Abstract
Introduction
Left ventricular assist device (LVAD) implantation has become a well-established treatment option for patients with end stage heart failure (HF) who are refractory to medical therapy. While LVADs implantation does effectively improve hemodynamic performance many patients still possess peripheral pathological adaptations often present in end-stage HF. Therefore, increased attention has been placed on investigating the effects of exercise training for patients with LVADs to improve clinical outcomes. However, the available evidence on exercise training for patients with LVADs is limited.
Areas Covered
The purpose of this narrative review is to summarize: 1) The evolution of LVAD technology and usage; 2) The physiological responses to exercise in patients with LVADs; 3) The available evidence regarding exercise training; 4) Potential strategies to implement exercise training programs for this patient population.
Expert opinion
The available evidence for exercise training to improve physical function and clinical outcomes for patients with LVADs is promising but limited. Future research is needed to further elucidate the ideal exercise training parameters, method of delivery for exercise training, and unique barriers and facilitators to exercise training for patients receiving LVAD implantation.
Keywords: LVAD, heart failure, exercise training, cardiac rehabilitation, exercise testing, left ventricular assistive devices, cardiac transplantation
1.0. Introduction
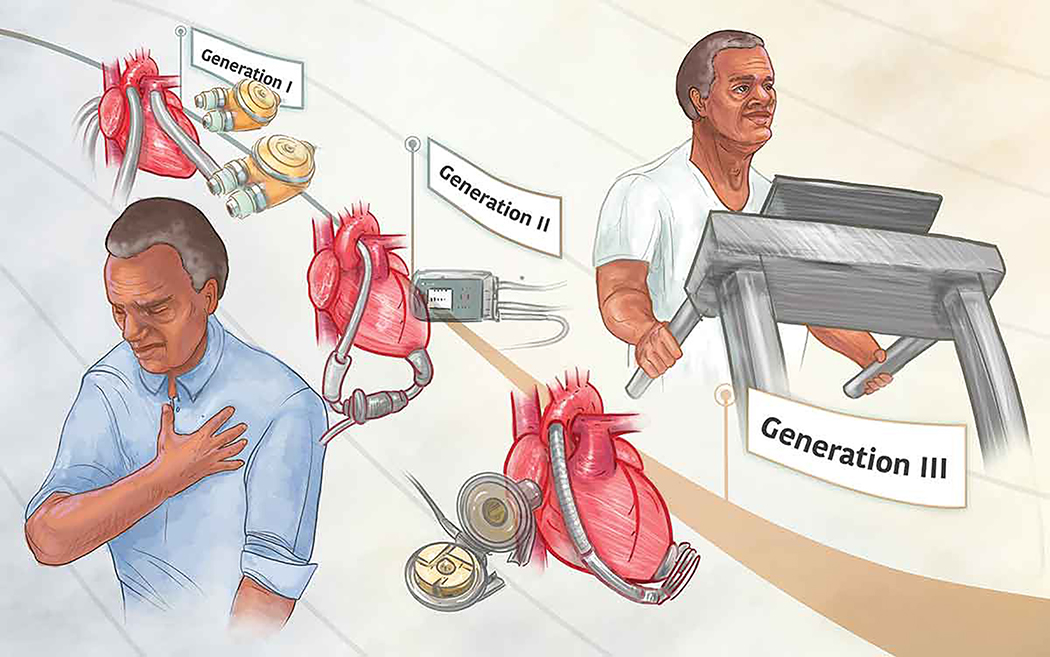
Left ventricular assist device (LVAD) implantation has become a well-established treatment option for patients with end stage heart failure (HF) who are refractory to medical therapy1–3. The usage of LVADs has continued to increase and evolve given that cardiac transplantation, the recommended treatment option for patients with end-stage HF refractory to medical therapy4–6, has a significant disparity between the number of available hearts and eligible candidates6,7. As such LVADs provide patients awaiting a donor heart a bridge to transplantation (BTT) and increased time to become eligible for transplantation1–3,8. As technology has advanced (Figure 1), LVADs have also become a destination therapy (DT) for patients either unwilling to undergo cardiac transplantation or who are deemed inappropriate candidates1–3,8. The number of LVAD implantations continues to increase each year, as well of the number of patients receiving LVADs as a DT8. In North America alone, between June 23, 2006 and December 31, 2017, a total of 20,130 patients have received a LVAD; the majority being continuous flow devices (CF LVAD)8 with over 2,000 are implanted annually8. Between 2012 and 2017, the rate of LVAD implantation as a DT increased from 46% to 73% in patients receiving axial CF LVADs and increased from 0% in 2012 to 27% in patients receiving centrifugal CF LVADs8.
Figure 1.
The evolution of Left Ventricular Assist Devices (LVAD). As LVADs have evolved they have become smaller in size, more reliable, and less restrictive. These advancements in device technology occurring with each generation have allowed patients with LVADs to exercise with greater ease.
The first generation of LVADs utilized either pneumatic or electrically driven membrane pumps to create pulsatile arterial flow3. These early devices were large, heavily instrumented, and had a limited battery life1,3. Patients were often confined to residing in hospitals while utilizing these devices. These factors present in first generation LVADs resulted in significant barriers to patient mobility, independence, and physical exercise, in addition to other complications such as infections and device malfunctions1,3.
Second generation LVADs were developed in the 1990s to address some of the barriers and risks present in first generation devices1,3. These devices used continuous flow pumps, were smaller in size, more durable, and less prone to device malfunction1,3. Second generation LVADs provided patients improved mobility and survival rates1,3. The durability of these devices also permitted a greater possibility to discharge home after implantation and even permitted some the opportunity to return to work1,3. The most often utilized second generation LVAD is the Heartmate II which received Food and Drug Administration (FDA) approval for clinical usage in 2008 and in 2010 as a DT1,3,8.
While the second generation LVADs had greater mechanical longevity and led to longer patient survival, they possessed a significant adverse event risk profile which was at least partially attributed to continuous blood flow interfacing with contact bearings in the pump rotor1,9,10. To address this issue, third generation LVADs were developed which are even smaller in size. The pumps in these devices generate flow using magnetically levitated rotors that rotate without generating friction, and by alternating rotor speeds they can also produce pulsatile flow1,9. The combination of these device characteristics reduced the risk of hemorrhage and thromboembolic complications1.
While peak oxygen consumption (VO2) typically improves following LVAD implantation when compared to pre-implantation values11–13, LVAD recipients still fail to attain age and sex predicted norms for peak VO214 with average values ranging from 11 to 20 mlO2•kg−1•min−1 14. Peak VO2 is one of the strongest independent risk factors for mortality15 and is an important component of the pre-operative assessment for surgery16; these factors support its use as a criterion for determining transplantation candidacy4–6,15,17. Peak VO2 is also strongly associated with survivorship post cardiac transplantation18,19. The prognostic value of peak VO2 on survivorship following LVAD implantation has yet to be determined. This may be due to a limited number of studies which have investigated exercise training in patients following LVAD implantation, the traditional use of LVAD as a BTT with only a recent emergence of LVAD as a DT, and that many patients still demonstrate relatively low values for peak VO2 despite improvements following surgery. Recent work has demonstrated that functional status determined by the New York Heart Association (NYHA) classification is associated with long-term survivorship following LVAD20. A retrospective study by Gosev et al. of 156 patients post LVAD implantation reported that at years 2 years post LVAD implantation 97% were NYHA Class I or II, and 92% were NYHA Class I or II at 4 years20. While NYHA classifications only serve as an approximate surrogate for peak VO2,21,22 the potential relationship between peak VO2 and survivorship following LVAD implantation is promising. While peak VO2 is an important indicator of central function [i.e., cardiac output (CO)], skeletal muscle strength is also an important outcome for this population. In fact, the presence of skeletal muscle weakness in patients with LVADs has been associated with poorer clinical outcomes, health status and quality of life23,24.
Impairments in peak VO2 and skeletal muscle strength observed in patients following LVAD implantation may be attributed to many factors such as device characteristics25–27, skeletal muscle abnormalities11,28,29, endothelial dysfunction30,31, and cardiac abnormalities present in the native heart11,28,29. It is important to acknowledge that LVAD implantation is used for patients with end stage heart failure. Therefore, while LVAD implantation may improve hemodynamic performance, these patients still possess the multitude of well recognized peripheral pathological adaptations present in end stage heart failure. As more patients have survived for longer periods of time utilizing LVADs either as a BTT or DT1,3,8, increased attention has been placed on exercise training to address these peripheral pathological adaptations and improve clinical outcomes24,32,33. However, the available evidence on exercise training in this population while promising is limited24,32–34. Additionally, there is limited available evidence identifying the most effective strategies or models to implement clinical exercise training programs for patients with LVADs24,32,33.
The purpose of this narrative review is to summarize the physiological responses to exercise in patients with LVADs, the available evidence regarding exercise training and to provide potential strategies to implement exercise training programs for this patient population.
2.0. The Physiology of Exercise in Patients with LVAD Support
The expected physiological response of the cardiovascular system to exercise is an increase in CO to match the rise in metabolic demand of the working muscles. This rise is CO is determined by increases in both heart rate (HR) and stroke volume (SV). Contributors to SV include preload, afterload, and contractility. At moderate-intensity exercise levels (i.e., ≈50–60% peak VO2) SV reaches a plateau (except in elite athletes) and the rise in CO is driven solely by increases in HR 35. These increases in CO are achieved through parasympathetic withdrawal and sympathetic activation. Additionally, during exercise, there is a rapid increase in CO to match the metabolic demand and a drop in total peripheral resistance (TPR) due to functional sympatholysis and metabolic autoregulation that lead to vasodilation within working skeletal muscle. These physiological changes should occur without any significant changes in ventricular filling pressures 29. In patients with HF, exercise tolerance is significantly limited due to deviations in normal cardiovascular, respiratory, neural, and muscle physiology 36,37. Cardiovascular limitations in HF include chronotropic incompetence, impaired contractility, increased preload, increased filling pressures, increased afterload and increased vascular resistance, all of which act to reduce CO and exercise tolerance. Patients with LVAD support are able improve hemodynamics and this improvement in turn has beneficial effects on peripheral limitations; however, many of these underlying limitations remain present. Due to differences in physiological responses to exercise in patients following LVAD implantation, clinicians must exercise caution when utilizing evidence to prescribe exercise training interventions from other populations such as healthy patients, and even patients with HF. In the following section we will describe some of the key unique differences in physiological responses to exercise in patients following LVAD implantation.
2.1. Cardiac Output in Patients with LVAD Support
Total cardiac output (TCO) in patients with LVAD support depend on two main components: 1) pump flow; and 2) CO of the natural heart. Pump flow in turn is dependent upon pump speed and the pressure difference across the pump (delta P) which is the pressure difference between the left ventricle (LV) and the aorta. This pressure difference is overcome by work of the LVAD. Increases in differential pressure will require greater work of the LVAD and thus reduced pump flow, while increases in pump speed will assist LVAD work and thus improve pump flow. High levels of delta P may be the result of high systemic pressure (afterload) or low ventricular pressure, or a combination of both 38. Regardless of the exact cause, the absolute pressure difference across the pump is what ultimately determines flow 38, while pump speed routinely remains constant.
Under resting conditions, TCO in patients with LVAD support will rely mainly on pump flow with minimal contributions from the native heart. If LV contraction can generate pressures sufficient enough to overcome systemic arterial pressure, the aortic valve (AV) will open and LV ejection will contribute to TCO 14,39. Brassard et al demonstrated AV opening with maximum exercise in 7 of 8 participants; however, there were no changes in LV dimensions and fractional shortening compared to rest 40. When studying the effects of reducing pump speed on peak VO2, Noor et al demonstrated that patients with LV ejection fractions (LVEF) >40% had minimal reductions in peak VO2 in response to speed reduction while those of LVEF <40% had significant drops 41. These data indicate the important role of native heart contractility in contributing to TCO during exercise in patients with LVAD support.
Given that LVAD speed is usually held constant, changes in afterload will alter LVAD pump flow. Thus, when LV pressure increases during systole there will be a decrease in delta P and an increase in flow. The opposite will occur in diastole. As a result, CF LVAD devices provide continuous, but not constant, blood flow 14.
After LVAD implantation, patients tend to show improvements in HF symptoms, augmentation in cardiac index, improved filling pressures, and improved exercise tolerance. Unexpectedly however, cardiorespiratory fitness measured by peak VO2 pre and post implantation have either shown no or minimal improvement 14,28,29,42,43. When considering submaximal exercise testing measures, such as the 6-minute walk test (6MWT), patients with LVAD support show more consistent improvements 42. Schmidt et al performed cardiopulmonary exercise testing (CPET) and noninvasive CO measurements (inert gas rebreathing method) in 20 patients approximately 6 weeks post-LVAD implantation 44. Differences between measured and predicted CO were assessed at rest, light, moderate, and heavy exercise. There was a significant increase in CO from rest to light exercise, but no further significant increases in CO were detected afterwards.
2.2. Effect of Preload and Afterload on LVAD Flow
Under healthy conditions, the heart is sensitive to increases in preload allowing an appropriate CO adjustment (Frank-Starling relationship), while simultaneously being less sensitive to changes in afterload. Preload and afterload sensitivity refer to the ability of the pump to generate changes in flow according to changes in preload and afterload. Mechanical pumps were found to have about half the sensitivity of the natural heart to preload and three times greater sensitivity to afterload 38,45. Both the decreased preload sensitivity and increased afterload sensitivity my help explain the limitations to maximum exercise observed in these patients.
LV preload is dependent on a multitude of factors including volume status, filling pressures, and right ventricular (RV) function. Right-sided HF was found to be strongly associated with increased morbidity and mortality post-LVAD implant 14. Adverse reactions of the RV due to interventricular ischemia and altered septal geometry in response to LVAD support have been shown 38,46,47. Poor RV function and decreased transpulmonary blood flow relative to LVAD flow will lead to decreased preload. Since LVADs have reduced sensitivity to changes in preload, LVAD pump speed may not adjust to the drop in filling pressure and result in LV emptying and suctioning and therefore reduced pump flows and hypotensive events. The propensity of suction events increases as pump speed increases, especially in patients with RV dysfunction. Previous work has demonstrated improved RV function post-LVAD implant, mainly due to reduced RV afterload 14. Whether RV function poses primary limitations to exercise performance in patients with LVAD support was investigated by Jaski et al, who showed that HF patients with pulsatile flow -LVAD support had decreases in RV dimensions, increases RV filling pressure and RV stroke volume in addition to increases in LV dimensions with exercise suggesting that RV function is not limiting exercise performance 48. Other studies tend to suggest similar conclusions 29, however, further investigation into the role of the RV on exercise performance are required.
In regards to afterload, upon examining the hemodynamic response to exercise in patients with LVAD support, Martina et al reported significant drops in systemic vascular resistance (SVR) that showed the strongest correlation to TCO (r = −0.72, p < .01), further stressing the importance of afterload sensitivity to maximum exercise performance but also indicating appropriate exercise-induced vasodilation in these patients 39.
Afterload during exercise is mainly dependent on peripheral vascular function and the ability of the blood vessels to vasodilate in response to increased metabolic demand. Witman et al demonstrated that flow-mediated dilation (FMD), a surrogate of endothelial-dependent vasodilation, was found to be reduced in patients with LVAD support compared to healthy controls and patients with HF with reduced ejection fraction (HFrEF) classified as NYHA stage II 31. When % FMD was normalized to shear rate, patients with CF-LVAD support had reduced vascular function when compared to patients with HFrEF stages III/IV as well. When comparing nitroglycerin-induced dilation, a surrogate of endothelial-independent vasodilation, there was no significant differences between both groups 31, suggesting reduced endothelial function in patients with CF-LVAD support. This attenuation in endothelial function may be the result of a near lack of pulsatility in CF-LVAD devices and is supported by reduced FMD in patients with CF-LVAD compared to those with pf-LVAD support 30. Interestingly, total vasodilatory capacity (reactive hyperemia – representing both endothelilal-dependent and -independent vasodilation) was not found to be different between patients with LVAD support, patients with HFrEF, and healthy controls and would indicate the absence of microvascular dysfunction 31,49. Further studies on the effect of vascular function on TCO and exercise tolerance in patients with CF-LVAD support are needed.
2.3. Respiratory Changes with Exercise in Patients with LVAD Support
Post-LVAD implant, patients demonstrate improved ventilation-perfusion coupling and ventilatory efficiency evident by reductions in the minute ventilation/carbon dioxide production (VE/VCO2) slope 14,29. Jung et al reported reductions in the VE/VCO2 slope with increasing pump speed during maximal exercise (41 ± 14.9 to 36 ± 11.7, p = 0.005) 26. Apostolo et al also demonstrated that increasing LVAD pump speed improved ventilatory efficiency (−1.9 ± 3.1, p = 0.031) 25. However, further reductions in alveolar-capillary gas diffusion measured by the diffusing capacity of the lungs for carbon monoxide (DLCO) and lung diffusing capacity for nitric oxide (DLNO) 16 hours after changing LVAD speed were also reported 25. This deterioration of lung diffusion was thought to be a consequence of higher left atrial pressures and lung fluid content 25. These findings warrant further caution when suggesting free application of pump speeds during exercise.
3.0. Existing Support for Exercise Training in LVAD Patients
The volume of investigative work examining the effects of exercise in LVAD patients is comparatively smaller than the work that has been completed in medically managed HF patients. However, among the existing retrospective and randomized controlled trials, it is evident that LVAD patients also respond favorably to regular exercise as indicated by increased peak VO2, 6MWT distance, leg strength, decreases in ventilatory inefficiency, and improvements in quality of life24,32,50, whether performed at home50,51 or in a clinical facility24,32,52,53 (Table 1). Given the very invasive nature of LVAD implantation surgery, inpatient therapy is a significant component of the recovery process. Much of the focus in inpatient therapy centers around re-establishing self-care, mobility, home management and device management. Thus, interventions may need to be highly individualized and are greatly dependent on the physical state of the patient. Due to the many complexities associated with inpatient therapy, this section will focus on investigations on home or facility based, outpatient exercise interventions. For information regarding inpatient therapy in patients with an LVAD, the reader is directed to the following paper.54
Table 1.
Exercise training interventions in patients with an LVAD
| Study | Participant Characteristics | Training Protocol | Outcomes | Events |
|---|---|---|---|---|
| Home Based | ||||
| Laoutaris et al. 201150 | LVAD/BiVAD recipients - EG=10, CG=5 patients, 38.3±15.9 yr old - Time since LVAD 6.3±4 months - All participated in an inpatient rehabilitation program (details not available) before discharge |
Exercise Group - Bike or treadmill exercise at home for 45 minutes, moderate intensity (12–14 Borg scale), 3–5 times/wk. - Performed high intensity inspiratory muscle training at 60% of maximal sustained inspiratory muscle strength 2–3 days/week - 10 wk training period Control Group - Advised to walk every day for 30–45 minutes. |
Exercise Group Peak VO2 (ml·kg−1·min−1)* Pre 16.8±3.7; Post 19.3±4.5 VE/VCO2* Pre 40±6.5; Post 35.9±5.6 6MWD (m)* Pre 462±88; Post 527±76 MLwHFQ* Pre 48.9±12.8; Post 38.2±11.6 SPimax (cmH2O/s/103) Pre 340±193; Post 484±195 Control Group Peak VO2 (ml−1·kg−1·min−1) Pre 14.9±4; Post 14.8±4.2 VEVCO2 Pre 41.4±8.1; Post 40.2±7.3 6MWD (m) Pre 430±41; 448±55 MLwHFQ Pre 49.8±9.5; Post 50.8±10.3 SPimax (cmH2O/s/103) Pre 326±140; Post 299±114 |
Not reported |
| Kugler et al. 201251 | 70 LVAD recipients - Intervention group: 52±2 yr old, 85.4% men - Control group: 51±2 yr old, 87.5% men - Recruited 6 weeks post implantation |
Exercise Group (n=34) - Home aerobic training, performed every other day, consisting of 6 minute warm-up, 20 min at 10% less than anaerobic threshold, 2 min cool down - Also received dietary counselling and psychosocial support. - Duration of 18 months Control Group (n=36) - Recommendation for healthy diet and exercise |
Exercise Group - Significant increase in peak workload and SF-36 score Control Group - No significant changes in peak workload or SF-36 score |
Not reported |
| Adamopoulos et al. 201372 | LVAD/BiVAD recipients 11 in the exercise group, 39.7±4.3 yr old, 90.9% men 11 in the control group, 40.9±4.9 yr old, 81.8% men |
Exercise Group - Home-based aerobic training, 4 days/wk, 45 min at an intensity of 12–14 on the Borg scale. - Supervised high-intensity IMT, 3 times/week. - Duration: 12 weeks Control Group advised to walk every day |
Exercise Group Peak VO2 (ml−1·kg−1·min−1)* Pre 12.9±1.2; Post 18.0±0.8 Control Group Peak VO2 (ml−1·kg−1·min−1)* Pre 12.0±0.8; Post 13.7±0.7 |
Not reported |
|
Center Based |
||||
| Hayes et al. et al.32 | - 14 patients who underwent LVAD implantation. - Recruited to the study when they were able to independently walk 70 meters - Randomized at a mean of 32 days after LVAD implantation |
All participants - Mobilization protocol: walk for 60 minutes at an RPE of 13 on the Borg scale, 5 days/wk until discharge. Exercise training protocol (n=7) - 3 days/wk for 8 week - 15 min stationary cycling at 50% of VO2 reserve; 15 min treadmill at a speed 60% of 6MWT walking speed. RPE maintained at 13 on the Borg scale - RE: 3 upper and 3 lower limb exercises, 2 sets of 10 reps Control group (n=7) - Advised to walk 5 days per week |
Exercise Group Peak VO2 (ml·kg−1·min−1)* Pre 10.5±2.3; Post 14.8±4.9 6MWD (m)* Pre 351±77; Post 531±131 SF-36* Pre 30.4±10.7; Post 59.6±24.2 Control Group Peak VO2 (ml·kg−1·min−1)* Pre 12.4±1.7; Post 15.3±4.4 6MWD (m)* Pre 367±129; Post 489±95 SF-36* Pre 36.7±12.2; Post 53.0±6.2 |
No adverse events |
| Kerrigan et al. 201424 | - 24 patients (7 women, 55±13 years old) - 1–6 months post LVAD implantation |
Exercise Training (n=16) - 3 sessions/wk for 6 weeks - 5-min warm up followed by 15 minutes of treadmill exercise and 15 minutes of a secondary modality (i.e., stationary cycle, arm ergometer, recumbent stepper). - 60% of heart rate reserved and encouraged to progress to 80% of heart rate reserve. Control group (n=7) - Continue to follow physician instructions regarding care, including standard recommendation to perform daily walking. |
Exercise Group Peak VO2 (ml·kg−1·min−1)* Pre 13.6±3.3; Post 15.3±4.4 6MWD (m)* Pre 350±64; Post 402±89 KCCQ: significant increase Significant improvement in leg strength Control Group Peak VO2 (ml·kg−1·min−1) Pre 11.2±2.0; Post 11.8±2.0 6MWD (m) Pre 336±59; Post 356±51 KCCQ: no change No change in leg strength |
1 untoward event that required an ED admission due to a syncopal episode occurring immediately following a completed exercise session. 3 other patients in the CR group required ED admission outside of exercise sessions. 4 control patients required ED admission |
| Karapolat et al. 201353 | 11 LVAD recipients - 45.57±14.05 yr old, 85.7% men - 2.8±2.13 months post implantation |
Exercise Training - Site based 8-week intervention, 3 days/wk - 30 min aerobic exercise at 60–70% of VO2max or 12–14 on Borg scale - Strengthening exercises (.25 – .5 kg, upper/lower body, 8 muscle groups) |
Peak VO2 (ml−1·kg−1·min−1)* Pre 14.7±3.6; Post 15.1±3.4 FeV1%* Pre 67.7±30.9; Post 77.9±26.9 FVC%* Pre 72.0±25.3; Post 82.2±22.8 SF-36 Improvements in physical function, pain, vitality, emotional role, mental health |
Not reported |
| Marko et al. 201552 | 41 LVAD recipients - 54.8±11.6 yr old, 80% men - 48±34 days post implantation |
Exercise Training - The exercise intervention lasted on average of 32±6 days - Interval cycle training: 1 min higher intensity, 20 second recovery, gradually increased interval repetitions and intensity across program (see article for details) - Walking: group walking taking place on paths covering different distances, elevations, and times (see article for details) - Coordination, strength and balance training |
Peak VO2 (ml·kg−1·min−1)* Pre 11.3±4.1; Post 14.5±5.2 VE/VCO2* Pre 37.8±7.9; Post 33.7±5.8 Improved leg strength |
1 training-related adverse event (non-sustained ventricular tachycardia) in ∼1,600 training sessions |
CR, cardiac rehabilitation; ED, emergency department; FeV1, forced expiratory volume; FVC, forced vital capacity; IMT, inspiratory muscle training; KCCQ, Kansas City Cardiomyopathy Questionnaire; MLwHFQ, Minnesota Living with Heart Failure Questionnaire; MWD, minute walk distance; MWT, minute walk test; RE, resistance exercise; SF-36, 36 Item Short Form Survey; SPimax, maximal inspiratory pressure; VE/VCO2, ventilation to carbon dioxide ration
significant difference from pre
Among the first randomized trials to investigate the effect of aerobic exercise training in LVAD (pulsatile or continuous flow devices) patients had the training group perform 45 minutes of home based moderate-intensity exercise (12–14 on the Borg scale), 3–5 days per week on a treadmill or bike, in addition to hospital based high-intensity (60% of maximal inspiratory strength) inspiratory muscle training (IMT) 3 times per week for 10 weeks50. This intervention lead to a 2.5 ml•kg−1•min−1 increase in peak VO2 (pre 16.8±3.7 ml•kg−1•min−1, post 19.3±4.5 ml•kg−1•min−1, P<0.01), −4.1 decrease in VE/VCO2 slope (pre 40±6.5, post 35.9±5.6, P<0.01) and a 65m increase in 6MWT distance (pre 462±88m, post 527±76m, P<0.01), in addition to improvements in quality of life50. Whereas the control group (received exercise recommendations) did not experience significant changes in these values. Others have also demonstrated the benefits of home-based exercise on functional status and quality of life (Table 1). In conjunction with routine phone call updates with clinical staff, long term (i.e., 18 months) adherence and continued functional benefits of aerobic exercise training is feasible in this population51. Despite the favorable responses observed in studies implementing home based exercise, the ability to interpret the volume of exercise (product of frequency, intensity, and time of exercise) necessary to accumulate the observed improvements is reduced. Moreover, the inclusion of concurrent therapy such as IMT50 and other health behavior modification interventions51 to the training intervention further complicates the effects of aerobic exercise on cardiorespiratory fitness. Particularly when IMT alone has previously been shown to be an effective intervention that leads to increases in physical function in HF patients55. Despite these limitations, these initial studies provide invaluable evidence supporting the implementation of aerobic exercise in LVAD patients.
In 2014, the Centers of Medicare and Medicaid Services in the United States expanded outpatient phase 2 cardiac rehabilitation to patients with heart failure and a reduced ejection fraction. Therefore, increasing opportunities for LVAD patients to receive supervised, high quality exercise interventions and education aimed at improving fitness, functional status and quality of life. Many investigations implemented center-based exercise interventions typically performed by non-LVAD patients with heart failure. Hayes et al., randomized patients with a continuous flow LVAD to a control or exercise training group, with both groups taking part in an initial daily walking intervention during their hospital stay at a self-perceived moderate intensity32. The control group participants were encouraged to build up to completing 60 minutes of walking daily at the end of their hospital stay whereas patients randomized to the training group performed 15 minutes of stationary bike exercise at 50% of VO2 reserve, 15 minutes of treadmill walking at 60% of the average speed during an initial 6MWT followed by strength training (3 upper and 3 lower limb strengthening exercises using weight machines and free weights, 2 sets of 10 repetitions)32. This was performed three days per week for 8 weeks. Interestingly, both training and control group participants demonstrated similar improvements in peak VO2, peak workload, 6MWT distance, and quality of life. These non-significant differences may have been attributed to only having 7 subjects within each group, but the authors also speculated that the training intensities performed by those in the exercise group may not have been significantly higher than the recommended intensity for those in the control group.
To address study limitations experienced during the early studies on exercise in patients with continuous flow LVAD, Kerrigan et al investigated the effects of applying an exercise model similar to outpatient cardiac rehabilitation24. Those randomized to the exercise intervention completed 18 sessions, performed 3 days per week for 6 weeks. Training consisted of a 5-minute warm-up, mainly treadmill exercise with a secondary modality (i.e., stationary cycle, arm ergometer, or recumbent stepper) for a total of 30 minutes at an intensity of 60% HR reserve with a goal of increasing intensity to 80% of HR reserve. After the 6 week intervention period, patients in the exercise group significantly increased peak VO2 (pre, 13.6±3.3 ml•kg−1•min−1; post, 15.3±4.4 ml•kg−1•min−1), 6MWT distance (pre, 350.1±64.7m; post, 402.4±89.3m), as well as a 17% increase in peak leg torque24. The increase in leg torque occurred even though resistance exercise was not incorporated into the training sessions. This observation is particularly meaningful given that low skeletal muscle mass is a major health concern associated with advanced stage HF. Furthermore, Kerrigan et al.’s study was the first to implement cardiac rehabilitation-based training in LVAD patients and demonstrated that the inclusion of LVAD patients in cardiac rehabilitation (CR) programs may be a valuable component in their continuum of care.
The key exercise intervention studies discussed in this section, collectively provide support for not only the center-based exercise training interventions, but also the efficacy of performing home based exercise. It is important to acknowledge that clinicians should exercise caution when utilizing
4.0. Training Considerations for LVAD Patients
The successful implementation of exercise training in outpatient clinical settings relies on understanding patients’ exercise tolerance56, thresholds at which symptoms generally manifest and the patients’ physical limitations posed by the LVAD11,14. Upon receiving cardiology clearance to initiate exercise, a preliminary physical function test is recommended to not only characterize fitness level, mean arterial blood pressure, flow, heart rate/rhythm responses to exertion, but to also utilize acquired data to guide the exercise prescription. Performing a CPET is safe in patients receiving optimal medical management and is considered the gold standard for fitness assessment15 Exercise intensities can be objectively determined such that patients exercise at the recommended moderate intensity (40–60% of HR reserve) during the initial phases of the program while gradually incorporating vigorous intensity exercise (60–80% of HR reserve) as activity tolerance increases. Many programs, however, may not be able to perform graded exercise tests prior to initiating an exercise training intervention. Therefore, the patient’s rating of perceived exertion representative of a moderate intensity is feasible to promote improvements in fitness50. The use of perceived exertion is especially relevant for patients that may have a significantly lower exercise capacity due to advanced pathophysiology, age, device characteristics, and/or the presence of co-morbidities57. Therefore, the initial duration of exercise may be dependent on the patients’ tolerance of activity and may accordingly need to be performed with intermittent bouts of activity and passive rest; patients can be progressed to continuous exercise at higher intensities as previously demonstrated24. Because there have not been studies to date that investigate the effects of different durations/intensities/volumes of exercise in a LVAD population, we are unable to provide recommendations on the “optimal” dose of exercise that correspond with favorable health outcomes.
Due to the advanced clinical condition of LVAD patients as well as the physical limitations associated with this device, health professionals overseeing the exercise program should be aware of common considerations when working with LVAD patients to ensure a safe exercise environment. Many patients may have difficulty maintaining balance and coordination during the initial phases of carrying the external battery unit57. Therefore, the exercise facility should be clear of unneeded obstacles that may lead to postural changes and falls that could result in disconnection from the LVAD external power supply. Similarly, patients should be oriented to exercise equipment and be mindful of potential hazards that could entangle the lines. Accordingly, driveline stabilization belts are highly recommended to be worn during exercise in order to minimize this risk57. When initiating exercise with LVAD patients’ warm-up/cool-down periods should be gradual and prolonged to provide adequate time for circulatory adjustments to activity. Patients should not perform running, rowing, cross trainer, abdominal exercises, bilateral arms moved above the head with weights, abduction with weights or swimming. Instead, activities that include treadmill, static bike, standing hamstring curls, leg press, bicep curls, core stability, IMT, or arm ergometry can be performed. During the course of exercise, if a patient presents with signs and symptoms indicative of inadequate cardiac or device function (i.e., feeling light headed, shortness of breath, chest pain/pressure, headache) or having the LVAD alarm sound or reading LVAD measures outside the normal parameters for flow, speed and watt operation, exercise should be stopped immediately. These considerations for exercise are certainly not comprehensive and for that reason the reader is directed to a recent European Society of Cardiology Position Statement57.
5.0. Methods of Delivery for Exercise Training in LVAD Patients
Similar to the evidence on the efficacy and safety of exercise training for patients with LVAD, the available evidence for the most effective method of delivery for exercise training is limited but promising. Ideally patients following LVAD implantation would receive structured exercise training through an onsite CR program. Cardiac rehabilitation is indicated both for patients with stable HFrEF and following cardiac transplantation17. Currently, LVAD implantation is not an eligible diagnosis for CR or Medicare coverage for CR. However, since many patients receive a LVAD as treatment for HFrEF they would be eligible for CR under that criteria and possibly others such as stable angina or acute myocardial infarction within the past year. Additionally, most patients with a LVAD would also be eligible for Medicare benefits thus providing health insurance coverage for CR58. However, the gap in CR participation rates found across other populations also appears to be observed in patients following LVAD implantation. A study by Bachmann et al investigated CR participation rates in Medicare beneficiaries following LVAD placement and found that only one-third of LVAD recipients attend CR59. Less than one third of patients who initiated CR following LVAD implantation attended the recommended 36 sessions - the average number of sessions attended was 24.559. The time between discharge and the initial CR session was also much longer in LVAD recipients (median 83 days) when compared to other patients such as those with ischemic heart disease (median 42 days)59. These findings are concerning since CR does appear to provide significant benefits on mortality and hospitalization risk in LVAD patients and to a similar degree as observed in other populations where these effects are well-established24,32,33,59. The benefits of CR on mortality and hospitalizations also appear to follow a dose-dependent response where the more visits a patient attends the greater the effect60,61. While this dose-dependent response for CR has yet to be examined in patients following LVAD implementation it has been demonstrated in patients with ischemic heart disease60,61 which is a frequent concomitant diagnosis in LVAD patients. Therefore, it is not only likely that it is important for patients following LVAD implantation to participate in CR but that they also participate in as many sessions as possible.
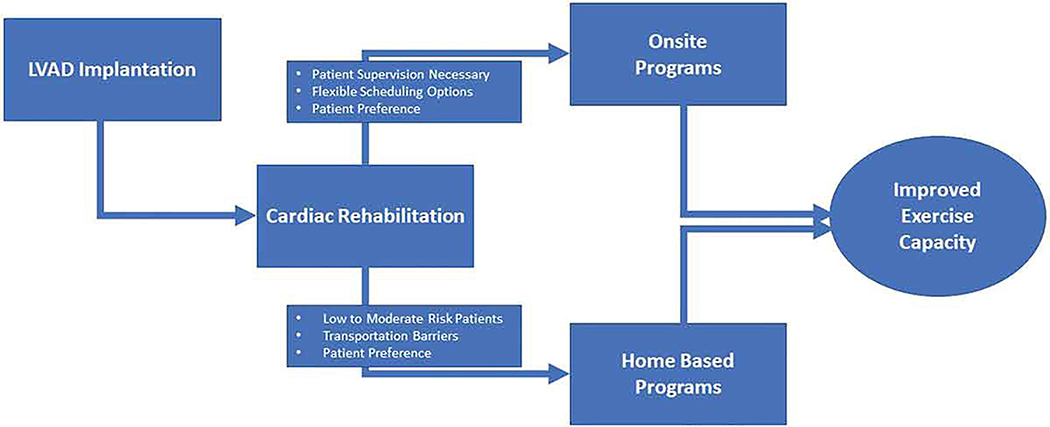
To improve CR participation rates in patients following LVAD implantation additional research will be required to identify the potential barriers and facilitators to participation for this population. Currently, there has been no published research investigating the barriers and facilitators to CR participation in LVAD patients specifically. However, previous research has identified several key barriers to CR participation in other populations which include but are not limited to: 1) transportation difficulties; 2) financial constraints; and 3) program availability56,62. Several strategies have been proposed to address these barriers to CR participation56. One of the more promising approaches to address this lack of participation has been the use of telemedicine for home-based CR63. Utilizing this approach effectively resolves any transportation barriers since the patient would exercise at home while being supervised through a video-conference interface with remote physiological monitoring63. When compared to traditional onsite CR programs, home-based CR programs have been shown to result in similar outcomes, higher participation rates, and lower costs63–65. Home-based CR is typically reserved for low to moderate risk patients56,63–65 and the literature investigating the use of this approach for LVAD patients is limited33. However, the current available evidence on home-based programs for LVAD patients suggests that it is effective and safe with similar adverse event rates when compared to onsite CR33. Telemedicine has also been used for several other aspects of the clinical care for LVAD patients such as monitoring hemodynamics, telemetry, pump parameters and coagulation values66,67. Additionally, providing the patient the option to choose the setting for CR (home-based vs onsite) and offering flexible scheduling options for sessions (early morning, evenings, and weekends) may also improve participation rates56. Therefore, either onsite or home-based supervision should be considered when implementing CR programs for LVAD patients (Figure 2) or perhaps even a combination.
Figure 2.
Methods of delivery for exercise training in LVAD patients.
6.0. Summary and Expert Opinion
Patients with LVADs are a growing clinical population with unique physiological adaptations, precautions, and clinical needs. Left ventricular assist device implantation does effectively improve hemodynamic performance1,3, however, these patients still possess many of the peripheral pathological adaptations often present in end-stage HF11,28. With advances in device technology, patients receiving LVADs are surviving longer and more patients are using LVADs as DT as well as a BTT1,3,8. These devices have become smaller, more reliable, less restrictive and patients are now able to exercise with greater ease, possibly even as outpatients. Additionally, advances in LVAD technology such as pumps with adaptive speed sensors and algorithms coordinated to the intensity of physical activity may improve exercise capacity and potentially the tolerance to exercise training68,69. There has been considerable progress in this technology and devices may be soon become available for clinical use68–71.
The evidence supporting the benefits of exercise training and CR across multiple cardiac conditions is strong and well-established17,56,60,61, however, presently it is limited but promising for patients receiving LVAD implantation. Additionally, the prognostic value of peak VO2 on survivorship outcomes following LVAD implantation remains undetermined. Despite the limited available evidence, CR does appear to be a safe and effective intervention to improve exercise capacity for patients with LVADs. Participation in exercise training and CR programs should be encouraged for this clinical population. Similar to patients with other cardiac conditions, a multimodal CR program utilizing a combination of aerobic exercise, strength training, balance training, and inspiratory muscle training may also be an ideal training approach for patients with LVADs. Additionally, there are also likely unique dietary and psychosocial considerations for LVAD patients with respect to exercise training; which have yet to be investigated in this population. Similar to other clinical populations, CR may also help facilitate positive behavior change towards long term exercise participation upon conclusion of the training program. For those reasons, interdisciplinary CR programs involving physical therapists, exercise physiologists, dietitians, and clinical psychologists should be considered for patients with LVADs to optimize clinical outcomes. The utilization and efficacy of interdisciplinary CR programs for patients with LVADs should also be further investigated.
There appears to be a gap in CR participation rates among LVAD patients which is similar to other cardiac conditions24. However, the barriers and facilitators to CR participation have yet to be investigated in the LVAD population specifically. The barriers to CR participation in LVAD patients may be different due to their unique needs and demographics8. Advancements in mobile and wearable biomedical technology may help address some of these barriers to CR participation in LVAD patients. These technological advances may help improve access to CR services by providing patients the convenience of performing CR at home and reducing costs both for CR facilities and patients. Wearable and mobile technologies also present exciting opportunities to provide maintenance programs for LVAD patients following completion of a supervised CR program. Supervised CR has been shown to improve clinical outcomes, notably peak exercise capacity in LVAD patients24. However, it is imperative that LVAD patients continue to participate in exercise training long-term to maintain peak exercise capacity following CR and potentially further improve. Creating immersive, iterative, and interactive user-interfaces and apps utilizing wearable and mobile technologies might provide the requisite positive user-experience that keeps LVAD patients motivated to participate in exercise training long-term. These technologies may also provide clinicians the ability to objectively monitor patient participation in exercise and detect changes in peak exercise capacity or tolerance, as well as device performance during exercise at follow-up visits.
There are also significant healthcare policy factors affecting the ability to improve exercise capacity in LVAD patients. Most notably, the lack of insurance coverage and eligibility for CR using LVAD implantation as a primary diagnosis58. While healthcare policy decisions are typically not topics of investigation for clinical research, the results from such studies may help influence policy decisions. Future studies investigating exercise training and CR for LVAD patients are not only important to determine safety and efficacy, they will also help improve access to clinical care. As this line of research develops, clinicians and clinical researchers should collaborate with policy makers to facilitate policy changes regarding eligibility and insurance coverage of CR for LVAD patients.
In summary, future research is needed to further elucidate the ideal exercise training parameter, and method of delivery for exercise training in patients following LVAD implantation. Additionally, future research is needed to investigate whether the strong and well-established relationship between peak VO2 on survivorship is maintained in patients following LVAD implantation. Future research should be also be directed towards identifying the unique barriers and facilitators to exercise training for patients receiving LVAD implantation. The use of mobile and wearable technology may provide unique opportunities to address these barriers to CR participation and provide strategies to encourage long term exercise training in LVAD patients. Hopefully future research may also help support policy changes that are more conducive to improving access to exercise training for LVAD patients.
Key Points.
Peak exercise capacity typically improves following LVAD implantation when compared to pre-implantation values, however LVAD recipients often fail to attain age and sex predicted normative values.
The available evidence for exercise training to improve physical function, exercise capacity, and clinical outcomes for patients with LVADs is promising but limited.
Cardiac rehab has been shown to be an effective model for delivering exercise training to improve peak exercise capacity, and other clinical outcomes in LVAD patients.
The prognostic value of peak VO2 on survivorship following LVAD implantation requires further inquiry, as the use of LVAD as a destination therapy continues to increase.
Clinicians and clinical researchers should collaborate with policy makers to identify and develop solutions to barriers regarding cardiac rehab participation by LVAD patients.
Acknowledgments
This review was supported in part by NIH training grant T32-HL139439 (AS)
Footnotes
Disclosures: The authors have nothing to disclose.
References
- 1.Prinzing A, Herold U, Berkefeld A, Krane M, Lange R, Voss B. Left ventricular assist devices-current state and perspectives. J Thorac Dis. 2016;8(8):E660–6. doi: 10.21037/jtd.2016.07.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pozzi M, Giraud R, Tozzi P, et al. Long-term continuous-flow left ventricular assist devices (LVAD) as bridge to heart transplantation. J Thorac Dis. 2015;7(3):532–542. doi: 10.3978/j.issn.2072-1439.2015.01.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez LE, Suarez EE, Loebe M, Bruckner BA. Ventricular assist devices (VAD) therapy: new technology, new hope? Methodist Debakey Cardiovasc J. 2013;9(1):32–37. http://www.ncbi.nlm.nih.gov/pubmed/23519193. Accessed May 6, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crespo-Leiro MG, Metra M, Lund LH, et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20(11):1505–1535. doi: 10.1002/ejhf.1236 [DOI] [PubMed] [Google Scholar]
- 5.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of Amer. J Am Coll Cardiol. 2017;70(6):776–803. doi: 10.1016/j.jacc.2017.04.025 [DOI] [PubMed] [Google Scholar]
- 6.Alraies MC, Eckman P. Adult heart transplant: Indications and outcomes. J Thorac Dis. 2014;6(8):1120–1128. doi: 10.3978/j.issn.2072-1439.2014.06.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers DC, Cherikh WS, Goldfarb SB, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth adult lung and heart-lung transplant report—2018; Focus theme: Multiorgan Transplantation. J Hear Lung Transplant. 2018;37(10):1169–1183. doi: 10.1016/j.healun.2018.07.020 [DOI] [PubMed] [Google Scholar]
- 8.Kormos RL, Cowger J, Pagani FD, et al. The Society of Thoracic Surgeons Intermacs Database Annual Report: Evolving Indications, Outcomes, and Scientific Partnerships. Ann Thorac Surg 2019;107(2):341–353. doi: 10.1016/j.athoracsur.2018.11.011* This annual report of the INTERMACS Database describes current trends in LVAD implantation and outcomes.
- 9.Moazami N, Fukamachi K, Kobayashi M, et al. Axial and centrifugal continuous-flow rotary pumps: A translation from pump mechanics to clinical practice. J Hear Lung Transplant. 2013;32(1):1–11. doi: 10.1016/j.healun.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 10.Kirklin JK, Naftel DC, Pagani FD, et al. Pump thrombosis in the Thoratec HeartMate II device: An update analysis of the INTERMACS Registry. J Hear Lung Transplant. 2015;34(12):1515–1526. doi: 10.1016/j.healun.2015.10.024 [DOI] [PubMed] [Google Scholar]
- 11.Fresiello L, Buys R, Jacobs S, et al. Exercise capacity in left ventricular assist device patients with full and partial support. Eur J Prev Cardiol. 2017;24(2):168–177. doi: 10.1177/2047487316656088 [DOI] [PubMed] [Google Scholar]
- 12.Rogers JG, Aaronson KD, Boyle AJ, et al. Continuous Flow Left Ventricular Assist Device Improves Functional Capacity and Quality of Life of Advanced Heart Failure Patients. J Am Coll Cardiol. 2010;55(17):1826–1834. doi: 10.1016/j.jacc.2009.12.052 [DOI] [PubMed] [Google Scholar]
- 13.Allen JG, Weiss ES, Schaffer JM, et al. Surviving 12 Months After Left Ventricular Assist. Hear Lung. 2011;29(3):278–285. doi: 10.1016/j.healun.2009.07.017.QUALITY [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung MH, Gustafsson F. Exercise in heart failure patients supported with a left ventricular assist device. J Hear Lung Transplant. 2015;34(4):489–496. doi: 10.1016/j.healun.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 15.Ross R, Blair SN, Arena R, et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement from the American Heart Association. Vol 134; 2016. doi: 10.1161/CIR.0000000000000461 [DOI] [PubMed] [Google Scholar]
- 16.Richardson K, Levett DZH, Jack S, Grocott MPW. Fit for surgery? Perspectives on preoperative exercise testing and training. Br J Anaesth. 2017;119:i34–i43. doi: 10.1093/bja/aex393 [DOI] [PubMed] [Google Scholar]
- 17.Thomas RJ, Balady G, Banka G, et al. 2018 ACC/AHA Clinical Performance and Quality Measures for Cardiac Rehabilitation: A Report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2018;71(16):1814–1837. doi: 10.1016/j.jacc.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 18.Yardley M, Gullestad L, Nytrøen K. Importance of physical capacity and the effects of exercise in heart transplant recipients. World J Transplant. 2018;8(1):1–12. doi: 10.5500/wjt.v8.i1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yardley M, Havik OE, Grov I, Relbo A, Gullestad L, Nytrøen K. Peak oxygen uptake and self-reported physical health are strong predictors of long-term survival after heart transplantation. Clin Transplant. 2016;30(2):161–169. doi: 10.1111/ctr.12672 [DOI] [PubMed] [Google Scholar]
- 20.Gosev I, Kiernan MS, Eckman P, et al. Long-Term Survival in Patients Receiving a Continuous-Flow Left Ventricular Assist Device. Ann Thorac Surg 2018;105(3):696–701. doi: 10.1016/j.athoracsur.2017.08.057** This study has provided preliminary data investigating the association between exercise capacity and mortality in patients with LVAD.
- 21.van den Broek SA, van Veldhuisen DJ, de Graeff PA, Landsman ML, Hillege H, Lie KI. Comparison between New York Heart Association classification and peak oxygen consumption in the assessment of functional status and prognosis in patients with mild to moderate chronic congestive heart failure secondary to either ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1992;70(3):359–363. doi: 10.1016/0002-9149(92)90619-a [DOI] [PubMed] [Google Scholar]
- 22.Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 Suppl):S24–30. doi: 10.1016/j.ahj.2009.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerrigan DJ, Williams CT, Ehrman JK, et al. Muscular strength and cardiorespiratory fitness are associated with health status in patients with recently implanted continuous-flow LVADs. J Cardiopulm Rehabil Prev. 2013;33(6):396–400. doi: 10.1097/HCR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 24.Kerrigan DJ, Williams CT, Ehrman JK, et al. Cardiac rehabilitation improves functional capacity and patient-reported health status in patients with continuous-flow left ventricular assist devices: The rehab-vad randomized controlled trial. JACC Hear Fail. 2014;2(6):653–659. doi: 10.1016/j.jchf.2014.06.011** This is one of the first clinical trials to implement a cardiac rehabilitation-based training in LVAD patients.
- 25.Apostolo A, Paolillo S, Contini M, et al. Comprehensive effects of left ventricular assist device speed changes on alveolar gas exchange, sleep ventilatory pattern, and exercise performance. J Hear Lung Transplant. 2018;37(11):1361–1371. doi: 10.1016/j.healun.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 26.Jung MH, Hansen PB, Sander K, et al. Effect of increasing pump speed during exercise on peak oxygen uptake in heart failure patients supported with a continuous-flow left ventricular assist device. A double-blind randomized study. Eur J Heart Fail. 2014;16(4):403–408. doi: 10.1002/ejhf.52 [DOI] [PubMed] [Google Scholar]
- 27.Jung MH, Houston B, Russell SD, Gustafsson F. Pump speed modulations and submaximal exercise tolerance in left ventricular assist device recipients: A double-blind, randomized trial. J Hear Lung Transplant. 2017;36(1):36–41. doi: 10.1016/j.healun.2016.06.020 [DOI] [PubMed] [Google Scholar]
- 28.Rosenbaum AN, Dunlay SM, Pereira NL, et al. Determinants of improvement in cardiopulmonary exercise testing after left ventricular assist device implantation. ASAIO J 2018;64(5):610–615. doi: 10.1097/MAT.0000000000000693 [DOI] [PubMed] [Google Scholar]
- 29.Loyaga-Rendon RY, Plaisance EP, Arena R, Shah K. Exercise physiology, testing, and training in patients supported by a left ventricular assist device. J Hear Lung Transplant. 2015;34(8):1005–1016. doi: 10.1016/j.healun.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 30.Amir O, Radovancevic B, Delgado RM, et al. Peripheral vascular reactivity in patients with pulsatile vs axial flow left ventricular assist device support. J Hear Lung Transplant. 2006;25(4):391–394. doi: 10.1016/j.healun.2005.11.439 [DOI] [PubMed] [Google Scholar]
- 31.Witman MAH, Garten RS, Gifford JR, et al. Further Peripheral Vascular Dysfunction in Heart Failure Patients With a Continuous-Flow Left Ventricular Assist Device: The Role of Pulsatility. JACC Hear Fail. 2015;3(9):703–711. doi: 10.1016/j.jchf.2015.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayes K, Leet AS, Bradley SJ, Holland AE. Effects of exercise training on exercise capacity and quality of life in patients with a left ventricular assist device: A preliminary randomized controlled trial. J Hear Lung Transplant. 2012;31(7):729–734. doi: 10.1016/j.healun.2012.02.021 [DOI] [PubMed] [Google Scholar]
- 33.Ganga H V, Leung A, Jantz J, et al. Supervised exercise training versus usual care in ambulatory patients with left ventricular assist devices: A systematic review. PLoS One. 2017;12(3):1–16. doi: 10.1371/journal.pone.0174323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ben Gal T, Piepoli MF, Corrà U, et al. Exercise programs for LVAD supported patients: A snapshot from the ESC affiliated countries. Int J Cardiol. 2015;201:215–219. doi: 10.1016/j.ijcard.2015.08.081 [DOI] [PubMed] [Google Scholar]
- 35.Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: A scientific statement from the American heart association. Circulation. 2013;128(8):873–934. doi: 10.1161/CIR.0b013e31829b5b44 [DOI] [PubMed] [Google Scholar]
- 36.Piña IL, Apstein CS, Balady GJ, et al. Exercise and heart failure: A statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention. Circulation. 2003;107(8):1210–1225. doi: 10.1161/01.CIR.0000055013.92097.40 [DOI] [PubMed] [Google Scholar]
- 37.Piepoli MF, Crisafulli A. Pathophysiology of human heart failure: Importance of skeletal muscle myopathy and reflexes. Exp Physiol. 2014;99(4):609–615. doi: 10.1113/expphysiol.2013.074310 [DOI] [PubMed] [Google Scholar]
- 38.Lim HS, Howell N, Ranasinghe A. The Physiology of Continuous-Flow Left Ventricular Assist Devices. J Card Fail 2017;23(2):169–180. doi: 10.1016/j.cardfail.2016.10.015 [DOI] [PubMed] [Google Scholar]
- 39.Martina J, Jonge N, Rutten M, et al. Exercise hemodynamics during extended continuous flow left ventricular assist device support: The response of systemic cardiovascular parameters and pump performance. Artif Organs. 2013;37(9):754–762. doi: 10.1111/aor.12151 [DOI] [PubMed] [Google Scholar]
- 40.Brassard P, Jensen AS, Nordsborg N, et al. Central and Peripheral Blood Flow During Exercise With a Continuous-Flow Left Ventricular Assist Device. Circ Hear Fail. 2011;4(5):554–560. doi: 10.1161/circheartfailure.110.958041 [DOI] [PubMed] [Google Scholar]
- 41.Noor MR, Bowles C, Banner NR. Relationship between pump speed and exercise capacity during HeartMate II left ventricular assist device support: Influence of residual left ventricular function. Eur J Heart Fail. 2012;14(6):613–620. doi: 10.1093/eurjhf/hfs042** This study describes the association between pump speed in CF-LVAD devices and exercise capacity, as well as the contributions of the native heart to total cardiac exercise during exercise in patients with CF-LVAD.
- 42.Schmidt T, Bjarnason-Wehrens B, Bartsch P, et al. Exercise Capacity and Functional Performance in Heart Failure Patients Supported by a Left Ventricular Assist Device at Discharge From Inpatient Rehabilitation. Artif Organs. 2018;42(1):22–30. doi: 10.1111/aor.12936 [DOI] [PubMed] [Google Scholar]
- 43.Jakovljevic DG, Yacoub MH, Schueler S, et al. Left Ventricular Assist Device as a Bridge to Recovery for Patients With Advanced Heart Failure. J Am Coll Cardiol. 2017;69(15):1924–1933. doi: 10.1016/j.jacc.2017.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt T, Bjarnason-Wehrens B, Mommertz S, et al. Changes in Total Cardiac Output and Oxygen Extraction During Exercise in Patients Supported With an HVAD Left Ventricular Assist Device. Artif Organs. 2018;42(7):686–694. doi: 10.1111/aor.13102 [DOI] [PubMed] [Google Scholar]
- 45.Salamonsen RF, Mason DG, Ayre PJ. Response of Rotary Blood Pumps to Changes in Preload and Afterload at a Fixed Speed Setting Are Unphysiological When Compared With the Natural Heart. Artif Organs. 2011;35(3). doi: 10.1111/j.1525-1594.2010.01168.x [DOI] [PubMed] [Google Scholar]
- 46.Daly RC, Chandrasekaran K, Cavarocchi NC, Tajik AJ, Schaff H V. Ischemia of the interventricular septum. A mechanism of right ventricular failure during mechanical left ventricular assist. J Thorac Cardiovasc Surg. 1992;103(6):1186–1191. [PubMed] [Google Scholar]
- 47.Moon MR, DeAnda A, Castro LJ, Daughters GT 2nd,, Ingels NBJ, Miller DC. Effects of mechanical left ventricular support on right ventricular diastolic function. J Heart Lung Transplant. 1997;16(4):398–407. [PubMed] [Google Scholar]
- 48.Jaski BE, Kim J, Maly RS, et al. Effects of exercise during long-term support with a left ventricular assist device. Results of the experience with left ventricular assist device with exercise (EVADE) pilot trial. Circulation. 1997;95(10):2401–2406. [DOI] [PubMed] [Google Scholar]
- 49.Lou X, Templeton DL, John R, Dengel DR. Effects of continuous flow left ventricular assist device support on microvascular endothelial function. J Cardiovasc Transl Res. 2012;5(3):345–350. doi: 10.1007/s12265-011-9321-z [DOI] [PubMed] [Google Scholar]
- 50.Laoutaris I d., Dritsas A, Adamopoulos S, et al. Benefits of physical training on exercise capacity, inspiratory muscle function, and quality of life in patients with ventricular assist devices long-term postimplantation. Eur J Prev Cardiol. 2011;18(1):33–40. doi: 10.1097/HJR.0b013e32833c0320 [DOI] [PubMed] [Google Scholar]
- 51.Kugler C, Malehsa D, Schrader E, et al. A multi-modal intervention in management of left ventricular assist device outpatients: Dietary counselling, controlled exercise and psychosocial support. Eur J Cardio-thoracic Surg. 2012;42(6):1026–1032. doi: 10.1093/ejcts/ezs206** This is one of the first clinical trials to investigate the use and effects of a home-based exercise training program in patients with LVAD.
- 52.Marko C, Danzinger G, Käferbäck M, et al. Safety and efficacy of cardiac rehabilitation for patients with continuous flow left ventricular assist devices. Eur J Prev Cardiol. 2015;22(11):1378–1384. doi: 10.1177/2047487314558772 [DOI] [PubMed] [Google Scholar]
- 53.Karapolat H, Engin C, Eroglu M, et al. Efficacy of the cardiac rehabilitation program in patients with end-stage heart failure, heart transplant patients, and left ventricular assist device recipients. Transplant Proc. 2013;45(9):3381–3385. doi: 10.1016/j.transproceed.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 54.Scheiderer R, Belden C, Schwab D, Haney C, Paz J. Exercise Guidelines for Inpatients Following Ventricular Assist Device Placement: A Systematic Review of the Literature. Cardiopulm Phys Ther J. 2013;(June(2)):35–42. doi: 10.1097/01823246-201324020-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cahalin LP, Arena R, Guazzi M, et al. Inspiratory muscle training in heart disease and heart failure: a review of the literature with a focus on method of training and outcomes. Expert Rev Cardiovasc Ther. 2013;11(2):161–177. doi: 10.1586/erc.12.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ades PA, Keteyian SJ, Wright JS, et al. Increasing Cardiac Rehabilitation Participation From 20% to 70%: A Road Map From the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc. 2017;92(2):234–242. doi: 10.1016/j.mayocp.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adamopoulos S, Corrà U, Laoutaris ID, et al. Exercise training in patients with ventricular assist devices: a review of the evidence and practical advice. A position paper from the Committee on Exercise Physiology and Training and the Committee of Advanced Heart Failure of the Heart Failure Associat. Eur J Heart Fail. 2019;21(1):3–13. doi: 10.1002/ejhf.1352* This position statement by the European Society of Cardiology provides a pragmatic guideline on how to implement an exercise training program for patients with LVAD as well as monitoring and safety considerations.
- 58.Social Security Administration. Disability Evaluation Under Social Security (Blue Book - October 2008). https://www.ssa.gov/disability/professionals/bluebook/4.00-Cardiovascular-Adult.htm#4_02. Published 2008. Accessed June 5, 2018.
- 59.Bachmann JM, Duncan MS, Shah AS, et al. Association of Cardiac Rehabilitation With Decreased Hospitalizations and Mortality After Ventricular Assist Device Implantation. JACC Hear Fail 2018;6(2):130–139. doi: 10.1016/j.jchf.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly medicare beneficiaries. Circulation. 2010;121(1):63–70. doi: 10.1161/CIRCULATIONAHA.109.876383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suaya JA, Stason WB, Ades PA, Normand SLT, Shepard DS. Cardiac Rehabilitation and Survival in Older Coronary Patients. J Am Coll Cardiol. 2009;54(1):25–33. doi: 10.1016/j.jacc.2009.01.078 [DOI] [PubMed] [Google Scholar]
- 62.Peters AE, Keeley EC. Trends and predictors of participation in cardiac rehabilitation following acute myocardial infarction: Data from the behavioral risk factor surveillance system. J Am Heart Assoc. 2018;7(1):1–6. doi: 10.1161/JAHA.117.007664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kraal JJ, Elske Van Den Akker-Van Marle M, Abu-Hanna A, Stut W, Peek N, Kemps HMC. Clinical and cost-effectiveness of home-based cardiac rehabilitation compared to conventional, centre-based cardiac rehabilitation: Results of the FIT@Home study. Eur J Prev Cardiol 2017;24(12):1260–1273. doi: 10.1177/2047487317710803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schopfer DW, Krishnamurthi N, Shen H, Duvernoy CS, Forman DE, Whooley MA. Association of Veterans Health Administration Home-Based Programs With Access to and Participation in Cardiac Rehabilitation. JAMA Intern Med. 2018;178(5):715–717. doi: 10.1001/jamainternmed.2017.8039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buckingham SA, Taylor RS, Jolly K, et al. Home-based versus centre-based cardiac rehabilitation: Abridged Cochrane systematic review and meta-analysis. Open Hear 2016;3(2). doi: 10.1136/openhrt-2016-000463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reiss N, Schmidt T, Boeckelmann M, et al. Telemonitoring of left-ventricular assist device patients-Current status and future challenges. J Thorac Dis 2018;10(Suppl 15):S1794–S1801. doi: 10.21037/jtd.2018.01.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lampert BC, Emani S. Remote hemodynamic monitoring for ambulatory left ventricular assist device patients. J Thorac Dis. 2015;7(12):2165–2171. doi: 10.3978/j.issn.2072-1439.2015.10.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmidt T, Reiss N, Deniz E, et al. Adaptive pump speed algorithms to improve exercise capacity in patients supported with a left-ventricular assist device. Stud Health Technol Inform 2017;(236):235–240. doi: 10.3233/978-1-61499-759-7-235 [DOI] [PubMed] [Google Scholar]
- 69.Vignati C, Apostolo A, Cattadori G, et al. Lvad pump speed increase is associated with increased peak exercise cardiac output and vo2, postponed anaerobic threshold and improved ventilatory efficiency. Int J Cardiol 2017;(230):28–32. doi: 10.1016/j.ijcard.2016.12.112 [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Koenig SC, Wu Z, Slaughter MS, Giridharan GA. Sensor-based physiologic control strategy for biventricular support with rotary blood pumps. ASAIO J. 2018;64(2):338–350. doi: 10.1097/MAT.0000000000000671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vollkron M, Schima H, Huber L, Benkowski R, Morello G, Wieselthaler G. Development of a reliable automatic speed control system for rotary blood pumps. J Hear Lung Transplant. 2005;24(11):1878–1885. doi: 10.1016/j.healun.2005.02.004 [DOI] [PubMed] [Google Scholar]
- 72.Adamopoulos S, Gouziouta A, Mantzouratou P, et al. Thyroid hormone signalling is altered in response to physical training in patients with end-stage heart failure and mechanical assist devices: Potential physiological consequences? Interact Cardiovasc Thorac Surg 2013;17(4):664–668. doi: 10.1093/icvts/ivt294 [DOI] [PMC free article] [PubMed] [Google Scholar]