Abstract
The reproducibility of contrast-enhanced ultrasound (CEUS) and standard B-mode ultrasound in the assessment of radiofrequency-ablated volume of benign thyroid nodules was compared. A preliminary study was conducted on consecutive patients who underwent radiofrequency ablation (RFA) of benign thyroid nodules between 2014 and 2016, with available CEUS and B-mode post-ablation checks. CEUS and B-mode images were retrospectively evaluated by two radiologists to assess inter- and intra-observer agreement in the assessment of ablated volume (Bland–Altman test). For CEUS, the mean inter-observer difference (95% limits of agreement) was 0.219 mL (-0.372–0.809 mL); for B-mode, the mean difference was 0.880 mL (-1.655–3.414 mL). Reproducibility was significantly higher for CEUS (85%) than for B-mode (27%). Mean intra-observer differences (95% limits of agreement) were 0.013 mL (0.803–4.097 mL) for Reader 1 and 0.031 mL (0.763–3.931 mL) for Reader 2 using CEUS, while they were 0.567 mL (-2.180–4.317 mL, Reader 1) and 0.759 mL (-2.584–4.290 mL, Reader 2) for B-mode. Intra-observer reproducibility was significantly higher for CEUS (96% and 95%, for the two readers) than for B-mode (21% and 23%). In conclusion, CEUS had higher reproducibility and inter- and intra-observer agreement compared to conventional B-mode in the assessment of radiofrequency-ablated volume of benign thyroid nodules.
Keywords: thyroid nodule, radiofrequency ablation, ultrasonography, contrast-enhanced ultrasound, observer variation
1. Introduction
Thyroid nodules are a common occurrence in the general population, with a prevalence between 20% and 70% [1,2,3,4,5]. The vast majority are benign and incidentally detected at ultrasound (US) examination. Although they are normally asymptomatic and can be managed conservatively (i.e., clinical and US follow-up), some of them may require treatment because of cosmetic reasons, subjective compressive symptoms (for compression on structures around the thyroid gland), or for an abnormally increased function of the nodule [1,6].
Surgery has been traditionally the main treatment option for thyroid nodules, carrying however several drawbacks, such as scars, need for general anesthesia, and risk of induced hypothyroidism [7]. Therefore, minimally invasive image-guided thermal ablation techniques have been increasingly applied in the last years in order to reduce the invasiveness of treatment in patients with benign thyroid nodules. Among image-guided thermal ablation techniques, laser and radiofrequency ablation (RFA) are the most commonly used [8,9]. Some guidelines and consensus documents suggested the application of image-guided thermal ablation as an alternative to surgery in patients with symptomatic thyroid nodules, even as first-line treatment [7,10,11].
Although thermal ablation is a safe and effective procedure, nodule regrowth can occur during follow-up, with a rate of 5.5% and 9% for RFA and laser, respectively reported in literature [12,13,14].
To find early signs of a potential future nodule regrowth would be of relevant clinical value. Usually, technique efficacy is defined as a volume reduction >50% of the initial volume and is evaluated at one year after treatment [15,16]. Recently, some authors introduced initial ablation ratio (IAR) as a quantitative early indicator correlated with volume reduction ratio during follow-up [15]. In their paper, Sim et al. evaluated the IAR identifying the ablated area on standard B-mode ultrasound images [16]. However, some time margins of the ablated area can be difficult to precisely delineate on B-mode ultrasound. In this scenario, contrast-enhanced ultrasound (CEUS) is largely used after ablation to better identify the ablated area and could improve IAR definition in thyroid ablations [17,18,19,20].
The aim of this study was to compare the reproducibility of CEUS and B-mode and to evaluate inter- and intra-observer agreement in the assessment of the radiofrequency-ablated volume of benign thyroid nodules, comparing CEUS and B-mode.
2. Experimental Section
2.1. Patients
The local ethics committee approved this study (San Raffaele Hospital Ethics Committee, registry number 63/INT/2020). This study was partially supported by Ricerca Corrente funding from Italian Ministry of Health to IRCCS Policlinico San Donato. Due to the retrospective nature of this study, specific informed consent was waived.
We retrospectively assessed consecutive patients with predominantly solid nonfunctioning thyroid nodules treated with RFA in a single center between January 2014 and December 2016. All patients declined or were not eligible for surgery. Benign proven thyroid nodules causing compressive symptoms and cosmetic concerns were considered for treatment.
Eligibility criteria for inclusion in the study were patients who underwent RFA of benign thyroid nodules and whose follow-up included CEUS and B-mode evaluation one year after treatment.
2.2. Preablation Assessment and Procedure
All patients had a cytologically proven benign, TI-RADS 2 category (benign findings, according to the Thyroid Imaging Reporting and Data System) [21] thyroid nodule determining pressure symptoms and/or cosmetic problems.
All procedures were performed under local anesthesia and conscious sedation by one interventional radiologist with 10 years of experience in image-guided percutaneous ablations.
Under the guidance of a high-resolution linear transducer (L3-12a linear transducer, RS80A with Prestige US System—Samsung Medical Imaging, Seoul, South Korea), we used an internally cooled, 18G electrode with a 0.5–1.5 cm active tip (AMICA, HS Hospital Service, Aprilia, Italy) and a free-hand “moving-shot” technique [22].
2.3. Follow-Up Examinations
All follow-up US scans were taken one, six, and twelve months after ablation in the same center by the same interventional radiologist who performed RFA, using a high-frequency linear probe (L3-12a linear transducer, RS80A with Prestige US System—Samsung Medical Imaging, Seoul, South Korea). Technical parameters, including gain adjustment, field of view, and transmission focusing, were optimized for thyroid imaging.
Two perpendicular images of the largest ablated area were acquired for each treated nodule with CEUS and B-mode.
CEUS evaluation was performed the same day of the B-mode scan, after intravenous administration of 4.8 mL of Sonovue (Bracco Imaging, Milan, Italy) with the same US scanner and linear probe, using a dedicated preset.
2.4. Radiofrequency-Ablated Volume Assessment
Two physicians (V.F. and C.B., with 5 and 6 years of experience in thyroid imaging respectively) retrospectively reviewed US images of the last check (one year after treatment) on the Picture Archiving and Communication System. They measured the ablated volume on gray-scale B-mode images in a first session and on CEUS images in a second session, after two weeks and randomization of the cases. To assess intra-observer agreement, a second reading session for both B-mode and CEUS scans was performed one month after the first one.
All measurements were blindly taken in random order and freehandedly. Three orthogonal nodule diameters, including the largest diameter, were measured on both B-mode and CEUS images. Nodule volume was calculated using the equation V = πabc/6, where V is the volume, a is the longest diameter, and b and c are the other two perpendicular diameters.
2.5. Statistical Analysis
Inter- and intra-observer agreement was assessed by using the Bland–Altman test. Mean difference (bias) and the 95% limits of agreement (mean difference ± 1.96 standard deviation) were determined. Statistical analysis was performed using commercial software packages (IBM SPSS Statistics for Windows, version 26.0, IBM Corp., Armonk, NY, USA).
3. Results
A total of thirty-four patients were treated between January 2014 and December 2016, twenty-three of whom met the inclusion criteria and were included. Patient and nodule characteristics are detailed in Table 1.
Table 1.
Patient and nodule characteristics.
| N | 23 | |
| Females/Males | 20/3 | |
| Mean age (± standard deviation) | 60 (±14) years | |
| Nodule location | Right | 9 |
| Isthmic | 3 | |
| Left | 11 | |
| Nodule mean volume before treatment (mL) | 23.90 ± 17.2 (range 7.3–62.5) | |
| Mean ablated volume (± standard deviation) assessed with CEUS (mL) | Reader 1 | 3.947 ± 4.243 |
| Reader 2 | 3.729 ± 4.196 | |
| Mean ablated volume (± standard deviation) assessed with B-mode (mL) | Reader 1 | 2.441 ± 1.735 |
| Reader 2 | 1.562 ± 1.965 | |
CEUS—contrast-enhanced ultrasound.
3.1. Inter-observer Agreement
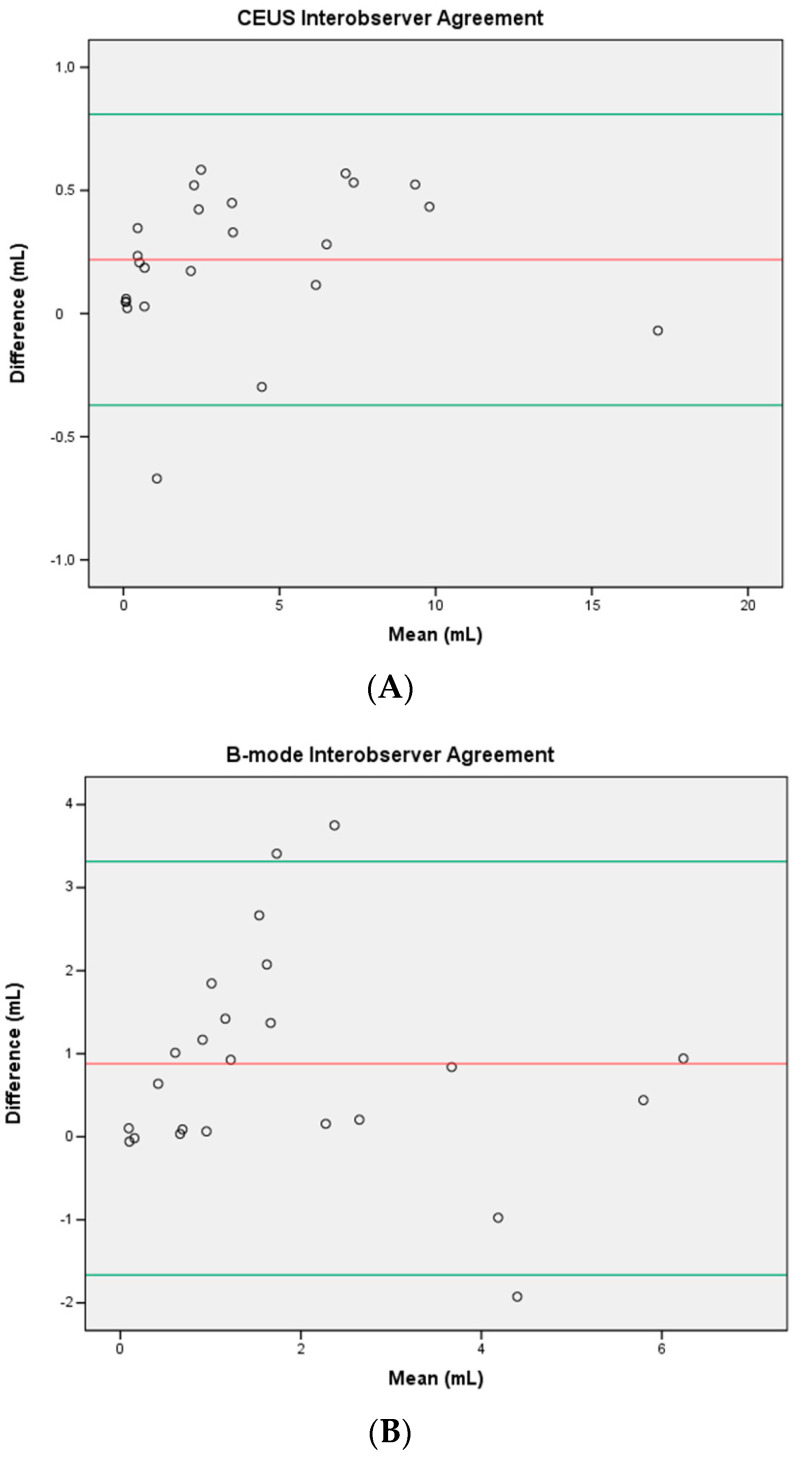
Regarding inter-observer agreement, CEUS showed a bias (95% limits of agreement) of 0.219 mL (-0.372 to 0.809 mL) and a coefficient of reproducibility (COR) of 0.593 mL (p = 0861). B-mode had a bias of 0.880 mL (-1.655 to 3.414 mL) and a COR of 2.558 mL (p = 0.115). CEUS showed a higher inter-observer reproducibility (85%) compared to B-mode (27%).
Reader 1 had higher mean volume estimation compared to Reader 2 using both CEUS and B-mode.
Plots are shown in Figure 1.
Figure 1.
Bland–Altman plots showing inter-observer reproducibility of measurements for CEUS (A) and B-mode (B) assessments. The x-axes show the mean of the volume measurements, and the y-axes show the differences between the measurements. Red lines = mean difference between readers, and green lines = 95% (1.96 SD) limits of agreement.
3.2. Intra-observer Agreement
Intra-observer agreement was better for CEUS compared to B-mode for both the readers: a bias (95% limits of agreement) of 0.013 mL (0.803 to 4.097 mL) was found for Reader 1 assessing his intra-observer reproducibility assessing ablated volume using CEUS, and 0.031 mL (0.763 to 3.931 mL) for Reader 2. For CEUS, COR was 0.159 mL for Reader 1 (p = 0.992) and 0.190 mL for Reader 2 (p = 0.980), and reproducibility was 96% and 95%, respectively.
The ablated volume assessed with B-mode showed a bias of 0.567 mL (-2.180 to 4.317 mL) and 0.759 mL (-2.584 to 4.290 mL) for Readers 1 and 2, respectively. For B-mode, COR was 2.203 mL for Reader 1 (p = 0.286) and 2.397 mL for Reader 2 (p = 0.169), and reproducibility was 21% and 23%, respectively.
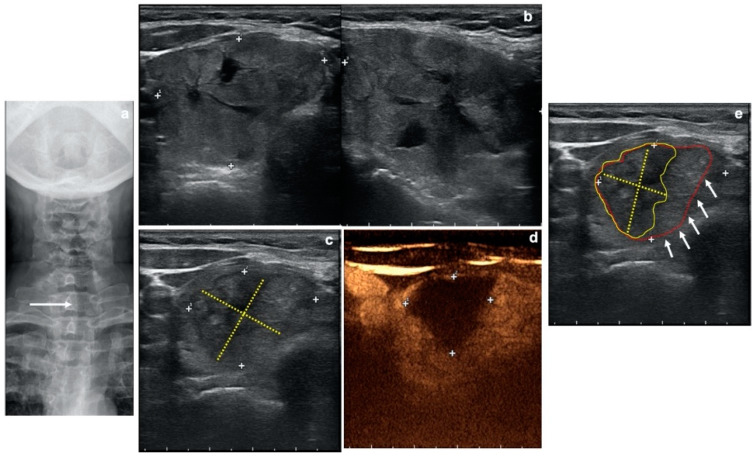
Plots are available as Supplementary Figures S1–4. Figure 2 shows a right nodule ablation results evaluation where B-mode US and CEUS are compared.
Figure 2.
Case of a 56-year-old woman treated with radiofrequency ablation. (a) Thoracic inlet X-ray showing a large retrosternal goiter with left deviation (white arrow) and indentation of the trachea. (b) Two-planes gray-scale ultrasound (US) showing pretreatment evaluation of an almost completely solid right thyroid lobe nodule (Volume: 28.8 mL). (c) Gray-scale US at 6 months after treatment showing volumetric reduction of the ablated nodule (yellow dotted lines = evaluation of the ablated area with B-mode US). (d) Contrast-enhanced ultrasound (CEUS) at 6 months after treatment demonstrating the area of ablation as an area with lack of enhancement (white crosses = evaluation of the ablated area with CEUS). (e) After CEUS-treated nodule evaluation, treatment results can be better reconsidered on the grey-scale US (c) showing real ablated nodule margins (yellow dotted lines and encircled area) as compared to previously measured margins (white arrows; red encircled area).
4. Discussion
Image-guided thermal ablation is becoming increasingly common as an alternative to surgery for the treatment of a variety of benign and malignant conditions [23,24,25,26,27,28,29,30,31,32]. When dealing with diseases in the neck, image-guided thermal ablations have been reported to be effective in the treatment of benign thyroid nodules, autonomously functioning nodules, metastatic lymph nodes, and recurrent thyroid cancers [9,12,33,34,35,36,37]. In the treatment of benign thyroid nodules, different techniques have been used, including laser and RFA, which are the most widely used techniques, and have been proven to be effective and with good results over time in terms of nodule volume reduction [31,33,34,38,39,40,41]. In order to evaluate the success of the procedure, it is crucial to follow treated nodules over time. In fact, some of them might regrow and need further ablation sessions.
US is the most widely used imaging modality for the assessment of thyroid nodules. With B-mode, a central hypoechoic area is considered as a direct sign of ablation zone (immediately after the procedure) and expression of tissue remodeling (during follow-up). From a recent study conducted with B-mode, the central ablated area can be used to determine a quantitative index to predict therapeutic success [16]. According to this study, IAR is the ratio of the ablated volume to total volume of the nodule. If IAR after RFA is <70%, the nodule is likely to regrow [16].
However, some limitations of US, such as low reproducibility and operator-depending performance and measuring, might reduce its accuracy in the evaluation of thyroid nodules’ ablated volume. Particularly, the ablated area can be difficult to clearly demarcate with B-mode, as it can appear as an isoechoic area in comparison with nonablated surrounding thyroid tissue. In this setting, CEUS could be helpful in achieving a better demarcation of the ablated area and to improve the reproducibility of the measurements. In fact, CEUS provides information regarding the vascularization of a human tissue and is widely used in the US assessment of ablations results in other organs [18,20,35,36,42]. Furthermore, it is applied in some centers to precisely delineate the ablated area in thyroid nodules treated with image-guided thermal ablation [34,37,43]. Notably, US contrast agents can be directly administered to complete a standard US examination and are extremely safe and well tolerated. In fact, US contrast agents are not excreted through the kidneys and can be safely administered to patients with renal insufficiency with no risk of contrast-related nephropathy or nephrogenic systemic fibrosis. There is no need for blood tests prior to injection, and there is no evidence of any effect on thyroid function, as they do not contain iodine. These contrast agents have a very low rate of anaphylactoid reactions (1:7000 patients, 0.014%), significantly lower than the rate with iodinated computer tomography (CT) contrast agents (35–95:100,000 patients, 0.035–0.095%), comparable to the rate of severe anaphylactoid reactions associated with gadolinium-based contrast agents at 0.001–0.01% [43].
In this study, we observed a significantly higher inter- and intra-observer reproducibility in measuring the ablated area with CEUS compared to those with B-mode. CEUS, in fact, better enhances vascularized tissue, which helps to clarify boundaries between viable and nonviable tissue. This could be helpful to obtain a more precise and reproducible measure of the ablated area. In our study, it helped in fact to reduce intra- and inter-observer variation. Some studies evaluated the inter-observer and intra-observer variations assessment of thyroid nodules with US [44,45,46]. All these studies were focused in evaluating the diagnostic performance of US in the diagnosis of malignancy among the investigated nodules, while, to the best of our knowledge, no study reported on the analysis of inter-observer and intra-observer agreement in the evaluation of the ablated area. Thus, our paper is the first to depict the potential role of adding CEUS to standard B-mode ultrasound to increase the reproducibility of the measurement of the ablated area after radiofrequency ablation of benign thyroid nodules.
This study has some limitations. First, it is a retrospective study, performed in a single center with a low number of cases. Larger prospective studies are necessary to confirm our preliminary data. Second, the real value of a precise evaluation of the ablated area is still under investigation, and its potential role in predicting a regrowth is still unproven. Therefore, the potential clinical value is still hypothetical. Third, the two readers had a quite similar experience in thyroid ultrasound, and the variability among operators with larger difference in experience should be further investigated.
5. Conclusions
US follow-up is crucial after RFA of benign thyroid nodules. CEUS, compared to conventional B-mode, better depicts the ablated area and for this reason reduces variation in its measurement, with a significantly higher inter- and intra-observer reproducibility. Thus, application of CEUS during the follow-up of patients treated with RFA for a benign thyroid nodule can be beneficial to achieve a better definition of the ablated area. Further studies on larger samples are needed to better understand the potential clinical value of these findings.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/5/1504/s1, Figure S1: Reader 1 CEUS, Figure S2: Reader 1 B-mode, Figure S3: Reader 2 CEUS, Figure S4: Reader 2 B-mode.
Author Contributions
Conceptualization, G.M.; data curation, S.S.; supervision, L.M.S. and G.M.; visualization, D.R.; writing—original draft, S.S.; writing—review and editing, F.S., D.R., V.F., C.B., M.A., L.M., L.M.S., and G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
S.S. declares having received payment for lectures including service on speakers’ bureaus from GE Healthcare. The remaining authors declare no conflict of interest.
References
- 1.Haugen B.R., Alexander E.K., Bible K.C., Doherty G.M., Mandel S.J., Nikiforov Y.E., Pacini F., Randolph G.W., Sawka A.M., Schlumberger M., et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gharib H., Papini E. Thyroid Nodules: Clinical Importance, Assessment, and Treatment. Endocrinol. Metab. Clin. North Am. 2007;36:707–735. doi: 10.1016/j.ecl.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Guth S., Theune U., Aberle J., Galach A., Bamberger C.M. Very High Prevalence of Thyroid Nodules Detected by High Frequency (13 MHz) Ultrasound Examination. Eur. J. Clin. Invest. 2009;39:699–706. doi: 10.1111/j.1365-2362.2009.02162.x. [DOI] [PubMed] [Google Scholar]
- 4.Papini E., Pacella C.M., Hegedus L. Diagnosis of Endocrine Disease: Thyroid Ultrasound (US) and US-Assisted Procedures: From the Shadows into an Array of Applications. Eur. J. Endocrinol. 2014;170:133–146. doi: 10.1530/EJE-13-0917. [DOI] [PubMed] [Google Scholar]
- 5.Filetti S., Durante C., Torlontano M. Nonsurgical Approaches to the Management of Thyroid Nodules. Nat. Clin. Pract. Endocrinol. Metab. 2006;2:384–394. doi: 10.1038/ncpendmet0215. [DOI] [PubMed] [Google Scholar]
- 6.Shin J.H., Baek J.H., Chung J., Ha E.J., Kim J.H., Lee Y.H., Lim H.K., Moon W.J., Na D.G., Park J.S., et al. Ultrasonography Diagnosis and Imaging-Based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J. Radiol. 2016;17:370–395. doi: 10.3348/kjr.2016.17.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J., Baek J.H., Lim H.K., Ahn H.S., Baek S.M., Choi Y.J., Choi Y.J., Chung S.R., Ha E.J., Hahn S.Y., et al. 2017 Thyroid Radiofrequency Ablation Guideline: Korean Society of Thyroid Radiology. Korean J. Radiol. 2018;19 doi: 10.3348/kjr.2018.19.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baek J.H., Lee J.H., Valcavi R., Pacella C.M., Rhim H., Na D.G. Thermal Ablation for Benign Thyroid Nodules: Radiofrequency and Laser. Korean J. Radiol. 2011;12:525–540. doi: 10.3348/kjr.2011.12.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mauri G., Gennaro N., Lee M.K., Baek J.H. Laser and Radiofrequency Ablations for Benign and Malignant Thyroid Tumors. Int. J. Hyperth. 2019;36:13–20. doi: 10.1080/02656736.2019.1622795. [DOI] [PubMed] [Google Scholar]
- 10.Dietrich C.F., Müller T., Bojunga J., Dong Y., Mauri G., Radzina M., Dighe M., Cui X.W., Grünwald F., Schuler A., et al. Statement and Recommendations on Interventional Ultrasound as a Thyroid Diagnostic and Treatment Procedure. Ultrasound Med. Biol. 2018;44:14–36. doi: 10.1016/j.ultrasmedbio.2017.08.1889. [DOI] [PubMed] [Google Scholar]
- 11.Papini E., Pacella C.M., Solbiati L.A., Achille G., Barbaro D., Bernardi S., Cantisani V., Cesareo R., Chiti A., Cozzaglio L., et al. Minimally-Invasive Treatments for Benign Thyroid Nodules: A Delphi-Based Consensus Statement from the Italian Minimally-Invasive Treatments of the Thyroid (MITT) Group. Int. J. Hyperth. 2019;36:376–382. doi: 10.1080/02656736.2019.1575482. [DOI] [PubMed] [Google Scholar]
- 12.Lim H.K., Lee J.H., Ha E.J., Sung J.Y., Kim J.K., Baek J.H. Radiofrequency Ablation of Benign Non-Functioning Thyroid Nodules: 4-Year Follow-up Results for 111 Patients. Eur. Radiol. 2013;23:1044–1049. doi: 10.1007/s00330-012-2671-3. [DOI] [PubMed] [Google Scholar]
- 13.Sim J.S., Baek J.H., Lee J., Cho W., Jung S.I. Radiofrequency Ablation of Benign Thyroid Nodules: Depicting Early Sign of Regrowth by Calculating Vital Volume. Int. J. Hyperth. 2017;33:905–910. doi: 10.1080/02656736.2017.1309083. [DOI] [PubMed] [Google Scholar]
- 14.Valcavi R., Riganti F., Bertani A., Formisano D., Pacella C.M. Percutaneous Laser Ablation of Cold Benign Thyroid Nodules: A 3-Year Follow-up Study in 122 Patients. Thyroid. 2010;20:1253–1261. doi: 10.1089/thy.2010.0189. [DOI] [PubMed] [Google Scholar]
- 15.Mauri G., Pacella C.M., Papini E., Solbiati L., Goldberg S.N., Ahmed M., Sconfienza L.M. Image-Guided Thyroid Ablation: Proposal for Standardization of Terminology and Reporting Criteria. Thyroid. 2019;29:611–618. doi: 10.1089/thy.2018.0604. [DOI] [PubMed] [Google Scholar]
- 16.Sim J.S., Baek J.H., Cho W. Initial Ablation Ratio: Quantitative Value Predicting the Therapeutic Success of Thyroid Radiofrequency Ablation. Thyroid. 2018;28:1443–1449. doi: 10.1089/thy.2018.0180. [DOI] [PubMed] [Google Scholar]
- 17.Sidhu P., Cantisani V., Dietrich C., Gilja O., Saftoiu A., Bartels E., Bertolotto M., Calliada F., Clevert D.-A., Cosgrove D., et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Short Version) Ultraschall der Medizin - Eur. J. Ultrasound. 2018;39:154–180. doi: 10.1055/s-0044-101254. [DOI] [PubMed] [Google Scholar]
- 18.Meloni M.F., Andreano A., Zimbaro F., Lava M., Lazzaroni S., Sironi S. Contrast Enhanced Ultrasound: Roles in Immediate Post-Procedural and 24-h Evaluation of the Effectiveness of Thermal Ablation of Liver Tumors. J. Ultrasound. 2012;15:207–214. doi: 10.1016/j.jus.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prada F., Del Bene M., Fornaro R., Vetrano I.G., Martegani A., Aiani L., Sconfienza L.M., Mauri G., Solbiati L., Pollo B., et al. Identification of Residual Tumor with Intraoperative Contrast-Enhanced Ultrasound during Glioblastoma Resection. Neurosurg. Focus. 2016;40 doi: 10.3171/2015.11.FOCUS15573. [DOI] [PubMed] [Google Scholar]
- 20.Mauri G., Porazzi E., Cova L., Restelli U., Tondolo T., Bonfanti M., Cerri A., Ierace T., Croce D., Solbiati L. Intraprocedural Contrast-Enhanced Ultrasound (CEUS) in Liver Percutaneous Radiofrequency Ablation: Clinical Impact and Health Technology Assessment. Insights Imaging. 2014;5:209–216. doi: 10.1007/s13244-014-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russ G., Bonnema S.J., Erdogan M.F., Durante C., Ngu R., Leenhardt L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid J. 2017;6:225–237. doi: 10.1159/000478927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S.J., Chung H.W., Baek J.H., Lee J.S., Lee S.H., Lee M.H., Shin M.J. Ultrasonography-Guided Radiofrequency Ablation of Malignant Musculoskeletal Soft-Tissue Tumors Using the “Moving-Shot” Technique at a Single-Institution Experience. Ultrasound Q. 2014;30:295–300. doi: 10.1097/RUQ.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 23.Shyn P.B., Mauri G., Alencar R.O., Tatli S., Shah S.H., Morrison P.R., Catalano P.J., Silverman S.G. Percutaneous Imaging-Guided Cryoablation of Liver Tumors: Predicting Local Progression on 24-Hour MRI. AJR. Am. J. Roentgenol. 2014;203:W181–W191. doi: 10.2214/AJR.13.10747. [DOI] [PubMed] [Google Scholar]
- 24.Shyn P.B., Tatli S., Sahni V.A., Sadow C.A., Forgione K., Mauri G., Morrison P.R., Catalano P.J., Silverman S.G. PET/CT-Guided Percutaneous Liver Mass Biopsies and Ablations: Targeting Accuracy of a Single 20 s Breath-Hold PET Acquisition. Clin. Radiol. 2014;69:410–415. doi: 10.1016/j.crad.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Mauri G., Nicosia L., Varano G.M., Bonomo G., Della Vigna P., Monfardini L., Orsi F. Tips and Tricks for a Safe and Effective Image-Guided Percutaneous Renal Tumour Ablation. Insights Imaging. 2017;8:357–363. doi: 10.1007/s13244-017-0555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacella C.M., Patelli G., Iapicca G., Manenti G., Perretta T., Ryan C.P., Esposito R., Mauri G. Transperineal Laser Ablation for Percutaneous Treatment of Benign Prostatic Hyperplasia: A Feasibility Study. Results at 6 and 12 Months from a Retrospective Multi-Centric Study. Prostate Cancer Prostatic Dis. 2019 doi: 10.1038/s41391-019-0196-4. [DOI] [PubMed] [Google Scholar]
- 27.de Baere T., Tselikas L., Gravel G., Hakime A., Deschamps F., Honoré C., Mir O., Lecesne A. Interventional Radiology: Role in the Treatment of Sarcomas. Eur. J. Cancer. 2018;94:148–155. doi: 10.1016/j.ejca.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Forner A., Llovet J.M., Bruix J. Hepatocellular Carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 29.Ljungberg B., Bensalah K., Canfield S., Dabestani S., Hofmann F., Hora M., Kuczyk M.A., Lam T., Marconi L., Merseburger A.S., et al. EAU Guidelines on Renal Cell Carcinoma: 2014 Update. Eur. Urol. 2015;67:913–924. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Monfardini L., Gennaro N., Della Vigna P., Bonomo G., Varano G., Maiettini D., Bonello L., Solbiati L., Orsi F., Mauri G. Cone-Beam CT-Assisted Ablation of Renal Tumors: Preliminary Results. Cardiovasc. Intervent. Radiol. 2019;42:1718–1725. doi: 10.1007/s00270-019-02296-5. [DOI] [PubMed] [Google Scholar]
- 31.Monfardini L., Orsi F., Caserta R., Sallemi C., Della Vigna P., Bonomo G., Varano G., Solbiati L., Mauri G. Ultrasound and Cone Beam CT Fusion for Liver Ablation: Technical Note. Int. J. Hyperth. 2018;0:1–5. doi: 10.1080/02656736.2018.1509237. [DOI] [PubMed] [Google Scholar]
- 32.Wu J., Bai H.X., Chan L., Su C., Zhang P.J., Yang L., Zhang Z. Sublobar Resection Compared with Stereotactic Body Radiation Therapy and Ablation for Early Stage Non–Small Cell Lung Cancer: A National Cancer Database Study. J. Thorac. Cardiovasc. Surg. 2020 doi: 10.1016/j.jtcvs.2019.11.132. [DOI] [PubMed] [Google Scholar]
- 33.Mauri G., Cova L., Ierace T., Baroli A., Di Mauro E., Pacella C.M., Goldberg S.N., Solbiati L. Treatment of Metastatic Lymph Nodes in the Neck from Papillary Thyroid Carcinoma with Percutaneous Laser Ablation. Cardiovasc. Intervent. Radiol. 2016;39:1023–1030. doi: 10.1007/s00270-016-1313-6. [DOI] [PubMed] [Google Scholar]
- 34.Pacella C.M., Mauri G., Achille G., Barbaro D., Bizzarri G., De Feo P., Di Stasio E., Esposito R., Gambelunghe G., Misischi I., et al. Outcomes and Risk Factors for Complications of Laser Ablation for Thyroid Nodules: A Multicenter Study on 1531 Patients. J. Clin. Endocrinol. Metab. 2015;100:3903–3910. doi: 10.1210/jc.2015-1964. [DOI] [PubMed] [Google Scholar]
- 35.Trimboli P., Castellana M., Sconfienza L.M., Virili C., Pescatori L.C., Cesareo R., Giorgino F., Negro R., Giovanella L., Mauri G. Efficacy of Thermal Ablation in Benign Non-Functioning Solid Thyroid Nodule: A Systematic Review and Meta-Analysis. Endocrine. 2020;67:35–43. doi: 10.1007/s12020-019-02019-3. [DOI] [PubMed] [Google Scholar]
- 36.Kim J.H., Yoo W.S., Park Y.J., Park D.J., Yun T.J., Choi S.H., Sohn C.H., Lee K.E., Sung M.W., Youn Y.K., et al. Efficacy and Safety of Radiofrequency Ablation for Treatment of Locally Recurrent Thyroid Cancers Smaller than 2 Cm. Radiology. 2015;276:909–918. doi: 10.1148/radiol.15140079. [DOI] [PubMed] [Google Scholar]
- 37.Cesareo R., Palermo A., Benvenuto D., Cella E., Pasqualini V., Bernardi S., Stacul F., Angeletti S., Mauri G., Ciccozzi M., et al. Efficacy of Radiofrequency Ablation in Autonomous Functioning Thyroid Nodules. A Systematic Review and Meta-Analysis. Rev. Endocr. Metab. Disord. 2019;20:37–44. doi: 10.1007/s11154-019-09487-y. [DOI] [PubMed] [Google Scholar]
- 38.Mauri G., Cova L., Monaco C.G., Sconfienza L.M., Corbetta S., Benedini S., Ambrogi F., Milani V., Baroli A., Ierace T., et al. Benign Thyroid Nodules Treatment Using Percutaneous Laser Ablation (PLA) and Radiofrequency Ablation (RFA) Int. J. Hyperth. 2017;33:295–299. doi: 10.1080/02656736.2016.1244707. [DOI] [PubMed] [Google Scholar]
- 39.Mauri G., Cova L., De Beni S., Ierace T., Tondolo T., Cerri A., Goldberg S.N., Solbiati L. Real-Time US-CT/MRI Image Fusion for Guidance of Thermal Ablation of Liver Tumors Undetectable with US: Results in 295 Cases. Cardiovasc. Intervent. Radiol. 2015;38:143–151. doi: 10.1007/s00270-014-0897-y. [DOI] [PubMed] [Google Scholar]
- 40.Mauri G., Nicosia L., Della vigna P., Varano G.M., Maiettini D., Bonomo G., Giuliano G., Orsi F., Solbiati L., De fiori E., et al. Percutaneous Laser Ablation for Benign and Malignant Thyroid Diseases. Ultrasonography. 2019;38:25–36. doi: 10.14366/usg.18034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu W., Zhou P., Zhao Y., Tian S., Wu X. Superb Microvascular Imaging Compared with Contrast-Enhanced Ultrasound for Assessing Laser Ablation Treatment of Benign Thyroid Nodules. Biomed Res. Int. 2018;2018 doi: 10.1155/2018/1025657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meloni M.F., Smolock A., Cantisani V., Bezzi M., D’Ambrosio F., Proiti M., Lee F., Aiani L., Calliada F., Ferraioli G. Contrast Enhanced Ultrasound in the Evaluation and Percutaneous Treatment of Hepatic and Renal Tumors. Eur. J. Radiol. 2015;84:1666–1674. doi: 10.1016/j.ejrad.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 43.Pacella C.M., Mauri G., Cesareo R., Paqualini V., Cianni R., De Feo P., Gambelunghe G., Raggiunti B., Tina D., Deandrea M., et al. A Comparison of Laser with Radiofrequency Ablation for the Treatment of Benign Thyroid Nodules: A Propensity Score Matching Analysis. Int. J. Hyperth. 2017;33:911–919. doi: 10.1080/02656736.2017.1332395. [DOI] [PubMed] [Google Scholar]
- 44.Choi S.H., Kim E.K., Kwak J.Y., Kim M.J., Son E.J. Interobserver and Intraobserver Variations in Ultrasound Assessment of Thyroid Nodules. Thyroid. 2010;20:167–172. doi: 10.1089/thy.2008.0354. [DOI] [PubMed] [Google Scholar]
- 45.Friedrich-Rust M., Meyer G., Dauth N., Berner C., Bogdanou D., Herrmann E., Zeuzem S., Bojunga J. Interobserver Agreement of Thyroid Imaging Reporting and Data System (TIRADS) and Strain Elastography for the Assessment of Thyroid Nodules. PLoS ONE. 2013;8:1–6. doi: 10.1371/journal.pone.0077927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dobruch-Sobczak K., Migda B., Krauze A., Mlosek K., Słapa R.Z., Wareluk P., Bakuła-Zalewska E., Adamczewski Z., Lewiński A., Jakubowski W., et al. Prospective Analysis of Inter-Observer and Intra-Observer Variability in Multi Ultrasound Descriptor Assessment of Thyroid Nodules. J. Ultrason. 2019;19:198–206. doi: 10.15557/JoU.2019.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.