Abstract
Bag3 is a Bag family co-chaperone that regulates the ATPase activity of Hsp70 (heat-shock protein 70) chaperones. Recent studies have demonstrated that Bag3 can initiate macroautophagy in co-operation with small heat-shock protein HspB8. In this issue of the Biochemical Journal, Fuchs and co-workers have discovered the IPV motif in Bag3 that is necessary for binding to HspB8. The authors have also identified HspB6 as a new binding partner for Bag3 and characterized further the binding of both HspB8 and HspB6 in Bag3-mediated clearance of aggregated polyglutamine-containing protein Htt43Q (huntingtin exon 1 fragment with 43 CAG repeats). It is clear from recent identification of a Bag3 mutation that causes a form of muscular dystrophy that the full function of Bag3 in disease is not clear. We will apply the findings of Fuchs et al. in this issue to reconcile the phenotypes of Bag3 homologue knockouts with the emerging role of Bag3 in autophagy.
Keywords: autophagy, Bag3, co-chaperone, HspB6, HspB8, muscular dystrophy, protein degradation
Heat-shock proteins (Hsps), also called molecular chaperones, perform folding, stabilization, trafficking and degradation functions to maintain cellular homoeostasis. Mutation, overexpression or deletion of chaperones results in destabilization and misfolding of proteins, which can result in disease. Chaperone proteins are therefore now garnering more attention for their roles in disease initiation and progression. Involvement of Hsp70 and Hsp90, sHsps (small Hsps) has been described recently in protein aggregation diseases such as Huntington’s, Alzheimer’s and prion diseases [1]. Increases in chaperone expression and activity are hypothesized to provide support for overexpressed or mutated proteins in cancer. Additionally, Hsps have been shown to contribute to the maintenance or restoration of tissue integrity and contractile function in cardiac and skeletal muscle tissue.
It is important to note that molecular chaperones act in concert with additional factors that are often called co-chaperones. The term ‘co-chaperone’ can be loosely defined as a non-substrate protein that binds to and affects the activity of chaperone proteins. These co-chaperones can modulate chaperone activity through conformational shifts that induce behaviours such as recruitment to a specific cellular compartment, introduction to substrate client proteins and/or nucleotide exchange. One example of a co-chaperone family that has been implicated recently in the development of disease is the Bag domain-containing protein family. The Bag family of proteins has been characterized traditionally by their binding and modulation of the ATPase activity of the Hsp70 chaperone family, despite their initial identification as interaction partners of the anti-apoptotic protein Bcl-2 [1]. Structural studies have shown that binding of the triple-helix Bag domain to the Hsc70 (heat-shock cognate 70) ATPase domain occurs in the C-terminal lobe of Hsp70 adjacent to the deep ATP-binding crevice. In contrast with Hsp70 co-chaperones such as the Hip and J domain proteins that enhance refolding of firefly luciferase by Hsp70, Bag1 inhibits this refolding to maintain heat-inactivated luciferase in an insoluble, enzymatically inactive state. Other Bag proteins have since been confirmed to bind to Hsp70 family proteins, and Bag2 appears to be the only family member that enhances rather than suppresses Hsp70 mediated refolding of denatured substrates. These and other observations have now resulted in general acceptance that the primary function of Bag domain-containing proteins is to regulate protein degradation rather than folding.
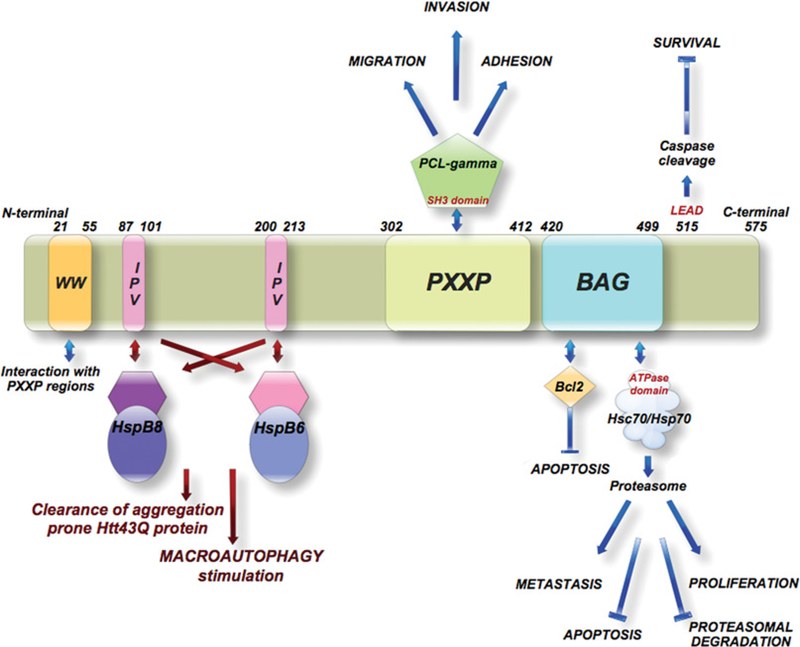
In the current issue of the Biochemical Journal, Fuchs et al. [2] have contributed a significant new finding to the growing body of knowledge about Bag3, a Bag-family member. As found with other co-chaperones, Bag3 has a modular structure that contains many domains to mediate potential interactions with chaperones and/or other proteins (Figure 1). The authors have identified the existence of two conserved IPV (Ile-Pro-Val) motifs in the Bag3 central region between the WW (Trp-Trp) domain and PXXP (proline-rich repeat) region. Although there has been no function identified yet for the WW domain, studies of Bag3 have implicated the PXXP region in diverse behaviours, including binding to the SH3 (Src homology 3)-containing protein PLC-γ (phospholipase C-γ) and stimulation of invasion and migration in models of cancer [3]. The authors present convincing data that the IPV motif is necessary for Bag3 binding to the sHsps HspB8 (Hsp22) and HspB6 (Hsp20). Interestingly, HspB8 and HspB6 do not have the IPV motif, although it is found in other sHsps, such as Hsp27 and αβ-crystallin [2]. The authors conclude from this study that HspB8 and HspB6 instead use the Bag3 IPV motif to form hetero-oligomers that are necessary for HspB8 and HspB6 chaperone activity.
Figure 1. Human Bag3 protein domains and their interactions.
The two newly identified IPV motifs are the site for binding for the complex formation of Bag3 with HspB6 and HspB8. Both sHsps interact through their hydrophobic groove to form the Bag3 complex. Flanking the IPV motifs are the WW domain, consisting of two tryptophan residues spaced 20–22 amino acids apart for which no function has been identified, and the proline-rich (PXXP) region documented to bind proteins containing an SH3 motif. The BAG domain consists of three anti-parallel α-helices, the last two of which bind with high affinity to the ATPase domain of Hsps 70 (Hsp70/Hsc70). Bag3 also associates with the anti-apoptotic protein Bcl-2 through its BAG domain. The C-terminal 515LEAD, conserved in higher mammals, is a functional caspase recognition and cleavage site.
The findings of these authors lead to several questions that still to be answered regarding the interaction of Bag3 with the sHsps. Most importantly, how does the interaction of Bag3 with the sHsps relate to the known Bag3 knockout, mutation and deletion phenotypes? The most obvious answer is that the sHsps and Bag3 co-operate in regulation of protein degradation that affects many cellular processes. Protein degradation through both the proteasome and autophagy pathways is regulated by Bag3 and its interactors [4]. Initially, studies using the Hsp90 inhibitor geldanamycin to induce client protein degradation demonstrated that Bag3 inhibited proteasomal degradation of ubiquitinated Hsp70 substrates [1]. A subsequent study by Gamerdinger et al. [4] that described the role of Bag3 in protein quality control during aging clarified further the role of Bag proteins in regulating degradation of proteins. The authors showed that the Bag proteins act as a molecular switch mechanism to determine whether proteins are degraded in a proteasomal or autophagic manner. In young cells, the proportion of Bag1 was high, which correlated with high proteasomal activity, whereas in old cells induction of Bag3 stimulated autophagic degradation of substrate proteins. Additionally, Bag3 was found to act in concert with the ubiquitin-binding protein SQSTM1 (sequestosome 1) to increase macroautophagic activity.
Until recently, evidence for participation of chaperone proteins in autophagy was limited to Hsp70 recognition of substrates containing a specific sequence, and subsequent delivery of those substrates to the lysosome for degradation [5]. In contrast, the primary role of sHsps, such as HspB8 and HspB6, was thought to prevent protein aggregation as a function of their chaperone activity, with no known direct involvement in autophagic degradation of proteins. Studies have now indirectly implicated HspB8 in the stimulation of macroautophagy through its interaction with Bag3, where it may act to present substrate proteins to the autophagic machinery for degradation [6]. Hsp70-driven chaperone-mediated autophagy (CMA) is performed on a smaller scale by inducing the translocation of specific substrates across the lysosomal membrane. Macroautophagy is thought to be a less specific means of large-scale degradation [5]. In macroautophagy, cellular structures are internalized in lysosomes and broken down by resident enzymes into their constituent building blocks that are then released and used by the cell to synthesize new structures.
Bag3 knockout mice show muscle defects resulting in fulminant myopathy, which is characterized by non-inflammatory myofibrillar degeneration [7,8]. It is enticing to think that the deteriorated state of the muscles in the Bag3-deficient mice may be due to a lack of Bag3-stimulated autophagy. Normal build-up of oxidatively damaged proteins in muscles during the aging process may require the larger-scale degradation method of macroautophagy. HspB6 is induced concurrently during skeletal muscle aging and may also contribute to stimulation of autophagy [5]. It can be hypothesized that the Bag3–sHsp complex stimulates the autophagic process after Bag3 is up-regulated in an HSF-1(heat-shock factor 1)-dependent manner in response to disrupted cellular homoeostasis from oxidative stress. However, in a Bag3-deficient environment autophagy may not be an option for protein degradation in response to protein misfolding or oxidation. This may explain the increased products of lipid peroxidation and elevated levels of oxidative stress-responsive enzymes, such as the superoxide dismutases in tissues from muscular dystrophy patients [9]. It can be hypothesized that deletion of Bag3 would hinder autophagy initiation and force the cells to instead choose death because they would have no mechanism to degrade the oxidized proteins. This cell death could then result in deteriorating muscle tissue, like that seen in Bag3 knockout mice and muscular dystrophy patients.
Another possible role for the Bag3–sHsp complex in autophagy stimulation is its interaction with calcium signalling pathways. Studies have recently shown that an mTOR (mammalian target of rapamycin)-independent pathway exists for the induction of autophagy [5]. This pathway reduces free inositol and Ins(1,4,5)P3 levels through the inhibition of IMPase (inositol monophosphatase) and is regulated by intracellular calcium and cAMP levels. Autophagy is inhibited when intracellular cAMP levels are increased, which leads to activation of PLC. This results in the release of calcium from endoplasmic reticulum stores to increase intracytosolic calcium levels. Conversely, low calcium levels induce autophagy. Previous studies have shown that Bag3 is induced by decreased calcium influx as a result of exposure to CAI (carboxyamido-triazole), an inhibitor of non-voltage-gated calcium channels [3]. It is tempting to imagine that signalling to initiate autophagy through the mTOR-independent pathway causes induction of Bag3 through modulation of intracytosolic calcium. Bag3 then would be able to stimulate downstream pathways to increase autophagic processes. It is reasonable to hypothesize that the stimulation of autophagy by Bag3 involves its binding to PLC-γ, both because the PXXP region that it binds is necessary for the stimulation of autophagy by Bag3 and because Bag3 binding to PLC-γ is increased in response to CAI treatment. However, siRNA (small interfering RNA)-mediated knockdown of PLC-γ did not reduce the ability of Bag3 to stimulate clearance of Htt43Q (huntingtin exon 1 fragment with 43 CAG repeats), indicating that PLC-γ may not contribute to Bag3 induction of autophagy. Further study of the PXXP domain-binding partners will be likely to reveal a new interaction critical for Bag3 stimulation of autophagy. Additional studies are also needed to dissect the exact role of sHsp binding to Bag3 in response to calcium flux.
Two other important questions remain regarding the Bag3 and sHsp interaction. First, it is important to determine whether the mutation of the IPV motifs that causes a dominant form of childhood muscular dystrophy [10] alters Bag3 binding to Hsp70, and, more importantly, whether Bag3 still is able to stimulate Hsp70 nucleotide exchange when it harbours the mutation. To date there have been no links between sHsp and Hsp70 function, although both are involved in management of protein aggregation. The separation of the Bag3 IPV motifs from the Bag domain may allow Bag3 to bind both a sHsp and Hsp70 simultaneously and provide the missing link between the two chaperone families. However, it is more likely that Bag3 binds them at separate times as a regulatory step in switching from proteasomal degradation to autophagy. This binding to one of the Bag3 domains may induce a conformational shift that would prevent binding at the other site. Unfortunately, no crystal structure of Bag3 or Bag3–Hsp70 has been published, which limits the analysis of any potential impact of the IPV mutation on the structure of Bag3.
Secondly, it is possible that the IPV motif is altering the oligomerization of Bag3 with partners other than sHsps. Previous studies have established that the IxI/V sequence is mostly conserved in the α-crystallin family of sHsps. Point mutations in the IPV motif found in members of the α-crystallin family cause changes in oligomerization status. This oligomerization is critical for their chaperone function in protein disaggregation and refolding or degradation [2]. Because this motif plays such an important role in the chaperone activity of these sHsps through regulation of their oligomerization, it can be hypothesized that Bag3 may also contain this motif to mediate other hetero- or homo-oligomerization that is important to its functions. There is evidence that Bag3 may have independent chaperone activity, but in most cases probably requires a partner protein such as Hsp70 or one of the sHsps to directly affect protein homoeostasis. The identification of the IPV motif has already yielded HspB6 as a new binding partner for Bag3 [2], but others may yet be identified. Future studies are needed to dissect the potential relationships of Bag3 with other sHsps, other proteins that interact with IPV motifs and, possibly, even oligomerization of Bag3 with itself.
Significant advances have been made to develop a new model for the role of Bag3 in protein quality control. Previously, Bag3 was characterized largely by its role as a nucleotide-exchange factor for Hsp70. However, this function could not easily explain the development of myopathy and skeletal muscle defects observed in Bag3 knockout mice and Drosophila respectively. New data now suggest a significant role for Bag3 in stimulating macroautophagy in concert with its newly established binding partners HspB6 and HspB8. Fuchs et al. [2] have contributed to this new hypothesis by identifying the IPV motif required for Bag3 binding to HspB8 and HspB6, both of whom are involved in protein quality control and clearance. It is imperative that future experiments dissect the contribution of this interaction to autophagy stimulation and also define further the role of Bag3 in autophagy signalling. These data, combined with previous studies will provide a new window into the pathology of skeletal muscle disease and a possible new target for therapeutic intervention.
Abbreviations used
- CAI
carboxyamido-triazole
- Hsc70
heat-shock cognate 70
- Hsp 70/90
heat-shock protein 70/90
- mTOR
mammalian target of rapamycin
- SH3
Src homology 3
- sHsp
small Hsp
- PLC-γ
phospholipase C-γ
- PXXP
proline-rich repeat
REFERENCES
- 1.Doong H, Vrailas A and Kohn EC (2002) What’s in the ‘BAG’? – A functional domain analysis of the BAG-family proteins. Cancer Lett. 188, 25–32 [DOI] [PubMed] [Google Scholar]
- 2.Fuchs M, Poirier DJ, Seguin S, Lambert H, Carra S, Charrette SJ and Landry J (2010) Identification of the key structural motifs involved in HspB8/HspB6–Bag3 interaction. Biochem. J. 425, 245–255 [DOI] [PubMed] [Google Scholar]
- 3.Doong H, Price J, Kim YS, Gasbarre C, Probst J, Liotta LA, Blanchette J, Rizzo K and Kohn E (2000) CAIR-1/BAG-3 forms an EGF-regulated ternary complex with phospholipase C-γ and Hsp70/Hsc70. Oncogene 19, 4385–4395 [DOI] [PubMed] [Google Scholar]
- 4.Gamerdinger M, Hajieva P, Kaya AM, Wolfrum U, Hartl FU and Behl C (2009) Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J. 28, 889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravikumar B, Futter M, Jahreiss L, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Narayanan U, Renna M et al. (2009) Mammalian macroautophagy at a glance. J Cell Sci. 122, 1707–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carra S, Seguin SJ, Lambert H and Landry J (2008) HspB8 chaperone activity toward poly(Q)-containing proteins depends on its association with Bag3, a stimulator of macroautophagy. J. Biol. Chem. 283, 1437–1444 [DOI] [PubMed] [Google Scholar]
- 7.Youn DY, Lee DH, Lim MH, Yoon JS, Lim JH, Jung SE, Yeum CE, Park CW, Youn HJ, Lee JS et al. (2008) Bis deficiency results in early lethality with metabolic deterioration and involution of spleen and thymus. Am. J. Physiol. Endocrinol. Metab. 295, E1349–E1357 [DOI] [PubMed] [Google Scholar]
- 8.Homma S, Iwasaki M, Shelton GD, Engvall E, Reed JC and Takayama S (2006) BAG3 deficiency results in fulminant myopathy and early lethality. Am. J. Pathol. 169, 761–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson MJ (2008) Redox regulation of skeletal muscle. IUBMB Life 60, 497–501 [DOI] [PubMed] [Google Scholar]
- 10.Selcen D, Muntoni F, Burton BK, Pegoraro E, Sewry C, Bite AV and Engel AG (2009) Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Ann. Neurol. 65, 83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]