Abstract
Background
Dramatic hemodynamic fluctuation occurs frequently during surgery for pheochromocytoma or paraganglioma. However, the criteria of intraoperative hemodynamic instability vary widely, and most of them were defined arbitrarily but not according to patients’ prognosis. The objective was to analyze the relationship between different thresholds and durations of intraoperative hyper−/hypotension and the risk of postoperative complications in patients undergoing surgery for pheochromocytoma or paraganglioma.
Methods
This was a retrospective single-center cohort study performed in a tertiary care hospital from January 1, 2005 to December 31, 2017. Three hundred twenty-seven patients who underwent surgery for pheochromocytoma or paraganglioma, of which the diagnoses were confirmed by postoperative pathologic examination, were enrolled. Those who were less than 18 years, underwent surgery involving non-tumor organs, or had incomplete data were excluded. The primary endpoint was a composite of the occurrence of AKI or other complications during hospital stay after surgery. Multivariate Logistic regression models were used to analyze the association between different thresholds and durations of intraoperative hyper−/hypotension and the development of postoperative complications.
Results
Forty three (13.1%) patients developed complications during hospital stay after surgery. After adjusting for confounding factors, intraoperative hypotension, defined as systolic blood pressure (SBP) of ≤95 mmHg for ≥20 min (OR 3.211; 99% CI 1.081–9.536; P = 0.006), SBP of ≤90 mmHg for ≥20 min (OR 3.680; 98.8% CI 1.107–12.240; P = 0.006), SBP of ≤85 mmHg for ≥10 min (OR 3.975; 98.3% CI 1.321–11.961; P = 0.003), and SBP of ≤80 mmHg for ≥1 min (OR 3.465; 95% CI 1.484–8.093; P = 0.004), were associated with an increased risk of postoperative complications. On the other hand, intraoperative hypertension was not significantly associated with the development of postoperative complications.
Conclusions
For patients undergoing surgery for pheochromocytoma or paraganglioma, intraoperative hypotension is associated with increased postoperative complications; and the harmful effects are level- and duration-dependent. The effects of intraoperative hypertension need to be studied further.
Keywords: Pheochromocytoma-paraganglioma, Intraoperative hypotension, Postoperative complications
Background
Pheochromocytoma and paraganglioma are uncommon neuroendocrine diseases with a combined annual incidence rate from 0.29 to 0.46 per 100,000 person-years [1]. Surgical resection is the mainstay for the management of these tumors. Because of excessive intraoperative catecholamine release, especially during endotracheal intubation, creation of pneumoperitoneum, manipulation of tumor, and dividing adrenal vein [2–4], dramatic hemodynamic fluctuation occurs frequently, making the perioperative management a great challenge for the anesthesiologists and intensivists.
Much attention has been paid to the hemodynamic management of these patients during surgery [5–7]. However, the criteria of intraoperative hemodynamic instability vary widely, including absolute thresholds and/or relative changes of systolic blood pressure (SBP), mean arterial pressure (MAP) and even heart rate (HR), leading to reported rates range from 22.7 to 91.1% [8–10]. More importantly, most of these criteria were defined arbitrarily but not according to the risks of postoperative morbidity and mortality.
In previous studies of patients following non-cardiac surgery, intraoperative hypotension is found to be associated with increased risks of acute kidney injury (AKI) [11, 12], myocardial injury [12, 13], delirium [14], stroke [15], and even death [16, 17]. On the other hand, intraoperative hypertension is also reported to be associated with adverse outcomes [18]; although there are evidences that the harmful effects of intraoperative hypertension might be less than those of intraoperative hypotension [19]. Extreme hyper- and hypotension are more likely to occur during surgery for pheochromocytoma or paraganglioma. We, therefore, hypothesized that intraoperative hyper- and/or hypotension were associated with the development of postoperative complications.
In this study, we aimed to analyze the relationship between different thresholds and durations of intraoperative hyper−/hypotension and the risk of postoperative complications in patients undergoing surgery for pheochromocytoma or paraganglioma.
Methods
This was a retrospective single-center cohort study. The database used in the present study was established years ago after approval by the Clinical Research Ethics Committee of Peking University First Hospital, Beijing, China (2016–1062) [10]. For the purpose of the present study, we updated the database after another ethical approval (2018–47). All data were retrospectively collected. Because of the retrospective nature and that all data of patients were collected from the medical records, the local Ethics Committee agreed to exempt the written informed consent. However, the privacy of all participants was strictly protected.
Patients
Potential participants were patients who underwent surgery for pheochromocytoma or paraganglioma, of which the diagnoses were confirmed by postoperative pathologic examination, from January 1, 2005 to December 31, 2017 in Peking University First Hospital. Patients who met any of the following criteria were excluded: (1) age less than 18 years; (2) surgery involving non-tumor organs; (3) incomplete data collected from the medical record system.
Data collection
Patients’ data were collected from the electronic medical record system of the hospital. Baseline and preoperative data included demographic characteristics (age, gender, body mass index [BMI]), previous comorbidity, American Society of Anesthesiology (ASA) classification, preoperative hemoglobin, baseline serum catecholamine level, size and location of tumor, medical treatment, as well as blood pressure and heart rate before surgery.
Intraoperative data included period of surgery, type and duration of anesthesia, type and duration of surgery, estimated blood loss, minimal hemoglobin, blood transfusion, positive fluid balance, hemodynamic parameters, use of antihypertensive drugs, and use of vasopressors.
Postoperative data included use and duration of vasopressors, intensive care unit (ICU) admission, use and duration of mechanical ventilation, length of stay in ICU and hospital after surgery, occurrence of complications during hospital stay, and in-hospital mortality.
The primary endpoint was a composite of the occurrence of AKI or other complications during hospital stay after surgery. AKI and its stage were diagnosed according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria using the maximal change in serum creatinine value compared with the preoperative baseline and urine output [20]. Other postoperative complications were defined as any deviation from the normal postoperative course which required pharmacological treatment or interventional procedures, i.e., grade II or higher on the Clavien-Dindo classification [21].
To ensure the accuracy of the outcome database, two investigators collected the information of postoperative outcomes separately and independently. They were blinded to intraoperative blood pressure management at the time of postoperative data collection. In case of any difference between the two investigators, final agreement was achieved by rechecking medical records and full discussion with a senior physician.
Preoperative antihypertensive treatment
For a patient with diagnosed or strongly suspected pheochromocytoma or paraganglioma, the attending physician/surgeon would initiate the α-blocker therapy with a starting dose while decreasing or ceasing other antihypertensive drugs used before. The dose of α-blockers was gradually adjusted to achieve a target blood pressure while avoiding orthostatic hypotension. During this process, a calcium channel blocker and/or a β-adrenergic receptor blocker might be added when considered necessary.
Intraoperative blood pressure management
In case of hypertension which commonly occurred during stimulation or tumor manipulation, anesthesia depth was firstly checked and deepened when necessary. When hypertension persisted, intravenous antihypertensives such as phentolamine, urapidil and/or nicardipine as well as a short-acting beta-blocker esmolol were administered. Intravenous antihypertensives were ceased shortly before ligation of tumor vessels. In case of hypotension which frequently occurred after tumor removal, intravascular volume was re-evaluated and fluid resuscitation was performed when considered necessary. When hypotension persisted, intravenous vasopressors such as phenylephrine, norepinephrine and/or epinephrine were administered to maintain adequate organ perfusion.
Processing of intraoperative blood pressure data
The data of blood pressure was captured every 10 s and stored by the electronic anesthesia information system in a real-time manner and were examined separately and independently by two investigators (NL and HK). During episodes when no blood pressure was recorded or when artifacts were marked by the anesthesiologists, the last blood pressure was used to replace the missing data.
For the purpose of analysis, absolute thresholds of SBP with different durations were used to define intraoperative hyper−/hypotension. We adopt this criterion because SBP is the primary target of intervention [22]. Specifically, SBPs of ≥160, 180 and 200 mmHg were used as thresholds for intraoperative hypertension and those of ≤100, 95, 90, 85, and 80 mmHg for intraoperative hypotension, with durations of 1, 5, 10, 20, 30, 40 and 50 min, respectively.
Statistical analysis
Patients’ data were analyzed according to the development of postoperative complications or not. Numeric data with normal distribution were compared using independent samples t rest; numeric data with non-normal distribution or ranked data were compared using Mann-Whitney U test. Categorical data were compared using chi-square test or Fisher’s exact test. Time-to-event data were analyzed by Kaplan-Meier estimator, with difference between groups compared by log- rank test. The association between different thresholds and durations of intraoperative SBP and the development of postoperative complications were analyzed using the Logistic regression models. Independent variables with P values < 0.10 in univariate analyses or were considered clinically important were included in the multivariate models to adjust for confounding factors. Two-sided P values of < 0.05 were considered as statistically significant. For multiple comparisons, the thresholds of P values were adjusted with Bonferroni correction. Statistical analyses were performed with the SPSS statistical package version 25.0 (IBM SPSS Inc., Chicago, IL, USA).
Results
Patients
From January 1, 2005 to December 31, 2017, 367 patients underwent surgery for pheochromocytoma or paraganglioma; among these, 327 met the inclusion/exclusion criteria and were included in final analysis (Fig. 1).
Fig. 1.
Flowchart of the study
Of the enrolled patients, 43 (13.1%) developed AKI or other complications during hospital stay after surgery (Table 1). When compared with patients who did not develop postoperative complications, those who did had a higher male ratio, more comorbidity of diabetes mellitus and previous stroke, larger tumor diameters, and more paragangliomas; they received more calcium channel blockers and combined antihypertensive therapies, had higher preoperative BP and HR, and underwent more surgery between 2010 and 2013 (Table 2). During surgery, patients who developed postoperative complications underwent longer anesthesia/surgery and more open surgery, lost more blood, had lower hemoglobin level, received more blood transfusion, had more positive fluid balance, and were given more antihypertensives and vasopressors (Table 2).
Table 1.
Occurrence and severity of individual complications following surgery
| Complications | Total number | Severity of postoperative complications a | |||||
|---|---|---|---|---|---|---|---|
| II | IIIa | IIIb | IVa | IVb | V | ||
| Stroke b | 2 | 2 | – | – | – | – | – |
| Respiratory complications | 6 | 4 | 2 | – | – | – | – |
| Pulmonary infection c | 4 | 4 | – | – | – | – | – |
| Pleural effusion d | 2 | – | 2 | – | – | – | – |
| Cardiovascular complications | 3 | 2 | 1 | – | – | – | – |
| New onset arrhythmia e | 1 | – | 1 | – | – | – | – |
| Acute myocardial infarction f | 2 | 2 | – | – | – | – | – |
| Surgery-related complications | 6 | 3 | – | 1 | 1 | 1 | – |
| Surgical bleeding g | 3 | – | – | 1 | 1 | 1 | – |
| Ileus h | 3 | 3 | – | – | – | – | – |
| Thromboembolic complications | 3 | 2 | – | – | 1 | – | – |
| Pulmonary embolism i | 1 | – | – | – | 1 | – | – |
| Deep venous thrombosis j | 2 | 2 | – | – | – | – | – |
| Hypoglycemia k | 9 | 9 | – | – | – | – | – |
| Urinary tract infection l | 1 | 1 | – | – | – | – | – |
Data are number
a According to Clavien-Dindo classification
b Persisted new focal neurologic deficit and confirmed by neurologic imaging
c Presence of at least one of the following manifestations, i.e., increased or color-changed sputum, new or changed pulmonary infiltrates, fever, and leukocyte count > 12,000/mm3, and required antibiotic therapy
d Confirmed by chest X-ray or ultrasound examination and required drainage, aspiration, and/or diuresis after albumin administration
e New onset atrial fibrillation or paroxysmal supraventricular tachycardia that necessitated antiarrhythmic therapy
f Concentration of cardiac troponin I exceed the diagnostic criteria for myocardial infarction as well as new Q waves (lasts for 0.03 s) or continuous (4 days) abnormal ST-T segment
g Bleeding after surgery that required secondary surgical hemostasis
h Lack of bowel movement, flatulence, and requirement of parenteral nutrition for more than 1 week after surgery
i Confirmed by computed tomographic pulmonary angiography
j Confirmed by deep venous ultrasonography
k Defined as a documented serum blood glucose level of less than 55 mg/dL
l Confirmed by urinalysis and urine culture and necessitated antibiotic therapy
Table 2.
Baseline and intraoperative variables
| Variables | Without postoperative complications (n = 284) | With postoperative complications (n = 43) | P value a |
|---|---|---|---|
| Age (years) | 46 ± 15 | 48 ± 16 | 0.379 |
| Male gender b | 119 (41.9%) | 28 (65.1%) | 0.004 |
| BMI (kg/m2) | 23.3 ± 3.5 | 23.2 ± 3.7 | 0.811 |
| Preoperative comorbidity | |||
| Diabetes mellitus | 38 (13.4%) | 12 (27.9%) | 0.014 |
| Coronary heart disease | 18 (6.3%) | 1 (2.3%) | 0.487 |
| Previous stroke | 9 (3.2%) | 7 (16.3%) | 0.002 |
| ASA classification b | 0.223 | ||
| 1 | 14 (4.9%) | 2 (4.7%) | |
| 2 | 210 (73.9%) | 26 (60.5%) | |
| 3 | 59 (20.8%) | 15 (34.9%) | |
| 4 | 1 (0.4%) | 0 (0.0%) | |
| Preoperative examination | |||
| Hemoglobin (g/L) | 134 ± 18 | 133 ± 24 | 0.851 |
| Serum catecholamine | |||
| Dopamine (pmol/L) | 0.10 (0.05 to 0.31) | 0.10 (0.06 to 0.44) | 0.412 |
| Norepinephrine (pmol/L) | 11.7 (4.3 to 26.7) | 15.3 (4.9 to 33.1) | 0.397 |
| Epinephrine (pmol/L) | 0.45 (0.09 to 0.90) | 0.66 (0.28 to 1.20) | 0.100 |
| Maximal tumor diameter (cm) b,c | 5.3 ± 2.2 | 6.7 ± 3.2 | 0.012 |
| Paraganglioma b | 53 (18.7%) | 16 (37.2%) | 0.005 |
| Preoperative antihypertensives | |||
| α-AR antagonist d | 246 (86.6%) | 37 (86.0%) | 0.918 |
| Selective α1-AR antagonist | 144 (58.5%) | 20 (54.1%) | 0.607 |
| β-AR antagonist | 65 (22.9%) | 12 (27.9%) | 0.470 |
| Calcium channel blocker | 73 (25.7%) | 20 (46.5%) | 0.005 |
| Combined b | 83 (29.2%) | 22 (51.2%) | 0.004 |
| Intravenous fluid | 140 (49.6%) | 22 (51.2%) | 0.853 |
| Preoperative SBP (mmHg) | 125 ± 17 | 131 ± 13 | 0.040 |
| Preoperative DBP (mmHg) | 77 ± 12 | 81 ± 10 | 0.025 |
| Preoperative HR (bpm) | 74 ± 11 | 78 ± 9 | 0.020 |
| Period of surgery b | 0.005 | ||
| 2005–2009 | 65 (22.9%) | 7 (16.3%) | |
| 2010–2013 | 61 (21.5%) | 19 (44.2%) | |
| 2014–2017 | 158 (55.6%) | 17 (39.5%) | |
| Duration of anesthesia (min) | 214 ± 82 | 308 ± 148 | < 0.001 |
| Type of anesthesia b | 0.055 | ||
| General | 188 (66.2%) | 22 (51.2%) | |
| Combined epidural-general e | 96 (33.8%) | 21 (48.8%) | |
| Duration of surgery (min) b | 129 ± 75 | 223 ± 148 | < 0.001 |
| Type of surgery b | 0.015 | ||
| Open | 89 (31.3%) | 23 (53.5%) | |
| Laparoscopic | 192 (67.6%) | 20 (46.5%) | |
| Transurethral | 3 (1.1%) | 0 (0.0%) | |
| Estimated blood loss (ml) | 100 (50 to 300) | 500 (100 to 1800) | < 0.001 |
| Minimal hemoglobin (g/L) | 110 ± 22 | 96 ± 22 | 0.001 |
| Blood transfusion b,f | 37 (13.0%) | 20 (46.5%) | < 0.001 |
| Positive fluid balance (ml) | 2200 (1500 to 3025) | 3150 (2350 to 5475) | 0.001 |
| Use of antihypertensives | 243 (85.6%) | 41 (95.3%) | 0.077 |
| Combined antihypertensives b,g | 173 (60.9%) | 34 (79.1%) | 0.021 |
| Use of vasopressors b | 129 (45.4%) | 33 (76.7%) | < 0.001 |
| Combined vasopressors h | 46 (16.2%) | 16 (37.2%) | 0.001 |
Data are presented as mean ± SD, number (%), or median (interquartile range)
BMI Body mass index, ASA American Society of Anesthesiologists, AR Adrenergic receptor, SBP Systolic blood pressure, DBP Diastolic blood pressure, HR Heart rate
a Comparison between patients with or without postoperative complications
b Variables adjusted in the multivariate model
c Confirmed by postoperative pathologic examination results
d Including phenoxybenzamine, doxazosin and terazosin. Forty four patients did not receive α-AR antagonist therapy due to normal blood pressure and serum catecholamine level before surgery. Diagnosis of pheochromocytoma was confirmed by postoperative pathologic examination
e These patients also received postoperative patient-controlled epidural analgesia (PCEA)
f Includes packed red blood cell, fresh frozen plasma, and/or concentrated platelet
g Combined use of two or more intravenous antihypertensive drugs, including phentolamine, urapidil, nicardipine and/or esmolol
h Combined use of two or more intravenous vasopressors, including phenylephrine, norepinephrine, and/or epinephrine
During the postoperative period, patients who developed postoperative complications received more vasopressors, were admitted to the ICU more frequently, underwent more and longer mechanical ventilation, and stayed longer in the ICU and hospital after surgery. No patient died during hospital stay (Table 3).
Table 3.
Postoperative outcomes
| Variables | Without postoperative complications (n = 284) | With postoperative complications (n = 43) | P value |
|---|---|---|---|
| Acute kidney injury a | – | 23 (53.5%) | – |
| Stage 1 | – | 19 (44.2%) | – |
| Stage 2 | – | 4 (9.3%) | – |
| Other postoperative complications | – | 30 (69.8%) | – |
| Use of vasopressors b | 47 (16.5%) | 19 (44.2%) | < 0.001 |
| Duration of vasopressors (hr) c | 18.2 (11.8, 24.7) | 22.1 (8.8, 35.4) | 0.546 |
| ICU admission | 174 (61.3%) | 41 (95.3%) | < 0.001 |
| Use of MV | 107 (37.7%) | 33 (76.7%) | < 0.001 |
| Duration of MV (hr) d | 4.1 (3.4, 4.8) | 14.7 (5.6, 23.5) | 0.003 |
| Length of ICU stay (day) e | 1.4 (1.2, 1.5) | 2.2 (1.8, 2.7) | < 0.001 |
| Hospital stay after surgery (day) | 5.9 (5.5, 6.2) | 9.5 (7.5, 11.5) | < 0.001 |
| In-hospital mortality | 0 (0.0%) | 0 (0.0%) | – |
Data were presented as number of patients (percentage) or mean (95% confidence interval)
ICU Intensive care unit, MV Mechanical ventilation
a Defined as increase in serum creatinine by ≥26.5 μmol/l within 48 h; or increase in serum creatinine to ≥1.5 times baseline, which is known or presumed to have occurred within the prior 7 days; or urine volume < 0.5 ml/kg/h for 6 h. Stage 1 was defined as serum creatinine 1.5–1.9 times baseline or ≥ 26.5 μmol/l increase or urine output < 0.5 ml/kg/h for 6–12 h; stage 2 was defined as serum creatinine 2.0–2.9 times baseline or urine output < 0.5 ml/kg/h for ≥12 h; stage 3 was defined as serum creatinine 3 times baseline or increase to ≥353.6 μmol/l or initiation of renal replacement therapy or urine output < 0.3 ml/kg/h ≥ 24 h or anuria ≥12 h
b Requirement of vasopressors (norepinephrine or epinephrine) to maintain systolic blood pressure ≥ 90 mmHg after surgery
c Results of patients who required vasopressors after surgery
d Results of patients who required mechanical ventilation in the ICU after surgery
e Results of patients who were admitted to the ICU after surgery
Intraoperative blood pressure
The median percentage of no blood pressure record, calculated as the cumulated duration of no blood pressure record divided by the total duration of anesthesia, was 1.10% (interquartile range [IQR] 0.74 to 1.68%). The median percentage of blood pressure with artifacts, calculated as the cumulated duration of blood pressure with artifacts divided by the total duration of anesthesia, was 0.27% (IQR 0.00 to 0.65%). The durations of SBP below different thresholds were significantly longer after tumor removal than before (Fig. 2).
Fig. 2.
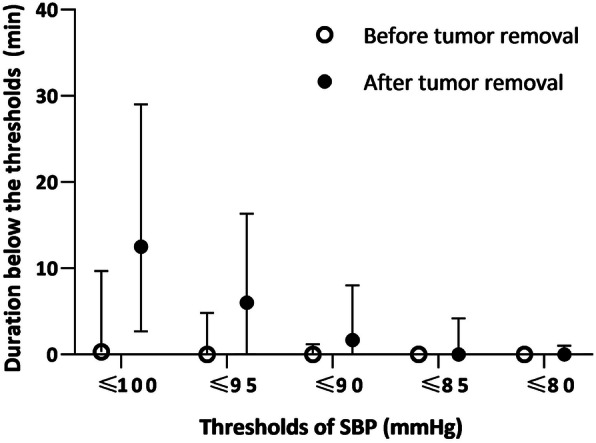
The duration of SBP below different thresholds before and after tumor removal. The spots and whiskers plots show medians (interquartile range). P < 0.001 for all thresholds of SBP. P < 0.01 (0.05/5) were considered statistically significant after Bonferroni correction. SBP, systolic blood pressure
Identification of potential confounding factors
Univariate analyses identified 24 variables with P values < 0.10 (Additional file 1: Supplementary Table 1). After testing for correlation, 12 variables were entered into the multivariate model to adjust for confounding factors, including male gender, ASA classification (3 + 4 vs. 1 + 2), maximal tumor diameter (cm), paraganglioma, preoperative combined antihypertensives, period of surgery, type of anesthesia (combined epidural-general vs. general), duration of surgery (minute), type of surgery (open vs. laparoscopic/transurethral), intraoperative blood transfusion, combined antihypertensives during surgery and use of vasopressor during surgery (Table 4).
Table 4.
Association between intraoperative hyper−/hypotension and postoperative complications
| Thresholds and durations | N | Univariate analysis | Multivariate analysis a | ||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (adjusted CI) | P value | ||
| SBP ≥200 mmHg | (95% CI), P < 0.05 | ||||
| ≥ 1 min | 97 | 1.485 (0.760–2.902) | 0.247 | 1.094 (0.497–2.407) | 0.824 |
| SBP ≥180 mmHg | (98.3% CI), P < 0.017 (0.05/3) b | ||||
| ≥ 1 min | 186 | 1.185 (0.616–2.281) | 0.611 | 0.735 (0.275–1.966) | 0.455 |
| ≥ 5 min | 99 | 1.130 (0.569–2.246) | 0.727 | 0.719 (0.268–1.930) | 0.425 |
| ≥ 10 min | 63 | 1.321 (0.613–2.847) | 0.478 | 0.792 (0.259–2.424) | 0.619 |
| SBP ≥160 mmHg | (99% CI), P < 0.01 (0.05/5) b | ||||
| ≥ 1 min | 270 | 1.099 (0.463–2.611) | 0.831 | 0.753 (0.175–3.240) | 0.617 |
| ≥ 5 min | 213 | 1.655 (0.801–3.423) | 0.174 | 0.963 (0.308–3.016) | 0.933 |
| ≥ 10 min | 161 | 1.217 (0.640–2.314) | 0.550 | 0.702 (0.254–1.940) | 0.370 |
| ≥ 20 min | 92 | 2.043 (1.055–3.958) | 0.034 | 1.142 (0.406–3.213) | 0.740 |
| ≥ 30 min | 53 | 2.294 (1.090–4.828) | 0.029 | 1.234 (0.387–3.933) | 0.641 |
| SBP ≤100 mmHg | (99.3% CI), P < 0.007 (0.05/7) b | ||||
| ≥ 1 min | 278 | 4.065 (0.950–17.390) | 0.059 | 3.141 (0.338–29.226) | 0.166 |
| ≥ 5 min | 243 | 2.335 (0.948–5.749) | 0.065 | 1.710 (0.416–7.037) | 0.306 |
| ≥ 10 min | 211 | 2.685 (1.201–6.002) | 0.016 | 1.839 (0.511–6.617) | 0.200 |
| ≥ 20 min | 162 | 3.017 (1.489–6.113) | 0.002 | 1.809 (0.573–5.714) | 0.164 |
| ≥ 30 min | 121 | 3.043 (1.547–5.882) | 0.001 | 1.871 (0.603–5.801) | 0.136 |
| ≥ 40 min | 83 | 4.293 (2.211–8.336) | < 0.001 | 2.535 (0.796–8.076) | 0.030 |
| ≥ 50 min | 58 | 4.392 (2.199–8.774) | < 0.001 | 2.625 (0.828–8.320) | 0.024 |
| SBP ≤95 mmHg | (99% CI), P < 0.01 (0.05/5) b | ||||
| ≥ 1 min | 245 | 2.253 (0.915–5.551) | 0.077 | 1.746 (0.446–6.834) | 0.293 |
| ≥ 5 min | 217 | 2.452 (1.096–5.487) | 0.029 | 1.807 (0.528–6.180) | 0.215 |
| ≥ 10 min | 170 | 2.695 (1.330–5.458) | 0.006 | 1.916 (0.640–5.735) | 0.126 |
| ≥ 20 min | 106 | 3.900 (2.008–7.547) | < 0.001 | 3.211 (1.081–9.536) | 0.006 |
| ≥ 30 min | 63 | 4.874 (2.465–9.634) | < 0.001 | 3.173 (1.012–9.950) | 0.009 |
| SBP ≤90 mmHg | (98.8% CI), P < 0.012 (0.05/4) b | ||||
| ≥ 1 min | 207 | 2.424 (1.120–5.248) | 0.025 | 1.653 (0.510–5.358) | 0.283 |
| ≥ 5 min | 147 | 2.588 (1.324–5.058) | 0.005 | 1.662 (0.579–4.769) | 0.226 |
| ≥ 10 min | 101 | 3.396 (1.762–6.546) | < 0.001 | 2.160 (0.757–6.159) | 0.065 |
| ≥ 20 min | 53 | 5.122 (2.540–10.332) | < 0.001 | 3.680 (1.107–12.240) | 0.006 |
| SBP ≤85 mmHg | (98.3% CI), P < 0.017 (0.05/3) b | ||||
| ≥ 1 min | 153 | 3.021 (1.512–6.033) | 0.002 | 1.855 (0.647–5.317) | 0.161 |
| ≥ 5 min | 93 | 3.516 (1.822–6.783) | < 0.001 | 2.039 (0.739–5.621) | 0.094 |
| ≥ 10 min | 60 | 4.692 (2.361–9.325) | < 0.001 | 3.975 (1.321–11.961) | 0.003 |
| SBP ≤80 mmHg | (95% CI), P < 0.05 | ||||
| ≥ 1 min | 98 | 5.062 (2.580–9.935) | < 0.001 | 3.465 (1.484–8.093) | 0.004 |
N Number, SBP Systolic blood pressure, OR Odds ratio, CI Confidence interval
a Independent factors with P values < 0.10 in univariate analyses or were considered clinically important were included in the multivariate logistic regression model. These included male gender, ASA classification (3 + 4 vs. 1 + 2), maximal tumor diameter (cm), paraganglioma, preoperative combined antihypertensives, period of surgery, type of anesthesia (combined epidural-general vs. general), duration of surgery (min), type of surgery (open vs. laparoscopic/transurethral), intraoperative blood transfusion, combined antihypertensives during surgery and use of vasopressors during surgery. History of diabetes mellitus and previous stroke were excluded due to correlation with ASA classification; preoperative calcium channel blocker, SBP, DBP and HR were excluded due to correlation with preoperative combined antihypertensives; duration of anesthesia and intraoperative positive fluid balance were excluded due to correlation with duration of surgery; intraoperative minimal hemoglobin and estimated blood loss were excluded due to correlation with intraoperative blood transfusion; use of antihypertensives during surgery was excluded due to correlation with combined antihypertensives during surgery; use of combined vasopressors during surgery were excluded due to correlation with use of vasopressors during surgery
b The threshold level of significance was corrected using the Bonferroni method
Association between intraoperative hyper−/hypotension and postoperative complications
Multivariate Logistic regression analyses showed that SBP of ≤95 mmHg for ≥20 min (OR 3.211; 99% CI 1.081–9.536; P = 0.006), SBP of ≤90 mmHg for ≥20 min (OR 3.680; 98.8% CI 1.107–12.240; P = 0.006), SBP of ≤85 mmHg for ≥10 min (OR 3.975; 98.3% CI 1.321–11.961; P = 0.003), and SBP of ≤80 mmHg for ≥1 min (OR 3.465; 95% CI 1.484–8.093; P = 0.004) were significantly associated with an increased risks of postoperative complications after Bonferroni correction. On the other hand, intraoperative hypertension, defined as SBP of different thresholds for different durations, was not significantly associated with the development of postoperative complications (Table 4).
Discussion
Results of this retrospective study showed that, in patients undergoing surgery for pheochromocytoma or paraganglioma, intraoperative hypotension was associated with an increased risk of postoperative complications; whereas intraoperative hypertension was not. The harmful effects of intraoperative hypotension seemed “dose-dependent”, i.e., higher threshold with longer duration or lower threshold with shorter duration was associated with an increased risk of postoperative complications. Our results provided further evidence regarding the blood pressure management for these patients during surgery.
To avoid ambiguity, postoperative complications and their severities were clearly defined in the present study. In our patients, 13.1% developed postoperative complications; this was consistent with the multicenter study of Brunaud et al. [9] who reported an incidence of 16% after laparoscopic adrenalectomy for pheochromocytoma. Among all individual complications, AKI was the most common one and occurred in 7.0% of our patients, higher than the reported rate of 2.8% after surgery for non-functional adrenal tumors [23], possibly due to more severe/frequent intraoperative hypotension [12, 24]. Other individual complications included hypoglycemia (2.8%), pulmonary infection (1.2%), and so forth (Table 1).
Cumulative evidence showed an association between intraoperative hypotension and postoperative morbidity in patients undergoing abdominal [25, 26], vascular [27] and cardiac surgeries [28]. Intraoperative hypotension was found to be related to the development of myocardial injury [12, 13, 26, 27, 29], AKI [11, 12, 29, 30], and even death [16, 17, 31] after surgery. However, evidence in patients undergoing surgery for pheochromocytoma or paraganglioma are limited. In line with the above results, our study found that intraoperative hypotension is an independent risk factor of postoperative complications. Gaujoux et al. [32] also reported that perioperative hemodynamic instability, defined as the need for a cumulative dose of norepinephrine of > 5 mg, is significantly associated with morbidity development after surgery for pheochromocytoma. Currently, there are no widely accepted definition of intraoperative hypotension. In a recent systematic review, elevated risks of end-organ injury were presented for prolonged exposure (≥10 min) to MAP < 80 mmHg, for shorter durations < 70 mmHg, and for any exposure < 55–50 mmHg [33]. In accordance with this trend, our study revealed that the risk of postoperative complications increased when SBP ≤95 mmHg for ≥20 min, SBP ≤90 mmHg for ≥20 min, SBP ≤85 mmHg for ≥10 min, and SBP ≤80 mmHg for ≥1 min (Table 4). However, it should be noted that our results do not demonstrate a causal relationship due to the retrospective study design. Furthermore, the external validity of the above thresholds and durations should consider treatment factors, such as fluid infusion and use of vasopressors.
The possible mechanisms underlying the development of end organ injuries from intraoperative hypotension may include the following. First of all, hypotension contributed to the imbalance of oxygen delivery-consumption in vital organs by decreasing oxygen supply [34]; the resulting ischemia-reperfusion injury then triggered inflammation responses which might be involved in the damage process [12, 17, 35]. In addition, intraoperative hypotension might be a marker of other intraoperative events and comorbidities that were associated with an increased risk of postoperative complications [36]. At last, improper use of catecholamine infusion during hypotension might have adverse effects on splanchnic blood flow which led to potentially deleterious consequences [37, 38].
Interestingly, our study did not find any significant association between intraoperative hypertension and postoperative complications. Similar results were also reported by others. For example, in a retrospective cohort study, Monk et al. [19] found that intraoperative hypotension, but not hypertension, was associated with increased 30-day mortality after non-cardiac surgery. One possible explanation is that patients with intraoperative hypertension often had experienced (and, thus, adapted to) hypertension before surgery; these might have made them more tolerable to intraoperative hypertension, but more vulnerable to hypotension. However, due to the retrospective nature of our study and the limited number of patients, the potential harmful effects of intraoperative hypertension cannot be excluded. Further studies are required to clarify whether and to what extent intraoperative hypertension affects outcomes in patients undergoing surgery for pheochromocytoma or paraganglioma.
In addition to the retrospective nature, there were some other limitations in our study. Firstly, given the long duration of this study, many innovations or new treatments were introduced during the studied period and might confound the results. However, after adjusting for period of surgery in the multivariate Logistic model, intraoperative hypotension remained an independent risk factor of postoperative complications (Table 4). Secondly, pheochromocytoma or paraganglioma are relatively rare diseases. Although the sample size was large in this study when compared with others, it was not enough to do further sensitivity analysis; for example, the impacts of hypotension with separate thresholds (SBP 95–90, 90–85, and 85–80 mmHg) for different durations (1–5, 5–10, and 10–20 min) on postoperative complications. Finally, patients’ data were collected until hospital discharge. In a prospective study, Woodfield et al. [39] found that about one-third of complications occurred between hospital discharge and 30 days after surgery. Our results might have underestimated the incidence of postoperative complications.
Conclusions
For patients undergoing surgery for pheochromocytoma or paraganglioma, intraoperative hypotension is associated with increased postoperative complications; and the harmful effects of intraoperative hypotension are level- and duration-dependent. The effects of intraoperative hypertension need to be studied further.
Supplementary information
Additional file 1 Supplementary Table 1. Univariate association between baseline/intraoperative variables and postoperative complications.
Acknowledgements
The authors gratefully acknowledge Dr. Qi Tang (MD, Department of Urology, Peking University First Hospital, Beijing, China) for his help in data collection.
Abbreviations
- AKI
Acute kidney injury
- AR
Adrenergic receptor
- ASA
American Society of Anesthesiologists
- BMI
Body mass index
- CI
Confidence interval
- DBP
Diastolic blood pressure
- HR
Heart rate
- ICU
Intensive care unit
- IQR
Interquartile range
- KDIGO
Kidney Disease Improving Global Outcomes
- MAP
Mean arterial pressure
- MV
Mechanical ventilation
- OR
Odds ratio
- PCEA
Patient-controlled epidural analgesia
- SBP
Systolic blood pressure
Authors’ contributions
NL: study development, data collection, data editing; first draft manuscript; HK: study development, data collection, manuscript editing; SLL: study design; SNZ: statistical analysis; ZZ: study design; DXW: study design, final version of manuscript. The authors read and approved the final manuscript.
Funding
The study was funded by the National Key R&D Program of China (2018YFC2001800). The study sponsor had no role in study design, data acquisition, analysis, interpretation of results, or in the writing of the report.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study protocol was approved by the Clinical Research Ethics Committee of Peking University First Hospital (No. 2016–1062 and 2018–47). The ethics committee agreed to exempt written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nan Li and Hao Kong contributed equally to this work.
Contributor Information
Nan Li, Email: iculinan@163.com.
Hao Kong, Email: konghao2438@126.com.
Shuang-Ling Li, Email: lishuangling888@hotmail.com.
Sai-Nan Zhu, Email: zhusainana77@163.com.
Zheng Zhang, Email: doczhz@aliyun.com.
Dong-Xin Wang, Email: wangdongxin@hotmail.com, Email: dxwang65@bjmu.edu.cn.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12871-020-01066-y.
References
- 1.Berends AMA, Buitenwerf E, de Krijger RR, Veeger NJGM, van der Horst-Schrivers ANA, Links TP, et al. Incidence of pheochromocytoma and sympathetic paraganglioma in the Netherlands: a nationwide study and systematic review. Eur J Intern Med. 2018;51:68–73. doi: 10.1016/j.ejim.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Kierman CM, Du L, Chen X, Broome JT, Shi C, Peters MF, et al. Predictors of hemodynamic instability during surgery for pheochromocytoma. Ann Surg Oncol. 2014;21(12):3865–3871. doi: 10.1245/s10434-014-3847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siddiqi H, Yang H, Laird A, Fox AC, Doherty GM, Miller BS, et al. Utility of oral nicardipine and magnesium sulfate infusion during preparation and resection of pheochromocytomas. Surgery. 2012;152(6):1027–1036. doi: 10.1016/j.surg.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Naranjo J, Dodd S, Martin YN. Perioperative management of pheochromocytoma. J Cardiothorac Vasc Anesth. 2017;31(4):1427–1439. doi: 10.1053/j.jvca.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 5.de Fourmestraux A, Salomon L, Abbou CC, Grise P. Ten year experience of retroperitoneal laparoscopic resection for pheochromocytomas: a dual-Centre study of 72 cases. World J Urol. 2015;33(8):1103–1107. doi: 10.1007/s00345-014-1397-z. [DOI] [PubMed] [Google Scholar]
- 6.Livingstone M, Duttchen K, Thompson J, Sunderani Z, Hawboldt G, Sarah Rose M, et al. Hemodynamic stability during Pheochromocytoma resection: lessons learned over the last two decades. Ann Surg Oncol. 2015;22(13):4175–4180. doi: 10.1245/s10434-015-4519-y. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal R, Mishra SK, Bhatia E, Mishra A, Chand G, Agarwal G, et al. Prospective study to compare perioperative hemodynamic alterations following preparation for pheochromocytoma surgery by phenoxybenzamine or prazosin. World J Surg. 2014;38(3):716–723. doi: 10.1007/s00268-013-2325-x. [DOI] [PubMed] [Google Scholar]
- 8.Chang RY, Lang BH, Wong KP, Lo CY. High pre-operative urinary norepinephrine is an independent determinant of peri-operative hemodynamic instability in unilateral pheochromocytoma/paraganglioma removal. World J Surg. 2014;38(9):2317–2323. doi: 10.1007/s00268-014-2597-9. [DOI] [PubMed] [Google Scholar]
- 9.Brunaud L, Nguyen-Thi PL, Mirallie E, Raffaelli M, Vriens M, Theveniaud PE, et al. Predictive factors for postoperative morbidity after laparoscopic adrenalectomy for pheochromocytoma: a multicenter retrospective analysis in 225 patients. Surg Endosc. 2016;30(3):1051–1059. doi: 10.1007/s00464-015-4294-7. [DOI] [PubMed] [Google Scholar]
- 10.Li N, Kong H, Li SL, Zhu SN, Wang DX. Combined epidural-general anesthesia was associated with lower risk of postoperative complications in patients undergoing open abdominal surgery for pheochromocytoma: a retrospective cohort study. PLoS One. 2018;13(2):e0192924. doi: 10.1371/journal.pone.0192924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallqvist L, Granath F, Huldt E, Bell M. Intraoperative hypotension is associated with acute kidney injury in noncardiac surgery: an observational study. Eur J Anaesthesiol. 2018;35(4):273–279. doi: 10.1097/EJA.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 12.Salmasi V, Maheshwari K, Yang D, Mascha EJ, Singh A, Sessler DI, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126(1):47–65. doi: 10.1097/ALN.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 13.Hallqvist L, Mårtensson J, Granath F, Sahlén A, Bell M. Intraoperative hypotension is associated with myocardial damage in noncardiac surgery: an observational study. Eur J Anaesthesiol. 2016;33(6):450–456. doi: 10.1097/EJA.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 14.Radinovic K, Markovic Denic L, Milan Z, Cirkovic A, Baralic M, Bumbasirevic V. Impact of intraoperative blood pressure, blood pressure fluctuation, and pulse pressure on postoperative delirium in elderly patients with hip fracture: a prospective cohort study. Injury. 2019;50(9):1558–1564. doi: 10.1016/j.injury.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 15.Sun LY, Chung AM, Farkouh ME, van Diepen S, Weinberger J, Bourke M, et al. Defining an intraoperative hypotension threshold in association with stroke in cardiac surgery. Anesthesiology. 2018;129(3):440–447. doi: 10.1097/ALN.0000000000002298. [DOI] [PubMed] [Google Scholar]
- 16.Mascha EJ, Yang D, Weiss S, Sessler DI. Intraoperative mean arterial pressure variability and 30-day mortality in patients having noncardiac surgery. Anesthesiology. 2015;123(1):79–91. doi: 10.1097/ALN.0000000000000686. [DOI] [PubMed] [Google Scholar]
- 17.Bijker JB, van Klei WA, Vergouwe Y, Eleveld DJ, van Wolfswinkel L, Moons KG, et al. Intraoperative hypotension and 1-year mortality after noncardiac surgery. Anesthesiology. 2009;111:1217–1226. doi: 10.1097/ALN.0b013e3181c14930. [DOI] [PubMed] [Google Scholar]
- 18.Reich DL, Bennett-Guerrero E, Bodian CA, Hossain S, Winfree W, Krol M. Intraoperative tachycardia and hypertension are independently associated with adverse outcome in noncardiac surgery of long duration. Anesth Analg. 2002;95(2):273–277. doi: 10.1097/00000539-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Monk TG, Bronsert MR, Henderson WG, Mangione MP, Sum-Ping ST, Bentt DR, et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. 2015;123(2):307–319. doi: 10.1097/ALN.0000000000000756. [DOI] [PubMed] [Google Scholar]
- 20.Kellum JA, Lameire N, Aspelin P, Barsoum RS, Mehta RL, Burdmann EA, et al. KDIGO clinical practice guideline for acute kidney injury 2012. Kidney Int. 2012;Suppl 2:1–138. [Google Scholar]
- 21.Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, et al. Extended Clavien-Dindo classification of surgical complications: Japan clinical oncology group postoperative complications criteria. Surg Today. 2016;46(6):668–685. doi: 10.1007/s00595-015-1236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strandberg TE, Pitkala K. What is the most important component of blood pressure: systolic, diastolic or pulse pressure? Curr Opin Nephrol Hypertens. 2003;12(3):293–297. doi: 10.1097/00041552-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Marcadis AR, Rubio GA, Khan ZF, Farra JC, Lew JI. High perioperative morbidity and mortality in patients with malignant nonfunctional adrenal tumors. J Surg Res. 2017;219:259–265. doi: 10.1016/j.jss.2017.05.116. [DOI] [PubMed] [Google Scholar]
- 24.Tang Y, Zhu C, Liu J, Wang A, Duan K, Li B, et al. Association of Intraoperative Hypotension with acute kidney injury after noncardiac surgery in patients younger than 60 years old. Kidney Blood Press Res. 2019;44(2):211–221. doi: 10.1159/000498990. [DOI] [PubMed] [Google Scholar]
- 25.Tassoudis V, Vretzakis G, Petsiti A, Stamatiou G, Bouzia K, Melekos M, et al. Impact of intraoperative hypotension on hospital stay in major abdominal surgery. J Anesth. 2011;25(4):492–499. doi: 10.1007/s00540-011-1152-1. [DOI] [PubMed] [Google Scholar]
- 26.Kheterpal S, O'Reilly M, Englesbe MJ, Rosenberg AL, Shanks AM, Zhang L, et al. Preoperative and intraoperative predictors of cardiac adverse events after general, vascular, and urological surgery. Anesthesiology. 2009;110:58–66. doi: 10.1097/ALN.0b013e318190b6dc. [DOI] [PubMed] [Google Scholar]
- 27.van Waes JA, van Klei WA, Wijeysundera DN, van Wolfswinkel L, Lindsay TF, Beattie WS. Association between intraoperative hypotension and myocardial injury after vascular surgery. Anesthesiology. 2016;124(1):35–44. doi: 10.1097/ALN.0000000000000922. [DOI] [PubMed] [Google Scholar]
- 28.Rettig TCD, Peelen LM, Geuzebroek GSC, van Klei WA, Boer C, van der Veer JW, et al. Impact of intraoperative hypotension during cardiopulmonary bypass on acute kidney injury after coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 2017;31(2):522–528. doi: 10.1053/j.jvca.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 29.Walsh M, Devereaux PJ, Garg AX, Kurz A, Turan A, Rodseth RN, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119(3):507–515. doi: 10.1097/ALN.0b013e3182a10e26. [DOI] [PubMed] [Google Scholar]
- 30.Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123(3):515–523. doi: 10.1097/ALN.0000000000000765. [DOI] [PubMed] [Google Scholar]
- 31.Gu WJ, Hou BL, Kwong JSW, Tian X, Qian Y, Cui Y, et al. Association between intraoperative hypotension and 30-day mortality, major adverse cardiacevents, and acute kidney injury after non-cardiac surgery: a meta-analysis of cohort studies. Int J Cardiol. 2018;258:68–73. doi: 10.1016/j.ijcard.2018.01.137. [DOI] [PubMed] [Google Scholar]
- 32.Gaujoux S, Bonnet S, Lentschener C, Thillois JM, Duboc D, Bertherat J, et al. Preoperative risk factors of hemodynamic instability during laparoscopic adrenalectomy for pheochromocytoma. Surg Endosc. 2016;30(7):2984–2993. doi: 10.1007/s00464-015-4587-x. [DOI] [PubMed] [Google Scholar]
- 33.Wesselink EM, Kappen TH, Torn HM, Slooter AJC, van Klei WA. Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br J Anaesth. 2018;121(4):706–721. doi: 10.1016/j.bja.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 34.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–2567. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 35.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Ischemia/reperfusion. Compr Physiol. 2016;7(1):113–170. doi: 10.1002/cphy.c160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brady K, Hogue CW. Intraoperative hypotension and patient outcome: does “one size fit all?”. Anesthesiology. 2013;119:495–497. doi: 10.1097/ALN.0b013e3182a10cce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilkman E, Kaukonen KM, Pettilä V, Kuitunen A, Varpula M. Association between inotrope treatment and 90-day mortality in patients with septic shock. Acta Anaesthesiol Scand. 2013;57(4):431–442. doi: 10.1111/aas.12056. [DOI] [PubMed] [Google Scholar]
- 38.Inoue T, Manley GT, Patel N, Whetstone WD. Medical and surgical management after spinal cord injury: vasopressor usage, early surgery and complications. J Neurotrauma. 2014;31(3):284–291. doi: 10.1089/neu.2013.3061. [DOI] [PubMed] [Google Scholar]
- 39.Woodfield JC, Jamil W, Sagar PM. Incidence and significance of postoperative complications occurring between discharge and 30 days: a prospective cohort study. J Surg Res. 2016;206(1):77–82. doi: 10.1016/j.jss.2016.06.07. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1 Supplementary Table 1. Univariate association between baseline/intraoperative variables and postoperative complications.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


