Abstract
Purpose:
We examined direct-to-consumer (DTC) websites for brand-name accelerated approval prescription drugs to determine whether and how accelerated approval is communicated to consumers.
Methods:
From the 34 brand-name prescription drugs under the Food and Drug Administration’s accelerated approval pathway presubmission requirement for promotional materials in December 2016, we identified a sample of 26 that had active DTC websites. Two raters independently coded the websites for the presence, placement, content, and readability of an accelerated approval disclosure.
Results:
Most (73%) of the websites contained an accelerated approval disclosure. Most of the disclosures (84%) included the basis for accelerated approval, whereas 68% stated that the clinical benefit of the product was unknown and 47% conveyed the need for additional research to confirm study findings. On average, the disclosures required at least a high school reading level and most conveyed the information in medical terms.
Conclusions:
DTC websites for brand-name accelerated approval prescription drugs do not consistently communicate the accelerated approval information for the product to consumers in a prominent, comprehensive, or readable manner.
Keywords: FDA, prescription drug, accelerated approval, disclosure, website
Introduction
To allow a faster approval pathway, the Food and Drug Administration (FDA) may grant accelerated approval to a prescription drug product for a serious or life-threatening disease based on surrogate or early clinical endpoints that are likely to predict clinical benefits.1 Accelerated approval can help make prescription drugs available to patients sooner.2 However, until there is confirmatory evidence, there remains some uncertainty as to whether the surrogate endpoints used for accelerated approval will be predictive of clinical benefit.3,4 Another concern is that less information is available with regard to dosing and risk information for accelerated approval products, as confirmatory studies are still ongoing. For instance, in 2013, FDA identified a life-threatening risk affecting more than one-third of patients taking an oncology product that had been granted accelerated approval in 2012.5 The product was temporarily withdrawn from the market and this risk information prompted FDA to revise and narrow the indication for the product, institute a new Risk Evaluation and Mitigation Strategy (REMS), and include new warning information in the product’s labeling. These concerns factor into FDA’s approach to limit accelerated approval to certain products that treat serious or life-threatening conditions as there is often “uncertainty about whether clinical benefit will be verified and the possibility of undiscovered risks.”6
The number of drugs approved under the accelerated approval regulatory pathway has been increasing.2 At the same time, prescription drugs are increasingly marketed online to consumers, including promotion of products indicated for serious or life-threatening medical conditions such as cancer.7–10 Information about the limitations inherent in the accelerated approval pathway may be relevant to patients making treatment decisions. Communicating these limitations in clear and understandable language may help consumers make informed decisions about their medical treatments in consultation with healthcare providers. We investigated whether and how direct-to-consumer (DTC) websites for brand-name accelerated approval products disclose accelerated approval information for the product to consumers.
Methods
We obtained a list of prescription drug products approved under the accelerated approval pathway that were subject to the presubmission requirement for promotional materials in December 2016 from the Office of Prescription Drug Promotion at FDA (n = 34). We searched for websites for each brand-name product, excluding those that (1) had no website, (2) had only a website for healthcare professionals or non-US audiences, or (3) had a website that consisted only of links to FDA-approved labeling such as the Prescribing Information (PI). The final sample consisted of brand-name accelerated approval products with US DTC websites (n = 26). We recorded the product’s indication and the accelerated approval statement from the PI.
Two raters independently searched all the websites and coded whether the websites contained an accelerated approval disclosure for the product (or for the relevant indication if more than one indication was promoted). We considered a website to include an accelerated approval disclosure for the product if it mentioned: (1) accelerated approval status (e.g., “This indication is approved under accelerated approval”), (2) the approval basis - the surrogate or clinical endpoint that supported the accelerated approval (e.g., “These indications are based on overall response rate”), (3) unknown outcomes (e.g., “Currently, no data have shown whether or not [Drug Name] improves disease-related symptoms or survival”), or (4) ongoing or future research (e.g., “There is an ongoing study to find out how [Drug Name] works over a longer period of time”). Inter-rater reliability, calculated with Gwet’s AC1, was high (AC1 = .94).11–12
Two raters then independently coded the disclosures for placement, content, and readability. The raters first randomly selected five disclosures to code, calculated inter-rater reliability, and refined the coding scheme. The raters then coded the remaining disclosures. All coding discrepancies were resolved through discussion between the two raters. For placement, the raters coded whether the accelerated approval disclosure was presented with the indication (AC1 = .88) and whether it was also presented along with the largest, most central claim on the website (AC1 = .87). For content, the raters coded whether the disclosure mentioned accelerated approval status (AC1 = 1.0), approval basis (AC1 = .93), unknown outcomes (AC1 = .75), and ongoing or future research (AC1 = 1.0). Finally, for readability, the raters coded whether the website used the exact language from the PI (which is generally not written in consumer-friendly language; AC1 = .80), whether the disclosure used at least some consumer-friendly language (for example, “patients’ tumors became smaller;” AC1 = .79), whether the disclosure included medical terms in any part (for example, “progression-free survival;” AC1 = .83), and whether any medical terms were defined (for example, using the terms “levels of iron in the blood” to communicate “serum ferritin levels;” AC1 = 1.0). For a more in-depth look at certain medical terms, the raters also coded whether the disclosure included “response/response rate” or “progression-free survival” (AC1 = 1.0). The codes were not mutually exclusive. We used Microsoft Word to determine (1) the disclosure length (number of words) and (2) the reading level (Flesch-Kincaid grade level and Flesch reading ease).
We conducted two supplementary analyses to determine (1) how many promotional pieces for accelerated approval products were internet-based and (2) whether the websites used the same accelerated approval disclosure as non-internet-based promotional pieces. We examined non-internet-based promotional pieces submitted to FDA under Form 2253 in the fourth quarter of 2016 for the sample of 34 products we previously identified as subject to the presubmission requirement for promotional materials. For products that had a website with an accelerated approval disclosure, we recorded whether the non-internet-based promotional pieces included the disclosure and whether it was the same, in whole or in part, as the website disclosure.
Results
Of the 26 accelerated approval drug websites in our sample, 77% (20/26) promoted oncology-related products. The remainder was spread among hematology (n = 2), gastroenterology (n = 2), cardiology (n = 1), and obstetrics (n = 1). Of the 26 accelerated approval drug websites in our sample, 73% (19/26) contained a disclosure communicating information related to the product’s accelerated approval (Figure 1). The seven websites without an accelerated approval disclosure were all for oncology prescription drugs.
Figure 1.
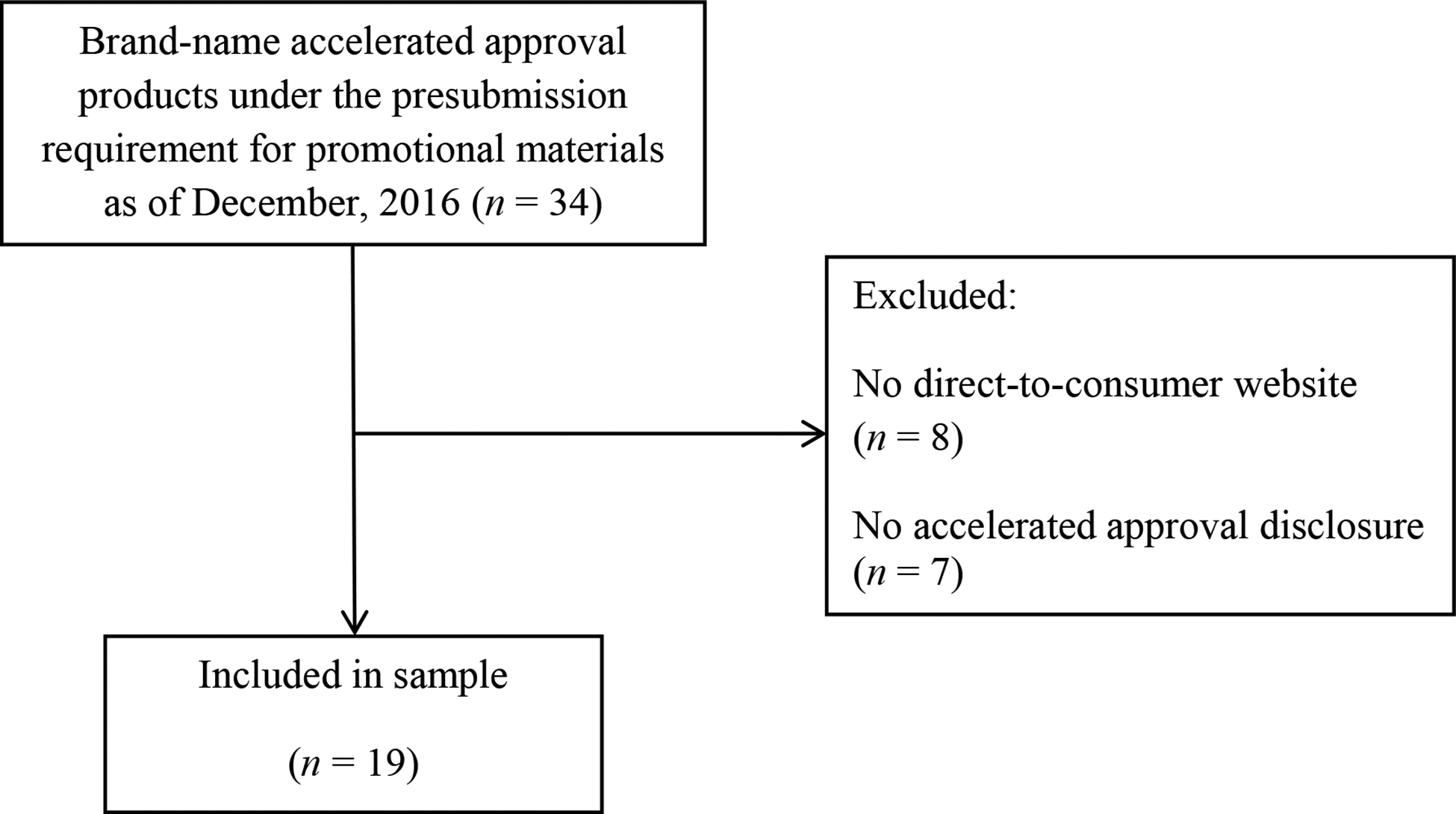
Content analysis sample.
All the accelerated approval disclosures (100%; 19/19) were presented in conjunction with the indication (Table 1). Only 10% (2/19) were also presented with the largest, most central claim. Only one disclosure (5%; 1/19) mentioned the accelerated approval status. Most (84%; 16/19) mentioned the approval basis, 68% (13/19) mentioned unknown outcomes, and 47% (9/19) mentioned ongoing or future research. Most (84%; 16/19) mentioned only one or two of these elements.
Table 1.
Characteristics of accelerated approval disclosures on direct-to-consumer websites for brand-name prescription drugs (n = 19).
| n | % | |
|---|---|---|
| Presented with the indication | 19 | 100 |
| Presented with largest, most central claim | 2 | 10 |
| Used exact language from the Prescribing Information | 4 | 21 |
| Elements mentioned | ||
| Accelerated approval status | 1 | 5 |
| Approval basis | 16 | 84 |
| Unknown outcomes | 13 | 68 |
| Ongoing or future research | 9 | 47 |
| Used consumer-friendly language | 10 | 53 |
| Used medical terms | 15 | 79 |
| Defined medical terms | 2 | 10 |
| Mean | Standard deviation | |
| Flesch-Kincaid grade level | 11.6 | 2.1 |
| Flesch reading ease test (0–100, higher = easier to read) | 39.0 | 16.0 |
| Number of words | 34.5 | 13.1 |
The disclosures had an average of 34.5 words (SD = 13.1, range = 14–62). Some of the accelerated approval disclosures (21%; 4/19) used language directly from the PI. We coded 53% (10/19) of the accelerated approval disclosures as using at least some consumer-friendly language. However, most (79%; 15/19) also used medical terms, with only 10% (2/19) including an explanation of the medical terms. For instance, of the 16 disclosures that mentioned the approval basis, 10 (62%) used the terms “response,” “response rate,” or “progression-free survival” to explain the approval basis without defining these medical terms. Moreover, the average Flesch-Kincaid grade level was 11.6 (SD = 2.1, range = 6.7–16.0) and the average Flesch reading ease test was 39.0 (SD = 16.0, range = 5.3–67.7). Thus, the accelerated approval disclosures, on average, required at least a high school reading level.
In the fourth quarter of 2016, 53% (n = 330) of the total promotional materials (n = 624) submitted to FDA on Form 2253 for our original list of accelerated approval products (n = 34) were internet-based. Of the 19 products that had DTC websites with accelerated approval disclosures, 13 had non-internet-based promotional pieces submitted during this time-frame (categorized as print ad, promotional label, direct mail, sales aid, detail aid, or exhibit), for a total of 144 pieces. We randomly selected 25% of the pieces for each product, for a sample of 37 non-internet-based pieces. Most (n = 20; 54%) did not include the accelerated approval disclosure because the pieces did not include the accelerated approval indication (e.g., a patient brochure for another indication, a co-pay card with no indication stated). Five pieces (13%) – all for the same product – were caregiver and patient testimonial scripts that did not include the accelerated approval disclosure. Eight pieces (22%) included the same disclosure as the website. Four pieces (11%) included a disclosure that overlapped with the website disclosure, but was either shorter, longer, or slightly different (e.g., “is approved on response” versus “is approved based on response”).
Discussion
We examined DTC websites for brand-name accelerated approval prescription drugs to determine whether and how information about accelerated approval for the product is communicated to consumers. Most, but not all, of the websites (73%) included a disclosure related to the product’s accelerated approval. Of the websites that included it, readability was low for the disclosures, and few presented the disclosure with the largest, most central claim. This suggests that even when a disclosure is included, it may be presented in a way that could cause consumers to overlook it. In addition, the omission of important context may impact consumers’ ability to understand the potential risks and benefits of these products. For instance, consumers viewing promotional materials for an oncology product approved under accelerated approval are unlikely to understand the limitations of what is known about the product if it is not explicitly stated that there are no data yet available to confirm clinical benefit (e.g., data on improved survival).
Content analysis can give a snapshot of how information is communicated; however, it cannot measure consumers’ exposure to this information or their comprehension of it. Qualitative and quantitative research is needed to determine whether patients read and understand these disclosures and whether the disclosures change consumers’ perceptions of the products or influence consumers’ decisions. Future research should examine the wording of disclosures and include comprehension testing of such language. This could help support clear recommendations on these important aspects of accelerated approval disclosures. This content analysis was also limited to websites. We found that roughly half of the promotional pieces for these products were internet-based. Examining accelerated approval status disclosures in other media, such as patient brochures, could also be informative. However, we note that when pharmaceutical companies include an accelerated approval disclosure in their DTC materials, they often use the same or similar disclosure across different types of DTC materials. For example, in the analyzed sample of non-internet-based DTC promotional materials, all materials that included an accelerated approval disclosure used the same (67%) or similar (33%) language as the product website.
The accelerated approval pathway allows prescription drugs intended to treat serious or life-threatening medical conditions to get to patients more quickly, but as this approval standard is based on surrogate or early clinical endpoints, there may be residual uncertainties about the risks and benefits of these products. While accelerated approval is a technical regulatory term, communicating the concept and the limitations of this approval may benefit patients who are considering expensive and physically costly treatments for serious or life-threatening medical conditions. Our findings here can help inform stakeholders and policymakers as they consider the most effective way to communicate this important information to consumers.
Bulleted ‘take-home’ messages, or key points:
Of a sample of 26 websites promoting brand-name accelerated approval prescription drugs to consumers, 19 (73%) contained a disclosure about accelerated approval.
Most disclosures explained accelerated approval using medical terms.
Disclosures often did not convey certain aspects of accelerated approval, such as the lack of confirmation of clinical benefit and the need for additional research. This may significantly impact consumers’ ability to understand the potential risks and benefits of these products.
Footnotes
This work has not been presented or posted previously.
Conflict of Interest Statement: The authors are employees of the US Food and Drug Administration. They have no conflicts of interest to report.
References
- 1.Food Federal, Drug & Cosmetic Act § 506(c), 21 U.S.C § 356 2017. [Google Scholar]
- 2.Beaver JA, Howie LJ, Pelosof L, Kim T, Liu J, Goldberg KB, Sridhara R, Blumenthal GM, Farrell AT, Keegan P, Pazdur R. A 25-year experience of US Food and Drug Administration accelerated approval of malignant hematology and oncology drugs and biologics: A review. JAMA Oncology 2018; doi: 10.1001/jamaoncol.2017.5618 [DOI] [PubMed] [Google Scholar]
- 3.Prasad V, Kim C, Burotto M, Vandross A. The strength of association between surrogate end points and survival in oncology: a systematic review of trial-level meta-analyses. JAMA Intern Med. 2015; 175(8): 1389–98. [DOI] [PubMed] [Google Scholar]
- 4.Naci H, Smalley KR, Kesselheim AS. Characteristics of preapproval and postapproval studies for drugs granted accelerated approval by the US Food and Drug Administration. JAMA 2017; 318(7): 626–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gainor JF, Chabner BA. Ponatinib: accelerated disapproval. Oncologist 2015; 20(8): 847–848. DOI: 10.1634/theoncologist.2015-0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Food and Drug Administration. Guidance for industry: Expedited programs for serious conditions—drugs and biologics 2014; Available at https://www.fda.gov/downloads/Drugs/Guidances/UCM358301.pdf
- 7.Kornfield R, Donohue J, Berndt ER, et al. Promotion of prescription drugs to consumers and providers, 2001–2010. PLoS One 2013; 8(3): e55504 10.1371/journal.pone.0055504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackey TK, Cuomo RE, Liang BA. The rise of digital direct-to-consumer advertising?: comparison of direct-to-consumer advertising expenditure trends from publicly available data sources and global policy implications. BMC Health Services Research 2015; 15(1): 1 DOI: 10.1186/s12913-015-0885-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan HW, Aikin KJ, Chung-Davies E, et al. Prescription drug promotion from 2001–2014: data from the U.S. Food and Drug Administration. PLoS One 2016; 11(5):e0155035 10.1371/journal.pone.0155035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan HW, Aikin KJ, Squiers LB. Quantitative information on oncology prescription drug websites. J Cancer Educ 2018; 33: 371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gwet K Kappa statistic is not satisfactory for assessing the extent of agreement between raters. Statistical Methods for Inter-Rater Reliability Assessment 2002; 1(6): 1–6. [Google Scholar]
- 12.Wongpakaran N, Wongpakaran T, Wedding D, Gwet KL. A comparison of Cohen’s Kappa and Gwet’s AC1 when calculating inter-rater reliability coefficients: a study conducted with personality disorder samples. BMC Med Res Methodol 2013; 13(1): 61. [DOI] [PMC free article] [PubMed] [Google Scholar]


