Abstract
Honey bees are key agricultural pollinators, but beekeepers continually suffer high annual colony losses owing to a number of environmental stressors, including inadequate nutrition, pressures from parasites and pathogens, and exposure to a wide variety of pesticides. In this review, we examine how two such stressors, pesticides and viruses, may interact in additive or synergistic ways to affect honey bee health. Despite what appears to be a straightforward comparison, there is a dearth of studies examining this issue likely owing to the complexity of such interactions. Such complexities include the wide array of pesticide chemical classes with different modes of actions, the coupling of many bee viruses with ectoparasitic Varroa mites, and the intricate social structure of honey bee colonies. Together, these issues pose a challenge to researchers examining the effects pesticide-virus interactions at both the individual and colony level.
Keywords: honey bee virus, virus tolerance/resistance, pesticide resistance, toxicology, insecticide–virus synergism
1. Introduction
Honey bees (Apis mellifera) are perhaps the most important insect for human well-being, helping to pollinate over $200 billion USD in agricultural crops per year [1]. Yet, despite their importance to agriculture, honey bees continue to suffer substantial annual colony losses in many countries, with American beekeepers alone losing more than 50% of their colonies in 2018 [2]. Despite considerable effort, no single ‘smoking gun’ for these losses has appeared; instead, high losses have been linked to several co-occurring biotic and abiotic environmental stressors. These include poor nutrition due to habitat loss and low floral quantity or quality in agriculture systems, parasite pressures from Varroa destructor mites, immune challenges from a suite of viruses and other pathogens, and exposure to numerous pesticides [3,4,5,6,7,8]. Adding further complexity to the issue, many of these stressors act simultaneously on honey bees and can exert additive or even synergistic effects [9,10,11,12,13,14,15,16]. For example, dietary pollen quality and quantity greatly affects immunocompetence, and bees with poor nutrition are more susceptible to parasites and pathogens [17,18,19].
In this review, we focus on the interaction between two stressors that has thus far received surprisingly little attention: that of pesticides and viruses. We first briefly discuss the broad range of chemical classes used by farmers, public health officials, and beekeepers to control pest populations, the modes of action by which these chemicals target insects, and the ways bees can be affected by sublethal doses. We then review our current knowledge of bee viruses, the immunological pathways used by bees to fight infection, and the ways viruses are transmitted between individuals, colonies, and even species. Finally, we examine how some pesticides do (or do not) promote viral replication or pathological effects at both the individual and colony level, and highlight areas of future research needed to fill knowledge gaps.
2. Pesticides
“Pesticide” is a broad term denoting any substance that is used to eliminate pest species and can include insecticides, herbicides, fungicides, and nematicides. Pesticides represent a diverse array of chemical classes with different modes of action, and as such, examining the effects of pesticides on honey bees is not a straightforward endeavor. Adding further complication, honey bees often encounter many different chemicals simultaneously [20,21,22,23] owing to their ubiquity in commercial pollination, their generalist foraging strategy, and their large foraging ranges that can cover hundreds of square kilometers [24]. These different chemicals, along with adjuvants and other additives in the applied formulations, can interact with one another to produce additive or sometimes synergistic effects in bees and other insects [12,25,26]. Much work has been done examining the acute toxicity and lethal dosages of these pesticides, as such measures are required by regulatory agencies for product registration [27], but bees often encounter pesticides at sublethal doses in their environment. Even these lower doses can produce various effects in bees, including impairments to behavior [28,29,30,31], learning and memory [32,33,34], longevity [35], and immune function [36]. Here, we briefly outline some of these chemical classes commonly encountered by bees, the sublethal effects they exert on bees, as well as the modes of actions of these chemicals in bees or other more common insect models, such as fruit flies and mosquitos.
2.1. General Background on Classes of Pesticides
Many commercial insecticides are synthetic analogs of naturally-occurring chemical compounds produced by plants and often act by disrupting the nervous system or muscle tissue function [37,38]. While a full discussion of all these compounds is beyond the scope of this review, comprehensive reviews can be found elsewhere [39,40]. Organophosphates and carbamates are widely used in agriculture and pest prevention and disrupt nerve function by inactivating acetylcholinesterase, an enzyme used to clear acetylcholine neurotransmitters from the synapse [40]. Both classes of chemicals have a broad range of toxicity towards honey bees [41], but one of the most commonly used in crop protection, chlorpyrifos, is highly toxic to bees [42] and often found in hive materials [43]. Even at doses far below the LD50 (i.e., the dosage that kills half of the subjects), chlorpyrifos has negative impacts on bees’ appetitive olfactory learning and memory [43]. Likewise, the organophosphate naled is mainly used to control mosquito populations, and incidental exposure in honey bees can lead to increased mortality and lower honey production [44]. Organophosphates and carbamates have been linked to many bee poisoning incidents in the UK [45].
Pyrethroids are another popular class of insecticides and are similar to the natural pyrethrin compounds produced in chrysanthemum plants. They target the insect nervous system by delaying the closure of voltage-gated sodium channels [40], leading to the loss of motor function, paralysis, and ultimately death (reviewed in [46]). They also display a broad range of toxic effects to honey bees [41,47]. Among the most common encountered by bees are bifenthrin and lambda-cyhalothrin. Bifenthrin is used in orchard agriculture and other sectors and at sublethal doses can impair larval development and queen fecundity [48]. Lambda-cyhalothrin, meanwhile, is used to protect a variety of crops and at sublethal doses has been shown to impair honey bee worker longevity, homing ability, and learning and memory [49].
The class of insecticides garnering most public and research attention in the past decade is the neonicotinoids, which are based on analogs to a natural plant-produced alkaloid, nicotine. These compounds act as acetylcholine receptor agonists to disrupt nerve function, and can be taken up systemically by crop plants via seed coatings and irrigation or applied as a foliar spray [40]. Among the most common neonicotinoids that bees encounter are imidacloprid, clothianidin, and thiamethoxam. There has been much concern about the negative effects these neonicotinoids may have on honey bees, but the evidence is somewhat mixed. While some studies have shown that neonicotinoid exposure can compromise foraging activity and survival in honey bees [30,50,51], the concentration of neonicotinoids in the nectar of treated plants may not be high enough to elicit such effects [52]. However, other studies have shown that bees housed near corn fields treated with neonicotinoids show reduced longevity and hygienic behavior [53].
In contrast to neurotoxic compounds that target adult insects, some insecticides target developing larvae by disrupting their molting and cuticle formation processes. These insect growth regulators (IGRs) are typically synthetic analogs of juvenile hormone or ecdysone, two hormones critical for development and molting, or they are chitin inhibitors that interfere with the formation of the exoskeleton. Thus, the production of viable new offspring can be greatly impeded. IGRs may have little to no lethal effects on adult honey bee workers [54], but they can disrupt their behavioral development [55,56] and have been shown to affect egg viability and larval development [56,57]. For a review of IGRs see [58].
Honey bees also encounter non-insect-targeting pesticides, such as herbicides and fungicides, which were long presumed to have little to no effect on bees. However, more recent studies suggest they pose some risk. Herbicides can indirectly impact honey bee health by limiting the availability of diverse floral sources [59], thus compromising the bees’ nutritional state, which can lead to further compromises to immune system function [60]. However, herbicides and fungicides can also exert more direct effects. For example, glyphosate is widely used to eliminate weeds in corn and soy production, but it can disrupt honey bee gut microbiota that play important roles in immunity and weight gain [61], as well as affect larval development [62] and worker behavior [63]. Interestingly, honey bee foragers show a preference for sucrose solution with low concentrations of glyphosate over control solution [64]. Atrazine, another popular herbicide that is frequently found in hive material [65], has been shown to reduce levels of some antioxidants in honey bees [66]. Azoles, a common class of fungicides, have been shown to affect ATP production in honey bee flight muscle [67], which in turn can impede bees’ abilities to thermoregulate [68]. Moreover, fungicides are often applied concurrently with insecticides in agriculture, and co-exposure experiments show synergistic effects on lethality and increased risk to honey bees [69].
It is also important to consider, however, that the findings discussed thus far represent just a snapshot of documented effects of specific pesticides on honey bees. Other effects may remain undiscovered because scientists have not yet looked for them. For example, neonicotinoids’ effects on honey bee health have been studied intensively, but older classes of pesticides like pyrethroids and organophosphates have received less attention. New information may also prompt re-examination of previously studied pesticides, as is the case with fungicides, which were considered harmless to bees but which are now understood to act synergistically with certain insecticides. Furthermore, some pesticides may elicit no effects in a particular study [70], but such non-effects may be less documented due to publication bias [71]. Undiscovered and non-effects could be important for understanding interactions between pesticides and the immune system, but scientists will have to overcome this information barrier.
2.2. How Bees Detoxify Pesticides
A pesticide’s toxicity and sublethal effects depend on how an organism metabolizes and detoxifies the particular compound. There are several families of enzymes involved in detoxification pathways, but the most prominent and well-studied are the cytochrome P450 monooxygenases [72]. Detoxification is typically initiated when a P450 enzyme targets specific chemical structures on the xenobiotic molecule and catalyzes its reduction into metabolites. These metabolites are then bound by other enzymes that allow for transport and eventual secretion of the metabolite. Some pesticides can interfere with the P450 function [73], and some synergists are specifically added to insecticide formulations for this purpose to increase toxicity towards target species [74]. A given pesticide’s toxicity is also not consistent among all honey bee colony members, with larvae being especially susceptible [48,62,75,76,77,78,79]. In fact, pesticide toxicity can vary with many factors, including caste [80], age [81], season [82,83], genetics [81], and nutritional state [83]. In this latter regard, plant pollens and nectars contain many phytochemicals that up-regulate the expression of detoxification genes and can increase bees’ tolerance to some pesticides [84,85]. It should also be noted that honey bees have fewer detoxification genes than solitary insect species [86,87].
2.3. Active Ingredients vs. Formulations
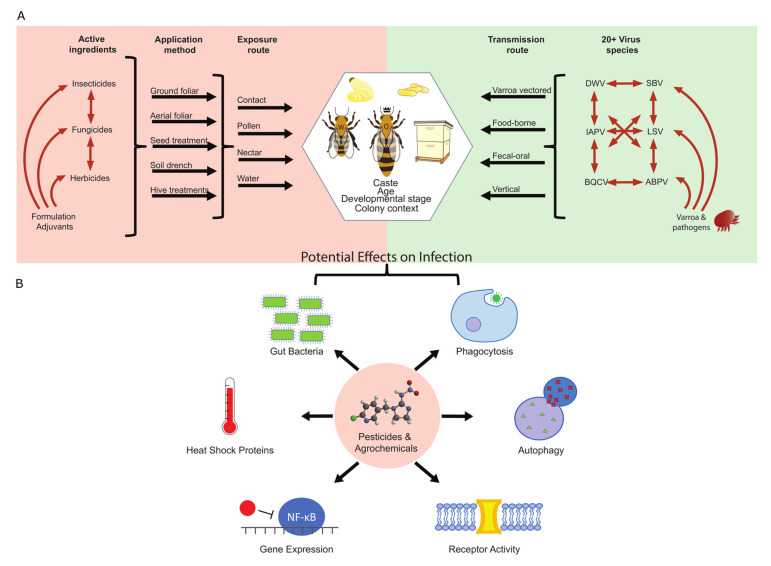
Pesticide toxicology studies in honey bees can be complicated by how the commercial products are formulated. While many experiments use pure active ingredients, real world applications of formulated product also contain adjuvants with potential effects on bees. These all have the potential to interact with each other, and even ingredients considered “inert” can negatively affect bees [26,75,88]. Identical active ingredients can also have different formulations for different applications. Each of these has its own potential risks and can all contribute to different potential routes of exposure to the bees—through direct drift of chemicals onto bees/hives, contamination of water, and contamination of diet via direct exposure or systemic plant movement (Figure 1A).
Figure 1.
(A) Pesticide × virus interactions require integration of many potential scenarios, and there are many challenges in understanding the relevance of potential interactions. Pesticide considerations: Honey bees can and have been exposed to many different pesticide types (insecticides, fungicides, herbicides, etc.), each containing multiple classes with different modes of action. Product formulations also contain adjuvants and “inert” chemicals that can interact with active ingredients to alter pesticide sensitivity in bees. One must also consider how application methods might affect how bees are exposed to the pesticides. Virus considerations: honey bees can be infected with more than 20 different types of viruses, often at the same time, and there are likely coinfection interactions (DWV: deformed wing virus; SBV: sacbrood virus; IAPV: Israeli acute paralysis virus; LSV: Lake Sinai virus; BQCV: black queen cell virus; ABPV: acute bee paralysis virus). At the same time, variable pressure from Varroa mites and other pathogens can affect these dynamics. The route of exposure to these viruses is likely to cause different responses in the infected bees. Overall considerations: for both pesticide and virus experiments, the context of investigation can also yield variable responses. Bees of different caste (queen vs. worker), developmental stage (egg, larva, pupa, and adult), and age can all experience these stressors differently. Further, most experimental manipulations of pesticide and virus stress are done outside of the colony context; honey bee colony units can respond differently than individuals or small groups in experimental settings. Thus, studying pesticide/virus interactions in honey bees sounds deceptively simple; however, the potential interactions make investigation a significant challenge. (B) Pesticide exposure can negatively impact many components and pathways of the immune system. Phagocytosis: pro-hemocyte differentiation can be impaired, resulting in fewer phagocytosing immune cells. The process of phagocytosis itself can also be affected; autophagy: Regulation of autophagy can be disrupted, potentially leading to apoptosis in cells; receptor activity: some insecticides target receptors that are also involved in antiviral defenses; gene expression: pesticides can alter expression of immune and detoxification genes. This includes upregulating inhibitors of the important immune system transcription factor, NF-κB; heat shock proteins: some pesticides downregulate expression of genes coding for heat shock proteins. These proteins can reduce viral load and also have functions in the RNAi antiviral pathway; gut bacteria: pesticides can also disrupt gut microbial communities, which are known to play roles in honey bee health and immunity.
2.4. Treating for Varroa mites
Honey bee exposure to pesticides is often incidental, being a consequence of farmers, public health officials, and even homeowners seeking to eliminate pests. However, beekeepers also intentionally apply some chemicals to treat for pests within bee colonies. These include treatments for small hive beetle (Aethina tumida), wax moths (Galleria mellonella and Achroia grisella), and tracheal mites (Acarapis woodi), but are mostly focused on control of Varroa mites. Several miticides have been used historically, including coumaphos (an organophosphate), τ-fluvalinate (a pyrethroid), amitraz (an adrenergic agonist), and several organic acids and essential oils (e.g., Thymol), though some of these, like coumaphos, have become uncommon due to resistance evolution [89]. Despite clear benefits in controlling parasites, each of these chemicals does pose some degree of risk for honey bees. For example, coumaphos exposure can lead to increased larval mortality [75,90,91], τ-fluvalinate exposure can affect learning and memory [92] and larval and adult survival [75,91], and thymol exposure can lead to brood removal by workers [93] and decreased larval survival at high doses [94]. Despite these risks, these treatments are still viewed as critical, as Varroa pose an outsized risk to honey bees and are a primary cause of annual colony losses [95,96]. Varroa mites have negative impacts on many important aspects of honey bee biology, including physiology [97], behavior [98], and nutritional state [99,100] (see [101,102] for comprehensive reviews). However, perhaps their biggest threat to honey bees come from their role as vectors for some of the most prolific and deadly honey bee viruses.
3. Honey Bee Viruses
Dozens of honey bee viruses have been characterized, with the vast majority being positive-sense single-stranded RNA viruses (+ssRNA). More exhaustive reviews than the one provided here can be found elsewhere, e.g., [103]. Among the most prevalent viruses, many belong to the Dicistroviridae family, including Israeli acute paralysis virus (IAPV), acute bee paralysis virus (ABPV), Kashmir bee virus (KBV), and black queen cell virus (BQCV) [104]. IAPV, ABPV, and KBV are very closely related and often considered as a virus complex [105]. Many other bee viruses belong to the Iflaviridae family, including deformed wing virus (DWV), sacbrood virus (SBV), and slow bee paralysis virus (SBPV). Varroa mites are vectors for many of these viruses, and two in particular, IAPV and DWV, have been associated with high colony losses [7]. IAPV symptoms include quivering, paralysis, and eventual death, and contracting the virus can be lethal to bees of all ages and developmental stages [106,107]. DWV infection during pupal development can lead to emerging adult workers with under-developed and non-functional wings, altered behavior, and decreased lifespan [108], which contribute to high winter colony losses. However, workers that contract the virus as adults do not appear to show overt symptoms [108]. DWV virulence also appears to be amplified by Varroa mites. Like most RNA viruses, there are many strains of DWV owing to high sequence variation (so called quasispecies) [108], and transmission via Varroa may be selecting for more virulent strains. Laboratory studies show that even when mites have a high diversity of replicating DWV strains, the infected pupae show increased replication for a single virulent strain [109,110]. This has also been observed in the field, where the high diversity of DWV strains in Hawaiian apiaries was greatly reduced when Varroa mites were introduced to the islands [111].
3.1. Sublethal Infection
Viral infections are extremely prevalent in honey bee colonies, with recent surveys showing DWV infection in 89% of US colonies [112] and 100% of English/Welsh apiaries [113]. Most bees harbor viral infections at subclinical levels, meaning the viral loads are such that individuals do not exhibit any overt symptoms. In many cases these low-level infections do not appear to negatively impact a colony’s growth rate, but adequately studying the impacts of asymptomatic infection is challenging due to the scarcity of proper non-infected control colonies. Typically, an individual bee’s immune system is able to control viral replication, but when particular environmental stressors increase in severity, they can compromise a bee’s immune system and release viral replication. For example, bees without access to diverse floral resources may lack important proteins and lipids, potentially allowing viral replication to increase [16]. Lab-reared adult workers that feed exclusively on sucrose end up with higher viral titers than workers whose diets are also supplemented with protein-rich pollen [16]. Sublethal infections also cause changes in gene expression patterns, some of which are due to the host’s antiviral response [114], but some may be due to virus manipulation of the host to promote transmission between colonies [115] (see Section 3.2).
3.2. Viral Transmission
Within a honey bee colony, viruses can be transmitted in a multitude of ways and seemingly exploit all routes of material exchange between colony members. Horizontal transmission between nestmates can occur when workers exchange saliva via food sharing (trophallaxis) and when they feed the queen and young larvae with a glandular secretion called royal jelly. Both saliva and royal jelly test positive for viral copies [114,116,117]. Horizontal transmission can also occur via sexual contact between drones (males) and the queen [114,118], and by physical contact between nestmates [117]. Vertical transmission is also present, as evidenced by viral copies in the queens ovaries and eggs [114,118]. However, Varroa mites remain the most problematic and well-documented route of bee viral transmission. Varroa can parasitize bees of all castes and developmental stages (excluding eggs and L1–L4) but given their nature as obligate parasites they primarily feed on the pupal stage when larvae are capped inside brood cells during their metamorphosis. Here, female mites lay eggs that will hatch before the end of pupation and the resulting offspring can spread to other larvae or adult bees within the hive, thereby exhibiting exponential population growth. Varroa mites also facilitate inter-colony viral transmission by attaching themselves to workers and drones that exit the hive and potentially switching hosts at floral forage patches. Further still, Varroa-vectored viruses can actually move between species [119,120], threatening vulnerable native bees [121]. Viruses can also transfer between hives without a Varroa vector, and viral infection may actually alter host behavior and chemical signature to help facilitate this process [115]. Within a hive, IAPV-infected workers receive fewer social interactions from their nestmates, but they are more likely than non-infected individuals to be permitted into foreign hives. Here, infected workers that leave the hive to forage are more likely than non-infected workers to return to a foreign hive, a process known as drifting. Drifting occurs under normal circumstances in densely packed apiaries, and drifters are typically turned away from foreign hives by a team of guard bees that monitor the hive entrance. However, infected bees are permitted into foreign hives at higher rates than is typical, possibly due to changes in their chemical signature encoded in their cuticle [115].
3.3. Antiviral Immune Pathways
Honey bees, like all insects, have several highly conserved innate immunological pathways to fight pathogens. This includes the RNA-interference (RNAi) pathway [122], which evolved specifically to combat viruses [123]. Here, the enzyme Dicer targets double-stranded RNA (dsRNA), as this type of molecule is typically only found in eukaryotic cells during viral replication. Dicer cleaves the dsRNA into smaller RNAs, after which these small RNAs are unwound and used as a template to find specific complementary sequences of single-stranded RNA (e.g., mRNA). These mRNAs are enzymatically cleaved, ultimately reducing viral replication and expression of viral genes. Researchers can exploit this RNAi pathway to knockdown gene expression by administering dsRNA to organisms, and feeding dsRNA to bees (both virus sequence-specific or non-specific alike) has been shown to reduce viral loads of bee pathogens like IAPV [124,125]
Insects also possess other conserved innate immune system pathways, including the immune deficiency (IMD), Toll, and Jak-STAT (Janus kinase and signal transducer and activator of transcription) pathways. These pathways have well-characterized responses against cellular pathogens like bacteria and fungi, but are also activated during viral infection [126,127]. Here, pathogen-associated molecular patterns (PAMPs) are first bound to pathogen-recognizing receptors, which initiate a cascade of reactions that often induces expression of antimicrobial peptides (AMPs). Both the Toll and IMD pathways share an important component, the transcription factor NF-κB (nuclear factor kappa-B), which translocates from the cytosol to the nucleus to upregulate AMP expression [128]. Prominent model systems like Drosophila melanogaster and various mosquito species have been used to show the IMD and Toll pathways playing direct antiviral roles [129,130,131,132]. For example, D. melanogaster injected with cricket paralysis virus (CrPV) experience depleted reserves of hemocytes (immune cells) over the course of infection, while IMD loss-of-function mutants are more sensitive to infection and display higher viral loads [129]. Similarly, Toll loss-of-function D. melanogaster mutants display higher viral loads and increased susceptibility compared to wildtype individuals when exposed to viruses [131].
Other immune system components involved in antiviral defense include autophagy, phagocytosis, and heat shock proteins [133,134,135]. Autophagy is an intracellular mechanism for degrading and recycling cytoplasmic components and occurs through the formation of double-membraned vesicles. These vesicles then fuse with lysosomes and their internal contents are degraded. This process is essential for fighting some insect viruses [133]. Phagocytosis is a cellular process by which certain hemocytes (i.e., macrophages/plasmatocytes) degrade dead, infected, or pathogenic cells, and this response can be crucial in eliminating virus-infected cells [134]. Heat shock proteins are part of the organismal stress responses, but their genes are also induced during viral infection to help reduce replication [135]. They also act as chaperones to help facilitate the RNAi antiviral pathway [136]. For more comprehensive reviews of insect antiviral pathways, see [126,127].
In addition to individual-level immune defenses, honey bees also employ colony-level defenses through a suite of behavioral and physiological traits termed social immunity. Individuals can prevent or slow the spread of pathogens by isolating or removing infected individuals, grooming each other for parasites, sterilizing the nest and food by distributing antimicrobial plant resins and secreting glucose oxidase enzymes throughout the hive material and food, and many other phenomena [137,138]. Social immunity can also protect future offspring through a process called trans-generational immune priming (TGIP) [139]. Here, a female that survives a pathogen attack can transfer PAMP-containing particles to her eggs [140], which primes the developing immune system to produce more resistant offspring. In honey bees, recent findings suggest the sterile worker caste may participate in TGIP by incorporating PAMP-containing particles into the royal jelly glandular secretions they feed to the queen and young larvae [141,142].
4. Interaction of Pesticides and Honey Bee Viruses
While honey bees and other insects have evolved mechanisms to detoxify xenobiotics like pesticides and fight pathogen threats like viruses, real-world conditions mean that honey bees are often exposed to these environmental stressors simultaneously. The question then becomes, does pesticide exposure lead to increased viral transmission or virulence? Perhaps unsurprising, given the wide variety of pesticides and pathogens encountered by bees in different contexts, investigations have provided mixed results, with many studies showing pesticides exerting additive or synergistic effects on virus-induced mortality and replication in honey bees [36,143,144,145,146,147], and others showing little to no effect of pesticide exposure on viral infections in honey bees and bumble bees [148,149,150,151].
4.1. How Pesticides Can Impact Antiviral Pathways
To understand why pesticides may increase susceptibility to viruses, it is important to know how they affect antiviral immune pathways (Figure 1B). Again, much of this work has been performed in other prominent insect models like D. melanogaster and various mosquito species. First, pesticide exposure can disrupt cellular immune defenses by affecting hemocyte differentiation and function. Larval D. melanogaster fed with acephate (an organophosphate) display altered differential hemocyte counts, with a relative decrease in phagocytosing macrophages/plasmatocytes [152]. Moreover, exposure to the neonicotinoid imidacloprid can reduce phagocytotic function of hemocytes [153,154], and also decrease the survival of fat body cells, which play critical roles in immune responses such as AMP production [155]. Some pesticides have also been shown to disrupt important intracellular pathways involved in immunity, like autophagy [156].
Second, numerous studies have highlighted the impact pesticides have on expression of detoxification and immune genes [155,157]. This includes downregulating gene expression of immunologically-relevant heat shock proteins [158]. One can additionally learn how pesticides affect immune function by comparing pesticide-resistant and wild-type strains of insects. Permethrin-resistant mosquitos upregulate expression of several detoxification and immune genes after exposure to the pesticide compared with wildtype mosquitos [159], and resistant mosquitos likewise upregulate detoxification and immune genes after Zika virus infection while wild-type mosquitos downregulate many of these genes after infection [160]. Effects on gene expression and subsequent protein levels may be pesticide- and tissue-specific. For example, mosquitos exposed to temephos (an organophosphate) show upregulation of dozens of proteins in the midgut, including many immune proteins [161].
Third, due to crosstalk and interconnectedness between various immune pathways [162], pesticides that affect gene expression in one pathway may elicit downstream effects in other pathways. For example, exposure to imidacloprid in D. melanogaster can down-regulate components of the IMD pathway that help to regulate the DUOX pathway [163,164]. The DUOX pathway produces hydrogen peroxide, which is used not only to kill pathogens but also to regulate gut microbiota composition [164], and disruption of this microbiome can increase susceptibility to some pathogens [165]. In honey bees, the herbicide glyphosate [61,166] is known to disrupt gut microbial communities, although exposure to imidacloprid may not produce such a change [167].
Finally, some pesticides can directly increase viral susceptibility in insects by targeting ion channels that play a role in antiviral defense. In an effort to combat pesticide-resistance in mosquito vectors of human diseases, promising new research has targeted inward rectifier potassium channels [168]. However, these channels have been shown to regulate infection of cardiotropic viruses in D. melanogaster [169], and honey bees show similar increases in mortality and viral replication when these receptors are blocked [170]. The development of novel insecticides is important for overcoming resistance but it poses a challenge to scientists who must once again discover all the off-target effects of a new chemical class.
So, does exposure to pesticides increase honey bees’ susceptibility to viruses? As previously mentioned, results are mixed and may depend on the specific pesticides and viruses being tested, the manner in which bees are exposed to pesticides, and whether researchers are focused on individual- or colony-level effects. To date, most studies examining pesticide-virus interactions in honey bees have focused on neonicotinoids, likely owing to the public concern that this class of chemicals may pose risks to pollinators. In a landmark study, Di Prisco et al. [36] showed that viral replication of DWV was increased in individual bees exposed to the neonicotinoids clothianidin and imidacloprid, but not in bees exposed to the organophosphate chlorpyrifos. Furthermore, clothianidin exposure upregulated an inhibitor of the important immunological transcription factor NF-κB, thereby providing a mechanistic explanation for how some pesticides can weaken host antiviral defenses and impart synergistic effects on viruses [36]. In subsequent studies, honey bees exposed to neonicotinoids like imidacloprid, clothianidin, and thiacloprid showed reduced hemocyte counts, reduced encapsulation response, and reduced antimicrobial activity [171], providing further insight into possible mechanisms of reduced antiviral defenses.
In another study looking at BQCV infection and thiacloprid exposure, researchers found additive effects of virus and pesticide in honey bee larvae when orally inoculated with high doses of the virus, but found no such effect in adult workers [146]. Several studies have also looked at the possible interactions between CBPV and various neonicotinoid pesticides, with varying results. Coulon et al. [143] orally administered thiamethoxam to summer-born worker bees and housed them with overtly infected bees, observing that high pesticide doses had a synergistic effect on virus-induced mortality without an increase in viral titers, while low pesticide doses exerted no synergistic effects on mortality but did cause an increase in viral titers. A follow-up study in winter-born bees showed no synergistic effect on mortality, but they did observe an increase in viral titers to a level typically seen in overt infections [144]. Similarly, Diao et al. [145] fed varying doses of imidacloprid to CBPV-inoculated spring bees and found that high pesticide doses elicited synergistic effects on viral titers and mortality, but that low pesticide doses had no discernable effect on these virus parameters. Other classes of pesticides remain less studied in terms of their effects on honey bee immunology. IGRs have been shown to have additive lethal effects with some insect pathogens [172,173,174], but to our knowledge there has been no demonstration of synergistic effects. Besides insecticides, other studies have observed synergistic effects on virus-induced mortality when honey bees are co-exposed to a fungicide [147] or a common adjuvant added to many pesticide products [88].
4.2. Laboratory- vs. Field-Based Studies
Despite evidence from laboratory experiments that pesticides can affect virus infections in bees, these findings do not always corroborate results from field-based studies that track changes in natural virus titers. Several large-scale studies have looked at honey bee hives placed near fields of clothianidin-treated canola (rape-seed) and found no increase in titers of several viruses [148,149], nor an increase in expression of immune genes [149], nor an increase in prevalence of overt viral symptoms [175]. A similar study in bumble bees also showed no increase in viral titers when hives were placed near clothianidin-treated fields [151]. In addition, some studies have shown that hives supplemented with imidacloprid in their diet did not experience significant population losses compared to control hives [176], and others found no correlation between colony health and viral prevalence at agricultural sites [150].
This apparent discrepancy between laboratory- and field-based pesticide exposure assays illustrates how, at the colony level, honey bees can be very resilient against environmental stressors and their responses to pesticides can be context-dependent. For example, imidacloprid exposure to laboratory-reared bees can down-regulate expression of immune and detoxification genes while hive-reared bees show up-regulation in several of these genes [177]. This discrepancy represents a major challenge for researchers trying to understand how bees respond to environmental stressors, and there are several factors that likely contribute to these differences in response. Firstly, honey bees live in highly structured societies, with a reproductive queen and a worker caste that progresses through age-related tasks in response to physiological, chemical, and social cues. While pesticide exposure can decrease lifespan of individual bees, a colony-level response can shift the demographics within a hive and mask many impacts [178]. Likewise, laboratory settings can better control extraneous variables, but a disruption to important social and chemical cues likely adds significant stress on the bees and may suppress their immunocompetence. Second, honey bees have a reduced number of immune and detoxification genes compared with solitary species [86,87], but, as already discussed, they also exhibit social immunity. Such social immunity mechanisms are likely lacking or absent in lab-reared bees that comprise a single age cohort. Finally, lab-reared bees are typically fed a restricted diet of sucrose solution, as opposed to the myriad nectars and pollens collected by hive-reared bees, and as already discussed a restricted diet can negatively impact immunity [16].
While field-based studies more accurately capture the day-to-day conditions experienced by bees, there are significant challenges in developing adequate experimental designs, especially in terms of sample size and proper controls. It is far easier to generate large samples of individual bees in a lab than it is to have a sufficient number of hives at various field sites. Two recent studies looking at the effects of field-realistic exposure to neonicotinoids on honey bee health illustrate the magnitude of experiments required to conduct adequate studies [53,179]. Trying to control variables like pesticide exposure is difficult, given that honey bees have large ranges and are generalist foragers. Hives placed in or near pesticide-treated fields will likely still gather materials from non-treated areas, and likewise, hives in or near untreated fields can still forage in treated areas and also be exposed to other pesticides applied by private citizens or public health officials. Treated and untreated sites can also differ in the types and diversity of plants available, adding further inconsistencies between sites. Adequate experiments require high site-type replication to overcome issues with such confounding variables, which places strains on research teams to manage such a large number of hives.
5. Future Directions
Despite the many challenges of studying pesticide-virus interactions in honey bees (Figure 1), continuing this research is critical for maintaining food security, particularly as agroecosystems face pressures from climate change. There are several aspects of this issue for which our knowledge must improve. First, we need a greater understanding of how innate immune system pathways such as Toll and IMD operate on a molecular level to fight viruses. We know that these pathways are activated upon viral infection and that knocking out certain components increases virus replication and lethality, but we still lack some mechanistic explanations for how the viruses are destroyed or prevented from replicating. Knowing these mechanisms may help researchers design treatments for current honey bee pathogens and novel pathogens that may arise in the future. Second, we must continue to identify immune and detoxification pathway components that are directly affected by pesticides in order to predict how exposure will affect host antiviral responses. Here, researchers could make uses of advances in machine learning and metabolic modeling to aid their search. Finally, we must carry out pesticide exposure and viral inoculation assays in both laboratory and field settings, as effects observed on individuals in the lab may be buffered by the group-level responses of whole colonies. In a perfect world, one would study a matrix of effects, examining a slew of viruses and pesticides administered in various combinations, doses, and exposure methods, but such endeavors are daunting. Perhaps instead, researchers can first turn to the plethora of immunological and detoxification studies performed in prominent insect models like D. melanogaster, mosquitos, and major crop pests. Here, an understanding of how these pathways are affected by pathogens and xenobiotics in one model organism can inform researchers’ hypotheses of how they may be affected in honey bees. Such hypotheses can first be tested in laboratory settings, and if effects are observed then the study can be expanded to determine if such effects persist at the colony level in the field. These types of studies will be particularly important going forward as new classes of “pollinator-friendly” pesticides are developed. Even those deemed relatively safe for honey bees may exert sublethal effects that impact honey bee health [25,180]. Challenges will always remain when studying a highly social species in landscapes filled with multiple co-occurring stressors, but honey bees’ great importance to human well-being necessitates that such efforts continue.
Acknowledgments
The authors thank J. Fine for helpful feedback on the original manuscript, and K. Dolezal for text editing.
Funding
This research was funded by the US Department of Agriculture grant 2019-67013-29300.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gallai N., Salles J.-M., Settele J., Vaissière B.E. Economic Valuation of the Vulnerability of World Agriculture Confronted with Pollinator Decline. Ecol. Econ. 2009;68:810–821. doi: 10.1016/j.ecolecon.2008.06.014. [DOI] [Google Scholar]
- 2.United States Department of Agriculture/National Agricultural Statistics Survey . Honey Bee Colonies. United States Department of Agriculture/National Agricultural Statistics Survey; Washington, DC, USA: 2019. [Google Scholar]
- 3.Naug D. Nutritional Stress Due to Habitat Loss May Explain Recent Honeybee Colony Collapses. Biol. Conserv. 2009;142:2369–2372. doi: 10.1016/j.biocon.2009.04.007. [DOI] [Google Scholar]
- 4.Paudel Y.P., Mackereth R., Hanley R., Qin W. Honey Bees (Apis Mellifera L.) and Pollination Issues: Current Status, Impacts, and Potential Drivers of Decline. J. Agric. Sci. 2015;7:93. doi: 10.5539/jas.v7n6p93. [DOI] [Google Scholar]
- 5.Steinhauer N., Kulhanek K., Antúnez K., Human H., Chantawannakul P., Chauzat M.-P., van Engelsdorp D. Drivers of Colony Losses. Curr. Opin. Insect Sci. 2018;26:142–148. doi: 10.1016/j.cois.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Smith K.M., Loh E.H., Rostal M.K., Zambrana-Torrelio C.M., Mendiola L., Daszak P. Pathogens, Pests, and Economics: Drivers of Honey Bee Colony Declines and Losses. EcoHealth. 2013;10:434–445. doi: 10.1007/s10393-013-0870-2. [DOI] [PubMed] [Google Scholar]
- 7.McMenamin A.J., Genersch E. Honey Bee Colony Losses and Associated Viruses. Curr. Opin. Insect Sci. 2015;8:121–129. doi: 10.1016/j.cois.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Potts S.G., Biesmeijer J.C., Kremen C., Neumann P., Schweiger O., Kunin W.E. Global Pollinator Declines: Trends, Impacts and Drivers. Trends Ecol. Evol. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Nazzi F., Brown S.P., Annoscia D., Del Piccolo F., Di Prisco G., Varricchio P., Della Vedova G., Cattonaro F., Caprio E., Pennacchio F. Synergistic Parasite-Pathogen Interactions Mediated by Host Immunity Can Drive the Collapse of Honeybee Colonies. PLoS Pathog. 2012;8:e1002735. doi: 10.1371/journal.ppat.1002735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng H.-Q., Gong H.-R., Huang S.-K., Sohr A., Hu F.-L., Chen Y.P. Evidence of the Synergistic Interaction of Honey Bee Pathogens Nosema Ceranae and Deformed Wing Virus. Vet. Microbiol. 2015;177:1–6. doi: 10.1016/j.vetmic.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Grassl J., Holt S., Cremen N., Peso M., Hahne D., Baer B. Synergistic Effects of Pathogen and Pesticide Exposure on Honey Bee (Apis Mellifera) Survival and Immunity. J. Invertebr. Pathol. 2018;159:78–86. doi: 10.1016/j.jip.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y.C., Yao J., Adamczyk J., Luttrell R. Synergistic Toxicity and Physiological Impact of Imidacloprid Alone and Binary Mixtures with Seven Representative Pesticides on Honey Bee (Apis Mellifera) PLoS ONE. 2017;12:e0176837. doi: 10.1371/journal.pone.0176837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aufauvre J., Biron D.G., Vidau C., Fontbonne R., Roudel M., Diogon M., Viguès B., Belzunces L.P., Delbac F., Blot N. Parasite-Insecticide Interactions: A Case Study of Nosema Ceranae and Fipronil Synergy on Honeybee. Sci. Rep. 2012;2:1–7. doi: 10.1038/srep00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Retschnig G., Neumann P., Williams G.R. Thiacloprid–Nosema Ceranae Interactions in Honey Bees: Host Survivorship but Not Parasite Reproduction Is Dependent on Pesticide Dose. J. Invertebr. Pathol. 2014;118:18–19. doi: 10.1016/j.jip.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Nazzi F., Pennacchio F. Disentangling Multiple Interactions in the Hive Ecosystem. Trends Parasitol. 2014;30:556–561. doi: 10.1016/j.pt.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 16.DeGrandi-Hoffman G., Chen Y., Huang E., Huang M.H. The Effect of Diet on Protein Concentration, Hypopharyngeal Gland Development and Virus Load in Worker Honey Bees (Apis Mellifera L.) J. Insect Physiol. 2010;56:1184–1191. doi: 10.1016/j.jinsphys.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Alaux C., Dantec C., Parrinello H., Le Conte Y. Nutrigenomics in Honey Bees: Digital Gene Expression Analysis of Pollen’s Nutritive Effects on Healthy and Varroa-Parasitized Bees. BMC Genomics. 2011;12:496. doi: 10.1186/1471-2164-12-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Pasquale G., Salignon M., Le Conte Y., Belzunces L.P., Decourtye A., Kretzschmar A., Suchail S., Brunet J.-L., Alaux C. Influence of Pollen Nutrition on Honey Bee Health: Do Pollen Quality and Diversity Matter? PLoS ONE. 2013;8:e72016. doi: 10.1371/journal.pone.0072016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolezal A.G., Carrillo-Tripp J., Judd T.M., Allen Miller W., Bonning B.C., Toth A.L. Interacting Stressors Matter: Diet Quality and Virus Infection in Honeybee Health. R. Soc. Open Sci. 2019;6:181803. doi: 10.1098/rsos.181803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson R.M., Ellis M.D., Mullin C.A., Frazier M. Pesticides and Honey Bee Toxicity–USA. Apidologie. 2010;41:312–331. doi: 10.1051/apido/2010018. [DOI] [Google Scholar]
- 21.Long E.Y., Krupke C.H. Non-Cultivated Plants Present a Season-Long Route of Pesticide Exposure for Honey Bees. Nat. Commun. 2016;7:1–12. doi: 10.1038/ncomms11629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chauzat M.-P., Faucon J.-P., Martel A.-C., Lachaize J., Cougoule N., Aubert M. A Survey of Pesticide Residues in Pollen Loads Collected by Honey Bees in France. J. Econ. Entomol. 2006;99:253–262. doi: 10.1093/jee/99.2.253. [DOI] [PubMed] [Google Scholar]
- 23.Mullin C.A., Frazier M., Frazier J.L., Ashcraft S., Simonds R., Pettis J.S. High Levels of Miticides and Agrochemicals in North American Apiaries: Implications for Honey Bee Health. PLoS ONE. 2010;5:e9754. doi: 10.1371/journal.pone.0009754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beekman M., Ratnieks F.L.W. Long-Range Foraging by the Honey-Bee, Apis mellifera L. Funct. Ecol. 2000;14:490–496. doi: 10.1046/j.1365-2435.2000.00443.x. [DOI] [Google Scholar]
- 25.Tosi S., Nieh J.C. Lethal and Sublethal Synergistic Effects of a New Systemic Pesticide, Flupyradifurone (Sivanto®), on Honeybees. Proc. R. Soc. B Biol. Sci. 2019;286:20190433. doi: 10.1098/rspb.2019.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullin C.A., Chen J., Fine J.D., Frazier M.T., Frazier J.L. The Formulation Makes the Honey Bee Poison. Pestic. Biochem. Physiol. 2015;120:27–35. doi: 10.1016/j.pestbp.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 27.US Environmental Protection Agency . Guidance on Exposure and Efffects Testing for Assessing Risks to Bees. US Environmental Protection Agency; Washington, DC, USA: 2016. [Google Scholar]
- 28.Decourtye A., Devillers J., Aupinel P., Brun F., Bagnis C., Fourrier J., Gauthier M. Honeybee Tracking with Microchips: A New Methodology to Measure the Effects of Pesticides. Ecotoxicology. 2011;20:429–437. doi: 10.1007/s10646-011-0594-4. [DOI] [PubMed] [Google Scholar]
- 29.Aliouane Y., El Hassani A.K., Gary V., Armengaud C., Lambin M., Gauthier M. Subchronic Exposure of Honeybees to Sublethal Doses of Pesticides: Effects on Behavior. Environ. Toxicol. Chem. 2009;28:113–122. doi: 10.1897/08-110.1. [DOI] [PubMed] [Google Scholar]
- 30.Henry M., Béguin M., Requier F., Rollin O., Odoux J.-F., Aupinel P., Aptel J., Tchamitchian S., Decourtye A. A Common Pesticide Decreases Foraging Success and Survival in Honey Bees. Science. 2012;336:348–350. doi: 10.1126/science.1215039. [DOI] [PubMed] [Google Scholar]
- 31.El Hassani A.K., Dacher M., Gary V., Lambin M., Gauthier M., Armengaud C. Effects of Sublethal Doses of Acetamiprid and Thiamethoxam on the Behavior of the Honeybee (Apis Mellifera) Arch. Environ. Contam. Toxicol. 2008;54:653–661. doi: 10.1007/s00244-007-9071-8. [DOI] [PubMed] [Google Scholar]
- 32.Decourtye A., Devillers J., Genecque E., Menach K.L., Budzinski H., Cluzeau S., Pham-Delègue M.H. Comparative Sublethal Toxicity of Nine Pesticides on Olfactory Learning Performances of the Honeybee Apis Mellifera. Arch. Environ. Contam. Toxicol. 2005;48:242–250. doi: 10.1007/s00244-003-0262-7. [DOI] [PubMed] [Google Scholar]
- 33.Williamson S.M., Wright G.A. Exposure to Multiple Cholinergic Pesticides Impairs Olfactory Learning and Memory in Honeybees. J. Exp. Biol. 2013;216:1799–1807. doi: 10.1242/jeb.083931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siviter H., Koricheva J., Brown M.J.F., Leadbeater E. Quantifying the Impact of Pesticides on Learning and Memory in Bees. J. Appl. Ecol. 2018;55:2812–2821. doi: 10.1111/1365-2664.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J.Y., Anelli C.M., Sheppard W.S. Sub-Lethal Effects of Pesticide Residues in Brood Comb on Worker Honey Bee (Apis Mellifera) Development and Longevity. PLoS ONE. 2011;6:e14720. doi: 10.1371/journal.pone.0014720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Prisco G., Cavaliere V., Annoscia D., Varricchio P., Caprio E., Nazzi F., Gargiulo G., Pennacchio F. Neonicotinoid Clothianidin Adversely Affects Insect Immunity and Promotes Replication of a Viral Pathogen in Honey Bees. Proc. Natl. Acad. Sci. USA. 2013;110:18466–18471. doi: 10.1073/pnas.1314923110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vijverberg H.P.M., Vanden Bercken J. Neurotoxicological Effects and the Mode of Action of Pyrethroid Insecticides. Crit. Rev. Toxicol. 1990;21:105–126. doi: 10.3109/10408449009089875. [DOI] [PubMed] [Google Scholar]
- 38.Simon-Delso N., Amaral-Rogers V., Belzunces L.P., Bonmatin J.M., Chagnon M., Downs C., Furlan L., Gibbons D.W., Giorio C., Girolami V., et al. Systemic Insecticides (Neonicotinoids and Fipronil): Trends, Uses, Mode of Action and Metabolites. Environ. Sci. Pollut. Res. 2015;22:5–34. doi: 10.1007/s11356-014-3470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson R.M. Honey Bee Toxicology. Annu. Rev. Entomol. 2015;60:415–434. doi: 10.1146/annurev-ento-011613-162005. [DOI] [PubMed] [Google Scholar]
- 40.Casida J.E., Durkin K.A. Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects. Annu. Rev. Entomol. 2013;58:99–117. doi: 10.1146/annurev-ento-120811-153645. [DOI] [PubMed] [Google Scholar]
- 41.Hardstone M.C., Scott J.G. Is Apis Mellifera More Sensitive to Insecticides than Other Insects? Pest Manag. Sci. 2010;66:1171–1180. doi: 10.1002/ps.2001. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez-Bayo F., Goka K. Pesticide Residues and Bees—A Risk Assessment. PLoS ONE. 2014;9:e94482. doi: 10.1371/journal.pone.0094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urlacher E., Monchanin C., Rivière C., Richard F.-J., Lombardi C., Michelsen-Heath S., Hageman K.J., Mercer A.R. Measurements of Chlorpyrifos Levels in Forager Bees and Comparison with Levels That Disrupt Honey Bee Odor-Mediated Learning Under Laboratory Conditions. J. Chem. Ecol. 2016;42:127–138. doi: 10.1007/s10886-016-0672-4. [DOI] [PubMed] [Google Scholar]
- 44.Zhong H., Latham M., Hester P.G., Frommer R.L., Brock C. Impact of Naled on Honey Bee Apis Mellifera L. Survival and Productivity: Aerial ULV Application Using a Flat-Fan Nozzle System. Arch. Environ. Contam. Toxicol. 2003;45:216–220. doi: 10.1007/s00244-002-0185-8. [DOI] [PubMed] [Google Scholar]
- 45.Barnett E.A., Charlton A.J., Fletcher M.R. Incidents of Bee Poisoning with Pesticides in the United Kingdom, 1994–2003. Pest Manag. Sci. 2007;63:1051–1057. doi: 10.1002/ps.1444. [DOI] [PubMed] [Google Scholar]
- 46.Soderlund D.M., Clark J.M., Sheets L.P., Mullin L.S., Piccirillo V.J., Sargent D., Stevens J.T., Weiner M.L. Mechanisms of Pyrethroid Neurotoxicity: Implications for Cumulative Risk Assessment. Toxicology. 2002;171:3–59. doi: 10.1016/S0300-483X(01)00569-8. [DOI] [PubMed] [Google Scholar]
- 47.Johnson R.M., Dahlgren L., Siegfried B.D., Ellis M.D. Acaricide, Fungicide and Drug Interactions in Honey Bees (Apis Mellifera) PLoS ONE. 2013;8:e54092. doi: 10.1371/journal.pone.0054092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai P.-L., Wang Q., Sun J.-H., Liu F., Wang X., Wu Y.-Y., Zhou T. Effects of Sublethal Concentrations of Bifenthrin and Deltamethrin on Fecundity, Growth, and Development of the Honeybee Apis Mellifera Ligustica. Environ. Toxicol. Chem. 2010;29:644–649. doi: 10.1002/etc.67. [DOI] [PubMed] [Google Scholar]
- 49.Liao C., He X., Wang Z., Barron A.B., Zhang B., Zeng Z., Wu X. Short-Term Exposure to Lambda-Cyhalothrin Negatively Affects the Survival and Memory-Related Characteristics of Worker Bees Apis Mellifera. Arch. Environ. Contam. Toxicol. 2018;75:59–65. doi: 10.1007/s00244-018-0514-1. [DOI] [PubMed] [Google Scholar]
- 50.Schneider C.W., Tautz J., Grünewald B., Fuchs S. RFID Tracking of Sublethal Effects of Two Neonicotinoid Insecticides on the Foraging Behavior of Apis Mellifera. PLoS ONE. 2012;7:e30023. doi: 10.1371/journal.pone.0030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guez D. A Common Pesticide Decreases Foraging Success and Survival in Honey Bees: Questioning the Ecological Relevance. Front. Physiol. 2013;4:37. doi: 10.3389/fphys.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cresswell J.E. A Meta-Analysis of Experiments Testing the Effects of a Neonicotinoid Insecticide (Imidacloprid) on Honey Bees. Ecotoxicology. 2011;20:149–157. doi: 10.1007/s10646-010-0566-0. [DOI] [PubMed] [Google Scholar]
- 53.Tsvetkov N., Samson-Robert O., Sood K., Patel H.S., Malena D.A., Gajiwala P.H., Maciukiewicz P., Fournier V., Zayed A. Chronic Exposure to Neonicotinoids Reduces Honey Bee Health near Corn Crops. Science. 2017;356:1395–1397. doi: 10.1126/science.aam7470. [DOI] [PubMed] [Google Scholar]
- 54.Rabea E.I., Nasr H.M., Badawy M.E.I. Toxic Effect and Biochemical Study of Chlorfluazuron, Oxymatrine, and Spinosad on Honey Bees (Apis Mellifera) Arch. Environ. Contam. Toxicol. 2010;58:722–732. doi: 10.1007/s00244-009-9403-y. [DOI] [PubMed] [Google Scholar]
- 55.Robinson G.E. Effects of a Juvenile Hormone Analogue on Honey Bee Foraging Behaviour and Alarm Pheromone Production. J. Insect Physiol. 1985;31:277–282. doi: 10.1016/0022-1910(85)90003-4. [DOI] [Google Scholar]
- 56.Tasei J.-N. Effects of Insect Growth Regulators on Honey Bees and Non-Apis Bees. A Review. Apidologie. 2001;32:527–545. doi: 10.1051/apido:2001102. [DOI] [Google Scholar]
- 57.Fine J.D., Mullin C.A., Frazier M.T., Reynolds R.D. Field Residues and Effects of the Insect Growth Regulator Novaluron and Its Major Co-Formulant N-Methyl-2-Pyrrolidone on Honey Bee Reproduction and Development. J. Econ. Entomol. 2017;110:1993–2001. doi: 10.1093/jee/tox220. [DOI] [PubMed] [Google Scholar]
- 58.Pener M.P., Dhadialla T.S. Chapter One—An Overview of Insect Growth Disruptors; Applied Aspects. In: Dhadialla T.S., editor. Advances in Insect Physiology. Volume 43. Academic Press; Cambridge, MA, USA: 2012. pp. 1–162. Insect Growth Disruptors. [DOI] [Google Scholar]
- 59.Johansen C.A. Pesticides and Pollinators. Annu. Rev. Entomol. 1977;22:177–192. doi: 10.1146/annurev.en.22.010177.001141. [DOI] [Google Scholar]
- 60.Alaux C., Ducloz F., Crauser D., Le Conte Y. Diet Effects on Honeybee Immunocompetence. Biol. Lett. 2010;6:562–565. doi: 10.1098/rsbl.2009.0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Motta E.V., Raymann K., Moran N.A. Glyphosate Perturbs the Gut Microbiota of Honey Bees. Proc. Natl. Acad. Sci. USA. 2018;115:10305–10310. doi: 10.1073/pnas.1803880115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vázquez D.E., Ilina N., Pagano E.A., Zavala J.A., Farina W.M. Glyphosate Affects the Larval Development of Honey Bees Depending on the Susceptibility of Colonies. PLoS ONE. 2018;13:e0205074. doi: 10.1371/journal.pone.0205074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herbert L.T., Vázquez D.E., Arenas A., Farina W.M. Effects of Field-Realistic Doses of Glyphosate on Honeybee Appetitive Behaviour. J. Exp. Biol. 2014;217:3457–3464. doi: 10.1242/jeb.109520. [DOI] [PubMed] [Google Scholar]
- 64.Liao L.-H., Wu W.-Y., Berenbaum M.R. Behavioral Responses of Honey Bees ( Apis Mellifera ) to Natural and Synthetic Xenobiotics in Food. Sci. Rep. 2017;7:1–8. doi: 10.1038/s41598-017-15066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ostiguy N., Drummond F.A., Aronstein K., Eitzer B., Ellis J.D., Spivak M., Sheppard W.S. Honey Bee Exposure to Pesticides: A Four-Year Nationwide Study. Insects. 2019;10:13. doi: 10.3390/insects10010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Helmer S.H., Kerbaol A., Aras P., Jumarie C., Boily M. Effects of Realistic Doses of Atrazine, Metolachlor, and Glyphosate on Lipid Peroxidation and Diet-Derived Antioxidants in Caged Honey Bees (Apis Mellifera) Environ. Sci. Pollut. Res. 2015;22:8010–8021. doi: 10.1007/s11356-014-2879-7. [DOI] [PubMed] [Google Scholar]
- 67.Mao W., Schuler M.A., Berenbaum M.R. Disruption of Quercetin Metabolism by Fungicide Affects Energy Production in Honey Bees (Apis Mellifera) Proc. Natl. Acad. Sci. USA. 2017;114:2538–2543. doi: 10.1073/pnas.1614864114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vandame R., Belzunces L.P. Joint Actions of Deltamethrin and Azole Fungicides on Honey Bee Thermoregulation. Neurosci. Lett. 1998;251:57–60. doi: 10.1016/S0304-3940(98)00494-7. [DOI] [PubMed] [Google Scholar]
- 69.Wade A., Lin C.-H., Kurkul C., Regan E.R., Johnson R.M. Combined Toxicity of Insecticides and Fungicides Applied to California Almond Orchards to Honey Bee Larvae and Adults. Insects. 2019;10:20. doi: 10.3390/insects10010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Forkpah C., Dixon L.R., Fahrbach S.E., Rueppell O. Xenobiotic Effects on Intestinal Stem Cell Proliferation in Adult Honey Bee (Apis Mellifera L) Workers. PLoS ONE. 2014;9:e91180. doi: 10.1371/journal.pone.0091180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson R.T., Dickersin K. Publication Bias against Negative Results from Clinical Trials: Three of the Seven Deadly Sins. Nat. Clin. Pract. Neurol. 2007;3:590–591. doi: 10.1038/ncpneuro0618. [DOI] [PubMed] [Google Scholar]
- 72.Li X., Schuler M.A., Berenbaum M.R. Molecular Mechanisms of Metabolic Resistance to Synthetic and Natural Xenobiotics. Annu. Rev. Entomol. 2007;52:231–253. doi: 10.1146/annurev.ento.51.110104.151104. [DOI] [PubMed] [Google Scholar]
- 73.Feyereisen R. Insect P450 Inhibitors and Insecticides: Challenges and Opportunities. Pest Manag. Sci. 2015;71:793–800. doi: 10.1002/ps.3895. [DOI] [PubMed] [Google Scholar]
- 74.Jones D.G. Piperonyl Butoxide. Elsevier; Amsterdam, The Netherlands: 1998. [Google Scholar]
- 75.Zhu W., Schmehl D.R., Mullin C.A., Frazier J.L. Four Common Pesticides, Their Mixtures and a Formulation Solvent in the Hive Environment Have High Oral Toxicity to Honey Bee Larvae. PLoS ONE. 2014;9:e77547. doi: 10.1371/journal.pone.0077547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cousin M., Silva-Zacarin E., Kretzschmar A., Maataoui M.E., Brunet J.-L., Belzunces L.P. Size Changes in Honey Bee Larvae Oenocytes Induced by Exposure to Paraquat at Very Low Concentrations. PLoS ONE. 2013;8:e65693. doi: 10.1371/journal.pone.0065693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gregorc A., Ellis J.D. Cell Death Localization in Situ in Laboratory Reared Honey Bee (Apis Mellifera L.) Larvae Treated with Pesticides. Pestic. Biochem. Physiol. 2011;99:200–207. doi: 10.1016/j.pestbp.2010.12.005. [DOI] [Google Scholar]
- 78.Mussen E.C., Lopez J.E., Peng C.Y. Effects of Selected Fungicides on Growth and Development of Larval Honey Bees, Apis Mellifera L. (Hymenoptera: Apidae) Environ. Entomol. 2004;33:1151–1154. doi: 10.1603/0046-225X-33.5.1151. [DOI] [Google Scholar]
- 79.Atkins E.L., Kellum D. Comparative Morphogenic and Toxicity Studies on the Effect of Pesticides on Honeybee Brood. J. Apic. Res. 1986;25:242–255. doi: 10.1080/00218839.1986.11100725. [DOI] [Google Scholar]
- 80.Dahlgren L., Johnson R.M., Siegfried B.D., Ellis M.D. Comparative Toxicity of Acaricides to Honey Bee (Hymenoptera: Apidae) Workers and Queens. J. Econ. Entomol. 2012;105:1895–1902. doi: 10.1603/EC12175. [DOI] [PubMed] [Google Scholar]
- 81.Rinkevich F.D., Margotta J.W., Pittman J.M., Danka R.G., Tarver M.R., Ottea J.A., Healy K.B. Genetics, Synergists, and Age Affect Insecticide Sensitivity of the Honey Bee, Apis Mellifera. PLoS ONE. 2015;10:e0139841. doi: 10.1371/journal.pone.0139841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smirle M.J., Winston M.L. Intercolony Variation in Pesticide Detoxification by the Honey Bee (Hymenoptera: Apidae) J. Econ. Entomol. 1987;80:5–8. doi: 10.1093/jee/80.1.5. [DOI] [Google Scholar]
- 83.Wahl O., Ulm K. Influence of Pollen Feeding and Physiological Condition on Pesticide Sensitivity of the Honey Bee Apis Mellifera Carnica. Oecologia. 1983;59:106–128. doi: 10.1007/BF00388082. [DOI] [PubMed] [Google Scholar]
- 84.Liao L.-H., Wu W.-Y., Berenbaum M.R. Impacts of Dietary Phytochemicals in the Presence and Absence of Pesticides on Longevity of Honey Bees (Apis Mellifera) Insects. 2017;8:22. doi: 10.3390/insects8010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mao W., Schuler M.A., Berenbaum M.R. CYP9Q-Mediated Detoxification of Acaricides in the Honey Bee (Apis Mellifera) Proc. Natl. Acad. Sci. USA. 2011;108:12657–12662. doi: 10.1073/pnas.1109535108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Honeybee Genome Sequencing Consortium Insights into Social Insects from the Genome of the Honeybee Apis Mellifera. Nature. 2006;443:931–949. doi: 10.1038/nature05260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Claudianos C., Ranson H., Johnson R.M., Biswas S., Schuler M.A., Berenbaum M.R., Feyereisen R., Oakeshott J.G. A Deficit of Detoxification Enzymes: Pesticide Sensitivity and Environmental Response in the Honeybee. Insect Mol. Biol. 2006;15:615–636. doi: 10.1111/j.1365-2583.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fine J.D., Cox-Foster D.L., Mullin C.A. An Inert Pesticide Adjuvant Synergizes Viral Pathogenicity and Mortality in Honey Bee Larvae. Sci. Rep. 2017;7:40499. doi: 10.1038/srep40499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pettis J.S. A Scientific Note on Varroa Destructor Resistance to Coumaphos in the United States. Apidologie. 2004;35:91–92. doi: 10.1051/apido:2003060. [DOI] [Google Scholar]
- 90.Haarmann T., Spivak M., Weaver D., Weaver B., Glenn T. Effects of Fluvalinate and Coumaphos on Queen Honey Bees (Hymenoptera: Apidae) in Two Commercial Queen Rearing Operations. J. Econ. Entomol. 2002;95:28–35. doi: 10.1603/0022-0493-95.1.28. [DOI] [PubMed] [Google Scholar]
- 91.Berry J.A., Hood W.M., Pietravalle S., Delaplane K.S. Field-Level Sublethal Effects of Approved Bee Hive Chemicals on Honey Bees (Apis Mellifera L) PLoS ONE. 2013;8:e76536. doi: 10.1371/journal.pone.0076536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Frost E.H., Shutler D., Hillier N.K. Effects of Fluvalinate on Honey Bee Learning, Memory, Responsiveness to Sucrose, and Survival. J. Exp. Biol. 2013;216:2931–2938. doi: 10.1242/jeb.086538. [DOI] [PubMed] [Google Scholar]
- 93.Floris I., Satta A., Cabras P., Garau V.L., Angioni A. Comparison between Two Thymol Formulations in the Control of Varroa Destructor: Effectiveness, Persistence, and Residues. J. Econ. Entomol. 2004;97:187–191. doi: 10.1603/0022-0493-97.2.187. [DOI] [PubMed] [Google Scholar]
- 94.Charpentier G., Vidau C., Ferdy J.-B., Tabart J., Vetillard A. Lethal and Sub-lethal Effects of Thymol on Honeybee (Apis Mellifera) Larvae Reared in Vitro. Pest Manag. Sci. 2014;70:140–147. doi: 10.1002/ps.3539. [DOI] [PubMed] [Google Scholar]
- 95.Guzmán-Novoa E., Eccles L., Calvete Y., Mcgowan J., Kelly P.G., Correa-Benítez A. Varroa Destructor Is the Main Culprit for the Death and Reduced Populations of Overwintered Honey Bee (Apis Mellifera) Colonies in Ontario, Canada. Apidologie. 2010;41:443–450. doi: 10.1051/apido/2009076. [DOI] [Google Scholar]
- 96.Le Conte Y., Ellis M., Ritter W. Varroa Mites and Honey Bee Health: Can Varroa Explain Part of the Colony Losses? Apidologie. 2010;41:353–363. doi: 10.1051/apido/2010017. [DOI] [Google Scholar]
- 97.Amdam G.V., Hartfelder K., Norberg K., Hagen A., Omholt S.W. Altered Physiology in Worker Honey Bees (Hymenoptera: Apidae) Infested with the Mite Varroa Destructor (Acari: Varroidae): A Factor in Colony Loss During Overwintering? J. Econ. Entomol. 2004;97:741–747. doi: 10.1603/0022-0493(2004)097[0741:APIWHB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 98.Kralj J., Brockmann A., Fuchs S., Tautz J. The Parasitic Mite Varroa Destructor Affects Non-Associative Learning in Honey Bee Foragers, Apis mellifera L. J. Comp. Physiol. A. 2007;193:363–370. doi: 10.1007/s00359-006-0192-8. [DOI] [PubMed] [Google Scholar]
- 99.Aronstein K.A., Saldivar E., Vega R., Westmiller S., Douglas A.E. How Varroa Parasitism Affects the Immunological and Nutritional Status of the Honey Bee, Apis Mellifera. Insects. 2012;3:601–615. doi: 10.3390/insects3030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dolezal A.G., Carrillo-Tripp J., Miller W.A., Bonning B.C., Toth A.L. Intensively Cultivated Landscape and Varroa Mite Infestation Are Associated with Reduced Honey Bee Nutritional State. PLoS ONE. 2016;11:e0153531. doi: 10.1371/journal.pone.0153531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rosenkranz P., Aumeier P., Ziegelmann B. Biology and Control of Varroa Destructor. J. Invertebr. Pathol. 2010;103:S96–S119. doi: 10.1016/j.jip.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 102.Nazzi F., Le Conte Y. Ecology of Varroa destructor, the Major Ectoparasite of the Western Honey Bee, Apis mellifera. Annu. Rev. Entomol. 2016;61:417–432. doi: 10.1146/annurev-ento-010715-023731. [DOI] [PubMed] [Google Scholar]
- 103.Grozinger C.M., Flenniken M.L. Bee Viruses: Ecology, Pathogenicity, and Impacts. Annu. Rev. Entomol. 2019;64:205–226. doi: 10.1146/annurev-ento-011118-111942. [DOI] [PubMed] [Google Scholar]
- 104.Bonning B.C. The Dicistroviridae: An Emerging Family of Invertebrate Viruses. Virol. Sin. 2009;24:415. doi: 10.1007/s12250-009-3044-1. [DOI] [Google Scholar]
- 105.De Miranda J.R., Cordoni G., Budge G. The Acute Bee Paralysis Virus–Kashmir Bee Virus–Israeli Acute Paralysis Virus Complex. J. Invertebr. Pathol. 2010;103:S30–S47. doi: 10.1016/j.jip.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 106.Maori E., Lavi S., Mozes-Koch R., Gantman Y., Peretz Y., Edelbaum O., Tanne E., Sela I. Isolation and Characterization of Israeli Acute Paralysis Virus, a Dicistrovirus Affecting Honeybees in Israel: Evidence for Diversity Due to Intra- and Inter-Species Recombination. J. Gen. Virol. 2007;88:3428–3438. doi: 10.1099/vir.0.83284-0. [DOI] [PubMed] [Google Scholar]
- 107.Boncristiani H.F., Evans J.D., Chen Y., Pettis J., Murphy C., Lopez D.L., Simone-Finstrom M., Strand M., Tarpy D.R., Rueppell O. In Vitro Infection of Pupae with Israeli Acute Paralysis Virus Suggests Disturbance of Transcriptional Homeostasis in Honey Bees (Apis Mellifera) PLoS ONE. 2013;8:e73429. doi: 10.1371/journal.pone.0073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Miranda J.R., Genersch E. Deformed Wing Virus. J. Invertebr. Pathol. 2010;103(Suppl. 1):S48–S61. doi: 10.1016/j.jip.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 109.Ryabov E.V., Wood G.R., Fannon J.M., Moore J.D., Bull J.C., Chandler D., Mead A., Burroughs N., Evans D.J. A Virulent Strain of Deformed Wing Virus (DWV) of Honeybees (Apis Mellifera) Prevails after Varroa Destructor-Mediated, or In Vitro, Transmission. PLoS Pathog. 2014;10:e1004230. doi: 10.1371/journal.ppat.1004230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yañez O., Chávez-Galarza J., Tellgren-Roth C., Pinto M.A., Neumann P., de Miranda J.R. The Honeybee (Apis Mellifera) Developmental State Shapes the Genetic Composition of the Deformed Wing Virus-A Quasispecies during Serial Transmission. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-62673-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martin S.J., Highfield A.C., Brettell L., Villalobos E.M., Budge G.E., Powell M., Nikaido S., Schroeder D.C. Global Honey Bee Viral Landscape Altered by a Parasitic Mite. Science. 2012;336:1304–1306. doi: 10.1126/science.1220941. [DOI] [PubMed] [Google Scholar]
- 112.Ryabov E.V., Childers A.K., Chen Y., Madella S., Nessa A., van Engelsdorp D., Evans J.D. Recent Spread of Varroa Destructor Virus-1, a Honey Bee Pathogen, in the United States. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-17802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kevill J.L., de Souza F.S., Sharples C., Oliver R., Schroeder D.C., Martin S.J. DWV-A Lethal to Honey Bees (Apis Mellifera): A Colony Level Survey of DWV Variants (A, B, and C) in England, Wales, and 32 States across the US. Viruses. 2019;11:426. doi: 10.3390/v11050426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen Y.P., Pettis J.S., Corona M., Chen W.P., Li C.J., Spivak M., Visscher P.K., DeGrandi-Hoffman G., Boncristiani H., Zhao Y., et al. Israeli Acute Paralysis Virus: Epidemiology, Pathogenesis and Implications for Honey Bee Health. PLoS Pathog. 2014;10:e1004261. doi: 10.1371/journal.ppat.1004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Geffre A.C., Gernat T., Harwood G.P., Jones B.M., Gysi D.M., Hamilton A.R., Bonning B.C., Toth A.L., Robinson G.E., Dolezal A.G. Honey Bee Virus Causes Context-Dependent Changes in Host Social Behavior. Proc. Natl. Acad. Sci. USA. 2020;117:10406–10413. doi: 10.1073/pnas.2002268117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen Y., Evans J., Feldlaufer M. Horizontal and Vertical Transmission of Viruses in the Honey Bee, Apis Mellifera. J. Invertebr. Pathol. 2006;92:152–159. doi: 10.1016/j.jip.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 117.Amiri E., Seddon G., Zuluaga Smith W., Strand M.K., Tarpy D.R., Rueppell O. Israeli Acute Paralysis Virus: Honey Bee Queen–Worker Interaction and Potential Virus Transmission Pathways. Insects. 2019;10:9. doi: 10.3390/insects10010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yue C., Schröder M., Gisder S., Genersch E. Vertical-Transmission Routes for Deformed Wing Virus of Honeybees (Apis Mellifera) J. Gen. Virol. 2007;88:2329–2336. doi: 10.1099/vir.0.83101-0. [DOI] [PubMed] [Google Scholar]
- 119.Levitt A.L., Singh R., Cox-Foster D.L., Rajotte E., Hoover K., Ostiguy N., Holmes E.C. Cross-Species Transmission of Honey Bee Viruses in Associated Arthropods. Virus Res. 2013;176:232–240. doi: 10.1016/j.virusres.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 120.Genersch E., Yue C., Fries I., de Miranda J.R. Detection of Deformed Wing Virus, a Honey Bee Viral Pathogen, in Bumble Bees (Bombus Terrestris and Bombus Pascuorum) with Wing Deformities. J. Invertebr. Pathol. 2006;91:61–63. doi: 10.1016/j.jip.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 121.Fürst M.A., McMahon D.P., Osborne J.L., Paxton R.J., Brown M.J.F. Disease Associations between Honeybees and Bumblebees as a Threat to Wild Pollinators. Nature. 2014;506:364–366. doi: 10.1038/nature12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Flenniken M.L., Andino R. Non-Specific DsRNA-Mediated Antiviral Response in the Honey Bee. PLoS ONE. 2013;8:e77263. doi: 10.1371/journal.pone.0077263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cerutti H., Casas-Mollano J.A. On the Origin and Functions of RNA-Mediated Silencing: From Protists to Man. Curr. Genet. 2006;50:81–99. doi: 10.1007/s00294-006-0078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maori E., Paldi N., Shafir S., Kalev H., Tsur E., Glick E., Sela I. IAPV, a Bee-Affecting Virus Associated with Colony Collapse Disorder Can Be Silenced by DsRNA Ingestion. Insect Mol. Biol. 2009;18:55–60. doi: 10.1111/j.1365-2583.2009.00847.x. [DOI] [PubMed] [Google Scholar]
- 125.Brutscher L.M., Daughenbaugh K.F., Flenniken M.L. Virus and DsRNA-Triggered Transcriptional Responses Reveal Key Components of Honey Bee Antiviral Defense. Sci. Rep. 2017;7:6448. doi: 10.1038/s41598-017-06623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kingsolver M.B., Huang Z., Hardy R.W. Insect Antiviral Innate Immunity: Pathways, Effectors, and Connections. J. Mol. Biol. 2013;425:4921–4936. doi: 10.1016/j.jmb.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Merkling S.H., van Rij R.P. Beyond RNAi: Antiviral Defense Strategies in Drosophila and Mosquito. J. Insect Physiol. 2013;59:159–170. doi: 10.1016/j.jinsphys.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 128.Khush R.S., Leulier F., Lemaitre B. Drosophila Immunity: Two Paths to NF-ΚB. Trends Immunol. 2001;22:260–264. doi: 10.1016/S1471-4906(01)01887-7. [DOI] [PubMed] [Google Scholar]
- 129.Costa A., Jan E., Sarnow P., Schneider D. The Imd Pathway Is Involved in Antiviral Immune Responses in Drosophila. PLoS ONE. 2009;4:e7436. doi: 10.1371/journal.pone.0007436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ferreira Á.G., Naylor H., Esteves S.S., Pais I.S., Martins N.E., Teixeira L. The Toll-Dorsal Pathway Is Required for Resistance to Viral Oral Infection in Drosophila. PLoS Pathog. 2014;10:e1004507. doi: 10.1371/journal.ppat.1004507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zambon R.A., Nandakumar M., Vakharia V.N., Wu L.P. The Toll Pathway Is Important for an Antiviral Response in Drosophila. Proc. Natl. Acad. Sci. USA. 2005;102:7257–7262. doi: 10.1073/pnas.0409181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xi Z., Ramirez J.L., Dimopoulos G. The Aedes Aegypti Toll Pathway Controls Dengue Virus Infection. PLoS Pathog. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shelly S., Lukinova N., Bambina S., Berman A., Cherry S. Autophagy Is an Essential Component of Drosophila Immunity against Vesicular Stomatitis Virus. Immunity. 2009;30:588–598. doi: 10.1016/j.immuni.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lamiable O., Arnold J., de Faria I.J.S., Olmo R.P., Bergami F., Meignin C., Hoffmann J.A., Marques J.T., Imler J.-L. Analysis of the Contribution of Hemocytes and Autophagy to Drosophila Antiviral Immunity. J. Virol. 2016;90:5415–5426. doi: 10.1128/JVI.00238-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McMenamin A.J., Daughenbaugh K.F., Flenniken M.L. The Heat Shock Response in the Western Honey Bee (Apis Mellifera) Is Antiviral. Viruses. 2020;12:245. doi: 10.3390/v12020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Iwasaki S., Kobayashi M., Yoda M., Sakaguchi Y., Katsuma S., Suzuki T., Tomari Y. Hsc70/Hsp90 Chaperone Machinery Mediates ATP-Dependent RISC Loading of Small RNA Duplexes. Mol. Cell. 2010;39:292–299. doi: 10.1016/j.molcel.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 137.Cremer S., Armitage S.A.O., Schmid-Hempel P. Social Immunity. Curr. Biol. 2007;17:R693–R702. doi: 10.1016/j.cub.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 138.Cremer S., Pull C.D., Fürst M.A. Social Immunity: Emergence and Evolution of Colony-Level Disease Protection. Annu. Rev. Entomol. 2018;63:105–123. doi: 10.1146/annurev-ento-020117-043110. [DOI] [PubMed] [Google Scholar]
- 139.López J.H., Schuehly W., Crailsheim K., Riessberger-Gallé U. Trans-Generational Immune Priming in Honeybees. Proc. R. Soc. Lond. B Biol. Sci. 2014;281:20140454. doi: 10.1098/rspb.2014.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Salmela H., Amdam G.V., Freitak D. Transfer of Immunity from Mother to Offspring Is Mediated via Egg-Yolk Protein Vitellogenin. PLoS Pathog. 2015;11:e1005015. doi: 10.1371/journal.ppat.1005015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Harwood G., Amdam G., Freitak D. The Role of Vitellogenin in the Transfer of Immune Elicitors from Gut to Hypopharyngeal Glands in Honey Bees (Apis Mellifera) J. Insect Physiol. 2019;112:90–100. doi: 10.1016/j.jinsphys.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 142.Maori E., Garbian Y., Kunik V., Mozes-Koch R., Malka O., Kalev H., Sabath N., Sela I., Shafir S. A Transmissible RNA Pathway in Honey Bees. Cell Rep. 2019;27:1949–1959. doi: 10.1016/j.celrep.2019.04.073. [DOI] [PubMed] [Google Scholar]
- 143.Coulon M., Schurr F., Martel A.-C., Cougoule N., Bégaud A., Mangoni P., Dalmon A., Alaux C., Le Conte Y., Thiéry R., et al. Metabolisation of Thiamethoxam (a Neonicotinoid Pesticide) and Interaction with the Chronic Bee Paralysis Virus in Honeybees. Pestic. Biochem. Physiol. 2018;144:10–18. doi: 10.1016/j.pestbp.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 144.Coulon M., Schurr F., Martel A.-C., Cougoule N., Begaud A., Mangoni P., Di Prisco G., Dalmon A., Alaux C., Ribiere-Chabert M. Influence of Chronic Exposure to Thiamethoxam and Chronic Bee Paralysis Virus on Winter Honey Bees. PLoS ONE. 2019;14:e0220703. doi: 10.1371/journal.pone.0220703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Diao Q., Li B., Zhao H., Wu Y., Guo R., Dai P., Chen D., Wang Q., Hou C. Enhancement of Chronic Bee Paralysis Virus Levels in Honeybees Acute Exposed to Imidacloprid: A Chinese Case Study. Sci. Total Environ. 2018;630:487–494. doi: 10.1016/j.scitotenv.2018.02.258. [DOI] [PubMed] [Google Scholar]
- 146.Doublet V., Labarussias M., de Miranda J.R., Moritz R.F.A., Paxton R.J. Bees under Stress: Sublethal Doses of a Neonicotinoid Pesticide and Pathogens Interact to Elevate Honey Bee Mortality across the Life Cycle. Environ. Microbiol. 2015;17:969–983. doi: 10.1111/1462-2920.12426. [DOI] [PubMed] [Google Scholar]
- 147.O’Neal S.T., Reeves A.M., Fell R.D., Brewster C.C., Anderson T.D. Chlorothalonil Exposure Alters Virus Susceptibility and Markers of Immunity, Nutrition, and Development in Honey Bees. J. Insect Sci. 2019;19:14. doi: 10.1093/jisesa/iez051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rolke D., Fuchs S., Grünewald B., Gao Z., Blenau W. Large-Scale Monitoring of Effects of Clothianidin-Dressed Oilseed Rape Seeds on Pollinating Insects in Northern Germany: Effects on Honey Bees (Apis Mellifera) Ecotoxicology. 2016;25:1648–1665. doi: 10.1007/s10646-016-1725-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Osterman J., Wintermantel D., Locke B., Jonsson O., Semberg E., Onorati P., Forsgren E., Rosenkranz P., Rahbek-Pedersen T., Bommarco R., et al. Clothianidin Seed-Treatment Has No Detectable Negative Impact on Honeybee Colonies and Their Pathogens. Nat. Commun. 2019;10:1–13. doi: 10.1038/s41467-019-08523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Simon-Delso N., San Martin G., Bruneau E., Minsart L.-A., Mouret C., Hautier L. Honeybee Colony Disorder in Crop Areas: The Role of Pesticides and Viruses. PLoS ONE. 2014;9:e103073. doi: 10.1371/journal.pone.0103073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wintermantel D., Locke B., Andersson G.K.S., Semberg E., Forsgren E., Osterman J., Pedersen T.R., Bommarco R., Smith H.G., Rundlöf M., et al. Field-Level Clothianidin Exposure Affects Bumblebees but Generally Not Their Pathogens. Nat. Commun. 2018;9:1–10. doi: 10.1038/s41467-018-07914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rajak P., Dutta M., Roy S. Altered Differential Hemocyte Count in 3rd Instar Larvae of Drosophila Melanogaster as a Response to Chronic Exposure of Acephate. Interdiscip. Toxicol. 2015;8:84–88. doi: 10.1515/intox-2015-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Walderdorff L., Laval-Gilly P., Wechtler L., Bonnefoy A., Falla-Angel J. Phagocytic Activity of Human Macrophages and Drosophila Hemocytes after Exposure to the Neonicotinoid Imidacloprid. Pestic. Biochem. Physiol. 2019;160:95–101. doi: 10.1016/j.pestbp.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 154.Walderdorff L., Laval-Gilly P., Bonnefoy A., Falla-Angel J. Imidacloprid Intensifies Its Impact on Honeybee and Bumblebee Cellular Immune Response When Challenged with LPS (Lippopolysacharide) of Escherichia Coli. J. Insect Physiol. 2018;108:17–24. doi: 10.1016/j.jinsphys.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 155.Li X., Liu J., Wang X. Exploring the Multilevel Hazards of Thiamethoxam Using Drosophila Melanogaster. J. Hazard. Mater. 2020;384:121419. doi: 10.1016/j.jhazmat.2019.121419. [DOI] [PubMed] [Google Scholar]
- 156.Pellacani C., Costa L.G. Role of Autophagy in Environmental Neurotoxicity. Environ. Pollut. 2018;235:791–805. doi: 10.1016/j.envpol.2017.12.102. [DOI] [PubMed] [Google Scholar]
- 157.Schmehl D.R., Teal P.E.A., Frazier J.L., Grozinger C.M. Genomic Analysis of the Interaction between Pesticide Exposure and Nutrition in Honey Bees (Apis Mellifera) J. Insect Physiol. 2014;71:177–190. doi: 10.1016/j.jinsphys.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 158.Koo J., Son T.-G., Kim S.-Y., Lee K.-Y. Differential Responses of Apis Mellifera Heat Shock Protein Genes to Heat Shock, Flower-Thinning Formulations, and Imidacloprid. J. Asia-Pac. Entomol. 2015;18:583–589. doi: 10.1016/j.aspen.2015.06.011. [DOI] [Google Scholar]
- 159.Reid W.R., Zhang L., Gong Y., Li T., Liu N. Gene Expression Profiles of the Southern House Mosquito Culex Quinquefasciatus during Exposure to Permethrin. Insect Sci. 2018;25:439–453. doi: 10.1111/1744-7917.12438. [DOI] [PubMed] [Google Scholar]
- 160.Zhao L., Alto B.W., Shin D., Yu F. The Effect of Permethrin Resistance on Aedes Aegypti Transcriptome Following Ingestion of Zika Virus Infected Blood. Viruses. 2018;10:470. doi: 10.3390/v10090470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Games P.D., Alves S.N., Katz B.B., Tomich J.M., Serrão J.E. Differential Protein Expression in the Midgut of Culex Quinquefasciatus Mosquitoes Induced by the Insecticide Temephos. Med. Vet. Entomol. 2016;30:253–263. doi: 10.1111/mve.12172. [DOI] [PubMed] [Google Scholar]
- 162.Kawai T., Akira S. Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 163.Daisley B.A., Trinder M., McDowell T.W., Welle H., Dube J.S., Ali S.N., Leong H.S., Sumarah M.W., Reid G. Neonicotinoid-Induced Pathogen Susceptibility Is Mitigated by Lactobacillus Plantarum Immune Stimulation in a Drosophila Melanogaster Model. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-02806-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chmiel J.A., Daisley B.A., Burton J.P., Reid G. Deleterious Effects of Neonicotinoid Pesticides on Drosophila Melanogaster Immune Pathways. mBio. 2019;10:e01395-19. doi: 10.1128/mBio.01395-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Raymann K., Moran N.A. The Role of the Gut Microbiome in Health and Disease of Adult Honey Bee Workers. Curr. Opin. Insect Sci. 2018;26:97–104. doi: 10.1016/j.cois.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dai P., Yan Z., Ma S., Yang Y., Wang Q., Hou C., Wu Y., Liu Y., Diao Q. The Herbicide Glyphosate Negatively Affects Midgut Bacterial Communities and Survival of Honey Bee during Larvae Reared in Vitro. J. Agric. Food Chem. 2018;66:7786–7793. doi: 10.1021/acs.jafc.8b02212. [DOI] [PubMed] [Google Scholar]
- 167.Raymann K., Motta E.V., Girard C., Riddington I.M., Dinser J.A., Moran N.A. Imidacloprid Decreases Honey Bee Survival Rates but Does Not Affect the Gut Microbiome. Appl. Env. Microbiol. 2018;84:e00545-18. doi: 10.1128/AEM.00545-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Swale D.R., Engers D.W., Bollinger S.R., Gross A., Inocente E.A., Days E., Kanga F., Johnson R.M., Yang L., Bloomquist J.R., et al. An Insecticide Resistance-Breaking Mosquitocide Targeting Inward Rectifier Potassium Channels in Vectors of Zika Virus and Malaria. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep36954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Eleftherianos I., Won S., Chtarbanova S., Squiban B., Ocorr K., Bodmer R., Beutler B., Hoffmann J.A., Imler J.-L. ATP-Sensitive Potassium Channel (KATP)–Dependent Regulation of Cardiotropic Viral Infections. Proc. Natl. Acad. Sci. USA. 2011;108:12024–12029. doi: 10.1073/pnas.1108926108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.O’Neal S.T., Swale D.R., Bloomquist J.R., Anderson T.D. ATP-Sensitive Inwardly Rectifying Potassium Channel Modulators Alter Cardiac Function in Honey Bees. J. Insect Physiol. 2017;99:95–100. doi: 10.1016/j.jinsphys.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 171.Brandt A., Gorenflo A., Siede R., Meixner M., Büchler R. The Neonicotinoids Thiacloprid, Imidacloprid, and Clothianidin Affect the Immunocompetence of Honey Bees (Apis Mellifera L.) J. Insect Physiol. 2016;86:40–47. doi: 10.1016/j.jinsphys.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 172.Bitsadze N., Jaronski S., Khasdan V., Abashidze E., Abashidze M., Latchininsky A., Samadashvili D., Sokhadze I., Rippa M., Ishaaya I., et al. Joint Action of Beauveria Bassiana and the Insect Growth Regulators Diflubenzuron and Novaluron, on the Migratory Locust, Locusta Migratoria. J. Pest Sci. 2013;86:293–300. doi: 10.1007/s10340-012-0476-4. [DOI] [Google Scholar]
- 173.Boucias D.G., Nordin G.L. Methoprene: Nucleopolyhedrosis Virus Interactions in Hyphantria Cunea (Drury) J. Kans. Entomol. Soc. 1980;53:56–60. [Google Scholar]
- 174.Furlong M.J., Groden E. Evaluation of Synergistic Interactions Between the Colorado Potato Beetle (Coleoptera: Chrysomelidae) Pathogen Beauveria Bassiana and the Insecticides, Imidacloprid, and Cyromazine. J. Econ. Entomol. 2001;94:344–356. doi: 10.1603/0022-0493-94.2.344. [DOI] [PubMed] [Google Scholar]
- 175.Cutler G.C., Scott-Dupree C.D., Sultan M., McFarlane A.D., Brewer L. A Large-Scale Field Study Examining Effects of Exposure to Clothianidin Seed-Treated Canola on Honey Bee Colony Health, Development, and Overwintering Success. PeerJ. 2014;2:e652. doi: 10.7717/peerj.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Paleolog J., Wilde J., Siuda M., Bąk B., Wójcik Ł., Strachecka A. Imidacloprid Markedly Affects Hemolymph Proteolysis, Biomarkers, DNA Global Methylation, and the Cuticle Proteolytic Layer in Western Honeybees. Apidologie. 2020;51:1–11. doi: 10.1007/s13592-020-00747-4. [DOI] [Google Scholar]
- 177.De Smet L., Hatjina F., Ioannidis P., Hamamtzoglou A., Schoonvaere K., Francis F., Meeus I., Smagghe G., de Graaf D.C. Stress Indicator Gene Expression Profiles, Colony Dynamics and Tissue Development of Honey Bees Exposed to Sub-Lethal Doses of Imidacloprid in Laboratory and Field Experiments. PLoS ONE. 2017;12:e0171529. doi: 10.1371/journal.pone.0171529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Henry M., Cerrutti N., Aupinel P., Decourtye A., Gayrard M., Odoux J.-F., Pissard A., Rüger C., Bretagnolle V. Reconciling Laboratory and Field Assessments of Neonicotinoid Toxicity to Honeybees. Proc. R. Soc. B Biol. Sci. 2015;282:20152110. doi: 10.1098/rspb.2015.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Woodcock B.A., Bullock J.M., Shore R.F., Heard M.S., Pereira M.G., Redhead J., Ridding L., Dean H., Sleep D., Henrys P., et al. Country-Specific Effects of Neonicotinoid Pesticides on Honey Bees and Wild Bees. Science. 2017;356:1393–1395. doi: 10.1126/science.aaa1190. [DOI] [PubMed] [Google Scholar]
- 180.Carleton J. Environmental Fate and Ecological Risk Assessment for Foliar, Soil Drench, and Seed Treatment Uses of the New Insecticide Flupyradifurone (BYI 02960) United States Environmental Protection Agency; Washington, DC, USA: 2014. [Google Scholar]