Abstract
Botulism is a rare but severe disease which is characterized by paralysis and inhibition of secretions. Only a few cases had been reported at the end of the 19th century in France. The disease was frequent during the second world war, and then the incidence decreased progressively. However, human botulism is still present in France with 10–25 cases every year. Food-borne botulism was the main form of botulism in France, whereas infant botulism (17 cases between 2004 and 2016) was rare, and wound and inhalational botulism were exceptional. Type B was the prevalent botulism type and was mainly due to consumption of home-made or small-scale preparations of cured ham and to a lesser extent other pork meat products. In the recent period (2000–2016), a wider diversity of botulism types from various food origin including industrial foods was reported. Severe cases of type A and F botulism as well as type E botulism were more frequent. Albeit rare, the severity of botulism justifies its continued surveillance and recommendations to food industry and consumers regarding food hygiene and preservation practices.
Keywords: botulism, food-borne botulism, infant botulism, Clostridium botulinum, botulinum neurotoxins, food poisoning
1. Introduction
Botulism is a serious disease of humans and vertebrate animals that is characterized by flaccid paralysis and inhibition of secretions. The most severe cases lead to respiratory distress and death. Botulism is caused by potent neurotoxins, called botulinum neurotoxins (BoNTs), which are produced by toxigenic Clostridium botulinum and more rarely by atypical strains from other clostridia (Clostridium baratii, Clostridium butyricum) and non-Clostridium species. BoNTs show a high immunological and genetic diversity. Indeed, BoNTs are divided into several toxinotypes (A, B, C, D, E, F, G, H, or F/A) based on their neutralization by specific corresponding antisera. In addition, each toxinotype is subdivided into subtypes based on amino acid variation (>2.6% in most cases). Up to now 41 subtypes have been identified [1]. More recently, a novel BoNT type called BoNT/X was characterized in a C. botulinum strain that also produces BoNT/B2 [2]. BoNT genes (bont) related sequences have been identified in a few non-clostridial strains such as bont/Wo or bont/I from Weisenella oryzae, a bacterium of fermented rice, bont/J (ebont/F or bont/En) from an Enterococcus faecalis strain in a cow, and Cp1 from Chryseobacterium piperi from sediment [3,4,5,6,7,8]. The activity of the non-clostridial BoNTs has not been characterized. Humans and animal species show variable sensitivity to the distinct toxinotypes. Human botulism is mainly caused by BoNT/A, B, E, and to a lower extent F, whereas BoNT/C and BoNT/D are prevalent in animal botulism and extremely rare in humans [9].
Naturally acquired botulism occurs in different clinical forms. Foodborne botulism results from the ingestion of preformed BoNT in food. Foods contaminated by C. botulinum spores that have not been or have been minimally processed and stored in conditions allowing C. botulinum growth, are at risk of containing BoNTs. Botulism by intestinal colonization is due to ingestion of C. botulinum spores or bacteria that develop and produce BoNT in situ in the intestine. This form is more frequent in infants up to 1 year (infant botulism) and occasionally in adults. Wound botulism is caused by C. botulinum growth and BoNT production in a wound. Drug users are at risk of wound botulism by injection with contaminated materials or drugs. Inhalational botulism, which results from aerosolization of BoNT, is very rare. Only, a few laboratory cases have been reported. Iatrogenic botulism is related to toxin overdoses for therapeutic or cosmetic purpose [10,11,12,13]. No iatrogenic botulism cases have been reported in France during the considered period.
Botulism in France was first investigated by certain hospital laboratories and mainly by the Anaerobe Laboratory (Institut Pasteur, Paris) under the direction of A. R. Prévot. The laboratory of anaerobic bacteria of Institut Pasteur was recognized as the National Reference Center of Botulism in 1978. Since 1986, human botulism is subjected to mandatory declaration in France. Since 1992, the survey of botulism based on clinical reporting and bacteriological investigations was performed by the RNSP (Réseau National de Santé Publique), which was converted into InVS (Institut national de Veille Sanitaire) in 1998, and then in SPF (Santé Publique France) in 2016.
Up to 1971, botulism cases were defined on clinical symptoms and investigation of suspected foods including detection of BoNT by mouse bioassay (MBA) and eventually isolation and characterization of C. botulinum. From 1971, BoNT investigation in the serum of patients by MBA was used for the diagnosis of botulism. Since 1998, the survey of botulism is coordinated by InVS/SPF and the National Reference Center based on the clinical declarations and investigations of biological (serum, feces) and food samples including BoNT identification by MBA as well as detection of toxigenic C. botulinum by molecular biology method in feces/food and isolation/characterization of C. botulinum strains.
This review is from published scientific papers from international and national literature.
2. Foodborne Botulism
The first detailed clinical description of botulism was performed by Kerner in 1817–1822 in the southwest German region of Wurtenberg. The disease was associated to the consumption of pork blood sausage and was initially termed sausage poisoning. The name botulism was coined later in the second half of the 19th century from the Latin word “botulus” meaning sausage [14,15]. Botulism was not reported or misdiagnosed during this period in Alsace which is close to the Wurtenberg region and where the consumption of pork sausages was common. The bacterial origin of botulism was determined by Emile Pierre van Ermengem, professor at the university of Ghent, who isolated the first toxigenic C. botulinum strain from the ham and one of the victims of a severe outbreak of botulism which occurred in a small Belgian village (Ellezelles) [16].
2.1. Period 1875–1944
The first most likely outbreak of human botulism in France was reported in 1875. Eleven prisoners who ate canned beef developed clinical signs of botulism, four of them died. The meat can was opened, stored at room temperature, and distributed to the prisoners 5 days later [17]. Botulism was rare in France before the second world war, probably because the consumption of canned foods was not traditional, although canned food products were developed by the French inventor Nicolas Appert in 1795–1806 [18]. From 1875 to 1936, 24 cases of botulism were recorded, and eight from 1936 to 1940 [19,20]. For example, one case was reported in a 42-year old man who ate home-made hare pâté [21], and an outbreak occurred in four people after consumption of canned smoked trout [22]. In 1935, two cases of botulism occurred after consumption of canned spinach from Morocco [23].
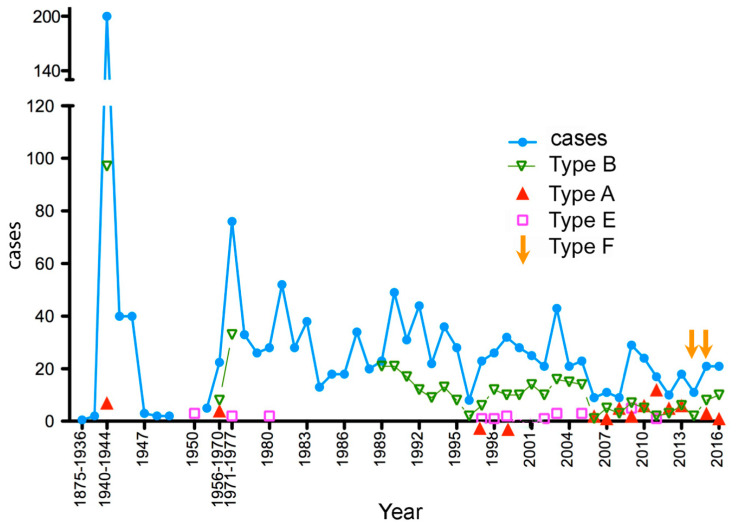
During the second world war (1940–1944), the incidence of botulism was very high (Figure 1). More than 500 outbreaks and 1000 cases were reported in France. Food deprivation and poor hygiene in the preparation of home-made preserved foods were the main factors responsible for the high incidence. Botulism type B was the most prevalent and most of the incriminated foods (93%) were from pork meat preparations, notably cured ham (90%). Only three outbreaks of botulism type A were identified in 1943. The mortality rate was low about 2% (15 deceased) [20,24].
Figure 1.
Incidence of human botulism in France, 1875–2016. The numbers indicated in the period ranges 1875–1936, 1940–1944, 1956–1970, and 1971–1977 are the annual mean values. Total cases (blue), type B botulism (green), type A botulism (red), type E botulism (purple), type F botulism (orange arrows) according to [17,19,24,25,26,27,28,29,30,31,32,33,34,35] and modified from [11].
2.2. Period 1945–1970
The incidence of human botulism strongly decreased after the second world war; 40 outbreaks in 1945, 40 in 1946, three in 1947, and two in 1948 [35]. In the period 1950–1954, five outbreaks including 26 cases were recorded [19]. The first outbreak of botulism type E in France was identified in 1951. Three people of the same family developed a botulism type E after the consumption of canned sardines [36]. Another case of botulism type E was reported in a man who ingested canned tuna in a restaurant in 1952 [37].
From 1956 to 1970, 73 outbreaks (149 cases) of human botulism were investigated. Except for two outbreaks (five cases of which two were fatal) which were type A botulism subsequently to ingestion of home-made beans, 70 outbreaks were type B botulism due to home-made preparations of cured ham, and in one outbreak (one case) following consumption of asparagus the botulism type was not determined [38,39]. Additional cases of botulism have been reported based on clinical symptoms. Thus, for this period the total number of outbreaks was 134 (5.5/year) including 337 (22.4/year) cases from which 17 deaths were reported [28,38].
2.3. Period 1971–1986
Since 1971, the diagnosis of botulism which was based on investigation of suspected foods, was improved by detection of BoNT in the serum of patients [40]. Thereby the incidence of human botulism was increased (70.8 annual cases between 1971 and 1978) due to the improved diagnosis of botulism but possibly also to the introduction of novel foods at risk of botulism such as industrial foods. During the period 1971–1986, 394 outbreaks and 821 cases of which at least 16 deaths were reported [33,41].
Botulism type B was the most prevalent type based on investigations of food and/or patient’s sera (97%). The food origin was identified in 41% of the outbreaks and was mainly home-made preparations of pork meat (about 73%), notably cured ham, and more rarely home-made preserved vegetables. In 33 outbreaks (167 cases) from 1971 to 1979, the origin of botulism was from commercial foods including industrial cans or preparations from small scale producers, and in four outbreaks (five cases) botulism resulted from meals at restaurant. They consisted of type B botulism except one type A outbreak (one case) with pork meat and one type E outbreak (two cases) with an industrial can of crab from Russia. Most of the other outbreaks (21 outbreaks, 64 cases) resulted from pork meat preparations (ham, pie). In two outbreaks in 1972 and 1984, industrial cans of asparagus from Spain caused five type B botulism cases (three deaths) [33,42]. A defect in the industrial sterilization process in 1984 was involved in C. botulinum growth in the cans containing unpeeled asparagus and likely contaminated by soil. It is noteworthy that the incidence of contaminated industrial cans was low. BoNT/B and C. botulinum B were detected in only two cans and not in 82 other investigated cans from the same company [42]. Industrial cheese was involved in two large outbreaks (32 and 37 cases) in 1973 and 1978 and in one additional outbreak in Switzerland. The cheeses were contaminated during the maturation process of 2–3 months at 12.5 °C on straw contaminated by C. botulinum B [33,43]. Other incriminated foods were seafood (three outbreaks, six cases), canned pears (one outbreak, one case), paella (one outbreak, one case), and undetermined in four outbreaks (13 cases) [33]. A home-made preparation of salted herrings was responsible of one outbreak (two cases) of botulism type E in 1980 [44]. A second case of botulism type E occurred in the same year due to a fresh water fish preparation [45].
One atypical botulism type was recorded in 1972. Four persons of the same family developed clinical symptoms of botulism, one person died. BoNT type C was identified in the serum of two patients. The consumption of a smoked chicken was suspected to cause this outbreak but has not been confirmed [46]. Botulism type C is common in birds but it is exceptional in humans [9]. In 1981, a fatal case of botulism occurred in a 21-year old woman who ate a home-made canned preparation of rabbit with pork fat. The incriminated food contained both BoNT/A and BoNT/B, and a bivalent C. botulinum strain producing 10-fold more BoNT/A than BoNT/B was isolated [47].
2.4. Period 1987–2016
The survey of human botulism became more efficient in France since the mandatory declaration of clinical and laboratory confirmed cases of botulism (1986) and national recordings by the governmental organizations (RNSP from 1992, InVS in 1998, and then SPF since 2016) (see above). The incidence of human botulism progressively decreased in the period 1987–2016 compared to the previous period (Figure 1). The botulism incidence was estimated to 0.018–0.033 cases per 100,000 population during the period 2013–2016 [29]. However, every year 5–28 outbreaks (annual incidence 13.4 outbreaks/year) of food-borne botulism (9–49 cases, annual incidence 24.3 cases/year) were observed. The incidence in the last 7 years (2010–2016) (9.4 outbreaks/year; 17.4 cases/year) was about two-fold lower than that in the first 7 years of this period (1987–1993) (18.1 outbreaks/year; 31.8 cases/year) confirming the overtime decline of human botulism in France.
The clinical signs and symptoms of human botulism recorded in the period 2007–2016 are shown in Table 1. The main symptoms include oculomotor disorders (diplopia, blurred vision, mydriasis) dry mouth, dysphagia, and in the more severe cases paralysis of limbs and diaphragm. The gastrointestinal disorders (vomiting, diarrhea), which only occurred in the first days of the disease and preceded the neurological symptoms, are not specific of botulism and rather reflect other food contaminants [13]. In contrast constipation, which is often observed throughout the course of the disease is related to BoNT effect on the intestinal motility. Similar clinical symptoms were reported about 108 cases between 1966 and 1990 in the Poitou Charentes region [48] as well as in 26 observations in northern France [49]. It is noteworthy that some mild form of botulism type B resulted only of oculomotor disorders [50,51].
Table 1.
Clinical signs and symptoms reported by patients with food-borne botulism, France 2007–2016 [29,30,31].
| Clinical Sign/Symptom | Number of Cases/Total Cases | % of Cases |
|---|---|---|
| Gastrointestinal disorders | ||
| Dry mouth | 70/133 | 52.6 |
| Vomiting | 64/134 | 47.8 |
| Constipation | 62/135 | 45.9 |
| Diarrhea | 32/139 | 23.0 |
| Nausea | 22/133 | 16.5 |
| Abdominal pain | 20/130 | 15.4 |
| Oculomotor disorders | ||
| Diplopia | 83/146 | 56.8 |
| Blurred vision | 63/139 | 45.3 |
| Mydriasis | 55/146 | 37.7 |
| Ptosis | 30/137 | 21.9 |
| Paralysis | ||
| Dysphagia | 91/134 | 67.9 |
| Food choking | 54/129 | 41.9 |
| Diaphragmatic paralysis | 36/130 | 27.7 |
| Limb paralysis | 33/126 | 26.2 |
| Dysarthria | 20/122 | 16.4 |
Botulism type B (85% of the identified botulism outbreaks) (Table 2) was the most prevalent type during the period 1987–2016. Botulism type A (9.4% of the identified outbreaks) was responsible for the most severe cases of botulism which most often results in hospitalization of the patients in intensive care units. Botulism type A was more frequent (28.4%) in the recent period 2006–2016 than in the previous periods. Two outbreaks of the rare C. baratii type F (F7) botulism were observed recently in 2014 and 2015 (Table 2). Type E botulism was rare (4.0% of the identified outbreaks).
Table 2.
Human botulism in France 1987–2016.
| Outbreaks Where Food Was Identified | ||||||||
|---|---|---|---|---|---|---|---|---|
| Year | Outbreaks | Cases | Deaths | Type (Outbreaks) |
Home-Made Ham or Ham from Small Scale Producers | Other Pork Meat Preparations Home-Made or from Small Scale Producers |
Other Food (Outbreaks) | References |
| 1987 | 20 | 34 | 1 | B (29) | 18 | 5 | [52] | |
| 1988 | 15 | 20 | 0 | E (1) | 1 (ham from Portugal) | |||
| 1989 | 18 | 23 | 0 | B (43) A (1) |
27 | 8 | [53] | |
| 1990 | 28 | 49 | 0 | home-made canned asparagus (1) | ||||
| 1991 | 19 | 31 | 0 | B (29) | 13 confirmed 3 suspected |
3 suspected | home-made beans (1) | [28,54] |
| 1992 | 15 | 44 | 0 | |||||
| 1993 | 12 | 22 | 1 | B (9) | 28 confirmed or suspected | 15 (suspected not confirmed) | [28] | |
| 1994 | 14 | 36 | 0 | B (13) | ||||
| 1995 | 10 | 28 | 1 | B (8) | ||||
| 1996 | 5 | 8 | 0 | B (2) | ||||
| 1997 | 14 | 23 | 1 | B (6) | 4 | [28,55,56,57] | ||
| A (2) | home-made beans (2) | |||||||
| E (2) | scallops (2) | |||||||
| 1998 | 17 | 26 | 0 | B (11) | 2 | [26,28,56,58] | ||
| E (1) | home-made marinated fish (1) | |||||||
| 1999 | 21 | 32 | 2 | B (13) | 2 | industrial chicken sausage (1) | ||
| A (2) | industrial fish soup (1) | |||||||
| AB (1) | industrial blood sausage? (1) | |||||||
| E (2) | marinated fish (1), restaurant? (1) | |||||||
| 2000 | 15 | 28 | 0 | B (10) | 1 | |||
| 2001 | 18 | 29 | 0 | B (14) | 4 | [27] | ||
| 2002 | 17 | 33 | 0 | B (11) | 4 | |||
| E (1) | chestnut jam (1) | |||||||
| 2003 | 22 | 43 | 0 | B (16) | 4 | home-made pork meat preparation (1) | industrial beef sausage (3) | [25,59] |
| E (3) | canned sardines? (1) | |||||||
| 2004 | 13 | 21 | 0 | B (12) | 2 | |||
| 2005 | 16 | 23 | 0 | B (14) | 5 | home-made pie (1) | boar meat preparation (1) | |
| 2006 | 5 | 9 | 0 | B (1) | ||||
| A (1) | home-made pie (1) | |||||||
| 2007 | 6 | 11 | 0 | B (5) | 2 | [30,60,61,62] | ||
| A (1) | ||||||||
| 2008 | 6 | 9 | 0 | B (3) | ||||
| A (2) | industrial enchiladas with chicken meat (1) subtype A7 home-made pumpkin jam (1) subtype A1(F) |
|||||||
| 2009 | 12 | 27 | 0 | B (7) | 2 1 | boar meat preparation (1) | ||
| A (2) | ||||||||
| E (1) | vacuum-packed hot-smoked whitefish (1) | |||||||
| 2010 | 7 | 24 | 1 | B (5) | 4 | home-made canned asparagus (1) subtype B2 |
[31,63,64] | |
| A (2) | home-made beans (1) subtype A2 | |||||||
| 2011 | 9 | 17 | 0 | B (2) | home-made canned spinach (1) subtype B2 | |||
| A (5) | commercial tapenades with olives and dried tomatoes (2) subtype A1(B) industrial fresh pasta carbonara (1) subtype A5 |
|||||||
| E (1) | ||||||||
| 2012 | 8 | 10 | 0 | B (3) | 2 | industrial pie (1) | ||
| A (4) | home-made canned eggplant (1) subtype A1(B) | |||||||
| 2013 | 11 | 18 | 0 | B (6) | 2, subtype B4 | chorizo (Portugal) (1) | [29,65,66,67,68,69] | |
| A (4) | ||||||||
| 2014 | 4 | 11 | 0 | B (2) | 1 | |||
| F (1) | ||||||||
| 2015 | 14 | 21 | 1 | B (8) | 2, subtype B4 | pie (Poland) (1) subtype B4 chorizo (Portugal) (1) |
||
| A (1) | home-made pheasant pâté (1) subtype A1 | |||||||
| F (1) | industrial ground meat, Bolognese sauce (1) subtype F7 | |||||||
| 2016 | 13 | 21 | 1 | B (10) | 4, subtype B4 | |||
| A (1) | ||||||||
| Total | 402 | 731 | 9 | B (254) | B (136) | B (19 confirmed, 18 suspected) | home-made food (5) industrial food (4) |
|
| A (28) | A (1) | home-made food (6) industrial food (5) |
||||||
| AB (2) | industrial food (1) | |||||||
| E (12) | E (2) | home-made food (2) industrial food (4) |
||||||
| F (2) | industrial food (1) | |||||||
1 A ham involved in an outbreak (2 cases) contained both BoNT/B and BoNT/E [62].
The food origin of botulism was identified in less than half of the outbreaks (185, 46.0% of the outbreaks). It could not be ruled out that other forms of botulism were involved. Wound botulism was excluded in the absence of wound. Food-borne botulism or possibly botulism by intestinal colonization, which also involves an initial oral contamination most probably by food, were the most likely forms of human botulism. The identification of contaminated food was often problematic. Indeed, the incubation period and the delay of suspicion/diagnosis of clinical botulism by physicians who often have no experience in this disease, induce a late investigation of the contaminated foods. Very often there was no leftover of the food responsible for botulism; the contaminated meals being totally consumed or the leftovers being discarded. Traditional hams which consist of large pieces of meat were the most often available food for investigation. Indeed, ham from home-made preparation or from small scale producers was involved in 73.5% of the botulism outbreaks with identified food source. Ham and other pork/boar meat preparations were responsible for most of the identified botulism outbreaks (83.7%) (Table 2). These outbreaks were type B botulism, more specifically B4, except one type A outbreak from home-made pie, and one type E outbreak from ham (Table 2). In another outbreak, the ham contained both BoNT/B and BoNT/E [62]. The other sources of botulism were home-made canned vegetables or fruits (beans, asparagus, eggplant, spinach, pumpkin, chestnut) in nine outbreaks, home-made meat or fish preparations in four outbreaks, and industrial foods (fish soup, chicken/beef sausage, chicken/enchiladas, ground meat, olives/dried tomatoes, fresh pasta carbonara) in 14 outbreaks (Table 2). Most of the outbreaks with non-pork meat were type A botulism. Home-made canned vegetables (beans, asparagus, spinach) and beef/chicken sausages were responsible for three and two type B botulism outbreaks, respectively. Two outbreaks of type E botulism were due to home-made marinated fish and commercial preserved fish/sea products were involved in at least four outbreaks. A vacuum-packed hot-smoked whitefish processed in Canada, purchased by a family in Finland, and later consumed in France caused type E botulism in three persons [61]. An industrial ground meat prepared in restaurant was identified as the source of one outbreak of C. baratii type F botulism [68]. The origin of the other C. baratii type F outbreak was not identified.
3. Infant Botulism and Botulism by Intestinal Colonization
The first case of infant botulism was identified in France in 2004. During the period 2004–2016, 15 infant botulism cases and two botulism cases by intestinal colonization in 12- and 18-months old infants were reported (Table 3). Nine were type A botulism and eight type B. The origin of these botulism cases was not identified. All the food samples possibly involved in these cases that have been investigated were negative. Six babies received honey. However, no C. botulinum spores were detected in any of the six honey samples. Environmental contamination was suspected in two cases. A 2-month old girl living close to a thermal power station that intermittently releases sprays of vapor and smoke/dust, developed several relapses of botulism. C. botulinum A2 resistant to β-lactams was isolated on stools up to 110 days after onset [70]. In the second outbreak, a 5-month old male infant exclusively breastfed was living close to a construction site. He presented clinical symptoms of botulism and BoNT/B was detected in his stools. C. botulinum B was isolated from stool as well as from three soil samples of the active construction site [71]. Exposure to dust containing C. botulinum spores was the only risk factor possibly involved in these two infant botulism cases.
Table 3.
Infant botulism and botulism by intestinal colonization. Infant botulism cases in France from 2004 to 2016 according to [29,31,70,71,72,73,74,75].
| Year | Sex | Age | Toxin in Serum | Toxin in Stool | Toxin in Stool Titer MLD/g | C. botulinum in Stool |
|---|---|---|---|---|---|---|
| 2004 | F | 17 d | B | nd | nd | A1 |
| 2005 | F | 6 m | B | nd | nd | B5 |
| 2006 | F | 2 m | A | A | 80 | A2 |
| 2007 | F | 5 m | A | A | nd | A2 |
| 2008 | F | 4 m | B | B | 20 | culture and PCR detection negative |
| 2009 | F | 2.5 m | A | A | 1000 | A1(B) |
| 2009 | F | 18 m | neg | neg | B2 | |
| 2009 | F | 6 m | neg | A | 20 | A2 |
| 2011 | F | 2.5 m | A | A | 100,000 | A2 |
| 2011 | F | 12 m | B | B | 600 | B2 |
| 2012 | F | 4 m | neg | A | 7000 | A1(B) |
| 2013 | F | 1 m | neg | B | 12,000 | Bf2 |
| 2013 | F | 3 m | neg | A | 4800–300,000 1 | A2 β-lactam resistant |
| 2013 | M | 6 m | neg | B | 340 | Bf |
| 2013 | M | 6 m | A | A | 10,240 | A2 |
| 2015 | M | 2 m | neg | B | 6000 | B5 |
| 2016 | F | 6 m | neg | B | 2000 | B2 |
1 several titrations during the course of the disease. nd, not done. neg, negative.
In addition, a 10-year boy with history of Meckel’s diverticulum and chronic constipation, presented dysarthria, dry mouth, hypotony, respiratory failure, and a cardiac arrest in 2011. Stool analyses were positive for C. butyricum E5 by PCR and DNA sequencing up to 2 months after discharge. Botulism by intestinal colonization with neurotoxigenic C. butyricum from undetermined origin was strongly suspected in this boy [31]. Botulism by intestinal colonization with C. butyricum type E was also found in two young boys having a Meckel’s diverticulum in Italy [76].
4. Wound Botulism and Inhalational Botulism
One case of wound botulism has been reported in a man with open leg fracture in 2008. The wound was suppurative and the patient developed signs of paralysis. BoNT/B was detected in the patient’s serum [30].
Two young men who inhaled cocaine, presented mild form of botulism. BoNT/B was identified in the serum of one of the two patients [77].
5. Discussion
Human botulism form and incidence are variable according to the countries and depend notably on the dietary habits. In France, food-borne botulism is the most prevalent form of botulism. In Europe, Italy has also a high incidence of human food-borne botulism [78]. In contrast to Italy where food-borne botulism is mainly caused by canned vegetables, the disease in France is mainly due to pork meat products, more specifically to cured ham, and the most prevalent form of botulism is type B. Pigs are often healthy carriers of C. botulinum B in their digestive tract [79,80,81,82]. Pork meat can be contaminated by intestinal C. botulinum spores, and preparations of uncooked and salted ham followed by storage at room temperature for several weeks or months facilitate C. botulinum growth and toxin production especially if the salt concentration is insufficient (lower than 5%) [83]. It is noteworthy that the C. botulinum strains isolated from ham were type B4 from group II (non-proteolytic strains), whereas the strains from canned vegetables were type B2 from C. botulinum group I (proteolytic strains). The incidence of human botulism was high at the beginning of the 20th century and then declined progressively (Figure 1). Improved sanitary conditions of ham preparation and storage likely contributed to the prevention of botulism outbreaks. Moreover, home-made preparations of ham and other pork products are less and less in use by the families in favor of industrial foods prepared in more strict safety conditions. Albeit botulism outbreaks can occur in all parts of France, type B botulism from ham and other pork meat preparations were mainly observed in the areas in the center of France where these foods are traditional [29,31].
BoNT/B concentrations in hams responsible for human botulism are variable from 4 to 60,000 mouse lethal doses (MLD)/g (Table 4). The toxin content can also differ in different parts of the same ham. For example, four samples of a same ham yielded <4, 4, 8, and 1000 MLD/g [29]. If we consider the common lowest BoNT/B concentration of 40 MLD/g (Table 2) and usual average portion of 100 g consumed by the patients, the minimal toxic dose by oral route is about 4000 MLD for an adult. Taking account of a 100-fold activation by trypsin of BoNT/B4 from C. botulinum group II as reported in ham sample tested by mouse bioassay before and after trypsinization [62] and of the value of 8.3 pg for 1 MLD50 of purified BoNT/B [84], the BoNT/B minimal toxic dose is estimated to 400,000 MLD or 3.2 μg by oral route to induce botulism symptoms in an adult. Minimal doses of 30–100 ng of BoNT/B have been reported to induce food-borne botulism in humans [85]. These values match our estimations without considering trypsin activation.
Table 4.
BoNT/B concentration in ham (mouse lethal dose (MLD)/g) responsible for human botulism (2003–2016). Clostridium botulinum B4 was identified in these samples [25,29,30,31].
| BoNT/B (MLD/g) | Number of Ham Samples (Total 26) |
|---|---|
| 60,000 | 1 |
| 40,000 | 1 |
| 20,000 | 4 |
| 12,000 | 1 |
| 10,000 | 1 |
| 8000 | 1 |
| 5000 | 1 |
| 4000 | 1 |
| 3000 | 1 |
| 2000 | 1 |
| 1000 | 1 |
| 400 | 1 |
| 300 | 1 |
| 200 | 2 |
| 140 | 1 |
| 40 | 4 |
| 20 | 1 |
| 10 | 1 |
| 4 | 1 |
The origin of contamination by C. botulinum E of two hams responsible for two outbreaks was undetermined. A possible explanation could be the use of marine salt possibly contaminated with C. botulinum E spores for the preparation of the hams [31].
Food-borne botulism type A was rare in France before 1997. It was more prevalent in the recent period 1997–2013 (Figure 1). The main origin was home-made canned vegetables (asparagus, beans, eggplant, pumpkin jam) and commercial foods (fish soup, enchiladas, tapenades with olives/dried tomatoes, fresh pasta carbonara). Two outbreaks were related to home-made meat preparations (pheasant, pork meat pie) (Table 2). The commercial foods responsible for food-borne botulism were consumed after the indicated expiry date and were stored at room temperature instead of cold conditions.
Type E botulism was not usual in France, and was mainly due to imported canned or preserved fish and sea food products. Very rare atypical botulism types were observed such as type C botulism in two patients [46], type AB [47], and C. baratii type F in two outbreaks [67,68]. Interestingly, an atypical non-neurotoxigenic Clostridium strain related to group III C. botulinum D/C was isolated from the blood of a 83-year old patient who died from a sepsis syndrome without the characteristic symptom of flaccid paralysis in 2010 [86].
Botulism outbreaks in France commonly concerned a low number of patients by outbreak. Independent unique cases are considered as distinct outbreaks including one case each. The size of the botulism outbreaks ranged from 1 to 6 as observed in the period 1998–2016 and most of the outbreaks (72%) consisted of only one patient (Table 5). The two largest outbreaks involved 32 and 37 cases and were due to industrial cheese [33,43].
Table 5.
| Number of Cases/Outbreak | Number of Outbreaks |
|---|---|
| 6 | 5 |
| 5 | 2 |
| 4 | 4 |
| 3 | 21 |
| 2 | 33 |
| 1 | 170 |
Infant botulism in France was rare (17 cases from 2004 to 2016) compared to the US (110–150 cases/year) [87] and Italy (36 cases between 1986 and 2015) [78]. The origin of infant botulism was undetermined except in two cases where environmental contamination was strongly suspected. It is not precluded that a higher number of undiagnosed cases could occur. Indeed, infant botulism is a rare disease and sometimes the pediatricians did not suspect this disease even in the presence of characteristic signs of paralysis and constipation. Moreover, some of them ruled out a diagnosis of infant botulism when the babies did not have honey, since they considered that honey was the unique source of contamination.
The other forms of botulism were exceptional in France. One wound botulism case and two botulism cases by inhalation in drug users were reported [30,77].
Food-borne botulism is the most severe food-borne poisoning and represents a public health concern in France. Albeit the incidence of food-borne botulism declined since the second world war which induced a high number of cases due to poor safety conditions of food preparations, botulism cases were still observed in the recent period. Home-made and small-scale preparations of cured ham were the main source of food-borne type B botulism in France. However, in the recent periods, a wider diversity of botulism types and food origin, including industrial products, were reported. Severe cases of botulism type A and F, as well as botulism type E were more frequent since 2000. It is noteworthy that the overall mortality of human botulism in France is low and was decreasing over time likely due to the predominance of botulism type B that is less severe than botulism types A and E as well as to better medical management including mechanical ventilation in the recent period (Table 6). Albeit rare, the severity of botulism justifies its continued surveillance and recommendations to industries and consumers regarding hygiene and food preservation practices.
Table 6.
| Years | Number of Cases | Deaths | % Death |
|---|---|---|---|
| 1956–1970 | 337 | 17 | 5.0 |
| 1971–1980 | 621 | 16 | 2.6 |
| 1981–1990 | 293 | 12 | 4.1 |
| 1991–2000 | 278 | 5 | 1.8 |
| 2001–2016 | 317 | 3 | 0.9 |
Author Contributions
Conceptualization, M.R.P.; Writing and editing, M.R.P.; review, C.R.-E. and E.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
Human botulism is a rare but severe disease which is still present in France with 10–25 annual cases. Food-borne botulism type B is the most prevalent form of botulism in France, mainly from home-made or small-scale preparations of pork meat products such as cured ham.
References
- 1.Peck M.W., Smith T.J., Anniballi F., Austin J.W., Bano L., Bradshaw M., Cuervo P., Cheng L.W., Derman Y., Dorner B.G., et al. Historical Perspectives and Guidelines for Botulinum Neurotoxin Subtype Nomenclature. Toxins. 2017;9:38. doi: 10.3390/toxins9010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang S., Masuyer G., Zhang J., Shen Y., Lundin D., Henriksson L., Miyashita S.I., Martinez-Carranza M., Dong M., Stenmark P. Identification and characterization of a novel botulinum neurotoxin. Nat. Commun. 2017;8:14130. doi: 10.1038/ncomms14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zornetta I., Azarnia Tehran D., Arrigoni G., Anniballi F., Bano L., Leka O., Zanotti G., Binz T., Montecucco C. The first non Clostridial botulinum-like toxin cleaves VAMP within the juxtamembrane domain. Sci. Rep. 2016;6:30257. doi: 10.1038/srep30257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunt J., Carter A.T., Stringer S.C., Peck M.W. Identification of a novel botulinum neurotoxin gene cluster in Enterococcus. FEBS Lett. 2018;592:310–317. doi: 10.1002/1873-3468.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang S., Lebreton F., Mansfield M.J., Miyashita S.I., Zhang J., Schwartzman J.A., Tao L., Masuyer G., Martinez-Carranza M., Stenmark P., et al. Identification of a Botulinum Neurotoxin-like Toxin in a Commensal Strain of Enterococcus faecium. Cell Host Microbe. 2018;23:169–176. doi: 10.1016/j.chom.2017.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansfield M.J., Wentz T.G., Zhang S., Lee E.J., Dong M., Sharma S.K., Doxey A.C. Bioinformatic discovery of a toxin family in Chryseobacterium piperi with sequence similarity to botulinum neurotoxins. Sci. Rep. 2019;9:1634. doi: 10.1038/s41598-018-37647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wentz T.G., Muruvanda T., Lomonaco S., Thirunavukkarasu N., Hoffmann M., Allard M.W., Hodge D.R., Pillai S.P., Hammack T.S., Brown E.W., et al. Closed Genome Sequence of Chryseobacterium piperi Strain CTM(T)/ATCC BAA-1782, a Gram-Negative Bacterium with Clostridial Neurotoxin-Like Coding Sequences. Genome Announc. 2017;5:e01296-17. doi: 10.1128/genomeA.01296-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong M., Stenmark P. Handbook of Experimental Pharmacology. Springer; Berlin/Heidelberg, Germany: 2019. The Structure and Classification of Botulinum Toxins; pp. 1–23. [DOI] [PubMed] [Google Scholar]
- 9.Rasetti-Escargueil C., Lemichez E., Popoff M.R. Public Health Risk Associated with Botulism as Foodborne Zoonoses. Toxins. 2019;12:17. doi: 10.3390/toxins12010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franz D.R., Pitt L.M., Clayton M.A., Hanes M.A., Rose K.J. Efficacy of prophylactic and therapeutic administration of antitoxin for inhalation botulism. In: DasGupta B.R., editor. Botulinum and Tetanus Neurotoxins. Plenum Press; New York, NY, USA: 1993. pp. 473–476. [Google Scholar]
- 11.Popoff M.R. Botulinum toxins, Diversity, Mode of Action, Epidemiology of Botulism in France. In: Nikolay S., editor. Botulinum Toxin. IntechOpen; London, UK: 2018. pp. 1–28. [DOI] [Google Scholar]
- 12.Popoff M.R., Mazuet C., Poulain B. The Prokaryotes: Human Microbiology. 4th ed. Volume 5. Springer; Berlin/Heidelberg, Germany: 2013. Botulism and Tetanus; pp. 247–290. [Google Scholar]
- 13.Sobel J. Botulism. Clin. Infect. Dis. 2005;41:1167–1173. doi: 10.1086/444507. [DOI] [PubMed] [Google Scholar]
- 14.Erbguth F.J., Naumann M. On the first systematic descriptions of botulism and botulinum toxin by Justinus Kerner (1786–1862) J. Hist. Neurosci. 2000;9:218–220. doi: 10.1076/0964-704X(200008)9:2;1-Y;FT218. [DOI] [PubMed] [Google Scholar]
- 15.Torrens J.K. Clostridium botulinum was named because of association with “sausage poisoning”. BMJ. 1998;316:151. doi: 10.1136/bmj.316.7125.151c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Ermengem E. Classics in infectious diseases. A new anaerobic bacillus and its relation to botulism. Rev. Infect. Dis. 1979;1:701–719. doi: 10.1093/clinids/1.4.701. Originally published as “Van Ermengem, E. Ueber einen neuen anaeroben Bacillus und seine Beziehungen zum Botulismus”. Zeitschrift fur Hygiene und Infektionskrankheiten1897, 26, 1–56. [DOI] [PubMed] [Google Scholar]
- 17.Du Mesnil O. Empoisonnement par de la viande de conserve. Ann. Santé Publique. 1875;43:472–478. [Google Scholar]
- 18.Garcia R., Adrian J. Nicolas Appert: Inventor and manufacturer. Food Rev. Int. 2009;25:115–125. doi: 10.1080/87559120802682656. [DOI] [Google Scholar]
- 19.Meyer K.F. The status of botulism as a world health problem. Bull. World Health Organ. 1956;15:281–298. [PMC free article] [PubMed] [Google Scholar]
- 20.Legroux R., Levaditi J.C., Jéramec C. Le botulisme en France pendant l’occupation. Presse Med. 1947;57:109–110. [Google Scholar]
- 21.Marie P.L. Un cas de botulisme. Bull. Soc. Med. Hôp. Paris. 1920;37:1471–1475. [Google Scholar]
- 22.De Saint-Martin Les manifestations oculaires de botulisme. Bull. Soc. Med. Hôp. Paris. 1920;37:52–55. [Google Scholar]
- 23.Dreyfus G., Ravina A., Weill J., Orinstein E., Wimphen A. Deux cas de botulisme consécutifs à l’ingestion d’épinards en conserve chez une fillette et son père diabétique. Bull. Soc. Med. Hôp. Paris. 1936;60:891–896. [Google Scholar]
- 24.Legroux R., Jeramec C., Levaditi J.C. Statistique du botulisme de l’occupation 1940–1944. Bull. Acad. Med. 1945;129:643–645. [PubMed] [Google Scholar]
- 25.Carlier J.P., Espié E., Popoff M.R. Le botulisme en France, 2003–2006. Bull. Epidemiol. Hebd. 2007;31:281–284. [Google Scholar]
- 26.Carlier J.P., Henry C., Lorin V., Popoff M.R. Le botulisme en France a la fin du deuxième millénaire (1998–2000) Bull. Epidemiol. Hebd. 2001;9:37–39. [Google Scholar]
- 27.Haeghebaert S., Carlier J.P., Popoff M.R. Caractéristiques épidémiologiques du botulisme humain en France, 2001 et 2002. Bul. Epidemiol. Hebd. 2003;29:129–130. [Google Scholar]
- 28.Haeghebaert S., Popoff M.R., Carlier J.P., Pavillon G., Delarocque-Astagneau E. Caractéristiques épidémiologiques du botulisme humain en France, 1991–2000. Bul. Epidémiol. Hebd. 2002;14:57–59. [Google Scholar]
- 29.Mazuet C., Silva J.D.N., Legeay C., Sautereau J., Popoff M.R. Le botulisme humain en France, 2013–2016. Bull. Epidémiol. Hebd. 2018;3:46–54. [Google Scholar]
- 30.Mazuet C., Bouvet P., King L.A., Popoff M.R. Le botulisme humain en France, 2007–2009. Bull. Epidémiol. Hebd. 2011;6:49–53. [Google Scholar]
- 31.Mazuet C., King L.A., Bouvet P., Legeay C., Sautereau J., Popoff M.R. Le botulisme humain en France, 2010–2012. Bull. Epidémiol. Hebd. 2014;6:106–114. [Google Scholar]
- 32.Sebald M. Le botulisme humain en France: 1970–1995: Les données du Centre de Référence sur les Anaérobies. Rev. Epidemiol. Santé Publique. 1996;44:S47. [Google Scholar]
- 33.Sebald M., Billon J., Cassaigne R., Rosset R., Poumeyrol G. Le botulisme en France. Incidence, mortalité, aliments responsables avec étude des foyers dus à un aliment qui n’est pas de préparation familiale. Med. Nutr. 1980;16:262–268. [Google Scholar]
- 34.Weinberg M., Nativelle R., Prévot A.R. Les Microbes Anaérobies. Masson et Cie; Paris, France: 1937. p. 1186. [Google Scholar]
- 35.Verge J. Le Botulisme. Rec. Med. Vet. 1951;127:767–828. [Google Scholar]
- 36.Prevot A.R., Huet M. Existence in France of human botulism due to fish and to Clostridium botulinum E. Bull. Acad. Natl. Med. 1951;135:432–435. [PubMed] [Google Scholar]
- 37.Prévot A.R., Loiseau J., Thévenrad A. Nouveau cas de botulisme humain d’origine pisciaire. Résultat du traitement par l’anatoxine E. Bull. Acad. Med. 1952;136:663–664. [Google Scholar]
- 38.Sebald M. On botulism in France from 1956 to 1970. Bull. Acad. Natl. Med. 1970;154:703–707. [PubMed] [Google Scholar]
- 39.Lamagnere J.P., Maupas P., Breteau M., Laugier J., Lamisse F., Gautier J., Desbuquois G. Botulism. Sem. Hop. 1973;49:1077–1085. [PubMed] [Google Scholar]
- 40.Sebald M., Saimot G. Toxémie botulique. Intérêt de sa mise en évidence dans le diagnostic du botulisme humain de type B. Ann. Microbiol. (Paris) 1973;124:61–69. [PubMed] [Google Scholar]
- 41.Olivares R., Hubert B. Le botulisme en 1985 et 1986. Bull. Epidémiol. Hebd. 1987;29:113–115. [Google Scholar]
- 42.Carré H., Gledel J., Poumeyrol M., Sebald M., Thomas G., Veit P. Enquète sur un foyer de botulisme. Nécessité du respect des bonnes pratiques professionnelles. Med. Nutr. 1987;23:391–397. [Google Scholar]
- 43.Sebald M., Jouglard J., Gilles G. B botulism in man due to cheese (author’s transl) Ann. Microbiol. (Paris) 1974;125A:349–357. [PubMed] [Google Scholar]
- 44.Blettery B., Soichot P., Virot C., Lorcerie B., Hillon P. Type E botulism. Two recent cases (author’s transl) Nouv. Presse Med. 1982;11:1131–1133. [PubMed] [Google Scholar]
- 45.Dine G., Soibinet C., Croix J.C. Second French case of botulism caused by fresh water fish. Nouv. Presse Med. 1981;10:339. [PubMed] [Google Scholar]
- 46.Maupas P., Lamagnere J.P., Lamisse F., Laugier J. Botulisme de type C. Intérêt de la recherche de toxémie. Med. Mal. Infect. 1976;6:207–210. doi: 10.1016/S0399-077X(76)80079-0. [DOI] [Google Scholar]
- 47.Poumeyrol M., Billon J., Delille F., Haas C., Marmonier A., Sebald M. Intoxication botulique mortelle due à une souche de Clostridium botulinum de type AB. Med. Mal. Infect. 1983;13:750–754. doi: 10.1016/S0399-077X(83)80014-6. [DOI] [Google Scholar]
- 48.Roblot P., Roblot F., Fauchère J.L., Devilleger A., Maréchaud R., Breux J.P., Grollier G., Becq-Giraudon B. Retrospective study of 108 cases of botulism in Poitiers France. J. Med. Microbiol. 1994;40:379–384. doi: 10.1099/00222615-40-6-379. [DOI] [PubMed] [Google Scholar]
- 49.Fourrier A., Delmer M., Wattel F., Furon D., Mouton Y. Cas récents de botulisme dans le nord de la France. A propos de 26 observations (1962–1971) Rev. Med. 1972;40:2615–2628. [Google Scholar]
- 50.Henry C., Moulin M., Richard G., Dor F. 12 cases of ambulatory botulism in Charollais and in Blanzy mining-basin. Bull. Soc. Ophtalmol. Fr. 1970;70:296–301. [PubMed] [Google Scholar]
- 51.Laroche L., Ollat H., Mignot B., Claux J.M., Saraux H. Ocular manifestations of botulism. Apropos of 3 cases. Bull. Soc. Ophtalmol. Fr. 1984;84:555–558. [PubMed] [Google Scholar]
- 52.Quenum B., Hubert B., Sebald M. Le botulisme en 1987 et en 1988. Bull. Epidemiol. Hebd. 1989;27:91. [Google Scholar]
- 53.Pelletier A., Hubert B., Sebald M. Le botulisme en 1989 et en 1990 en France. Bull. Epidemiol. Hebd. 1991;27:91. [Google Scholar]
- 54.Marchetti P., Lepoutre A. Le botulisme en 1991 et en 1992 en France. Bull. Epidemiol. Hebd. 1993;4:15. [Google Scholar]
- 55.Abgueguen P., Delbos V., Chennebault J.M., Fanello S., Brenet O., Alquier P., Granry J.C., Pichard E. Nine cases of foodborne botulism type B in France and literature review. Eur. J. Clin. Microbiol. Infect. Dis. 2003;22:749–752. doi: 10.1007/s10096-003-1019-y. [DOI] [PubMed] [Google Scholar]
- 56.Boyer A., Salah A. Le botulisme en France: Épidémiologie et clinique. Ann. Med. Interne. 2002;153:300–310. [PubMed] [Google Scholar]
- 57.Salomon J., Delarocque-Astagneau E., Popoff M.R., Carlier J.P. Le botulisme en France en 1997. Bull. Epidemiol. Hebd. 1998;46:201. [Google Scholar]
- 58.Boyer A., Girault C., Bauer F., Korach J.M., Salomon J., Moirot E., Leroy J., Bonmarchand G. Two cases of foodborne botulism type E and review of epidemiology in France. Eur. J. Clin. Microbiol. Infect. Dis. 2001;20:192–195. doi: 10.1007/s100960100466. [DOI] [PubMed] [Google Scholar]
- 59.Espié E., Vaillant V., de Valk H., Popoff M.R. France recalls internationally distributed halal meat products from the plant implicated as the source of a type B botulism outbreak. Eurosurveill. Wkly. 2003;7:030918. [Google Scholar]
- 60.King L.A. Two severe cases of botulism associated with industrially produced chicken enchiladas, France, August 2008. Eurosurveillance. 2008;13:1–2. doi: 10.2807/ese.13.37.18978-en. [DOI] [PubMed] [Google Scholar]
- 61.King L.A., Niskanen T., Junnikkala M., Moilanen E., Lindstrôm M., Korkeala H., Korhonen T., Popoff M., Mazuet C., Callon H., et al. Botulism and hot-smoked whitefish: A family cluster of type E botulism in France, September 2009. Eurosurveillance. 2009;14:1–3. doi: 10.2807/ese.14.45.19394-en. [DOI] [PubMed] [Google Scholar]
- 62.Mazuet C., Sautereau J., Legeay C., Bouchier C., Bouvet P., Popoff M.R. An Atypical Outbreak of Food-Borne Botulism Due to Clostridium botulinum Types B and E from Ham. J. Clin. Microbiol. 2015;53:722–726. doi: 10.1128/JCM.02942-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pingeon J., Vanbockstael C., Popoff M., King L., Deschamps B., Pradel G., Dupont H., Spanjaard A., Houdard A., Mazuet C., et al. Two outbreaks of botulism associated with consumption of green olive paste, France, September 2011. Eurosurveillance. 2011;16:1–3. doi: 10.2807/ese.16.49.20035-en. [DOI] [PubMed] [Google Scholar]
- 64.Oriot C., D’Aranda E., Castanier M., Glaizal M., Galy C., Faivre A., Poisnel E., Truong T.K., Mercury P., Hayek-Lanthois M., et al. One collective case of type A foodborne botulism in Corsica. Clin. Toxicol. (Phila) 2011;49:752–754. doi: 10.3109/15563650.2011.606222. [DOI] [PubMed] [Google Scholar]
- 65.Caparros L., Bourget S., Hida H. Trois cas de botulisme alimentaire en France. J. Pharm. Clin. 2016;35:164–168. [Google Scholar]
- 66.Langlois M.E., Blanc-Lasserre K., Reynaud C., Bouteloup M., Blanc Q. Clostridium baratii botulism. Presse Med. 2017;46:342–344. doi: 10.1016/j.lpm.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 67.Mazuet C., Legeay C., Sautereau J., Bouchier C., Criscuolo A., Bouvet P., Trehard H., Da Silva J.N., Popoff M.R. Characterization of Clostridium baratii Type F Strains Responsible for an Outbreak of Botulism Linked to Beef Meat Consumption in France. PLOS Curr. Outbreaks. 2017 doi: 10.1371/currents.outbreaks.6ed2fe754b58a5c42d0c33d586ffc606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mazuet C., Sautereau J., Legeay C., Bouchier C., Bouvet P., Jourdan da Silva N., Castor C., Popoff M.R. Characterization of the first Clostridium baratii strain responsible for an outbreak of botulism type F in France. Clin. Microbiol. Case Rep. 2015;1:1–4. [Google Scholar]
- 69.Trehard H., Poujol I., Mazuet C., Blanc Q., Gillet Y., Rossignol F., Popoff M.R., Jourdan Da Silva N. A cluster of three cases of botulism due to Clostridium baratii type F, France, August 2015. Euro Surveill. 2016;21:2–5. doi: 10.2807/1560-7917.ES.2016.21.4.30117. [DOI] [PubMed] [Google Scholar]
- 70.Mazuet C., Yoon E.J., Boyer S., Pignier S., Blanc T., Doehring I., Meziane-Cherif D., Dumant-Forest C., Sautereau J., Legeay C., et al. A penicillin- and metronidazole-resistant Clostridium botulinum strain responsible for an infant botulism case. Clin. Microbiol. Infect. 2016;22:644.e7–644.e12. doi: 10.1016/j.cmi.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 71.Bernardor J., Neveu J., Haas H., Pitelet G., Popoff M.R., Mazuet C., Berard E., Boulay C., Chabrol B. Infant botulism: Two case reports and electroneuromyogram findings. Arch. Pediatr. 2018 doi: 10.1016/j.arcped.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 72.Hoarau G., Pelloux I., Gayot A., Wroblewski I., Popoff M.R., Mazuet C., Maurin M., Croize J. Two cases of type A infant botulism in Grenoble, France: No honey for infants. Eur. J. Pediatr. 2012;171:589–591. doi: 10.1007/s00431-011-1649-5. [DOI] [PubMed] [Google Scholar]
- 73.King L.A., Popoff M.R., Mazuet C., Espié E., Vaillant V., de Valk H. Infant botulism in France, 1991–2009. Arch. Pediatr. 2010;17:1288–1292. doi: 10.1016/j.arcped.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 74.Paricio C., Bey K.J., Teyssier G., Ughetto A., Ros A., Rayet I., Lavocat M.P. Botulism in a neonate. Arch. Pediatr. 2006;13:146–148. doi: 10.1016/j.arcped.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 75.Patural H., Goffaux P., Paricio C., Emeriaud G., Teyssier G., Barthelemy J.C., Pichot V., Roche F. Infant botulism intoxication and autonomic nervous system dysfunction. Anaerobe. 2009;15:197–200. doi: 10.1016/j.anaerobe.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 76.Fenicia L., Franciosa G., Pourshaban M., Aureli P. Intestinal toxemia botulism in two young people, caused by Clostridium butyricum Type E. Clin. Infect. Dis. 1999;29:381–387. doi: 10.1086/313497. [DOI] [PubMed] [Google Scholar]
- 77.Roblot F., Popoff M., Carlier J.P., Godet C., Abbadie P., Matthis S., Eisendorn A., Le Moal G., Becq-Giraudon B., Roblot P. Botulism in patients who inhale cocaine: The first cases in France. Clin. Infect. Dis. 2006;43:e51–e52. doi: 10.1086/506567. [DOI] [PubMed] [Google Scholar]
- 78.Anniballi F., Auricchio B., Fiore A., Lonati D., Locatelli C.A., Lista F., Fillo S., Mandarino G., De Medici D. Botulism in Italy, 1986 to 2015. Eurosurveillance. 2017;22 doi: 10.2807/1560-7917.ES.2017.22.24.30550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cotta M.A., Whitehead T.R., Zeltwanger R.L. Isolation, characterization and comparison of bacteria from swine faeces and manure storage pits. Environ. Microbiol. 2003;5:737–745. doi: 10.1046/j.1467-2920.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- 80.Dahlenborg M., Borch E., Radstrom P. Prevalence of Clostridium botulinum types B, E and F in faecal samples from Swedish cattle. Int. J. Food Microbiol. 2003;82:105–110. doi: 10.1016/S0168-1605(02)00255-6. [DOI] [PubMed] [Google Scholar]
- 81.Klarmann D. The detection of Clostridium botulinum in fecal samples of cattle and swine and in the raw material and animal meal of different animal body rendering plants. Berl. Munch. Tierarztl. Wochenschr. 1989;102:84–86. [PubMed] [Google Scholar]
- 82.Myllykoski J., Nevas M., Lindstrôm M., Korkeala H. The detection and prevalence of Clostridium botulinum in pig intestinal samples. Int. J. Food Microbiol. 2006;110:172–177. doi: 10.1016/j.ijfoodmicro.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 83.Peck M.W. Clostridium botulinum and the safety of minimally heated, chilled foods: An emerging issue? J. Appl. Microbiol. 2006;101:556–570. doi: 10.1111/j.1365-2672.2006.02987.x. [DOI] [PubMed] [Google Scholar]
- 84.Weisemann J., Krez N., Fiebig U., Worbs S., Skiba M., Endermann T., Dorner M.B., Bergstrom T., Munoz A., Zegers I., et al. Generation and Characterization of Six Recombinant Botulinum Neurotoxins as Reference Material to Serve in an International Proficiency Test. Toxins. 2015;7:5035–5054. doi: 10.3390/toxins7124861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peck M.W. Biology and genomic analysis of Clostridium botulinum. Adv. Microb. Physiol. 2009;55:183–265, 320. doi: 10.1016/S0065-2911(09)05503-9. [DOI] [PubMed] [Google Scholar]
- 86.Bouvet P., Ruimy R., Bouchier C., Faucher N., Mazuet C., Popoff M.R. An Atypical Clostridium Strain Related to the Clostridium botulinum Group III Strain Isolated from a Human Blood Culture. J. Clin. Microbiol. 2014;52:339–343. doi: 10.1128/JCM.00390-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Centers for Disease Control and Prevention National Botulism Surveillance. [(accessed on 11 April 2020)]; Available online: https://www.cdc.gov/botulism/surveillance.html.