Abstract
Several imaging studies have attempted to characterize the contribution of glutamatergic dysfunction to functional dysconnectivity of large-scale brain networks using ketamine models. However, findings from BOLD imaging studies are conflicting, in part because the signal stems from a complex interaction between blood flow, blood volume, and oxygen consumption.
We used arterial spin labelling imaging to measure regional cerebral blood flow (rCBF) in a group of healthy volunteers during a saline and during a ketamine infusion. We examined changes in rCBF and interregional connectivity patterns, as well as their associations with clinical symptom severity and Glx (glutamate+glutamine) assessed with magnetic resonance spectroscopy.
We report a regionally selective pattern of rCBF changes following ketamine administration and complex changes in interregional connectivity patterns. We also found that the increase in rCBF in the bilateral putamen and left hippocampus was positively correlated with ketamine induced clinical symptom severity while anterior cingulate rCBF during the ketamine challenge was negatively correlated with change in hippocampal Glx.
Our study adds to the efforts to empirically confirm putative links between an NMDA receptor blockage and dysconnectivity of large-scale brain networks, specifically the salience, executive control and default mode networks, suggesting that a glutamatergic imbalance may contribute to dysconnectivity. Development of glutamatergic compounds that alleviate disease burden, possibly through normalizing glutamate excess related increased rCBF, is direly needed.
INTRODUCTION
Schizophrenia now is conceptualized as a dysconnectivity disorder, where the synchronization of functional brain networks is disrupted (Friston et al., 2016; Friston and Frith, 1995; Pettersson-Yeo et al., 2011). A number of imaging studies have attempted to characterize the contribution of glutamatergic dysfunction to functional dysconnectivity using ketamine, a non-competitive N-methyl-d-aspartate (NMDA) receptor blocker that transiently induces a behavioural phenotype similar to that seen in the illness (Kraguljac et al., 2018; Lahti et al., 1995; Lahti et al., 2001b; Parwani et al., 2005; Weiler et al., 2000), without long term adverse effects (Lahti et al., 2001a). These studies often use blood oxygen level dependent signal (BOLD) imaging during a resting state which have yielded inconsistent results (Driesen et al., 2013; Grimm et al., 2015; Hoflich et al., 2015; Scheidegger et al., 2012; Wong et al., 2016).
Discrepancies in findings may at least in part be attributable to intrinsic properties of the BOLD contrast, which measures a complex signal related to neural activity (Detre and Wang, 2002; Kwong et al., 1992; Ogawa et al., 1993). In contrast, arterial spin labelling (ASL) measures regional cerebral blood flow (rCBF), a single physiological parameter that is temporally stable and relatively straightforward to interpret (Stewart et al., 2014). Thus, ASL is ideally suited to study the acute effects of psychoactive compounds on neuronal activity.
Studies investigating effects of ketamine on rCBF, both with PET and ASL imaging, consistently report increases in rCBF in prefrontal, orbitofrontal, and cingulate cortices (Holcomb et al., 2005; Holcomb et al., 2001) as well as subcortical regions including the thalamus, caudate, and putamen (Bojesen et al., 2018; Langsjo et al., 2003; Pollak et al., 2015), but a decrease in rCBF in the hippocampus (Pollak et al., 2015) and cerebellum (Holcomb et al., 2001) has also been reported. These are all areas of the brain commonly implicated in the schizophrenia pathophysiology (Lahti et al., 2006; Medoff et al., 2001) and antipsychotic drug action (Bolding et al., 2012; Hadley et al., 2014; Lahti et al., 2009; Lahti et al., 2005). Regionally selective patterns of ketamine-related blood flow changes suggest that experimentally induced NMDA receptor hypofunction may affect neuronal activity on a network level. Network level changes in ASL imaging can be characterized by assessing interregional connectivity patterns of rCBF across subjects, a proxy of brain connectivity.
Here, we used ASL imaging in a group of healthy controls during a saline infusion and during a ketamine infusion to assess the effects of experimentally induced NMDA receptor hypofunction on rCBF patterns and interregional connectivity patterns. We hypothesized that ketamine would be associated with an increase of rCBF in the prefrontal, orbitofrontal, and cingulate cortices, thalamus and putamen as well as altered interregional connectivity. Because we have recently reported an association between hippocampal Glx (glutamate+glutamine) measured with magnetic resonance spectroscopy (MRS) and changes in resting state functional connectivity during a ketamine challenge in the same subjects (Kraguljac et al., 2017), and because glutamatergic neurotransmission plays a key role in the regulation of cerebral blood flow, we also chose to probe associations between rCBF and the extent of ketamine induced glutamate dysfunction (i.e. increase in hippocampus Glx). Additionally, we conducted exploratory analyses investigating the relationships between changes in rCBF and psychosis severity.
METHODS
Subjects
We recruited 19 healthy volunteers who gave written informed consent for this University of Alabama at Birmingham Institutional Review Board approved study. Experiments were performed in accordance with relevant guidelines/ regulations. Exclusion criteria were a history of an Axis I disorder or a psychotic disorder in a first-degree family member, significant medical or neurological conditions (including hypertension requiring prescription of blood pressure medications), lifetime use of psychotropic medications, prior exposure to ketamine, and pregnancy or breastfeeding.
Subjects meeting eligibility criteria during a phone screen were invited to complete a Diagnostic Interview for Genetic Studies and a psychiatric assessment and physical exam conducted by a board certified psychiatrist (NVK). Urine drug screens and, if applicable, pregnancy tests were completed during the screen and before each ketamine infusion. The Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1980) and Young Mania Rating Scale (YMRS) (Young et al., 1978) were used to assess mood symptoms before each infusion. The Clinician Administered Dissociative States Scale (CADSS) (Bremner et al., 1998) and the Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham, 1962) were completed before and after the ketamine challenge (subjects were asked to retrospectively report symptoms at the time drug effects were the most prominent).
To ensure drug tolerability and minimize novelty, subjects received an intravenous racemic ketamine challenge (0.27mg/kg bolus over 10 minutes, followed by a continuous infusion of 0.25mg/kg/hour for 50 minutes) in the Clinical Research Unit at least one week prior to imaging. Ten milliliters of blood were collected immediately after completion of the bolus and 50 minutes after start of the challenge during the drug tolerability assessment rather than scanning to avoid potential delays in our scanning timeline due to complications during the blood draws. Blood samples were centrifuged to obtain plasma and stored at −40°C. Ketamine plasma levels were assayed (Nathan Klein Institute) using a liquid chromatographic procedure. During the ketamine challenge, vital signs including heart rate, blood pressure, peripheral oxygen saturation, and respiratory rate (CO2 monitoring during scanning) were monitored by an anaesthesiology fellow under supervision of a board certified anaesthesiologist according to the standards for basic anaesthetic monitoring. Monitoring was continued for one hour after infusion completion. Prior to discharge into the care of an accompanying driver, subjects were medically cleared by the fellow and psychiatrist.
Two subjects withdrew from the study because they developed emesis, and two subjects revoked consent after the initial ketamine challenge, citing time constraints as their reason (both denied adverse drug effects); 15 subjects completed imaging.
To limit effects of nicotine intoxication or withdrawal, subjects were allowed, but not encouraged, to smoke up to one hour prior to image acquisition. Following anatomical, spectroscopy, resting state fMRI (for spectroscopy and resting state fMRI results see (Kraguljac et al., 2017)) and ASL scans during a saline infusion (flow rate of 0.01ml/s), subjects were given a short break. After repeat anatomical scans, subjects received a ketamine challenge consisting of a bolus (0.27mg/kg over 10 minutes), followed by a continuous infusion (0.25mg/kg/hour, flow rate of 0.01ml/s). ASL acquisition was started approximately 65 minutes after start of the ketamine challenge.
Imaging Parameters
Imaging was performed on a 3T head-only scanner (Magnetom Allegra, Siemens) with a circularly polarized transmit/receive head coil. A high-resolution structural scan was acquired to aid anatomical localization and image registration (MPRAGE, TR/TE/TI=2300/3.93/1100msec, 1mm isotropic voxels).
We acquired a 2D pCASL scan (TR/ TE= 5230/ 53ms, excitation flip angle= 90°, in-plane resolution= 3.8×3.8mm2, matrix= 64×64, Bandwidth 3004 Hz/ Px, 24 slices, slice thickness= 5mm, label time 1s, delay time 1s, labeling offset 8cm (Chen et al., 2012), total scan time 5 minutes 24 seconds) with 30 pairs of labeled and unlabeled images. Subjects were instructed to keep their eyes open during the scan.
A spectroscopic voxel was placed in the left hippocampus (2.7×1.5×1cm). Manual shimming was performed to optimize field homogeneity across the voxel. We used the CHESS technique for water suppression. Spectra were acquired using a PRESS sequence (TR/TE= 2000/80ms to optimize the glutamate signal (Schubert et al., 2004) and minimize macromolecule contribution; 1200 Hz spectral bandwidth; 1024 points; 640 averages, and 8 averages without water suppression). Voxel placement for the second acquisition was guided by an image of the voxel placement during the first scan.
pCASL data processing
Raw pCASL scans were skull stripped with FSL BET (Smith, 2002). We performed motion correction in two steps: (1) unlabeled and labeled images were aligned and motion corrected separately with as described by Wang et al. (Wang et al., 2008) (2) DVARS based motion correction was applied as described in Tanenbaum et al. (Tanenbaum et al., 2015).
Functional images were registered to the high resolution structural T1 scan and smoothed with a 3mm gaussian kernel. We calculated rCBF in ml/min/100g as described in (Wang et al., 2008) for the whole brain. To transform images to MNI space, functional images were segmented to create a DARTEL template (Ashburner, 2007), which was used for non-linear registration such that the value of the voxel rather than the size was preserved. Prior to statistical analyses, we applied a gray matter mask including subcortical areas resulting from segmentation to the images, as gray matter rCBF was our focus of interest.
MRS data processing
As previously reported (Kraguljac et al., 2017; Kraguljac et al., 2019), MRS data were quantified in the time domain with AMARES (Vanhamme et al., 1997) in jMRUI (version 5.2). We included prior knowledge form in vitro and in vivo metabolite spectra in the model. No spectra exceeded a (1) line width of the magnitude signal during manual shimming >20 Hz at FWHM and (2) Cramer-Rao lower bounds (CRLB) >20%, which were our exclusion criteria. Glutamate+glutamine (Glx) was quantified with respect to creatine. Structural scans were segmented into grey matter (GM), white matter (WM), and cerebrospinal fluid to calculate voxel tissue fractions.
Statistical analyses
Statistics were done in SPM12 for image analyses and in SPSS for other calculations. To investigate change in blood flow between saline and ketamine infusions, we used paired t-tests to compare ketamine and saline conditions and accounted for multiple comparisons with family wise error correction (FWE), pFWE< 0.05.
As a post hoc analysis, we examined interregional connectivity patterns across subjects using Interregional Correlation Analyses (Azari et al., 1992). We extracted rCBF values from the regions relevant to ketamine action both during the saline and ketamine condition using FSLmaths. Regions of interest (ROIs) were defined as the conjunction of areas of the brain that showed statistically significant changes in rCBF during the ketamine challenge and anatomical ROIs per the Harvard-oxford structural atlas (http://neuro.debian.net/pkgs/fsl-harvard-oxford-atlases.html) using FSLeyes (Table 1). Interregional connectivity between two ROIs was defined as the partial correlation between rCBF values across subjects using whole brain rCBF as a covariate (Horwitz and Rapoport, 1988). This was calculated separately for saline and ketamine conditions. ROIs showing correlated patterns of rCBF were considered to be connected. In order to test whether the interregional correlations were significantly different between saline and ketamine conditions, Fisher’s z transformation was applied to convert the correlation coefficients to z values. A z statistic was used to determine the significance of the between-condition differences in interregional correlations.
Table 1:
Regions of interest (ROI)
| Region of interest | MNI Coordinates1 | Number of voxels | ||
|---|---|---|---|---|
| x | y | z | ||
| Anterior cingulate cortex | 5 | 32 | 22 | 540 |
| Posterior cingulate cortex | −1 | −27 | 33 | 1035 |
| Paracingulate cortex | 1 | 29 | 34 | 2343 |
| Frontal pole | 8 | 56 | 10 | 2244 |
| Dorsolateral prefrontal cortex L | −47 | 17 | 21 | 2610 |
| Dorsolateral prefrontal cortex R | 49 | 18 | 18 | 3465 |
| Amygdala L | −22 | −4 | −18 | 359 |
| Amygdala R | 21 | −3 | −18 | 350 |
| Hippocampus L | −28 | −15 | −21 | 262 |
| Hippocampus R | 21 | −7 | −25 | 36 |
| Insula L | −32 | 22 | 0 | 141 |
| Insula R | 33 | 22 | 0 | 104 |
| Pallidum L | −20 | −5 | −2 | 465 |
| Pallidum R | 21 | −6 | −2 | 301 |
| Putamen L | −26 | −1 | 0 | 1015 |
| Putamen R | 26 | 2 | −1 | 1040 |
| Thalamus L | −13 | −19 | 7 | 256 |
| Thalamus R | 15 | −20 | 5 | 231 |
Abbreviations: L, Left; R, Right
Area of peak activation
In an exploratory fashion, we also investigated the relationship between changes in rCBF, Glx and clinical variables using Pearson’s correlation coefficients.
RESULTS
Demographics, clinical observations, and laboratory results
Fifteen subjects (10 male/ 5 female) aged 24.80+/− 3.49 years completed scanning. One subject smoked 3 cigarettes per day; all others denied smoking. None had HDRS or YMRS scores in the clinical range. BPRS and CADSS scores were significantly higher during the ketamine challenge compared to the saline infusion (Table 2). Ketamine plasma levels were 74.27+/− 22.08ng/ml and 97.47+/− 19.59ng/ml immediately after completion of the bolus and 50 minutes after start of infusion, respectively.
Table 2:
Clinical measures1, cerebral blood flow, image quality measures
| Saline | Ketamine | t-score | p value | |
|---|---|---|---|---|
| Clinical measures | ||||
| BPRS2 | ||||
| Total | 20.60 (0.74) | 32.73 (4.94) | −9.742 | < .01 |
| Positive | 3.00 (0.00) | 5.87 (1.69) | −6.590 | < .01 |
| Negative | 3.13 (0.35) | 6.87 (1.96) | −7.047 | < .01 |
| CADSS | ||||
| Total score | 0.07 (0.02) | 13.60 (6.50) | −8.049 | < .01 |
| Amnesia | 0.07 (0.02) | 2.07 (1.71) | −4.472 | < .01 |
| Derealization | 0.00 (0.00) | 7.27 (3.92) | −7.183 | < .01 |
| Depersonalization | 0.00 (0.00) | 3.47 (2.10) | −6.394 | < .01 |
| Confusion | 0.00 (0.00) | 0.13 (3.52) | −1.468 | .16 |
| Cerebral blood flow3 | ||||
| Whole brain | 39.50 (13.48) | 60.06 (11.85) | −7.202 | < .01 |
| Gray matter | 69.03 (25.64) | 119.20 (25.30) | −8.789 | < .01 |
| Image quality measures | ||||
| Mean framewise displacement | 0.32 (0.13) | 0.30 (0.21) | 0.224 | .83 |
| DVARS | 19.70 (2.45) | 15.22 (3.02) | 4.268 | < .01 |
Abbreviations: BPRS Brief Psychiatric Rating Scale; CADSS Clinician Administered Dissociative States Scale; HRSD Hamilton Rating Scale for Depression; YMRS Young Mania Rating Scale
Mean (SD) unless indicated otherwise, n= 15
Brief Psychiatric Rating Scale (1 – 7 scale); positive (conceptual disorganization, hallucinatory behavior, and unusual thought content); negative (emotional withdrawal, motor retardation, and blunted affect)
measured in ml/min/100g
Cerebral blood flow
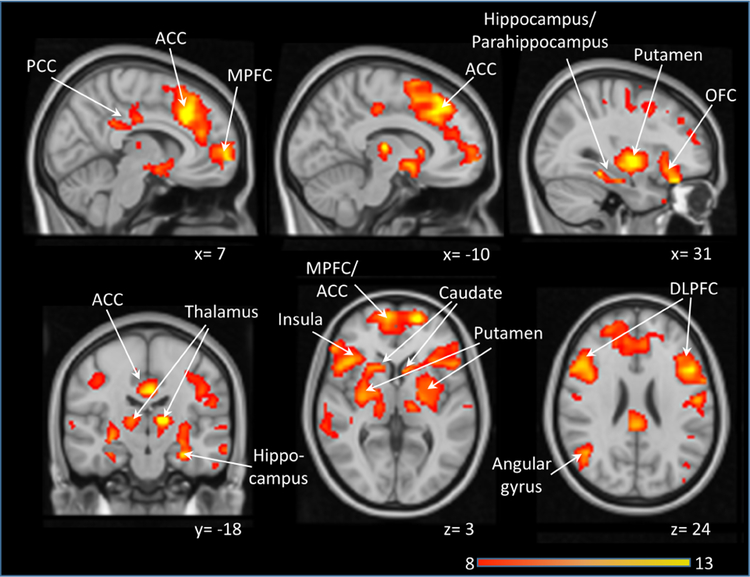
During the ketamine infusion, both whole brain and gray matter rCBF were significantly increased (Table). Voxelwise analysis comparing rCBF during a saline infusion and ketamine challenge demonstrated regionally selective increases in cortical areas including the medial prefrontal cortex, hippocampus, posterior cingulate cortex and angular gyrus (areas of the default mode network), the ACC and insula (areas of the salience network), the dorsolateral prefrontal cortex (a region of the executive control network), the orbitofrontal cortex and amygdala, as well as subcortical areas including the caudate, putamen, and thalamus (Figure 1). We found no areas with significantly decreased rCBF during the ketamine challenge compared to the saline infusion.
Figure 1:
Areas of increased regional cerebral blood flow (rCBF) during a ketamine challenge compared with a saline infusion (pFWE < 0.05). No areas showed a decrease in blood flow. Significant clusters were overlaid on a single subject T1 template. Numbers indicate Montreal Neurological Institute (MNI) coordinates. Color bar indicates t values. Abbreviations: ACC: Anterior cingulate cortex; DLPFC: dorsolateral prefrontal cortex; MPFC: Medial prefrontal cortex; OFC: orbitofrontal cortex; PCC: posterior cingulate cortex.
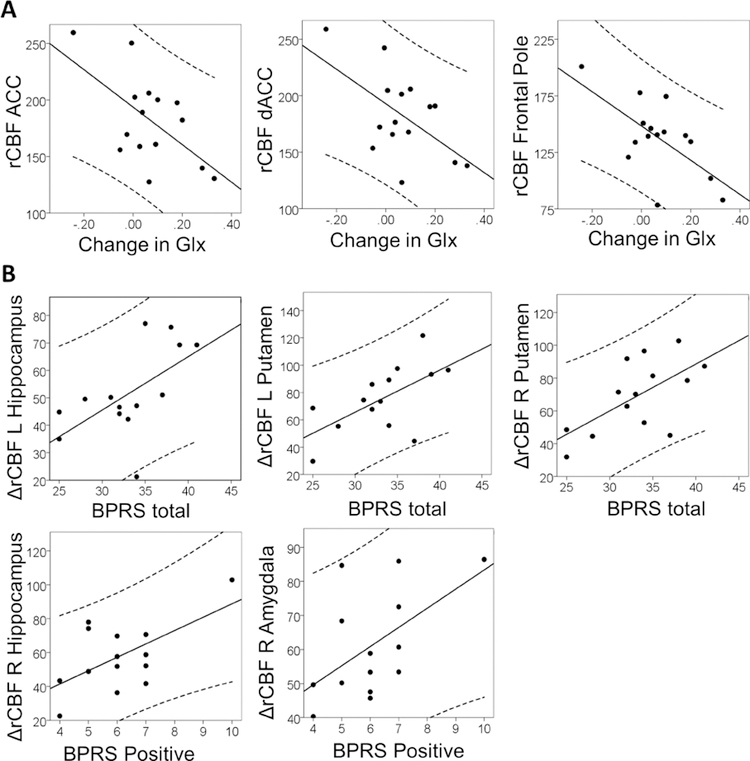
As previously reported, the signal to noise ratio (saline: 12.57+/− 1.76; ketamine 11.89+/− 1.85; p= 0.23) and full with at half maximum (saline: 7.42+/−1.32; ketamine 7.63+/− 1.46; p= 0.50), did not differ between spectroscopy acquisitions. rCBF in the ACC and frontal pole during the ketamine challenge negatively correlated with change in hippocampus Glx (r= − 0.60; p = 0.02 and r= −0.64; p= 0.01 respectively; Figure 2A), whereas the increase in BPRS total scores was correlated with change in rCBF in the bilateral putamen (left: r= 0.62; p= .02 and right: r= 0.63; p= .02) and left hippocampus (r= 0.59; p= .03) (Figure 2B).
Figure 2:
Top row. Relationship between regional cerebral blood flow (rCBF, measured in ml/min/100g) during the ketamine challenge and change in hippocampal Glx (glutamate+glutamine). Bottom row. Relationship between change in rCBF during the ketamine challenge and clinical symptom severity.
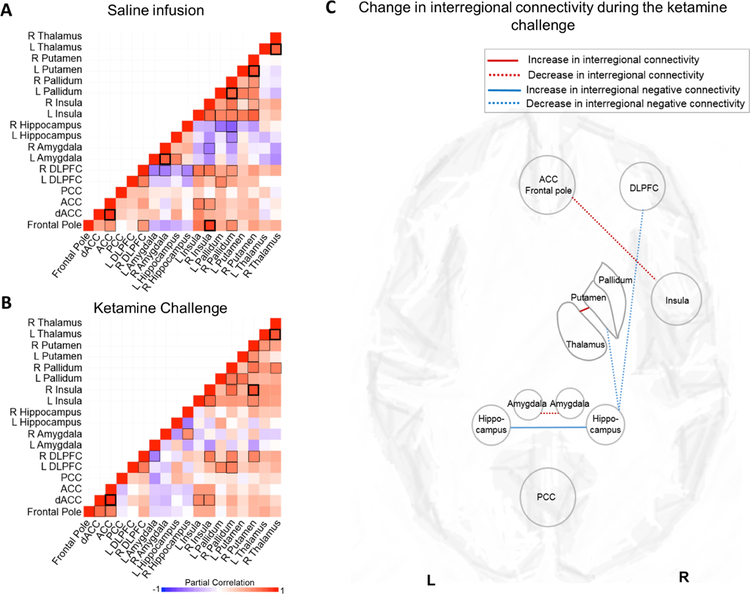
Post hoc interregional connectivity analyses revealed a complex pattern of ketamine related rCBF changes (Figure 3, note that due to the small sample size, a number of significant correlations in rCBF patterns across brain areas did not survive Benjamini-Hochberg corrections for multiple comparisons). Positive correlations between the ACC/ frontal pole and insula as well as the bilateral amygdala decreased, and positive correlations between the thalamus and putamen increased, while negative correlations between the hippocampus and the DLPFC as well as the putamen decreased, and correlations between the bilateral hippocampi became negative (Figure 3).
Figure 3:
Interregional connectivity maps. Plots show partial correlations in regional cerebral blood flow (rCBF, measured in ml/min/100g) of areas relevant to ketamine drug action, controlled for global CBF. A. Interregional connectivity maps during a saline infusion. Plots show partial correlations in regional cerebral blood flow (rCBF, measured in ml/min/100g) in the regions of interest. Positive correlations are depicted in red, negative correlations are depicted in purple. Back squares indicate significant correlations (p < .05), bold black squares indicate correlations that remain significant after Benjamini-Hochberg correction for multiple comparisons. B. Interregional connectivity maps during a ketamine challenge. Plots show partial correlations in regional cerebral blood flow (rCBF, measured in ml/min/100g) in the regions of interest. Positive correlations are depicted in red, negative correlations are depicted in purple. Back squares indicate significant correlations (p < .05), bold black squares indicate correlations that remain significant after Benjamini-Hochberg correction for multiple comparisons. C. Schematic depicting changes in interregional connectivity during a ketamine infusion compared to a saline infusion between regions of interest. Lines depict significant changes in interregional connectivity. Abbreviations: ACC: Anterior cingulate cortex; DLPFC: dorsolateral prefrontal cortex; PCC: posterior cingulate cortex; L: left; R: right
DISCUSSION
In this multimodal neuroimaging study of experimentally induced NMDA receptor hypofunction, we report a regionally selective pattern of rCBF changes following ketamine administration. Specifically, rCBF increased in the prefrontal, orbitofrontal and cingulate cortices as well as the insula, angular gyrus, caudate, putamen, thalamus and hippocampus. No areas show a decrease in rCBF. Interregional connectivity analyses of these regions demonstrate a complex pattern of ketamine related changes in interrelationships of rCBF patterns between brain regions across subjects. We further find that the increase in rCBF in the bilateral putamen and left hippocampus is positively correlated with ketamine induced clinical symptom severity while ACC rCBF during the ketamine challenge is negatively correlated with change in hippocampal Glx.
Following ketamine administration, we report increased rCBF in a number of cortical and subcortical brain regions that is largely consistent with prior reports in the literature. Spatial patterns echo the functional anatomy of large-scale brain networks, specifically the default mode network (medial prefrontal cortex, posterior cingulate cortex, angular gyrus, and hippocampus), the salience network (ACC and insula) and the cognitive control network (dorsolateral prefrontal cortex), as well as the motor loop, all which have been reported to be dysfunctional in patients with schizophrenia (Baker et al., 2014; Hadley et al., 2014; Kraguljac et al., 2016; Menon, 2011; Palaniyappan et al., 2013; Walther et al., 2017). To better capture the complexity of NMDA receptor hypofunction effects at the brain network level, we chose to complement our analyses with an exploration of changes in interregional connectivity. Here, we find decreased interregional connectivity within the salience network, which is consistent with a recent resting state fMRI study that showed a decrease in salience network functional connectivity during a ketamine infusion (Mueller et al., 2018). We also report a decrease in negative interregional connectivity between the hippocampus and dorsolateral prefrontal cortex, suggesting a possible ketamine induced imbalance between the default mode and cognitive control networks at rest. This NMDA receptor hypofunction related disruption in anticorrelated large-scale functional networks has previously been demonstrated during a working memory task (Anticevic et al., 2012). The same hyperconnectivity between the hippocampus and dorsolateral prefrontal cortex has also been shown in schizophrenia patients while performing a working memory task (Wolf et al., 2009). Taken together, findings lend further empirical support to the putative link between NMDA receptor hypofunction and large-scale functional brain network dysconnectivity. Similarly, recent reports from our group suggest that alterations in the relationship between cortical glutamate and task related BOLD response is disrupting the dynamics of major neural networks, both in first episode psychosis patients (Overbeek et al., 2018) and unmedicated patients with schizophrenia (Cadena et al., 2018). At the subcortical level, we unexpectedly discovered a ketamine related increase in interregional connectivity between the thalamus and putamen. Interestingly, these structures are linked by glutamatergic projections (Alexander et al., 1990) and are part of the cortico-striatal-thalamic circuits that regulate motor behavior, reward processing, and higher order cognitive processes. Disruption of these circuits have been reported in patients with schizophrenia (Bernard et al., 2017; Lottman et al., 2017; Siegel et al., 1993; Woodward et al., 2012), but to our knowledge, no studies to date have directly examined the contribution of glutamate excess to cortico-striatal-thalamic dysconnectivity in the illness.
We did not discover areas of rCBF reductions that have been reported in some (Holcomb et al., 2001; Pollak et al., 2015), but not all prior studies. This could in part be explained by our experimental design. Pollack and colleagues report dose-related decreases in hippocampal rCBF, where the lower dose of ketamine (target serum level of 50–75ng/ml) showed a decrease in hippocampus rCBF but not the higher dose (target serum level of 150ng/ml) (Pollak et al., 2015). Furthermore, ASL imaging in our study took place approximately 65 minutes after the bolus was started. It is possible that we were outside the time window capturing an initial decrease in rCBF, as the rCBF response to ketamine has been shown to be dynamic (Bojesen et al., 2018; Holcomb et al., 2001). Similarly, rCBF studies in schizophrenia are somewhat inconsistent in the reports of rCBF reductions vs elevations. Speculatively, the ketamine model may better replicate the early stages of the illness and/ or those who are not currently exposed to antipsychotic medications, as these may reduce/ normalize rCBF.
It is interesting that we found a dissociation between the areas of the brain where rCBF changes mapped onto clinical symptom severity and areas that showed a linear relationship with hippocampal glutamate excess. Several studies reported that ketamine decreases dopamine receptor availability in the striatum providing experimental confirmation that NMDA receptor hypofunction affects dopamine function (Breier et al., 1998; Smith et al., 1998; Vollenweider et al., 2000). Notably, the degree of decrease in striatal dopamine receptor availability was correlated with clinical symptom severity (Breier et al., 1998; Vollenweider et al., 2000), which is consistent with our finding of an association between the extent of increase in rCBF in the bilateral putamen and symptom severity. The only other area of the brain where we found this association was the hippocampus. This regional specificity in rCBF increase related to clinical symptom severity fits well with a pathophysiological model of schizophrenia positing that dysregulation of glutamatergic transmission in the hippocampus (Lieberman et al., 2018) results in downstream subcortical elevation of dopamine function (Lodge and Grace, 2011). On the other hand, we did not replicate a correlation between rCBF in the ACC and clinical symptom severity, which has been found in many (Breier et al., 1997; Holcomb et al., 2005; Holcomb et al., 2001; Vollenweider et al., 1997), but not all (Bojesen et al., 2018; Pollak et al., 2015), previous ketamine studies, and in drug free patients with schizophrenia (Lahti et al., 2006). Instead, we did discover a negative relationship between increase in hippocampus Glx and rCBF in the frontal pole and ACC during the ketamine challenge. The only other study concurrently examining ketamine induced rCBF changes and glutamate metabolism in humans found a relationship between rCBF and glutamate levels in the ACC before and immediately after ketamine administration, albeit without a corresponding increase in ACC glutamatergic metabolites (Bojesen et al., 2018) that would be expected based on prior studies (Rowland et al., 2005; Stone et al., 2012). In a preclinical model, ketamine related extracellular glutamate excess in the hippocampus has been shown to drive local increase in blood flow (Schobel et al., 2013). Even though we failed to replicate the association between local rCBF and glutamate excess, possibly because MRS measures whole tissue glutamate as opposed to extracellular glutamate, our findings suggest that hippocampal glutamate excess is associated with rCBF patterns in the ACC, a hub area of the salience network that is critically implicated in the schizophrenia pathophysiology. One could speculate that medications targeting the glutamate system could help alleviate network level metabolic alterations.
Recently, efforts to identify MRI based measures with sufficient effect size and cross-site reliability to serve as glutamatergic target engagement biomarkers in early-phase clinical trials have been published (Javitt et al., 2018). In that study, the sensitivity of three putative biomarkers in response to ketamine induced NMDA receptor hypofunction was tested in healthy volunteers. Interestingly, only the resting state fMRI marker showed a robust effect and strong cross-site reliability, suggesting it may have utility for multisite target engagement studies. The failure of the other target engagement biomarkers is a testament to the intricacies of conducting multisite neuroimaging studies, where even subtle differences in measurement error can have significant effects on cross-site reliability (Kraguljac and Lahti, 2018). While rCBF was not one of the biomarkers tested, a number of factors suggest that ASL imaging may have potential utility for glutamatergic target engagement studies where successful candidate drugs would blunt the effects of ketamine related increase in rCBF. First, ketamine causes a robust increase in rCBF; second, there is high consistency in regional patterns and magnitude of increase between our data and the existing literature; and third, ASL allows absolute quantification of rCBF making it less dependent on scanner effects. But of course, the utility of this, and other BOLD metrics, e.g. amplitude of low frequency fluctuations (ALFF), for target engagement biomarker development will have to be empirically tested in a multi-site setting.
Our findings need to be interpreted in the context of a number of limitations. While we were the first to demonstrate complex effects of NMDA receptor hypofunction on interregional connectivity using ASL imaging, our sample size was modest, results did not survive correction for multiple comparison testing and therefore need to be considered preliminary. Additionally, we were not able to directly assess the relationship between changes in interregional connectivity patterns and symptom severity, as it measures interregional connectivity patterns of rCBF across subjects, rather than within a single subject. All imaging was obtained during a continuous ketamine infusion. However, because ASL images were acquired approximately 45 minutes after hippocampal Glx was quantified, it is possible that some of the associations between glutamate excess and elevated rCBF were disguised by the temporal dissociation of measurements. Also, our ASL sequence had a short labeling duration, future studies may benefit from implementing recommendations for ASL sequence parameters from a recent white paper (Alsop et al., 2015). Here, we used a spectroscopy sequence that is optimized for detection of glutamate, where glutamine contributes approximately 15% to the combined glx peak, it is therefore likely that glutamate is the primary metabolite driving associations with rCBF. In addition, we did not control for or record caffeine consumption or time of day of the scan, which could have affected results. Lastly, we did not include a placebo control in this experiment as successful blinding in these type of studies is notoriously difficult to achieve due to ketamine’s unique side effect profile (Perlis et al., 2010). A study arm with an active placebo could mitigate these concerns (Enck et al., 2013), but any drug producing similar side effects could also confound imaging data, making interpretation of findings difficult.
In summary, we demonstrate complex network level changes in rCBF patterns related to ketamine induced NMDA receptor blockage in healthy human subjects. Our study adds to the efforts to empirically confirm putative links between an imbalance in glutamate metabolism and dysconnectivity of large-scale brain networks that are thought to be central to the schizophrenia pathophysiology. Development of glutamatergic compounds that alleviate disease burden across symptom dimensions, possibly through normalizing glutamate excess related increased rCBF, is direly needed. In this context, ASL shows promise for target engagement biomarker development.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Acknowledgements
This work was supported by the National Institute of Mental Health (K23MH106683, NVK; R01MH102951, ACL; T35HL007473, BJE), the UAB Civitan International Research Center and UAB Center for Clinical and Translational Science (NVK).
ACL has received an investigator initiated grant from Janssen Pharmaceuticals.
Footnotes
Conflict of Interest
All other authors declare no conflicts of interest, including relevant financial interests, activities, relationships, and affiliations.
REFERENCES
- Alexander GE, Crutcher MD, DeLong MR, 1990. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res 85, 119–146. [PubMed] [Google Scholar]
- Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJ, Wang DJ, Wong EC, Zaharchuk G, 2015. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73(1), 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Gancsos M, Murray JD, Repovs G, Driesen NR, Ennis DJ, Niciu MJ, Morgan PT, Surti TS, Bloch MH, Ramani R, Smith MA, Wang XJ, Krystal JH, Corlett PR, 2012. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci U S A 109(41), 16720–16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, 2007. A fast diffeomorphic image registration algorithm. Neuroimage 38(1), 95–113. [DOI] [PubMed] [Google Scholar]
- Azari NP, Rapoport SI, Salerno JA, Grady CL, Gonzalez-Aviles A, Schapiro MB, Horwitz B, 1992. Interregional correlations of resting cerebral glucose metabolism in old and young women. Brain Res 589(2), 279–290. [DOI] [PubMed] [Google Scholar]
- Baker JT, Holmes AJ, Masters GA, Yeo BT, Krienen F, Buckner RL, Ongur D, 2014. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry 71(2), 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Russell CE, Newberry RE, Goen JR, Mittal VA, 2017. Patients with schizophrenia show aberrant patterns of basal ganglia activation: Evidence from ALE meta-analysis. Neuroimage Clin 14, 450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojesen KB, Andersen KA, Rasmussen SN, Baandrup L, Madsen LM, Glenthoj BY, Rostrup E, Broberg BV, 2018. Glutamate Levels and Resting Cerebral Blood Flow in Anterior Cingulate Cortex Are Associated at Rest and Immediately Following Infusion of S-Ketamine in Healthy Volunteers. Front Psychiatry 9, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolding MS, White DM, Hadley JA, Weiler M, Holcomb HH, Lahti AC, 2012. Antipsychotic Drugs Alter Functional Connectivity between the Medial Frontal Cortex, Hippocampus, and Nucleus Accumbens as Measured by H215O PET. Front Psychiatry 3, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier A, Adler CM, Weisenfeld N, Su TP, Elman I, Picken L, Malhotra AK, Pickar D, 1998. Effects of NMDA antagonism on striatal dopamine release in healthy subjects: application of a novel PET approach. Synapse 29(2), 142–147. [DOI] [PubMed] [Google Scholar]
- Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D, 1997. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry 154(6), 805–811. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM, 1998. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). Journal of traumatic stress 11(1), 125–136. [DOI] [PubMed] [Google Scholar]
- Cadena EJ, White DM, Kraguljac NV, Reid MA, Gawronski BA, Lahti AC, 2018. A longitudinal multimodal neuroimaging study to examine relationships between resting state glutamate and task related BOLD response in schizophreia. Frontiers in Psychiatry, in press. [DOI] [PMC free article] [PubMed]
- Chen Y, Wang DJ, Detre JA, 2012. Comparison of arterial transit times estimated using arterial spin labeling. MAGMA 25(2), 135–144. [DOI] [PubMed] [Google Scholar]
- Detre JA, Wang J, 2002. Technical aspects and utility of fMRI using BOLD and ASL. Clin Neurophysiol 113(5), 621–634. [DOI] [PubMed] [Google Scholar]
- Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D’Souza DC, Gueorguieva R, He G, Ramachandran R, Suckow RF, Anticevic A, Morgan PT, Krystal JH, 2013. Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry 18(11), 1199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enck P, Bingel U, Schedlowski M, Rief W, 2013. The placebo response in medicine: minimize, maximize or personalize? Nature reviews. Drug discovery 12(3), 191–204. [DOI] [PubMed] [Google Scholar]
- Friston K, Brown HR, Siemerkus J, Stephan KE, 2016. The dysconnection hypothesis (2016). Schizophr Res 176(2–3), 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, 1995. Schizophrenia: a disconnection syndrome? Clin Neurosci 3(2), 89–97. [PubMed] [Google Scholar]
- Grimm O, Gass N, Weber-Fahr W, Sartorius A, Schenker E, Spedding M, Risterucci C, Schweiger JI, Bohringer A, Zang Z, Tost H, Schwarz AJ, Meyer-Lindenberg A, 2015. Acute ketamine challenge increases resting state prefrontal-hippocampal connectivity in both humans and rats. Psychopharmacology (Berl) 232(21–22), 4231–4241. [DOI] [PubMed] [Google Scholar]
- Hadley JA, Nenert R, Kraguljac NV, Bolding MS, White DM, Skidmore FM, Visscher KM, Lahti AC, 2014. Ventral tegmental area/midbrain functional connectivity and response to antipsychotic medication in schizophrenia. Neuropsychopharmacology 39(4), 1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1980. Rating depressive patients. The Journal of clinical psychiatry 41(12 Pt 2), 21–24. [PubMed] [Google Scholar]
- Hoflich A, Hahn A, Kublbock M, Kranz GS, Vanicek T, Windischberger C, Saria A, Kasper S, Winkler D, Lanzenberger R, 2015. Ketamine-Induced Modulation of the Thalamo-Cortical Network in Healthy Volunteers As a Model for Schizophrenia. Int J Neuropsychopharmacol 18(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb HH, Lahti AC, Medoff DR, Cullen T, Tamminga CA, 2005. Effects of noncompetitive NMDA receptor blockade on anterior cingulate cerebral blood flow in volunteers with schizophrenia. Neuropsychopharmacology 30(12), 2275–2282. [DOI] [PubMed] [Google Scholar]
- Holcomb HH, Lahti AC, Medoff DR, Weiler M, Tamminga CA, 2001. Sequential regional cerebral blood flow brain scans using PET with H2(15)O demonstrate ketamine actions in CNS dynamically. Neuropsychopharmacology 25(2), 165–172. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Rapoport SI, 1988. Partial correlation coefficients approximate the real intrasubject correlation pattern in the analysis of interregional relations of cerebral metabolic activity. J Nucl Med 29(3), 392–399. [PubMed] [Google Scholar]
- Javitt DC, Carter CS, Krystal JH, Kantrowitz JT, Girgis RR, Kegeles LS, Ragland JD, Maddock RJ, Lesh TA, Tanase C, Corlett PR, Rothman DL, Mason G, Qiu M, Robinson J, Potter WZ, Carlson M, Wall MM, Choo TH, Grinband J, Lieberman JA, 2018. Utility of Imaging-Based Biomarkers for Glutamate-Targeted Drug Development in Psychotic Disorders: A Randomized Clinical Trial. JAMA Psychiatry 75(1), 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, Carle M, Frolich MA, Tran S, Yassa MA, White DM, Reddy A, Lahti AC, 2018. Mnemonic Discrimination Deficits in First-Episode Psychosis and a Ketamine Model Suggests Dentate Gyrus Pathology Linked to N-Methyl-D-Aspartate Receptor Hypofunction. Biol Psychiatry Cogn Neurosci Neuroimaging 3(3), 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kraguljac NV, Frolich MA, Tran S, White DM, Nichols N, Barton-McArdle A, Reid MA, Bolding MS, Lahti AC, 2017. Ketamine modulates hippocampal neurochemistry and functional connectivity: a combined magnetic resonance spectroscopy and resting-state fMRI study in healthy volunteers. Mol Psychiatry 22(4), 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, Lahti AC, 2018. Paving the Way for Targeted Drug Development in Schizophrenia. JAMA Psychiatry 75(1), 19–20. [DOI] [PubMed] [Google Scholar]
- Kraguljac NV, Morgan CJ, Reid MA, White DM, Jindal RD, Sivaraman S, Martinak BK, Lahti AC, 2019. A longitudinal magnetic resonance spectroscopy study investigating effects of risperidone in the anterior cingulate cortex and hippocampus in schizophrenia. Schizophr Res [DOI] [PMC free article] [PubMed]
- Kraguljac NV, White DM, Hadley JA, Visscher K, Knight D, ver Hoef L, Falola B, Lahti AC, 2016. Abnormalities in large scale functional networks in unmedicated patients with schizophrenia and effects of risperidone. Neuroimage Clin 10, 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, et al. , 1992. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A 89(12), 5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA, 1995. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 13(1), 9–19. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Warfel D, Michaelidis T, Weiler MA, Frey K, Tamminga CA, 2001a. Long-term outcome of patients who receive ketamine during research. Biological psychiatry 49(10), 869–875. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R, 2006. Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology 31(1), 221–230. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Cropsey KL, 2009. Modulation of limbic circuitry predicts treatment response to antipsychotic medication: a functional imaging study in schizophrenia. Neuropsychopharmacology 34(13), 2675–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Medoff DR, Tamminga CA, Holcomb HH, 2005. Functional effects of single dose first- and second-generation antipsychotic administration in subjects with schizophrenia. Psychiatry Res 139(1), 19–30. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA, 2001b. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology 25(4), 455–467. [DOI] [PubMed] [Google Scholar]
- Langsjo JW, Kaisti KK, Aalto S, Hinkka S, Aantaa R, Oikonen V, Sipila H, Kurki T, Silvanto M, Scheinin H, 2003. Effects of subanesthetic doses of ketamine on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology 99(3), 614–623. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Girgis RR, Brucato G, Moore H, Provenzano F, Kegeles L, Javitt D, Kantrowitz J, Wall MM, Corcoran CM, Schobel SA, Small SA, 2018. Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol Psychiatry 23(8), 1764–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA, 2011. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci 32(9), 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottman KK, Kraguljac NV, White DM, Morgan CJ, Calhoun VD, Butt A, Lahti AC, 2017. Risperidone Effects on Brain Dynamic Connectivity-A Prospective Resting-State fMRI Study in Schizophrenia. Front Psychiatry 8, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff DR, Holcomb HH, Lahti AC, Tamminga CA, 2001. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus 11(5), 543–550. [DOI] [PubMed] [Google Scholar]
- Menon V, 2011. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15(10), 483–506. [DOI] [PubMed] [Google Scholar]
- Mueller F, Musso F, London M, de Boer P, Zacharias N, Winterer G, 2018. Pharmacological fMRI: Effects of subanesthetic ketamine on resting-state functional connectivity in the default mode network, salience network, dorsal attention network and executive control network. Neuroimage Clin 19, 745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, Ugurbil K, 1993. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J 64(3), 803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall J, Gorham D, 1962. The brief psychiatric rating scale. Psychology Report 10, 799–812. [Google Scholar]
- Overbeek G, Gawne TJ, Reid MA, Salibi N, Kraguljac NV, White DM, Lahti AC, 2018. Relationship between cortical excitation and inhibition and task-induced activation and deactivation: A combined MR Spectroscopy and functional MRI study at 7T in first episode psychosis. Biological Psychiatry: CNNI, in press. [DOI] [PMC free article] [PubMed]
- Palaniyappan L, Simmonite M, White TP, Liddle EB, Liddle PF, 2013. Neural primacy of the salience processing system in schizophrenia. Neuron 79(4), 814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parwani A, Weiler MA, Blaxton TA, Warfel D, Hardin M, Frey K, Lahti AC, 2005. The effects of a subanesthetic dose of ketamine on verbal memory in normal volunteers. Psychopharmacology (Berl) 183(3), 265–274. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Ostacher M, Fava M, Nierenberg AA, Sachs GS, Rosenbaum JF, 2010. Assuring that double-blind is blind. The American journal of psychiatry 167(3), 250–252. [DOI] [PubMed] [Google Scholar]
- Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A, 2011. Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev 35(5), 1110–1124. [DOI] [PubMed] [Google Scholar]
- Pollak TA, De Simoni S, Barimani B, Zelaya FO, Stone JM, Mehta MA, 2015. Phenomenologically distinct psychotomimetic effects of ketamine are associated with cerebral blood flow changes in functionally relevant cerebral foci: a continuous arterial spin labelling study. Psychopharmacology (Berl) 232(24), 4515–4524. [DOI] [PubMed] [Google Scholar]
- Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E, Barrow R, Yeo R, Lauriello J, Brooks WM, 2005. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry 162(2), 394–396. [DOI] [PubMed] [Google Scholar]
- Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H, Boesiger P, Henning A, Seifritz E, 2012. Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One 7(9), e44799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, Inbar BP, Corcoran CM, Lieberman JA, Moore H, Small SA, 2013. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 78(1), 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert F, Gallinat J, Seifert F, Rinneberg H, 2004. Glutamate concentrations in human brain using single voxel proton magnetic resonance spectroscopy at 3 Tesla. Neuroimage 21(4), 1762–1771. [DOI] [PubMed] [Google Scholar]
- Siegel BV Jr., Buchsbaum MS, Bunney WE Jr., Gottschalk LA, Haier RJ, Lohr JB, Lottenberg S, Najafi A, Nuechterlein KH, Potkin SG, et al. , 1993. Cortical-striatal-thalamic circuits and brain glucose metabolic activity in 70 unmedicated male schizophrenic patients. Am J Psychiatry 150(9), 1325–1336. [DOI] [PubMed] [Google Scholar]
- Smith GS, Schloesser R, Brodie JD, Dewey SL, Logan J, Vitkun SA, Simkowitz P, Hurley A, Cooper T, Volkow ND, Cancro R, 1998. Glutamate modulation of dopamine measured in vivo with positron emission tomography (PET) and 11C-raclopride in normal human subjects. Neuropsychopharmacology 18(1), 18–25. [DOI] [PubMed] [Google Scholar]
- Smith SM, 2002. Fast robust automated brain extraction. Hum Brain Mapp 17(3), 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SB, Koller JM, Campbell MC, Black KJ, 2014. Arterial spin labeling versus BOLD in direct challenge and drug-task interaction pharmacological fMRI. PeerJ 2, e687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ, Krystal JH, Nutt D, Barker GJ, 2012. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry 17(7), 664–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanenbaum AB, Snyder AZ, Brier MR, Ances BM, 2015. A method for reducing the effects of motion contamination in arterial spin labeling magnetic resonance imaging. J Cereb Blood Flow Metab 35(10), 1697–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhamme L, van den Boogaart A, Van Huffel S, 1997. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 129(1), 35–43. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Scharfetter C, Antonini A, Maguire P, Missimer J, Angst J, 1997. Metabolic hyperfrontality and psychopathology in the ketamine model of psychosis using positron emission tomography (PET) and [18F]fluorodeoxyglucose (FDG). Eur Neuropsychopharmacol 7(1), 9–24. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vontobel P, Oye I, Hell D, Leenders KL, 2000. Effects of (S)-ketamine on striatal dopamine: a [11C]raclopride PET study of a model psychosis in humans. J Psychiatr Res 34(1), 35–43. [DOI] [PubMed] [Google Scholar]
- Walther S, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Viher PV, 2017. Aberrant Hyperconnectivity in the Motor System at Rest Is Linked to Motor Abnormalities in Schizophrenia Spectrum Disorders. Schizophr Bull 43(5), 982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Aguirre GK, Rao H, Wang J, Fernandez-Seara MA, Childress AR, Detre JA, 2008. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging 26(2), 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler MA, Thaker GK, Lahti AC, Tamminga CA, 2000. Ketamine effects on eye movements. Neuropsychopharmacology 23(6), 645–653. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Vasic N, Sambataro F, Hose A, Frasch K, Schmid M, Walter H, 2009. Temporally anticorrelated brain networks during working memory performance reveal aberrant prefrontal and hippocampal connectivity in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 33(8), 1464–1473. [DOI] [PubMed] [Google Scholar]
- Wong JJ, O’Daly O, Mehta MA, Young AH, Stone JM, 2016. Ketamine modulates subgenual cingulate connectivity with the memory-related neural circuit-a mechanism of relevance to resistant depression? PeerJ 4, e1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Karbasforoushan H, Heckers S, 2012. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry 169(10), 1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA, 1978. A rating scale for mania: reliability, validity and sensitivity. The British journal of psychiatry : the journal of mental science 133, 429–435. [DOI] [PubMed] [Google Scholar]