Abstract
Standard chemotherapy is commonly used in clinical practice for the treatment of non-small cell lung cancer (NSCLC). However, its therapeutic efficacy remains low. Combination therapy for cancer treatment has attracted attention in recent years. The present study aimed to investigate the antitumor effect of the combination treatment with gemcitabine and sorafenib on NSCLC in vitro and in vivo, and to determine its underlying molecular mechanisms. The anti-NSCLC effects of combination therapy were analyzed by flow cytometry analysis, MTT, western blotting, reverse transcription-quantitative PCR, wound healing and Transwell invasion assays. A549 cells subjected to combination treatment with gemcitabine and sorafenib demonstrated a more irregular cellular morphology and lower cell viability compared with the monotherapy groups. Combination of gemcitabine and sorafenib significantly induced cell cycle arrest and apoptosis in A549 cells. Additionally, combination therapy was demonstrated to restrain the migration and invasion of tumor cells by suppressing epithelial-to-mesenchymal transition (EMT) of A549 cells. In vivo analyses confirmed that co-treatment with gemcitabine and sorafenib decreased NSCLC tumor growth and tumor weight in nude mice. Taken together, the results of the present study suggested that combination treatment with gemcitabine and sorafenib exerted a synergistic inhibitory effect on NSCLC in vitro and in vivo via the EMT process.
Keywords: gemcitabine, sorafenib, synergistic effect, EMT, NSCLC
Introduction
Lung cancer notably contributes to tumor-associated mortality worldwide which is accounting for 18.4% of all such deaths and responsible for more than 1.8 million deaths each year (1,2). Non-small cell lung cancer (NSCLC) accounts for ~85% of histological subtypes among all types of lung cancer (3). Chemotherapy is the most commonly used therapeutic regimen for the majority of patients with NSCLC, and significant progress has been achieved with targeted or immunotherapeutic agents for the treatment of NSCLC (4). However, the 5-year survival rate of patients with NSCLC still remains very low (5). The high mortality and low survival rate of patients with NSCLC are largely attributed to the capability of NSCLC cells to invade normal tissues, resulting in metastasis to remote sites (6). Thus, the development of novel strategies on anti-invasion and antimetastatic treatment for NSCLC are urgently required. The complex pathogenesis of NSCLC indicates that the combination of two or more antitumor agents possessing different molecular mechanisms may be a therapeutic option for efficient treatment (7).
Gemcitabine is a deoxycytidine analogue that has been approved as a first-line therapy drug for NSCLC for almost a decade (8). Gemcitabine has also been approved for the treatment of ovarian, advanced lung and pancreatic cancer due to its profound antitumor activity (9–11). However, drug resistance and the adverse side effects (such as anemia and leukopenia) associated with gemcitabine limit its chemotherapeutic efficacy (12). Previous studies have identified potential agents that may be used in combination with gemcitabine as effective chemotherapeutic regimes (13,14). For example, combination of gemcitabine and carboplatin has been verified as an effective adjuvant chemotherapeutic strategy for patients with completely resected NSCLC in a phase II study (15). In addition, gemcitabine in combination with nab-paclitaxel has been used in patients with metastatic breast cancer (14).
Sorafenib is a non-selective multi-kinase inhibitor that has the ability to suppress tumor cell proliferation and angiogenesis, primarily by restraining the activities of targets in tumor cells, such as the RAF/mitogen-activated protein kinase 1 (MEK)/ERK signaling pathway, vascular endothelial growth factor receptor-3 and platelet-derived growth factor receptor β (16). Sorafenib has also been reported to inhibit transforming growth factor-β1-induced epithelial-to-mesenchymal transition (EMT) in A549 cells (17). The antitumor effect of sorafenib in preclinical NSCLC models has been frequently reported (18,19). Sorafenib treatment is associated with adverse side effects, including hand-foot skin diseases, hypertension and diarrhea (20), which results in implementation of low doses, thus effecting efficacy. Thus, development of novel strategies are required in order to relieve the side effects or enhance the antitumor activity of sorafenib (21,22).
Combination treatment with various agents is a potential strategy to improve the synergistic therapeutic efficacy, as different agents exhibit their therapeutic effects via different molecular mechanisms, leading to a synergistic anticancer response (23). In addition, combination therapy has been demonstrated to overcome the harmful side effects associated with high-dose drugs (24). For example, a randomized phase II study revealed that combination treatment with gemcitabine and sorafenib is well tolerated and was demonstrated to be efficient in patients with advanced NSCLC compared with other combination treatments (25). However, the molecular mechanisms underlying the combination treatment with gemcitabine and sorafenib in NSCLC remain largely unknown. The present study aimed to investigate the effect of combination therapy with gemcitabine and sorafenib for NSCLC in vitro and in vivo.
Materials and methods
Materials and antibodies
Sorafenib was purchased from Dalian Meilun Biotech Co., Ltd., and gemcitabine was purchased from Shanghai Yuanye Biotech Co., Ltd. MTT was supplied by Sigma-Aldrich; Merck KGaA. Primary antibodies against N-cadherin, E-cadherin and Twist-1 were purchased from Cell Signaling Technology, Inc., whereas the antibody against GAPDH and horseradish peroxidase-conjugated secondary antibodies against rabbit or mouse IgG were purchased from Weiao Biotechnology Co., Ltd.
Cell culture
A549, H1975 and H1650 cell lines were purchased from The Cell Bank of Type Culture Collection of the Chinese Academy of Sciences. Cells were maintained in RPMI-1640 medium (Corning Inc.) supplemented with 10% heat-inactivated FBS (Gibco; Thermo Fisher Scientific, Inc.), and 100 µg/ml penicillin and 100 U/ml streptomycin (Beyotime Institute of Biotechnology) at 37°C with 5% CO2.
MTT assay
A549, H1975 and H1650 cells were seeded into 96-well plates at a density of 4×103 cells/well and treated with 0–100 µg/ml gemcitabine or 0–50 µM sorafenib at 37°C for 48 h. Subsequently, the cells were incubated with 0.5 mg/ml MTT at 37°C for 4 h to determine cell viability. Following the MTT incubation, the purple formazan crystals were dissolved in 150 µl DMSO, and cell viability was subsequently analyzed using a microplate reader (BioTek Instruments, Inc.) at a wavelength of 570 nm.
Morphological observation
A549 cells were incubated with 10 µg/ml gemcitabine or 10 µM sorafenib at 37°C for 48 h. The morphology of the tumor cells was observed using an inverted microscope (Nikon, Japan). Magnification, ×400.
Flow cytometric analysis of the cell cycle
Following treatment with 10 µg/ml gemcitabine and 10 µM sorafenib at 37°C for 48 h, A549 cells were collected and subsequently fixed with 70% ethanol at 4°C for 12 h. The samples were washed with pre-cooled PBS and incubated with 100 µg/ml of RNaseA (Nanjing KeyGen Biotech Co., Ltd.) at 37°C for 30 min. The cells were subsequently stained with 50 µg/ml of propidium iodide (Nanjing KeyGen Biotech Co., Ltd.) at 37°C for 15 min and cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences). The results were analyzed using Flow Jo software version 7.6.1 (TreeStar).
Flow cytometric analysis of apoptosis
Following treatment with 10 µg/ml gemcitabine and 10 µM sorafenib at 37°C for 48 h, A549 cells were collected using centrifugation at 800 × g at 4°C for 10 min. Then, cells were stained with annexin V-fluorescein isothiocyanate (FITC) and PI using the Annexin V-FITC Apoptosis Detection kit (Nanjing KeyGen Biotech Co., Ltd.). Briefly, the cells were incubated with Annexin V-FITC/PI at 37°C for 20 min, and apoptotic cells were subsequently analyzed using a FACSCalibur flow cytometer (BD Biosciences); Annexin V+/PI+ and Annexin V+/PI− cells were considered to be apoptotic. A549 cells treated with 100 nM docetaxel at 37°C for 48 h were used as positive control. The data was analyzed using CellQuest software version 3.1 (BD Biosciences).
Western blotting
Total protein was extracted from A549 cells and tumor tissue homogenates using RIPA cell lysis buffer (Nanjing KeyGen Biotech Co., Ltd.), and quantified using the BCA Protein Quantitation kit (Nanjing KeyGen Biotech Co., Ltd.). Equal amounts of total protein (20 µg) were separated by 12% SDS-PAGE and subsequently transferred to polyvinylidene difluoride membranes. Membrane blocking and incubation with the primary and secondary antibodies were performed as previously described (26). Protein bands were visualized using the Enhanced Chemiluminescent Detection kit (Pierce; Thermo Fisher Scientific, Inc.). The intensity of resulting bands were quantified using ImageJ version 1.47 (National Institutes of Health).
Reverse-transcription quantitative (RT-qPCR)
Total RNA was extracted from A549 cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.), reverse transcribed into cDNA using the SuperScript First Strand Synthesis kit (Invitrogen; Thermo Fisher Scientific, Inc.) at 42°C for 50 min according to the manufacturer's protocol. qPCR was detected using the BeyoFast™ SYBR Green qPCR Mix (Beyotime Institute of Biotechnology; cat. no. D7260) on Applied Biosystems® 7500 Real-Time PCR Systems (Thermo Fisher Scientific, Inc.). The thermocycling conditions were used as follows: 95°C for 20 sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 30 sec. The following primers sequences were used for qPCR: E-cadherin forward, 5′-TGCGCGTGAAGGTTTGCCAGT-3′ and reverse, 5′-ACGTTGTCCCGGGTGTCATCCT-3′; N-cadherin forward, 5′-CATCATCATCCTGCTTATCCTTGT-3′ and reverse, 5′-GGTCTTCTTCTCCTCCACCTTCT-3′; Twist-1 forward, 5′-GCATGCATTCTCAAGAGGT-3′ and reverse, 5′-CAGTTTGATCCCAGCGTTTT-3′; and GAPDH forward, 5′-GGACCTGACCTGCCGTCTAG-3′ and reverse, 5′-GTAGCCCAGGATGCCCTTGA-3′. Relative expression levels were quantified using the 2−ΔΔCq method (27) and normalized to the internal reference gene GAPDH.
Wound healing assay
Cell migration was assessed using the wound healing assay. Briefly, A549 cells were cultured with 1640 medium (containing 10% FBS) in a 12-well plates until they reached a confluent monolayer, and the cell monolayers were subsequently scratched using a 10 µl pipette tip. Culture medium was replaced with fresh culture medium (no FBS) containing 10 µg/ml gemcitabine or 10 µM sorafenib, followed by incubation for 0, 12, 24 and 48 h at 37°C. Migratory cells were counted in five randomly selected fields using an inverted light microscope at ×200 magnification (Nikon).
Invasion assay
Chamber inserts (8-µm pore; Corning, Inc.) were precoated with 300 µg/ml Matrigel for 30 min at 37°C according to the manufacturer's instructions. A total of ~5×103 A549 cells were plated in the upper chambers of Transwell plates in RPMI-1640 supplemented with 10% FBS and incubated in the presence of 10 µg/ml gemcitabine or 10 µM sorafenib. A total of 500 µl RPMI-1640 medium supplemented with 20% FBS was placed in the lower chambers. Following incubation at 37°C for 48 h, invasive cells on the lower chambers were stained with 0.1% crystal violet at 37°C for 15 min. Stained cells were washed twice with PBS and counted in randomly selected 5 fields using an inverted microscope (Nikon), magnification, ×400. Subsequently, crystal violet was dissolved using 33% acetic acid, and the invasive ability of the cells was measured at a wavelength of 590 nm using a microplate reader (BioTek Instruments, Inc.).
Tumor xenograft experiments
All experimental protocols involving animals were approved by the Institutional Animal Committee of Shanghai Ninth People's Hospital (Shanghai, China). A549 cells (1×106 cells/ml in 1 ml PBS) were subcutaneously injected into the right thigh of BALB/c nude mice (4–6 weeks old; 18–22 g, Charles River Laboratories, Inc.). The mice were housed at 24–26°C with 50–70% humidity and 12-h day/night cycle and provided with free access to food and water. The mice were randomized into four groups (6 mice/group) as follows: i) Vehicle (methylcellulose/Tween 80, intraperitoneal injection); ii) sorafenib (40 mg/kg/day, intraperitoneal injection); iii) gemcitabine (50 mg/kg/day, intraperitoneal injection); and iv) sorafenib (40 mg/kg/day, intraperitoneal injection) + gemcitabine (50 mg/kg/day, intraperitoneal injection). The body weights of the mice was measured twice/week, the tumor size was measured every other day using calipers, and tumor volumes were determined using the following formula: Volume=length × (width)2/2. The mice were sacrificed before the end of the experiment if the maximum tumor diameter reached 2 cm, and all mice were sacrificed after 28 days using CO2.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software, Inc.). Combination index (CI) of gemcitabine and sorafenib was calculated by CalcuSyn 2.0 software (Biosoft). CI values <1, =1 and >1 indicated synergism, additive effect and antagonism, respectively. Data are presented as the mean ± standard deviation. Differences between two groups were analyzed using unpaired Student's t-test (2-tailed), whereas one-way analysis of variance followed by Tukey's post hoc test was used to compare differences among multiple groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Dose-dependent cytotoxicity of gemcitabine and sorafenib to A549 cells
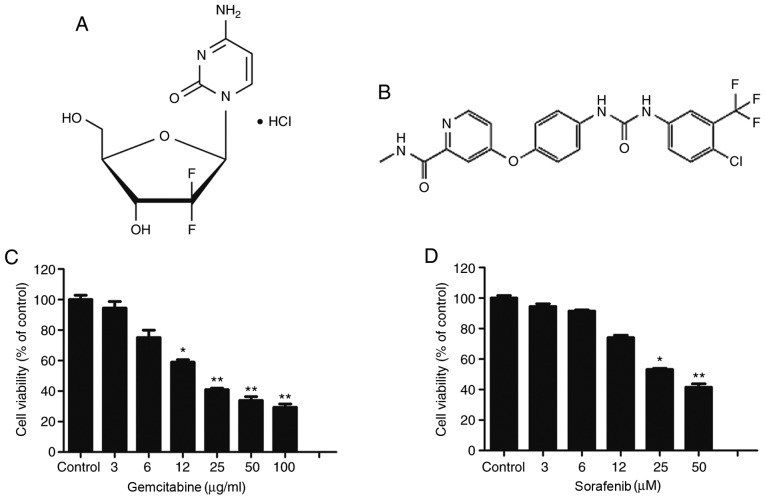
Fig. 1A and B illustrate the molecular structures of gemcitabine and sorafenib, respectively. Gemcitabine is a deoxycytidine analogue that has been approved as a first-line therapy drug for NSCLC. Conversely, sorafenib is a non-selective multi-kinase inhibitor, which has been approved for the treatment of hepatocellular carcinoma. The present study assessed the cytotoxic effects of gemcitabine and sorafenib as single agents on A549 cells using the MTT assay. The results demonstrated that treatment of A549 cells with varying concentrations of gemcitabine (0–100 µg/ml) or sorafenib (0–100 µM) for 48 h inhibited the viability of tumor cells in a dose-dependent manner (P<0.05), and the half maximal inhibitory concentration values of gemcitabine and sorafenib in A549 cells were 19.2 µg/ml and 44.8 µM, respectively (Fig. 1C and D).
Figure 1.
Dose-dependent cytotoxic effect of gemcitabine and sorafenib on A549 cells. (A and B) The molecular structure of (A) gemcitabine and (B) sorafenib. (C and D) A549 cells were treated with (C) 0–100 µg/ml of gemcitabine and (D) 0–100 µM of sorafenib for 48 h, and cell viability was determined by an MTT assay. *P<0.05 vs. Control, **P<0.01 vs. Control.
Synergistic antitumor effects of gemcitabine and sorafenib on A549 cells
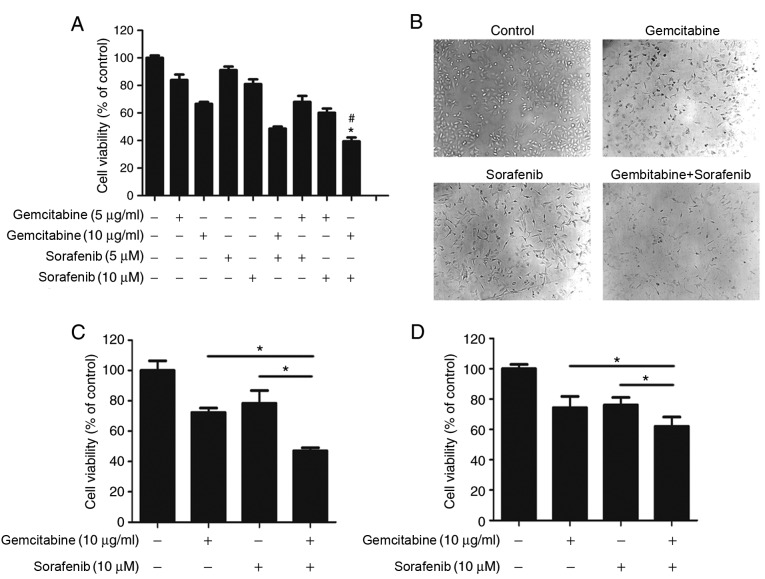
To determine whether a coadjutant antitumor effect existed between gemcitabine and sorafenib, A549 cells were treated with different concentrations of gemcitabine (5 µg/ml or 10 µg/ml) and sorafenib (5 µM or 10 µM) either individually or in combination. The results demonstrated that combination treatment significantly inhibited A549 cell viability compared with gemcitabine or sorafenib treatment alone (P<0.05, Fig. 2A), and the CI value calculated by Calcusyn 2.0 software was 0.65 with 10 µg/ml gemcitabine and 10 µM sorafenib. Subsequently, cell morphology was assessed, which demonstrated regular spindle-shaped cells in the control group, whereas irregular shapes were observed in cells treated with gemcitabine or sorafenib alone. Cells in the combination treatment group displayed multiple changes compared with the control group, including decreased volume and irregular shapes (Fig. 2B). The effect of the combination treatment was also assessed in NSCLC H1650 and H1975 cell lines. The results demonstrated stronger cytotoxicity in H1650 and H1975 cells in the combination treatment group than sorafenib and gemcitabine treated groups (P<0.05), Fig. 2C and D). Overall, cell viability analysis indicated that gemcitabine and sorafenib exerted a synergistic effect on NSCLC cells.
Figure 2.
Synergistic antitumor effects of gemcitabine and sorafenib on A549 cells. (A) A549 cells were treated with 5 or 10 µg/ml gemcitabine and 5 or 10 µM sorafenib, either alone or in combination for 48 h, and cell viability was determined by an MTT assay. (B) A549 cells were treated with a combination of 10 µg/ml gemcitabine and 10 µM sorafenib for 48 h, and cell morphology was subsequently observed using an inverted microscope. (C) H1975 and (D) H1650 cells were treated with 10 µg/ml gemcitabine and 10 µM sorafenib, either alone or in combination for 48 h, and cell viability was determined by an MTT assay. *P<0.05 compared vs. gemcitabine group; #P<0.05 compared vs. sorafenib group.
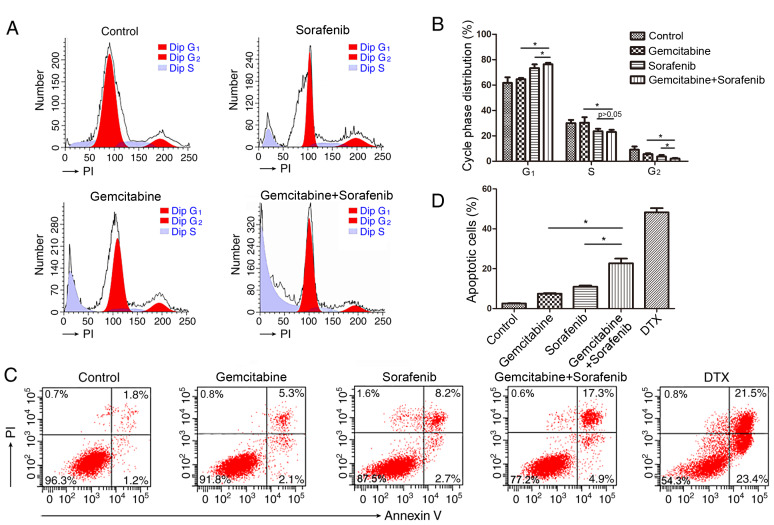
Combination of gemcitabine and sorafenib induces cell cycle arrest and apoptosis in A549 cells. Previous studies have reported that both gemcitabine and sorafenib disturb the cell cycle of tumor cells (21,28). Thus, the present study investigated the combined effects of gemcitabine and sorafenib on the cell cycle. The results demonstrated that co-treatment induced a significant increase in the number of cells in the G1 phase, while decreasing the number of cells in the S phase compared with the control group, indicating that a G1-phase arrest was induced in A549 cells by the combination treatment (P<0.05), Fig. 3A and B). Furthermore, a higher proportion of apoptotic cells (22.2%) was detected in the combination treatment group compared with gemcitabine (7.4%) or sorafenib (10.9%) monotherapy (P<0.05, Fig. 3C-D).
Figure 3.
Co-treatment with gemcitabine and sorafenib induces apoptosis and cell cycle arrest in A549 cells. A549 cells were treated with 10 µg/ml gemcitabine and 10 µM sorafenib, either alone or in combination for 48 h. (A) Cell cycle distribution was determined by flow cytometry analysis. (B) The quantification of cells in different phases of the cell cycle is presented in bar charts. (C) The percentage of apoptotic cells was determined by flow cytometry analysis. PI, propidium iodide. (D) The percentage of A549 cells positive for Annexin V staining was presented in bar charts. *P<0.05.
Effects of combination treatment on A549 cell migration
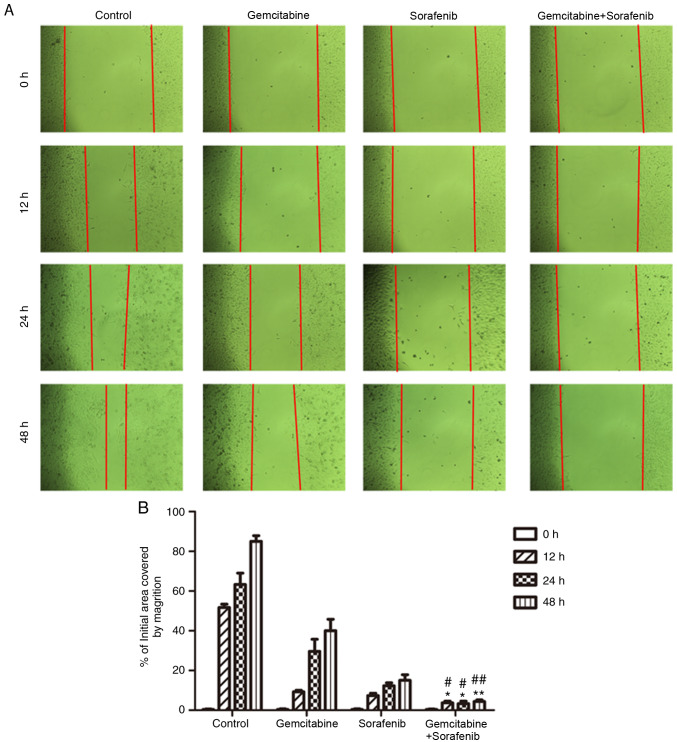
The wound healing assay was performed to detect the effects of combination treatment on the migratory ability of A549 cells. The results demonstrated that the scratch of cell monolayers in the negative control group was almost healed, and that of monotherapy groups was gradually closed following treatment for 48 h (Fig. 4A). However, the scratch of the combination treatment group still remained wide apart at 48 h (P<0.05, Fig. 4B). Taken together, the results indicated that the migratory ability of A549 cells was notably inhibited following co-treatment with gemcitabine and sorafenib.
Figure 4.
Effects of co-treatment with gemcitabine and sorafenib on migration of A549 cells. (A) A549 cells were treated with a combination of 10 µg/ml gemcitabine and 10 µM sorafenib for 48 h, and cell migration was determined by a wound healing assay using an inverted microscope (magnification, ×200). (B) Quantification of A549 migratory cells. *P<0.05 vs. gemcitabine group; **P<0.01 vs. gemcitabine group; #P<0.05 vs. sorafenib group; ##P<0.01 vs. sorafenib group.
Effects of combination treatment on the invasive ability of A549 cells
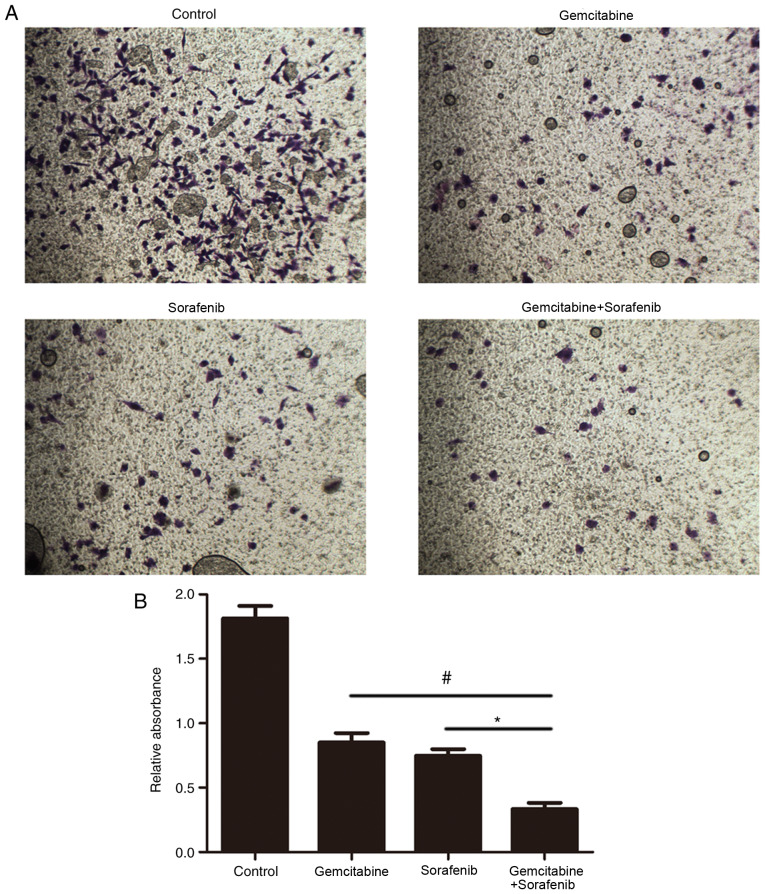
The effect of combination treatment on cell invasion was subsequently investigated. The results demonstrated that the number of invasive A549 cells in the combination treatment group was significantly decreased compared with the control and monotherapy groups (P<0.05, Fig. 5A and B). These results suggested that combination treatment with gemcitabine and sorafenib may inhibit the invasion of A549 cells.
Figure 5.
Effects of co-treatment with gemcitabine and sorafenib on invasion of A549 cells. (A) The result of cell invasion. (B) Quantification of A549 invasive cells. *P<0.05, #P<0.05.
Combination treatment suppresses the EMT of A549 cells
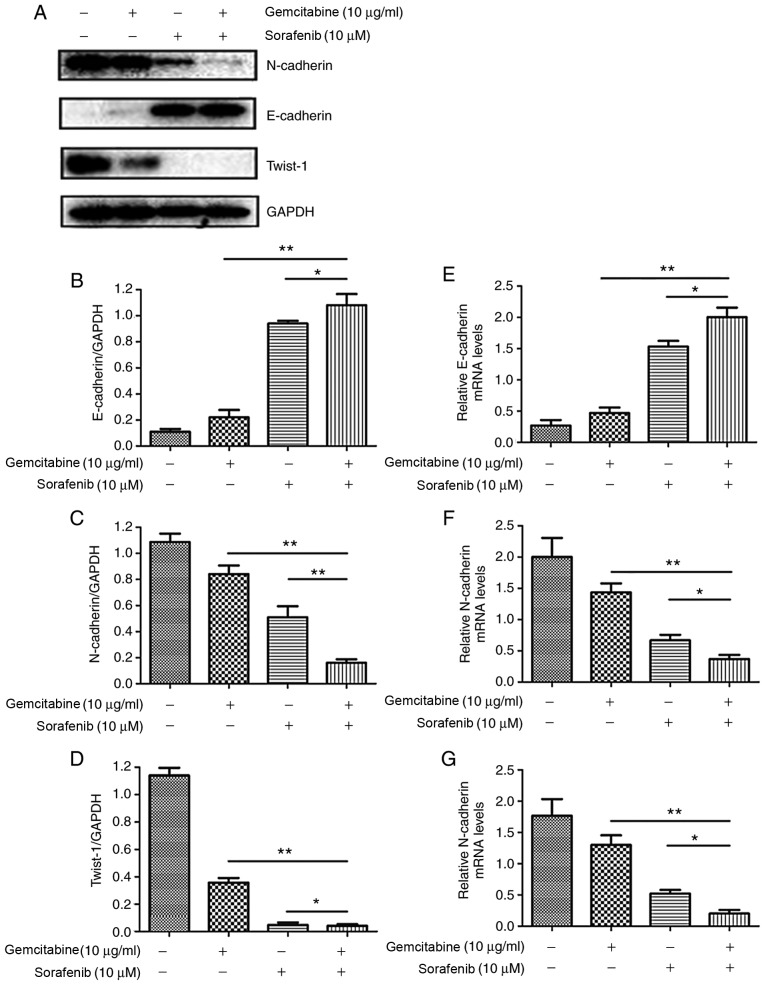
In order to further investigate the molecular mechanisms underlying the effects of combination treatment on the migration and invasion of A549 cells, expression levels of EMT-associated proteins were assessed by western blotting. The results demonstrated that co-treatment with gemcitabine and sorafenib upregulated the expression of the epithelial marker E-cadherin, whereas the expression levels of the mesenchymal markers N-cadherin and Twist-1 were downregulated compared with the basal level of the monotherapy and the negative control groups (P<0.05, Fig. 6A-D). Furthermore, RT-qPCR analysis indicated that N-cadherin and Twist-1 mRNA expression levels were significantly lower in the combination treatment group compared with the control and monotherapy groups, whereas the expression of E-cadherin was increased (P<0.05, Fig. 6E-G).
Figure 6.
Combination treatment with gemcitabine and sorafenib induces EMT inhibition of A549 cells. A549 cells were treated with a combination of 10 µg/ml gemcitabine and 10 µM sorafenib for 48 h. (A) Protein expression levels of EMT-associated markers N-cadherin, E-cadherin and Twist-1 were assessed by western blot analysis. (B-D) Quantitative analysis of (B) E-cadherin, (C) N-cadherin and (D) Twist-1. (E-G) mRNA expression levels of (E) E-cadherin, (F) N-cadherin and (G) Twist-1. *P<0.05, **P<0.01. EMT, epithelial-to-mesenchymal transition.
Synergistic antitumor effects of combined treatment in NSCLC xenograft model
To further determine whether gemcitabine and sorafenib exerted synergistic anti-NSCLC effects in vivo, nude mice were subcutaneously injected with A549 cells to establish a subcutaneous xenotransplanted tumor model. The results demonstrated that co-treatment with gemcitabine and sorafenib significantly inhibited tumor growth, as the average tumor volumes were decreased by 54.1 and 41.8% compared with gemcitabine or sorafenib alone, respectively (P<0.05, Fig. 7A and B). Furthermore, the combination treatment group exhibited significantly decreased tumor weight compared with the monotherapy groups (P<0.05, Fig. 7C), whereas no significant differences were observed regarding the body weight (P>0.05, Fig. 7D). The protein expression levels of N-cadherin and Twist-1 in the tumor tissues of the combination treatment group were significantly decreased in the co-treatment group compared with the monotherapy groups, whereas E-cadherin expression increased (P<0.05, Fig. 7E-H). Taken together, these results suggested that combination treatment with gemcitabine and sorafenib exerted antitumor effects via the EMT process in vivo.
Figure 7.
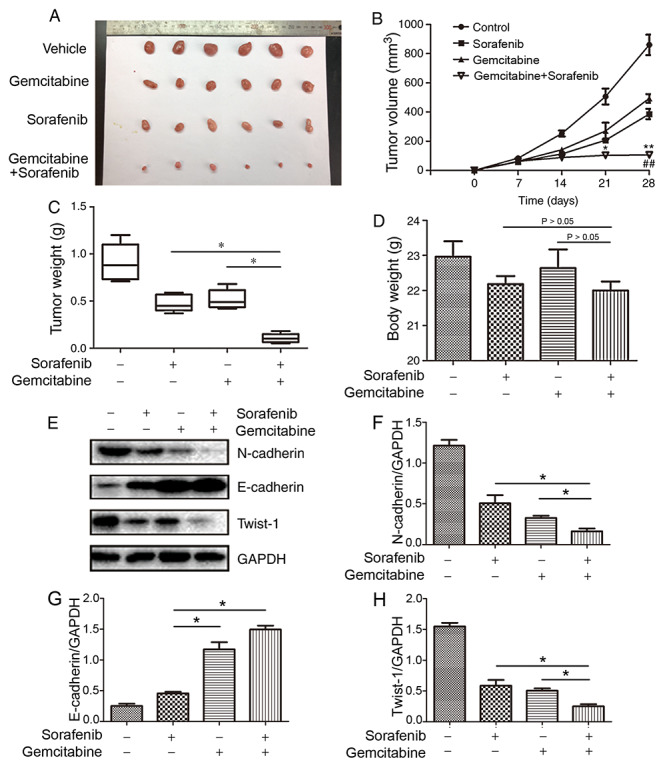
Synergistic antitumor effect of co-treatment with gemcitabine and sorafenib in non-small cell lung cancer xenograft model. (A) The images of tumor diameters of the different groups. (B) Tumor volume (*P<0.05 compared vs. gemcitabine group; **P<0.01 compared vs. gemcitabine group; ##P<0.01 compared vs. sorafenib group). (C) Tumor weight (*P<0.05) and (D) body weight of the different groups (P>0.05). (E) Protein expression levels of N-cadherin, E-cadherin and Twist-1 in tumor tissues. (F-H) Quantitative analysis of (F) N-cadherin, (G) E-cadherin and (H) Twist-1. *P<0.05.
Discussion
Invasion and metastasis greatly contribute to the poor prognosis of patients with NSCLC. The majority of cases of relapsed NSCLC can be attributed to intractable late-stage metastatic disease (29). Distant metastases are confirmed in almost half of all clinically diagnosed NSCLC cases (30). Multilevel cross-reactions among the different targets in the metastatic progression of lung cancer cells have been identified, and suppression of one target allows others to act as immune escape molecular mechanisms for tumor cells (31). Combination treatment consisting of two or more anticancer agents is considered to be more effective in inhibiting tumor progression compared with single-targeted agents (32). A previous study has reported that combination treatment of C2-ceramide and sorafenib has a synergistic interaction in Bel-7402 cells via the PI3K/AKT/mTOR and ERK signaling pathways (31). Similarly, Li et al (33) have demonstrated that the combination of gemcitabine and sorafenib exhibits a synergistic effect in A549 cells by inhibiting epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI)-sensitive and EGFR-TKI-resistant cells. In addition, gemcitabine inhibits micrometastasis of NSCLC by targeting epithelial cellular adhesion molecule-positive circulating tumor cells via the HGF/cMET signaling pathway (34). Previous studies have reported that sorafenib suppresses cell migration and invasion by inhibiting the MET and MEK/ERK signaling pathways (35,36). Li et al (37) have confirmed that sorafenib and gemcitabine or pemetrexed have synergistic effects by inducing apoptosis and cell cycle arrest, and inhibiting proliferation of lung cancer cells. However, the underlying molecular mechanisms of gemcitabine in combination with sorafenib on cell migration and invasion in NSCLC remain unclear. The present study investigated whether gemcitabine and sorafenib exerted a synergistic inhibitory effect on NSCLC in vitro and in vivo, and aimed to determine the potential molecular mechanisms underlying these interactions. The results demonstrated that co-treatment with gemcitabine and sorafenib markedly induced the cytotoxicity and cell cycle arrest in A549 cells. In addition, the combination treatment was demonstrated to significantly inhibit the invasion and migration of NSCLC cells by Transwell invasion and wound healing assays. Taken together, the results of the present study suggested that combination treatment with gemcitabine and sorafenib inhibited the metastatic capability of NSCLC cells, indicating its potential as a novel treatment option for NSCLC.
The EMT process serves an important role in cancer metastasis and invasion (38). During cancer progression, cells lose the cell-matrix contact, cell-cell connections and normal epithelial polarity, while simultaneously adopting mesenchymal characteristics that allow migration into the surrounding matrices (39,40). During EMT, the expression levels of mesenchymal markers such as N-cadherin are upregulated, whereas the epithelial cell markers such as E-cadherin are downregulated (41). Twist-1 is a transcriptional factor that belongs to the basic helix-loop-helix family; previous studies have reported that Twist-1 is an EMT inducer in the progression of NSCLC (42,43). In the present study, inhibition of EMT was observed in vitro and in vivo following combination treatment, as demonstrated by the notably downregulated expression of N-cadherin and Twist-1, as well the markedly upregulated expression of E-cadherin, at both the mRNA and protein levels.
In conclusion, the results of the present study demonstrated that combination treatment with gemcitabine and sorafenib exerted antimetastatic and anti-invasive effects by inhibiting EMT in A549 cells. In addition, combination treatment demonstrated synergistic cytotoxicity to NSCLC cells in vitro and in vivo. Thus, combination treatment with gemcitabine and sorafenib may provide valuable insights into the development of synergetic anticancer agents.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- NSCLC
non-small cell lung cancer
- EMT
epithelial-to-mesenchymal transition
Funding
The present study was funded by the National Natural Science Foundation of China (grant no. 81703779), the Fund of Science and Technology Commission Shanghai Municipality (grant no. 17401900800) and the Fund of Shanghai Jiao Tong University School of Medicine (grant no. JDYX2017QN007).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
SSJ and YFY conceived and designed the present study. SSJ, RW, FHW, XZ and SNL performed the experiments. SSJ wrote the paper. RW, XZ, SNL, FHW and YFY reviewed and edited the manuscript. All authors read and approved the final manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
All experimental protocols involving animals were approved by the Institutional Animal Committee of Shanghai Ninth People's Hospital (Shanghai, China) (approval no. SH9H-2019-A296-1).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kobayashi K, Seike M, Zou F, Noro R, Chiba M, Ishikawa A, Kunugi S, Kubota K, Gemma A. Prognostic significance of NSCLC and response to EGFR-TKIs of EGFR-mutated NSCLC based on PD-L1 expression. Anticancer Res. 2018;38:753–762. doi: 10.21873/anticanres.12281. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Vasconcellos VF, Marta GN, da Silva EM, Gois AF, de Castria TB, Riera R. Cisplatin versus carboplatin in combination with third-generation drugs for advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2020;1:CD009256. doi: 10.1002/14651858.CD009256.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 6.Bremnes RM, Veve R, Hirsch FR, Franklin WA. The E-cadherin cell-cell adhesion complex and lung cancer invasion, metastasis, and prognosis. Lung Cancer. 2002;36:115–124. doi: 10.1016/S0169-5002(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 7.Qiang H, Chang Q, Xu J, Qian J, Zhang Y, Lei Y, Han B, Chu T. New advances in antiangiogenic combination therapeutic strategies for advanced non-small cell lung cancer. J Cancer Res Clin Oncol. 2020;146:631–645. doi: 10.1007/s00432-020-03129-6. [DOI] [PubMed] [Google Scholar]
- 8.Kohutek F, Stratena M, Rosik A, Tamasova M, Bystricky B. First-line treatment of nonsquamous NSCLC using gemcitabine: A retrospective study of real-life practice. Lung Cancer Manag. 2016;5:123–130. doi: 10.2217/lmt-2016-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu BD, Guo J, Ye YZ, Du T, Cheng CS, Jiang Q, Liu RN, Zhang YB. Specific inhibitor of Notch3 enhances the sensitivity of NSCLC cells to gemcitabine. Onco Rep. 2018;40:155–164. doi: 10.3892/or.2018.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komiyama S, Kugimiya T, Takeya C, Takahashi R, Kubushiro K. Platinum-resistant recurrent ovarian cancer with long survival on bevacizumab and gemcitabine. J Obstet Gynaecol Res. 2018;44:1330–1334. doi: 10.1111/jog.13664. [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, Lee SC, Oh SY, Song SY, Lee N, Nam EM, Lee S, Hwang IG, Lee HR, Lee KT, et al. Attenuated FOLFIRINOX in the salvage treatment of gemcitabine-refractory advanced pancreatic cancer: A phase II study. Cancer Commun (Lond) 2018;38:32. doi: 10.1186/s40880-018-0304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yalçin S, Erkan M, Ünsoy G, Parsian M, Kleeff J, Gündüz U. Effect of gemcitabine and retinoic acid loaded PAMAM dendrimer-coated magnetic nanoparticles on pancreatic cancer and stellate cell lines. Biomed Pharmacother. 2014;68:737–743. doi: 10.1016/j.biopha.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Xu B, Tao ZZ. Piceatannol enhances the antitumor efficacy of gemcitabine in human A549 non-small cell lung cancer cells. Onco Res. 2014;22:213–217. doi: 10.3727/096504015X14386062091398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshitomi S, Taira N, Doihara H, Mizoo T, Nogami T, Iwamoto T, Motoki T, Shien T, Ogasawara Y, Matsuoka J, et al. A phase 1, dose-finding and pharmacokinetic study of gemcitabine with nab-paclitaxel in patients with metastatic breast cancer. Cancer Chemother Pharmacol. 2016;78:289–294. doi: 10.1007/s00280-016-3091-x. [DOI] [PubMed] [Google Scholar]
- 15.Sakurai R, Tomizawa Y, Yoshii A, Miura Y, Tsurumaki H, Kaira K, Sunaga N, Kawashima O, Hisada T, Yamada M, Saito R. A phase II study of biweekly gemcitabine and carboplatin in completely resected stage IB-IIIA non-small cell lung cancer. Cancer Chemother Pharmacol. 2018;81:103–109. doi: 10.1007/s00280-017-3439-x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Gold KA, Kim E. Sorafenib in non-small cell lung cancer. Expert OpinInvestig Drugs. 2012;21:1417–1426. doi: 10.1517/13543784.2012.699039. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Chen YL, Ji G, Fang W, Gao Z, Liu Y, Wang J, Ding X, Gao F. Sorafenib inhibits epithelial-mesenchymal transition through an epigenetic-based mechanism in human lung epithelial cells. PLoS One. 2013;8:e64954. doi: 10.1371/journal.pone.0064954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takezawa K, Okamoto I, Yonesaka K, Hatashita E, Yamada Y, Fukuoka M, Nakagawa K. Sorafenib inhibits non-small cell lung cancer cell growth by targeting B-RAF in KRAS wild-type cells and C-RAF in KRAS mutant cells. Cancer Res. 2009;69:6515–6521. doi: 10.1158/0008-5472.CAN-09-1076. [DOI] [PubMed] [Google Scholar]
- 19.Ngamphaiboon N, Dy GK, Ma WW, Zhao Y, Reungwetwattana T, DePaolo D, Ding Y, Brady W, Fetterly G, Adjei AA. A phase I study of the histone deacetylase (HDAC) inhibitor entinostat, in combination with sorafenib in patients with advanced solid tumors. Invest New Drugs. 2015;33:225–232. doi: 10.1007/s10637-014-0174-6. [DOI] [PubMed] [Google Scholar]
- 20.Jean GW, Mani RM, Jaffry A, Khan SA. Toxic effects of sorafenib in patients with differentiated thyroid carcinoma compared with other cancers. JAMA Oncol. 2016;2:529–534. doi: 10.1001/jamaoncol.2015.5927. [DOI] [PubMed] [Google Scholar]
- 21.Pignochino Y, Dell'Aglio C, Basiricò M, Capozzi F, Soster M, Marchiò S, Bruno S, Gammaitoni L, Sangiolo D, Torchiaro E, et al. The combination of sorafenib and everolimus abrogates mTORC1 and mTORC2 upregulation in osteosarcoma preclinical models. Clin Cancer Res. 2013;19:2117–2131. doi: 10.1158/1078-0432.CCR-12-2293. [DOI] [PubMed] [Google Scholar]
- 22.Pal HC, Baxter RD, Hunt KM, Agarwal J, Elmets CA, Athar M, Afaq F. Fisetin, a phytochemical, potentiates sorafenib-induced apoptosis and abrogates tumor growth in athymic nude mice implanted with BRAF-mutated melanoma cells. Oncotarget. 2015;6:28296–28311. doi: 10.18632/oncotarget.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swarnakar NK, Thanki K, Jain S. Enhanced antitumor efficacy and counterfeited cardiotoxicity of combinatorial oral therapy using Doxorubicin- and Coenzyme Q10-liquid crystalline nanoparticles in comparison with intravenous Adriamycin. Nanomedicine. 2014;10:1231–1241. doi: 10.1016/j.nano.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Das M, Jain R, Agrawal AK, Thanki K, Jain S. Macromolecular bipill of gemcitabine and methotrexate facilitates tumor-specific dual drug therapy with higher benefit-to-risk ratio. Bioconjug Chem. 2014;25:501–509. doi: 10.1021/bc400477q. [DOI] [PubMed] [Google Scholar]
- 25.Gridelli C, Morgillo F, Favaretto A, de Marinis F, Chella A, Cerea G, Mattioli R, Tortora G, Rossi A, Fasano M, et al. Sorafenib in combination with erlotinib or with gemcitabine in elderly patients with advanced non-small-cell lung cancer: A randomized phase II study. Ann Oncol. 2011;22:1528–1534. doi: 10.1093/annonc/mdq630. [DOI] [PubMed] [Google Scholar]
- 26.Jiang S, Fan J, Wang Q, Ju D, Feng M, Li J, Guan ZB, An D, Wang X, Ye L. Diosgenin induces ROS-dependent autophagy and cytotoxicity via mTOR signaling pathway in chronic myeloid leukemia cells. Phytomedicine. 2016;23:243–252. doi: 10.1016/j.phymed.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Hamed SS, Straubinger RM, Jusko WJ. Pharmacodynamic modeling of cell cycle and apoptotic effects of gemcitabine on pancreatic adenocarcinoma cells. Cancer Chemother Pharmacol. 2013;72:553–563. doi: 10.1007/s00280-013-2226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia Q, Zhang X, Chen Q, Chen X, Teng J, Wang C, Li M, Fan L. Down-regulation of tissue factor inhibits invasion and metastasis of non-small cell lung cancer. J Cancer. 2020;11:1195–1202. doi: 10.7150/jca.37321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgensztern D, Ng SH, Gao F, Govindan R. Trends in stage distribution for patients with non-small cell lung cancer: A national cancer database survey. J Thorac Oncol. 2010;5:29–33. doi: 10.1097/JTO.0b013e3181c5920c. [DOI] [PubMed] [Google Scholar]
- 31.Jiang S, Wang Q, Feng M, Li J, Guan Z, An D, Dong M, Peng Y, Kuerban K, Ye L. C2-ceramide enhances sorafenib-induced caspase-dependent apoptosis via PI3K/AKT/mTOR and Erk signaling pathways in HCC cells. Appl Microbiol Biotechnol. 2017;101:1535–1546. doi: 10.1007/s00253-016-7930-9. [DOI] [PubMed] [Google Scholar]
- 32.Petrelli A, Giordano S. From single- to multi-target drugs in cancer therapy: When aspecificity becomes an advantage. Curr Med Chem. 2008;15:422–432. doi: 10.2174/092986708783503212. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Pan YY, Zhang Y. Synergistic interaction between sorafenib and gemcitabine in EGFR-TKI-sensitive and EGFR-TKI-resistant human lung cancer cell lines. Oncol Lett. 2013;5:440–446. doi: 10.3892/ol.2012.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao ZJ, Guo YH, Zhao Z, Yao JT, Xu R, Nan KJ. Gemcitabine inhibits the micrometastasis of non-small cell lung cancer by targeting the EpCAM-positive circulating tumor cells via the HGF/cMET pathway. Int J Oncol. 2014;45:651–658. doi: 10.3892/ijo.2014.2464. [DOI] [PubMed] [Google Scholar]
- 35.Cervello M, Bachvarov D, Lampiasi N, Cusimano A, Azzolina A, McCubrey JA, Montalto G. Molecular mechanisms of sorafenib action in liver cancer cells. Cell Cycle. 2012;11:2843–2855. doi: 10.4161/cc.21193. [DOI] [PubMed] [Google Scholar]
- 36.Pasqualetti G, Ricciardi S, Mey V, Del Tacca M, Danesi R. Synergistic cytotoxicity, inhibition of signal transduction pathways and pharmacogenetics of sorafenib and gemcitabine in human NSCLC cell lines. Lung Cancer. 2011;74:197–205. doi: 10.1016/j.lungcan.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Wang S, Su ZF, Yuan Y. Synergistic effects of sorafenib in combination with gemcitabine or pemetrexed in lung cancer cell lines with K-ras mutations. Contemp Oncol (Pozn) 2016;20:33–38. doi: 10.5114/wo.2016.58499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo R, Hu T, Liu Y, He Y, Cao Y. Long non-coding RNA PRNCR1 modulates non-small cell lung cancer cells proliferation, apoptosis, migration, invasion and EMT through PRNCR1/miR-126-5p/MTDH axis. Biosci Rep BSR20193153. 2020 doi: 10.1042/BSR20193153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee MY, Chou CY, Tang MJ, Shen MR. Epithelial- mesenchymal transition in cervical cancer: Correlation with tumor progression, epidermal growth factor receptor overexpression, and snail up-regulation. Clin Cancer Res. 2008;14:4743–4750. doi: 10.1158/1078-0432.CCR-08-0234. [DOI] [PubMed] [Google Scholar]
- 40.Garg M. Epithelial, mesenchymal and hybrid epithelial/mesenchymal phenotypes and their clinical relevance in cancer metastasis. Expert Rev Mol Med. 2017;19:e3. doi: 10.1017/erm.2017.6. [DOI] [PubMed] [Google Scholar]
- 41.He R, Zhang FH, Shen N. LncRNA FEZF1-AS1 enhances epithelial-mesenchymal transition (EMT) through suppressing E-cadherin and regulating WNT pathway in non-small cell lung cancer (NSCLC) Biomed Pharmacother. 2017;95:331–338. doi: 10.1016/j.biopha.2017.08.057. [DOI] [PubMed] [Google Scholar]
- 42.Tsai CH, Lin LT, Wang CY, Chiu YW, Chou YT, Chiu SJ, Wang HE, Liu RS, Wu CY, Chan PC, et al. Over-expression of cofilin-1 suppressed growth and invasion of cancer cells is associated with up-regulation of let-7 microRNA. Biochim Biophys Acta. 2015;1852:851–861. doi: 10.1016/j.bbadis.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Li C, Yang Y, Liu X, Li R, Zhang M, Yin Y, Qu Y. Synaptotagmin 7 in twist-related protein 1-mediated epithelial-Mesenchymal transition of non-small cell lung cancer. EBioMedicine. 2019;46:42–53. doi: 10.1016/j.ebiom.2019.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
SSJ and YFY conceived and designed the present study. SSJ, RW, FHW, XZ and SNL performed the experiments. SSJ wrote the paper. RW, XZ, SNL, FHW and YFY reviewed and edited the manuscript. All authors read and approved the final manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.