their legs and wings under CO2-anesthesia. Then, the forced salivation assay was performed according to Heitmann et al. [37], with saliva collection for 45–60 min. The mosquito bodies and legs plus wings were placed into separate 2 mL screw cap tubes with two 3-mm steel beads and 560 µL AVL viral lysis buffer and carrier RNA (QIAGEN, Hilden, Germany) and stored at −70 °C until RNA extraction. The saliva samples were inoculated directly onto a 96-well-plate cell monolayer of Vero cells. For virus inoculation, Vero cells were maintained in MEM supplemented with 2% FCS and 1% antimicrobials (gentamicin/amphotericin and penicillin/streptomycin; Merck, St. Louis, MO, USA). After seven days, the plates were fixed with 7.5% neutral buffered formalin (Carl Roth, Karlsruhe, Germany) and stained with crystal violet (Carl Roth, Karlsruhe, Germany). If a sample portrayed a distinct cytopathogenic effect, 140 µL of the cell culture supernatant was removed prior to staining and added to a 2-mL tube with 560 µL AVL viral lysis buffer and carrier RNA. RNA was extracted using the QIAamp Viral RNA Mini Kit (QIAGEN, Hilden, Germany), eluted in 50 µL of elution buffer, and stored at −70 °C.
Mosquito bodies and legs plus wings were homogenized separately in the 2 mL screw cap tubes at 30 MHz for two minutes (TissueLyser II; QIAGEN, Hilden, Germany). Afterward, they were centrifuged in a 5430R centrifuge (Eppendorf, Hamburg, Germany) at room temperature for one minute at 13000 rpm. Nucleic acid was extracted from 200 µL of the supernatant with the BioSprint 96 (QIAGEN, Hilden, Germany) using the NucleoMag VET kit (MACHEREY-NAGEL, Düren, Germany). RNA extracts were eluted in 100 µL elution buffer and stored at −70 °C. Amplification of RNA (5 µL) from mosquito bodies, legs plus wings, and saliva cell culture supernatants was performed with a WNV-specific RT-qPCR assay [33] using the AgPath-ID One-Step RT-PCR Reagents (ThermoFischer Scientific, Darmstadt, Germany) and the CFX96TM Real-Time PCR Detection System (Bio-Rad Laboratories, Feldkirchen, Germany). For RNA quantification via RT-qPCR, a standard curve based on synthetic WNV RNA was run in parallel using 10-fold serial dilutions [33].
2.4. Vector Competence Indices
The feeding rate refers to the number of engorged females out of the total number of females exposed to the blood meal. The survival rate is the number of females surviving within a given period out of the total number of fed females. The infection rate describes the number of WNV-positive bodies in relation to the total number of mosquitoes examined. The dissemination rate is calculated as the number of specimens with WNV-positive legs plus wings out of the number of WNV-positive bodies. The transmission rate is the percentage of mosquitoes with infected bodies and legs plus wings that also had viable virus in their saliva. Transmission efficiency is the percentage of mosquitoes having viable virus in their saliva in relation to the total number of mosquitoes examined.
2.5. Data Analysis
All statistical analyses and graphical displays were performed with R version 3.6.0 (26 April 2019) [38] and the package “lsmeans” [39]. The effect of species, temperature, and/or sampling date (14/15 and 20/21 dpi), including their interactions (explanatory variables) on feeding, survival, infection, dissemination, and transmission rates and on transmission efficiencies (response variables), were investigated using generalized binomial regression models (glm). The final models were determined using stepwise backward elimination, leading to different models for the six response variables (Supplementary Materials Tables S1 and S2). Least-squares means [40] were used for testing linear contrasts among predictions with Tukey adjustment for p-values [41]. Results were deemed statistically relevant when the p-values (summarized in Supplementary Materials) were less than 0.05. Individual samples where the legs plus wings were virus-positive, but not the bodies, were not included in the analyses.
2.6. Ethics Statement
Blood for mosquito feeding was collected from animals kept at the FLI. Animals were held and sampled according to national and European legislation (Directive 2010/63/EU on the protection of animals used for scientific purposes). The procedures were approved by the competent authority of the Federal State of Mecklenburg-Western Pomerania, Germany (reference number: 7221.3-2-041/17, approved 12 Feb 2018).
3. Results
3.1. Feeding and Survival Rates
In total, 1217 mosquitoes were included in the WNV vector competence experiments (summarized in Table 2). Mosquito numbers were obtained via multiple replications of the infection experiments. The number of mosquitoes tested per time point fluctuates between mosquito populations, as the availability of mosquitoes for the saliva assays was strongly dependent on their feeding and survival rate. The 33.2% feeding rate of the field-acquired Cx. pipiens biotype pipiens (G) was significantly lower (p < 0.001) than the 82.8% rate of the laboratory-reared Cx. pipiens biotype pipiens (S). All other mosquito species had similar feeding rates varying between 53.1% and 56.7%. The Ae. albopictus population had the lowest survival rates 14/15 dpi (42.9%) and 14/15–20/21 dpi (58.1%). All other mosquito populations had survival rates ranging from 60.9% (Cx. pipiens biotype molestus from Serbia) to 90.4% (Cx. pipiens biotype pipiens from Germany) 14/15 dpi. Survival rates remained consistent 14/15–20/21 dpi, ranging from 65.6% (Cx. pipiens biotype molestus from Serbia) to 100.0% (Cx. pipiens biotype pipiens from Germany).
Table 2.
Mosquito feeding and survival rates, up to 14/15 days post infection (dpi) and 14/15–20/21 dpi, with German West Nile virus lineage 2 at different temperatures. CI stands for confidence interval.
| Mosquito Species | Incubation Temperature (°C) | Feeding Rate (%) (95% CI) |
Survival Rate 14/15 dpi (Excluding Day 0 Samples) (%) (95% CI) |
Survival Rate 14/15–20/21 dpi (Excluding Day 0 Samples and Mosquitoes Tested 14/15 dpi) (%) (95% CI) |
|---|---|---|---|---|
|
Culex pipiens biotype pipiens (S) |
25 | 48/58 (82.8) (70.6–91.4) |
41/46 (89.1) (76.4–96.4) |
17/21 (81.0) (58.1–94.6) |
|
Cx. pipiens biotype pipiens (G) |
18 | 147/443 (33.2) (28.8–37.8) |
47/52 (90.4) (77.8–95.9) |
23/23 (100.0) (72.6–97.8) |
| 25 | 49/55 (89.1) (79.0–96.8) |
26/29 (89.7) (85.2–100.0) |
||
| 28 | 21/30 (70.0) (50.6–85.3) |
9/12 (75.0) (42.8–94.5) |
||
|
Cx. pipiens biotype molestus (W) |
25 | 34/64 (53.1) (40.2–65.7) |
23/30 (76.7) (57.7–90.1) |
15/16 (93.8) (69.8–99.8) |
|
Cx. pipiens biotype molestus (N) |
25 | 159/299 (53.2) (47.3–58.9) |
92/151 (60.9) (52.7–68.8) |
40/61 (65.6) (52.3–77.3) |
|
Aedes albopictus (J) |
25 | 200/353 (56.7) (51.3–61.9) |
84/196 (42.9) (35.8–50.1) |
25/43 (58.1) (42.1–73.0) |
3.2. Vector Competence of Cx. pipiens and Ae. albopictus at 25 °C
In all infection experiments, the mosquitoes fed on a blood meal containing on average 7.3 (end of feeding period) to 8.2 (beginning of feeding period) log10 TCID50/mL of the German WNV strain. Infection, dissemination, and transmission rates and transmission efficiencies are summarized in Table 3. Both Cx. pipiens biotypes had high infection rates 14/15 dpi ranging from 64.5% to 100.0%. However, unlike Cx. pipiens biotype pipiens (S and G), the infection rates of Cx. pipiens biotype molestus (W and N) decreased to 6.7% and 15.0% 20/21 dpi. This resulted in statistically significant differences between the infection rates of both Cx. pipiens biotype pipiens colonies (S and G) and those of the German Cx. pipiens biotype molestus (W) (p = 8.1 × 10−3 and p = 2.5 × 10−2, respectively) and Serbian Cx. pipiens biotype molestus (N) (p = 1.5 × 10−2 and p = 3.9 × 10−3, respectively) 20/21 dpi. Aedes albopictus was the only species that had low infection rates (9.8% and 20.0%) at both sampling dates (14/15 and 20/21 dpi). These were significantly lower than those of the two Cx. pipiens biotype pipiens colonies (S and G) (p = 1.1 × 10−2 and p = 5.8 × 10−3, respectively) 20/21 dpi. However, the dissemination and transmission rates did not differ significantly between the different mosquito populations. All mosquito populations derived from field-collected egg rafts were tested negative for WNV prior to the infection experiments.
Table 3.
Infection, dissemination, and transmission rates and transmission efficiencies 14/15 and 20/21 days post infection at an incubation temperature of 25 °C. CI stands for confidence interval.
| Mosquito Species | Days Post Infection | Infection Rate (%) (95% CI) |
Dissemination Rate (%) (95% CI) |
Transmission Rate (%) (95% CI) |
Transmission Efficiency (%) (95% CI) |
|---|---|---|---|---|---|
|
Culex pipiens biotype pipiens (S) |
14/15 | 20/20 (100.0) (83.2–100.0) |
19/20 (95.0) (75.1–99.9) |
10/19 (52.6) (28.9–75.6) |
10/20 (50.0) (27.2–72.8) |
| 20/21 | 16/17 (94.1) (71.3–99.9) |
16/16 (100.0) (79.4–100.0) |
9/16 (60.0) (29.9–80.2) |
9/17 (52.9) (27.8–77.0) |
|
|
Cx. pipiens biotype pipiens (G) |
14/15 | 13/20 (65.0) (40.8–84.6) |
10/13 (76.9) (46.2–95.0) |
8/10 (80.0) (44.4–97.5) |
8/20 (40.0) (19.1–63.9) |
| 20/21 | 20/26 (76.9) (56.4–91.0) |
16/20 (80.0) (56.3–94.3) |
12/16 (75.0) (47.6–92.7) |
12/26 (46.2) (26.6–66.6) |
|
|
Cx. pipiens biotype molestus (W) |
14/15 | 7/7 (100.0) (59.0–100.0) |
6/7 (85.7) (42.1–99.6) |
2/6 (33.3) (4.3–77.7) |
2/7 (28.6) (3.7–71.0) |
| 20/21 | 1/15 (6.7) (0.2–31.9) |
1/1 (100.0) (2.5–100.0) |
1/1 (100.0) (2.5–100.0) |
1/15 (6.7) (0.2–31.9) |
|
|
Cx. pipiens biotype molestus (N) |
14/15 | 20/31 (64.5) (45.4–80.8) |
16/20 (80.0) (56.3–94.3) |
4/16 (25.0) (7.3–52.4) |
4/31 (12.9) (3.6–29.8) |
| 20/21 | 10/40 (15.0) (12.7–41.2) |
4/10 (40.0) (12.2–73.8) |
2/4 (50.0) (6.8–93.2) |
2/40 (5.0) (0.6–16.9) |
|
|
Aedes albopictus (J) |
14/15 | 4/41 (9.8) (2.7–23.1) |
4/4 (100.0) (39.8–100) |
4/4 (100.0) (39.8–100) |
4/41 (9.8) (2.7–23.1) |
| 20/21 | 5/25 (20.0) (6.8–40.7) |
4/5 (80.0) (28.4–99.5) |
0/4 (0.0) (0.0–60.2) |
0/25 (0.0) (0.0–13.7) |
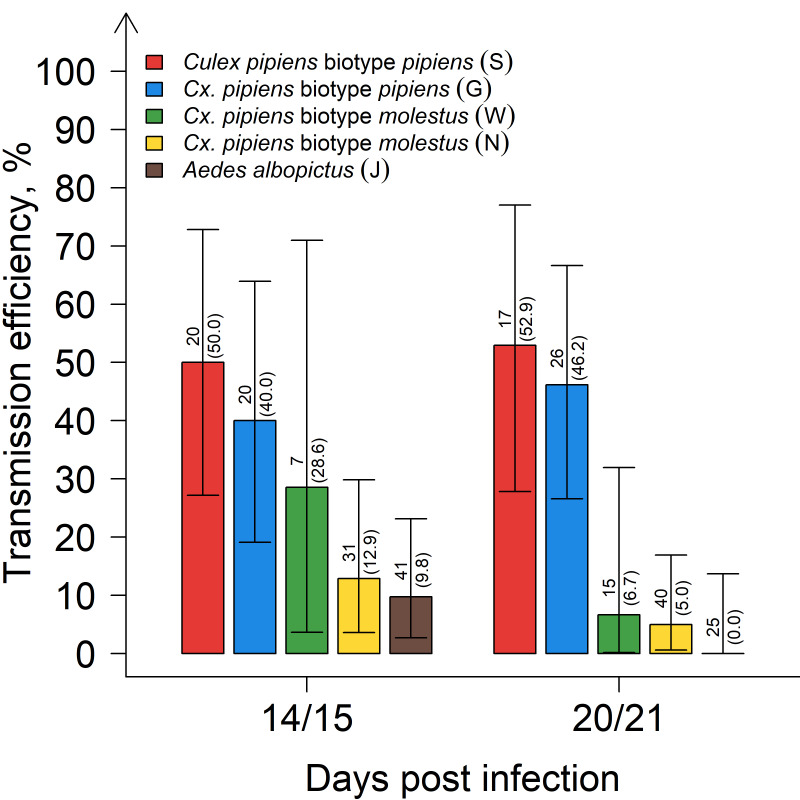
When comparing the transmission efficiencies (Figure 1) of the German Cx. pipiens biotype pipiens (S and G) with the Serbian Cx. pipiens biotype molestus (N), significant differences were observed independent of the sampling date (p < 0.001, respectively). Differences were also found between the German Cx. pipiens biotype pipiens (S and G) and the German Cx. pipiens biotype molestus (W), even though these were not statistically significant (p = 5.4 × 10−2 and p = 1.5 × 10−1, respectively). Transmission efficiencies of Ae. albopictus were also significantly lower than those of the Cx. pipiens biotype pipiens (S and G) (p < 0.001, respectively).
Figure 1.
Transmission efficiencies for Cx. pipiens biotype pipiens, Cx. pipiens biotype molestus, and Ae. albopictus 14/15 and 20/21 days post infection at an incubation temperature of 25 °C. Error bars represent 95% confidence intervals. The number above each bar indicates the number of mosquitoes tested per species/time point, and in brackets, the percentage tested WNV-positive.
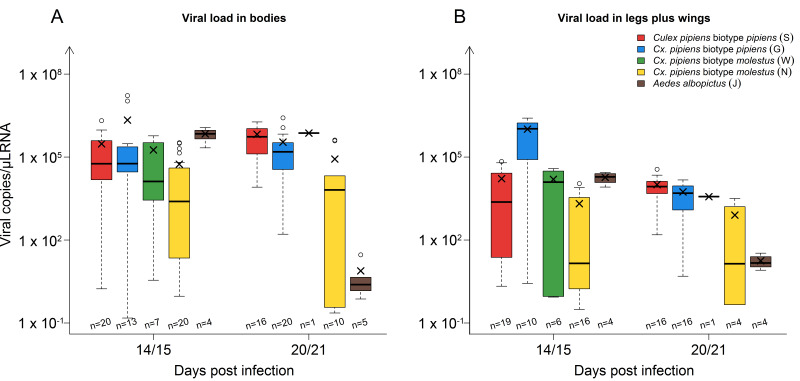
The viral loads (virus RNA copies/µL RNA) in the mosquito bodies and legs plus wings 14/15 and 20/21 dpi are depicted in Figure 2. The mean viral load in the Culex strains remained fairly stable from 14/15 to 20/21 dpi. The values in the bodies ranged from 5.5 × 104 to 2.2 × 106 copies/µL RNA and in the legs plus wings from 8.0 × 102 to 1.0 × 106 copies/µL RNA. Aedes albopictus, however, showed a time-dependent decline in the mean viral load both in the mosquito bodies (6.9 × 105 to 7.7 copies/µL RNA) and in the legs plus wings (1.9 × 104 to 1.8 × 101 copies/µL RNA). The viral loads found in the mosquitoes directly after blood feeding ranged between 1.8 × 101 to 8.8 × 102 (cycle threshold of 24.4–30.1). By contrast, all non-engorged day 0 samples lacked flavivirus-specific RNA.
Figure 2.
Viral load in mosquito bodies (A) and legs plus wings (B) 14/15 and 20/21 days post infection in the different strains: Culex pipiens biotype pipiens, Cx. pipiens biotype molestus, and Aedes albopictus. The horizontal black lines represent the medians, the black ×’s the means, the boxes show the interquartile ranges, and the whiskers the minimum and maximum values. Data falling outside the interquartile ranges are plotted as outliers (o). The number of samples tested per species/time point are indicated above the x-axis.
3.3. Vector Competence of Cx. pipiens Biotype pipiens at Three Different Temperature Regimes (18 °C, 25 °C, and 28 °C)
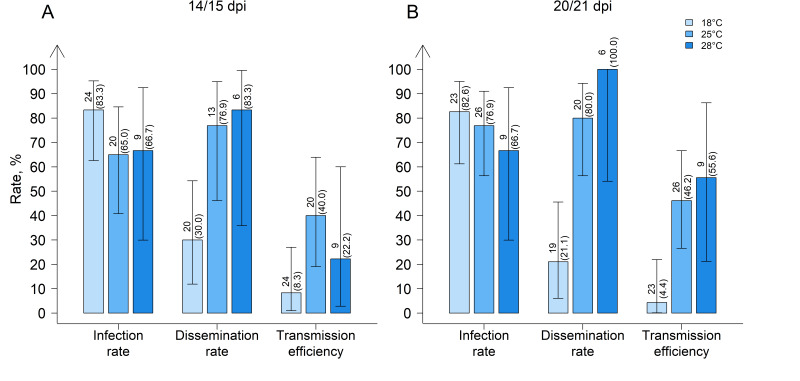
German Cx. pipiens biotype pipiens from “Groß Kreutz” (G), Brandenburg, showed higher dissemination and transmission efficiencies at 25 °C and 28 °C than at 18 °C (Figure 3). The dissemination rates (14/15 and 20/21 dpi) at 28 °C (83.3% and 100.0%) and 25 °C (76.9% and 80.0%) were significantly higher than at 18 °C (30.0% and 21.1%) (p < 0.001 and p = 5.0 × 10−3, respectively). Similarly, the transmission efficiencies (14/15 and 20/21 dpi) were significantly higher at 28 °C (22.2% and 55.6%) and 25 °C (40.0% and 46.2%) than at 18 °C (8.3% and 4.4%) (p < 0.001 and p = 1.0 × 10−2, respectively).
Figure 3.
Infection rate, dissemination rate, and transmission efficiency of field-collected Culex pipiens biotype pipiens from “Groß Kreutz”, Brandenburg, Germany, (A) 14/15 and (B) 20/21 days post infection (dpi) at three different temperature regimes. Error bars represent 95% confidence intervals. The number above each bar indicates the number of mosquitoes tested per species/time point, and in brackets, the percentage tested WNV-positive.
4. Discussion
This study demonstrates that German Cx. pipiens mosquitoes and Serbian Cx. pipiens biotype molestus fulfill the criteria for WNV vectors, portraying high virus susceptibility and efficient virus transmission, even at temperate climate conditions (e.g., 18 °C), which are common in Germany during the summer season [42]. The results also revealed, for the first time, the WNV vector competence of an established Ae. albopictus population in Germany [31]. The virus titers in the blood meals used in this study of 7.3–8.2 log10 TCID50/mL are commonly found in viremic birds in nature, with most species, however, not reaching peak viremia levels above 8 log10 TCID50/mL [17]. Therefore, the results of this study can relate to typical scenarios found in the field. When discussing these results, one must, however, bear in mind that the different blood sources used (chicken vs. bovine) may have had a modest effect on the vector competence of mosquito populations. The same is true for the laboratory colonization of the mosquito colonies used in this study [17].
Culex pipiens biotype pipiens is highly ubiquitous throughout Germany and, due to its ornithophilic feeding preference, suitable as a primary amplifier in the enzootic transmission cycle of WNV [32]. In general, the high susceptibility of Cx. pipiens biotype pipiens for WNV described within this study correlates with the results from several other studies of European mosquitoes [17]. These studies have described a virus and/or mosquito strain-dependent difference in the vector competence of mosquitoes [17]. This current study also reinforces this idea as the transmission efficiencies of the two German Cx. pipiens biotype pipiens populations (50.0% and 40.0% 14/15 dpi, respectively) infected with the German WNV lineage 2 strain were higher than those of Dutch (10.3% [25], 6% [17,23], and 10% [24]) and Italian (2% [24]) populations of the same species infected with a Greek WNV lineage 2 strain (GenBank accession no. HQ537483) after incubation at 23 °C or 25 °C. The transmission efficiencies of German Cx. pipiens biotype pipiens after infection with an Italian WNV lineage 1 strain (GenBank accession no. HM991273/HM641225) were even lower [27]. Further evidence supporting the role of Culex pipiens biotype pipiens as a WNV vector in the field was provided in 2019, where WNV was detected for the first time in German mosquitoes (in five Cx. pipiens biotype pipiens pools and two Cx. pipiens biotype pipiens/molestus pools) [43]. Keeping all these variations in mind, Cx. pipiens can be designated as an important vector for WNV in Europe.
The German and Serbian Cx. pipiens biotype molestus on the other hand had lower transmission efficiencies for WNV than the two Cx. pipiens biotype pipiens colonies, particularly at a later point in time post infection. Interestingly though, the tested Cx. pipiens biotype molestus populations showed infection rates (100.0% and 64.5%, respectively) and transmission efficiencies (28.6% and 12.9%, respectively) that were higher than those of Dutch Cx. pipiens biotype molestus infected with a Greek WNV lineage 2 strain (GenBank accession no. HQ537483.1) (24% and 10%, respectively) 14/15 dpi [17,23]. Although Cx. pipiens biotype molestus does not appear to be an essential amplifier in the transmission cycle between birds, it may act as a “bridge vector” due to its mammalophilic feeding-preference, infecting humans with WNV. Nonetheless, the limited number of females tested enables only a preliminary indication of the vector competence of this biotype.
Aedes albopictus is a vastly spreading, invasive mosquito species from tropical Asia and the Pacific and is a known secondary vector of many arboviruses [44,45,46,47]. In this study, the Ae. albopictus population from Jena, Germany, was susceptible to WNV lineage 2, although with significantly lower infection rates and transmission efficiencies (9.8% 14/15 dpi for both) than Cx. pipiens biotype pipiens. It was also less susceptible to the German WNV lineage 2 strain than Italian Ae. albopictus to an Italian WNV lineage 1 strain (GenBank accession no. HQ537483.1 [48]), with infection rates of 9.8% compared to 80% [45]. The difference, however, may have been due to a lower incubation temperature (25 °C vs. 27 °C). Furthermore, the Italian Ae. albopictus infected with the Italian WNV lineage 1 strain could still transmit virus 21 dpi [45] unlike the Ae. albopictus in this study. The German Ae. albopictus showed a strong decline in viral RNA copies/µL in the bodies and legs plus wings from 14/15 to 20/21 dpi. This could indicate a decline of intracellular virus replication in the mosquitoes followed by an absence of infectious virus particles in their saliva. Even though Ae. albopictus showed the lowest transmission efficiencies, the species may still be a relevant vector of WNV for humans due to its high abundance in human settlements and the intake of multiple blood meals from different hosts in a short period of time [49]. Aedes albopictus should therefore be considered a WNV vector in regions of Germany, where it succeeded in establishing itself and has reached high seasonal population densities [50].
With 19.3 °C and 19.2 °C, respectively, the average summer temperatures in 2018 and 2019 were unusually high for Germany, i.e., 2.1–2.2 °C warmer than normal (1981–2010) [42]. For a total of 74 and 52 days, respectively, the maximum daily temperature reached or exceeded 25 °C [51]. To better understand the ongoing epidemiology and predict the spread of WNV throughout Germany, it is essential to further test the temperature dependency of WNV transmission per mosquito species and population. This study focused on the most susceptible taxon, Cx. pipiens biotype pipiens. Transmission efficiencies were similar at incubation temperatures of 25 °C and 28 °C, however, they were significantly lower at 18 °C. This could primarily be due to the positive correlation between intracellular virus replication and temperature [17,52]. It is also possible that the midgut and salivary gland escape barriers only impede WNV vector competence at low temperatures but not at 25 °C or 28 °C. High temperatures can destabilize the midgut barrier and induce changes in the regulation of a mosquito’s immune system and RNA-interference pathways [53]. Other vector competence studies have described a complete lack of WNV transmission at incubation temperatures below 21 °C [27]. This was refuted by our results where several mosquitoes had WNV in their saliva after incubation at 18 °C. More frequent climate extremes associated with increased temperatures are anticipated in the near future [54] and will probably result in WNV propagation throughout Germany due to longer WNV transmission and mosquito breeding seasons with greater population densities [52].
5. Conclusions
Both Cx. pipiens biotypes (pipiens and molestus) and Ae. albopictus were proven vector-competent for the German WNV lineage 2 strain in the laboratory, suggesting a role as WNV vectors also in the field. Culex pipiens biotype pipiens appears to be highly adapted to temperate climate conditions found in Central and Northern Europe, still supporting WNV transmission at 18 °C. The expansion of WNV throughout Germany is facilitated through the expected increasing temperatures and the presence of highly potent WNV vectors and susceptible hosts in Germany.
Acknowledgments
We are grateful to René Schöttner, Oliver Tauchmann, and Cornelia Steffen (FLI, Greifswald-Insel Riems, Germany) for excellent technical assistance in the laboratory. Furthermore, we want to thank René Klein, Marlene Hausner, and Maxi Uecker (FLI, Greifswald-Insel Riems, Germany) for rearing the mosquitoes and identifying the larvae to species level. A big thank you goes to Ariel Viña-Rodríguez (FLI, Greifswald-Insel Riems, Germany) for providing us with the synthetic WNV RNA for the standard curve. We would like to thank Dušan Petrić (Faculty of Agriculture, University of Novi Sad, Novi Sad, Republic of Serbia) for supplying the Serbian Cx. pipiens biotype molestus colony. We are thankful to Anna Heitmann and Stephanie Jansen (Bernhard-Nocht-Institut, Hamburg, Germany) for their advice in designing the study. Finally, the authors would like to thank Bärbel Hammerschmidt (FLI, Greifswald-Insel Riems, Germany) for supplying chicken and bovine blood for the blood meals.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4915/12/5/561/s1, Table S1: Overview on explanatory variables included in the final generalized binomial regression models (glm) for the investigated rates at 25 °C, Table S2: Overview on explanatory variables included in the final generalized binomial regression models (glm) for Culex pipiens biotype pipiens (G) at three different temperature regimes for the investigated rates, Table S3: p-values of the fixed effects in the least-square means analysis when comparing the rates between all species at 25 °C in the final generalized binomial regression models specified in Supplementary Materials Table S1. p-value adjustment was performed using the Tukey method, Table S4: p-values of the interaction term in the least-square means analysis for the infection and transmission rate at 25 °C, Table S5: p-values of the fixed effect temperature in the least-square means analysis when comparing the rates at temperatures of 18 °C, 25 °C, and 28 °C in the final generalized binomial regression models specified in Supplemental Table S2. p-value adjustment was performed using the Tukey method.
Author Contributions
Conceptualization, M.H.G., C.S., U.Z., H.K., C.M.H., and A.V.; Methodology, C.M.H., A.V., U.Z., H.K., and C.S.; Validation, C.M.H. and A.V.; Formal analysis, J.S., and C.M.H.; Investigation, C.M.H., A.V., and C.R.; Resources, U.Z., M.H.G., C.S., H.K., and D.W., Writing-original draft preparation, C.M.H. and A.V.; Writing-review and editing, U.Z., C.R., H.K., D.W., J.S., C.S., and M.H.G.; Visualization, J.S. and C.M.H.; Funding acquisition, M.H.G. and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the German Federal Ministry of Food and Agriculture (BMEL) through the Federal Office for Agriculture and Food (BLE), grant numbers 2819113919 and 2818SE001.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chancey C., Grinev A., Volkova E., Rios M. The global ecology and epidemiology of West Nile virus. Biomed. Res. Int. 2015;2015:376230. doi: 10.1155/2015/376230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Komar N., Langevin S., Hinten S., Nemeth N., Edwards E., Hettler D., Davis B., Bowen R., Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pérez-Ramírez E., Llorente F., Jiménez-Clavero M.A. Experimental infections of wild birds with West Nile virus. Viruses. 2014;6:752–781. doi: 10.3390/v6020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smithburn K.C., Hughes T.P., Burke A.W., Paul J.H. A neurotropic virus isolated from the blood of a native of Uganda. Am. J. Trop. Med. Hyg. 1940;20:471–492. doi: 10.4269/ajtmh.1940.s1-20.471. [DOI] [Google Scholar]
- 5.Owen J., Moore F., Panella N., Edwards E., Bru R., Hughes M., Komar N. Migrating birds as dispersal vehicles for West Nile virus. Ecohealth. 2006;3:79–85. doi: 10.1007/s10393-006-0025-9. [DOI] [Google Scholar]
- 6.Asnis D.S., Conetta R., Teixeira A.A., Waldman G., Sampson B.A. The West Nile virus outbreak of 1999 in New York: The flushing hospital experience. Clin. Infect. Dis. 2000;30:413–418. doi: 10.1086/313737. [DOI] [PubMed] [Google Scholar]
- 7.McLean R.G. West Nile Virus in North American Birds. USDA National Wildlife Research Center-Staff Publications; Fort Collins, CO, USA: 2006. [Google Scholar]
- 8.Danis K., Papa A., Theocharopoulos G., Dougas G., Athanasiou M., Detsis M., Baka A., Lytras T., Mellou K., Bonovas S., et al. Outbreak of West Nile virus infection in Greece, 2010. Emerg. Infect. Dis. 2011;17:1868–1872. doi: 10.3201/eid1710.110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sirbu A., Ceianu C.S., Panculescu-Gatej R.I., Vázquez A., Tenorio A., Rebreanu R., Niedrig M., Nicolescu G., Pistol A. Outbreak of West Nile virus infection in humans, Romania, July to October 2010. Eurosurveillance. 2011;16:19762. [PubMed] [Google Scholar]
- 10.Barzon L., Pacenti M., Franchin E., Pagni S., Lavezzo E., Squarzon L., Martello T., Russo F., Nicoletti L., Rezza G., et al. Large human outbreak of West Nile virus infection in north-eastern Italy in 2012. Viruses. 2013;5:2825–2839. doi: 10.3390/v5112825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziegler U., Lühken R., Keller M., Cadar D., van der Grinten E., Michel F., Albrecht K., Eiden M., Rinder M., Lachmann L., et al. West Nile virus epizootic in Germany, 2018. Antivir. Res. 2019;162:39–43. doi: 10.1016/j.antiviral.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler U., Santos P.D., Groschup M.H., Hattendorf C., Eiden M., Höper D., Eisermann P., Keller M., Michel F., Klopfleisch R., et al. West Nile virus epidemic in Germany triggered by epizootic emergence, 2019. Viruses. 2020;12:448. doi: 10.3390/v12040448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcus I. West Nile Virus 48: Europe (Germany) ECDC. [(accessed on 20 January 2020)];2019 Available online: http://www.promedmail.org.
- 14.Kilpatrick A.M., Kramer L.D., Campbell S.R., Alleyne E.O., Dobson A.P., Daszak P. West Nile virus risk assessment and the bridge vector paradigm. Emerg. Infect. Dis. 2005;11:425–429. doi: 10.3201/eid1103.040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turell M.J., Sardelis M.R., Dohm D.J., O’Guinn M.L. Potential North American vectors of West Nile virus. Ann. N. Y. Acad. Sci. 2001;951:317–324. doi: 10.1111/j.1749-6632.2001.tb02707.x. [DOI] [PubMed] [Google Scholar]
- 16.Andreadis T.G. The contribution of Culex pipiens complex mosquitoes to transmission and persistence of West Nile virus in North America. J. Am. Mosq. Control Assoc. 2012;28:137–151. doi: 10.2987/8756-971X-28.4s.137. [DOI] [PubMed] [Google Scholar]
- 17.Vogels C.B., Göertz G.P., Pijlman G.P., Koenraadt C.J. Vector competence of European mosquitoes for West Nile virus. Emerg. Microbes Infect. 2017;6:e96. doi: 10.1038/emi.2017.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balenghien T., Vazeille M., Grandadam M., Schaffner F., Zeller H., Reiter P., Sabatier P., Fouque F., Bicout D.J. Vector competence of some French Culex and Aedes mosquitoes for West Nile virus. Vector Borne Zoonotic Dis. 2008;8:589–595. doi: 10.1089/vbz.2007.0266. [DOI] [PubMed] [Google Scholar]
- 19.Fortuna C., Remoli M.E., Di Luca M., Severini F., Toma L., Benedetti E., Bucci P., Montarsi F., Minelli G., Boccolini D., et al. Experimental studies on comparison of the vector competence of four Italian Culex pipiens populations for West Nile virus. Parasites Vectors. 2015;8:463. doi: 10.1186/s13071-015-1067-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brustolin M., Talavera S., Santamaría C., Rivas R., Pujol N., Aranda C., Marquès E., Valle M., Verdún M., Pagès N., et al. Culex pipiens and Stegomyia albopicta (= Aedes albopictus) populations as vectors for lineage 1 and 2 West Nile virus in Europe. Med. Vet. Entomol. 2016;30:166–173. doi: 10.1111/mve.12164. [DOI] [PubMed] [Google Scholar]
- 21.Fros J.J., Miesen P., Vogels C.B., Gaibani P., Sambri V., Martina B.E., Koenraadt C.J., van Rij R.P., Vlak J.M., Takken W., et al. Comparative usutu and West Nile virus transmission potential by local Culex pipiens mosquitoes in north-western Europe. One Health. 2015;1:31–36. doi: 10.1016/j.onehlt.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fros J.J., Geertsema C., Vogels C.B., Roosjen P.P., Failloux A.B., Vlak J.M., Koenraadt C.J., Takken W., Pijlman G.P. West Nile virus: High transmission rate in north-western European mosquitoes indicates its epidemic potential and warrants increased surveillance. PLoS Negl. Trop. Dis. 2015;9:e0003956. doi: 10.1371/journal.pntd.0003956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogels C.B., Fros J.J., Göertz G.P., Pijlman G.P., Koenraadt C.J. Vector competence of northern European Culex pipiens biotypes and hybrids for West Nile virus is differentially affected by temperature. Parasites Vectors. 2016;9:393. doi: 10.1186/s13071-016-1677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vogels C.B.F., Göertz G.P., Pijlman G.P., Koenraadt C.J.M. Vector competence of northern and southern European Culex pipiens pipiens mosquitoes for West Nile virus across a gradient of temperatures. Med. Vet. Entomol. 2017;31:358–364. doi: 10.1111/mve.12251. [DOI] [PubMed] [Google Scholar]
- 25.Koenraadt C.J.M., Möhlmann T.W.R., Verhulst N.O., Spitzen J., Vogels C.B.F. Effect of overwintering on survival and vector competence of the West Nile virus vector Culex pipiens. Parasites Vectors. 2019;12:147. doi: 10.1186/s13071-019-3400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leggewie M., Badusche M., Rudolf M., Jansen S., Börstler J., Krumkamp R., Huber K., Krüger A., Schmidt-Chanasit J., Tannich E., et al. Culex pipiens and Culex torrentium populations from Central Europe are susceptible to West Nile virus infection. One Health. 2016;2:88–94. doi: 10.1016/j.onehlt.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jansen S., Heitmann A., Lühken R., Leggewie M., Helms M., Badusche M., Rossini G., Schmidt-Chanasit J., Tannich E. Culex torrentium: A potent vector for the transmission of West Nile virus in Central Europe. Viruses. 2019;11:492. doi: 10.3390/v11060492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker N., Petrić D., Zgomba M., Boase C., Dahl C., Madon M., Kaiser A. Mosquitoes and Their Control. 2nd ed. Springer; Berlin/Heidelberg, Germany: 2010. [Google Scholar]
- 29.Harbach R.E., Harrison B.A., Gad A.M. Culex (Culex) molestus Forskal (Diptera: Culicidae): Neotype 538 designation, description, variation, and taxonomic status. Proc. Entomol. Soc. Wash. 1984;86:521–542. [Google Scholar]
- 30.Kampen H., Schuhbauer A., Walther D. Emerging mosquito species in Germany-a synopsis after 6 years of mosquito monitoring (2011–2016) Parasitol. Res. 2017;116:3253–3263. doi: 10.1007/s00436-017-5619-3. [DOI] [PubMed] [Google Scholar]
- 31.Kuhlisch C., Kampen H., Walther D. The Asian tiger mosquito Aedes albopictus (Diptera: Culicidae) in central Germany: Surveillance in its northernmost distribution area. Acta Trop. 2018;188:78–85. doi: 10.1016/j.actatropica.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 32.Rudolf M., Czajka C., Börstler J., Melaun C., Jöst H., von Thien H., Badusche M., Becker N., Schmidt-Chanasit J., Krüger A., et al. First nationwide surveillance of Culex pipiens complex and Culex torrentium mosquitoes demonstrated the presence of Culex pipiens biotype pipiens/molestus hybrids in Germany. PLoS ONE. 2013;8:e71832. doi: 10.1371/journal.pone.0071832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eiden M., Viña-Rodríguez A., Hoffmann B., Ziegler U., Groschup M.H. Two new real-time quantitative reverse transcription polymerase chain reaction assays with unique target sites for the specific and sensitive detection of lineages 1 and 2 West Nile virus strains. J. Vet. Diagn. Investig. 2010;22:748–753. doi: 10.1177/104063871002200515. [DOI] [PubMed] [Google Scholar]
- 34.Viña-Rodríguez A., Sachse K., Ziegler U., Chaintoutis S.C., Keller M., Groschup M.H., Eiden M. A novel pan-flavivirus detection and identification assay based on RT-qPCR and microarray. Biomed. Res. Int. 2017;2017:e4248756. doi: 10.1155/2017/4248756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayr A., Bachmann P.A., Bibrack B., Wittmann G. Virologische Arbeitsmethoden, (Zellkulturen-Bebrütete Hühnereier-Versuchstiere) Volume 1 VEB Gustav Fischer Verlag; Jena, Germany: 1974. [Google Scholar]
- 36.Ziegler U. Personal Communication. Friedrich-Loeffler-Institut; Greifswald-Insel Riems, Germany: 2019. [Google Scholar]
- 37.Heitmann A., Jansen S., Lühken R., Leggewie M., Schmidt-Chanasit J., Tannich E. Forced salivation as a method to analyze vector competence of mosquitoes. J. Vis. Exp. 2018;138:e57980. doi: 10.3791/57980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Development Core Team . R: A Language and Environment for Statistical Computing. R Development Core Team; Vienna, Austria: 2019. [Google Scholar]
- 39.Lenth R.V. Least-squares means: The R package lsmeans. J. Stat. Softw. 2016;69:1–33. doi: 10.18637/jss.v069.i01. [DOI] [Google Scholar]
- 40.Harvey W. Least-Squares Analysis of Data with Unequal Subclass Numbers. USDA National Agricultural Library; Washington, DC, USA: 1960. pp. 20–28. [Google Scholar]
- 41.Tukey W.J. The philosophy of multiple comparisons. Stat. Sci. 1991;6:100–116. doi: 10.1214/ss/1177011945. [DOI] [Google Scholar]
- 42.Deutscher Wetterdienst . Yearbook 2018 of the Deutscher Wetterdienst. Deutscher Wetterdienst; Offenbach am Main, Germany: 2018. Annual report 2018; p. 16. [Google Scholar]
- 43.Kampen H., Holicki C.M., Ziegler U., Groschup M.H., Tews B.A., Werner D. West Nile virus mosquito vectors (Diptera: Culicidae) in Germany. Viruses. 2020;12:493. doi: 10.3390/v12050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tiawsirisup S., Platt K.B., Evans R.B., Rowley W.A. A comparision of West Nile virus transmission by Ochlerotatus trivittatus (COQ.), Culex pipiens (L.), and Aedes albopictus (Skuse) Vector Borne Zoonotic Dis. 2005;5:40–47. doi: 10.1089/vbz.2005.5.40. [DOI] [PubMed] [Google Scholar]
- 45.Fortuna C., Remoli M.E., Severini F., Di Luca M., Toma L., Fois F., Bucci P., Boccolini D., Romi R., Ciufolini M.G. Evaluation of vector competence for West Nile virus in Italian Stegomyia albopicta (= Aedes albopictus) mosquitoes. Med. Vet. Entomol. 2015;29:430–433. doi: 10.1111/mve.12133. [DOI] [PubMed] [Google Scholar]
- 46.Turell M.J., O’Guinn M.L., Dohm D.J., Jones J.W. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J. Med. Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- 47.Sardelis M.R., Turell M.J., O’Guinn M.L., Andre R.G., Roberts D.R. Vector competence of three North American strains of Aedes albopictus for West Nile virus. J. Am. Mosq. Control Assoc. 2002;18:284–289. [PubMed] [Google Scholar]
- 48.Magurano F., Remoli M.E., Baggieri M., Fortuna C., Marchi A., Fiorentini C., Bucci P., Benedetti E., Ciufolini M.G., Rizzo C., et al. Circulation of West Nile virus lineage 1 and 2 during an outbreak in Italy. Clin. Microbiol. Infect. 2012;18:E545–E547. doi: 10.1111/1469-0691.12018. [DOI] [PubMed] [Google Scholar]
- 49.Delatte H., Desvars A., Bouétard A., Bord S., Gimonneau G., Vourc’h G., Fontenille D. Blood-feeding behavior of Aedes albopictus, a vector of chikungunya on la reunion. Vector Borne Zoonotic Dis. 2010;10:249–258. doi: 10.1089/vbz.2009.0026. [DOI] [PubMed] [Google Scholar]
- 50.Thomas S.M., Tjaden N.B., Frank C., Jaeschke A., Zipfel L., Wagner-Wiening C., Faber M., Beierkuhnlein C., Stark K. Areas with high hazard potential for autochthonous transmission of Aedes albopictus-associated arboviruses in Germany. Int. J. Environ. Res. Public Health. 2018;15:1270. doi: 10.3390/ijerph15061270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deutscher Wetterdienst . The Weather in Germany in Summer 2019. Deutscher Wetterdienst; Offenbach am Main, Germany: 2019. [Google Scholar]
- 52.Ewing D.A., Cobbold C.A., Purse B.V., Nunn M.A., White S.M. Modelling the effect of temperature on the seasonal population dynamics of temperate mosquitoes. J. Biol. 2016;400:65–79. doi: 10.1016/j.jtbi.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Murdock C.C., Paaijmans K.P., Cox-Foster D., Read A.F., Thomas M.B. Rethinking vector immunology: The role of environmental temperature in shaping resistance. Nat. Rev. Microbiol. 2012;10:869–876. doi: 10.1038/nrmicro2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vasseur D.A., DeLong J.P., Gilbert B., Greig H.S., Harley C.D.G., McCann K.S., Savage V., Tunney T.D., O’Connor M.I. Increased temperature variation poses a greater risk to species than climate warming. Proc. R. Soc. B Biol. Sci. 2014;281:20132612. doi: 10.1098/rspb.2013.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.