Abstract
While both men and women gain weight as a side effect of antipsychotic treatment, studies in mice have found only female mice are susceptible to weight gain. Therefore, to we set out to identify a strain of male mice that gain significant weight in response to APs which could better model AP-induced weight gain observed in humans. These studies determined that male Balb/c mice developed late onset olanzapine-induced weight gain. Patients often take APs for many years and thus understanding AP-mediated changes in energy expenditure and body weight regulation is particularly important.
Keywords: Antipsychotic, Olanzapine, Side-effects, Weight gain, Food intake, Obesity, Energy expenditure
Introduction
Approximately four million people in the USA are currently prescribed antipsychotic (AP) medications to manage a range of illnesses including schizophrenia, bipolar disorder, depression, anxiety, dementia, insomnia and post-traumatic stress disorder 1–6. Despite their therapeutic efficacy, APs cause serious side effects of excessive weight gain 7–11 and associated metabolic disease 12. Both men and women gain significant amounts of weight in response to APs 13–16. However, in contrast to the clinical data, rodent studies show highly reproducible sex-specific differences in weight gain in repsonse to APs. While olanzapine treatment consistently results in significant weight gain in female rodents,17–30 male rodents appear to be protected from AP-induced weight gain 17,28,29,31,32. To date, the underlying reasons for this sexual dimorphism in rodent’s response to APs are not yet known 33–35.
Genetic background of mice plays a significant role in the susceptibiliy to of many metabolic traits ranging from obesity 36, non-alcoholic hepatosteotosis 37 and cardiometabolic diseases 38. For example, high fat diet feeding results in significant weight gain in male C57BL/6 36,39 DBA/2 36,39 129X139, and FVB/N39 mice while male Balb/c 39, I/STN 36, SWR/J 36 and SJL/J 36 are notably protected from diet-induced obesity. To develop a consistent model for AP-induced weight gain in mice, Morgan et al., tested the effect of olanzapine treatment in female mice from eight inbred strains (A/J, C57BL/6J, 129S1SvlmJ, NOD/ShiLtJ, NZO/HlLtJ, CAST/EiJ, PWK/PhJ, WSB/EiJ) 40. This study found that C57BL/6J mice were highly susceptible to olanzapine-induced weight gain and thus C57BL/6J mice have been widely used to model for AP-induced weight gain in numerous studies 29,30,35,40. However, this female C57BL/6J model is often criticized for lack of translational relevance due to the finding male C57BL/6J are protected from AP-induced weight gain 29 while both men and women gain weight in repsonse to APs 13–16. Therefore, to we set out to identify a strain of male mice that gains significant weight in response to APs which could provide a better model of AP-induced weight gain observed in humans.
Materials and Methods
All procedures were approved by the University of California San Diego IACUC.
Male mouse strains were purchased from The Jackson Laboratory (Sacramento, CA) at 9 weeks of age: C57BL6/J (000664), Balb/cJ (000651), DBA/1J (000670), CBA/J (000656), AKR/J (000648), SJL/J (000686), C3H/HeJ (000659), 129X1/SvJ (000691). Mice were acclimatized to the laboratory conditions for 7 days. Animals were maintained in a 12-hour light:dark cycle with a humidity between 60-70%. Three days before the start of the study, mice were switched from normal chow (Rodent chow, 5001, Labdiet, CA) to high fat diet (HFD, 45% calories from fat, D09092903, Research Diets) and singly housed in standard laboratory cages. At the start of the study, mice were randomized (n=6/group) to receive either the 45% HFD with or without olanzapine (54mg/kg, D161110301, Research Diets). This robust model of administering olanzapine in 45% HFD to initiate weight gain has been widely used in similar studies29,30,40. Careful dosing studies established this dose of olanzapine in the diet resulted in a plasma concentrations (10–25 ng/mL) corresponding with the therapeutic range in humans 40. Food and water were provided ad libitum. Food intake was measured daily. Mice were housed in cages with highly absorbent paper to facilitate accurate determination of any spilled food (Kimberly Clark Wypal X60). Body weight was measured every other day for up to 28 days.
RNA extraction and quantitative PCR: Mice were sacrificed (non-fasted), and the hypothalamus, gonadal white and interscapular brown adipose tissues were collected, frozen in liquid nitrogen and stored at −80°C until further analyses. Total RNA was extracted using Trizol (Invitrogen) and RNeasy Extraction Kit (Qiagen). RNA concentration and quality were assessed using Nanodrop. cDNA was synthesized from 500 ng of RNA using High Capacity cDNA transcription kit (Thermo Fisher). qPCR was performed using Step One Plus (Applied Biosciences). Gene expression was normalized to housekeeping genes hypoxanthine-guanine phosphoribosyltransferase (Hprtl) and phosphoglycerate kinase 1 (Pgk1) for the hypothalamus and Hprtl and ATP synthase F1 subunit epsilon (Atp5e) for the adipose tissues. Primer sequences are detailed in Table 1.
Table 1.
Primer sequences used for qPCR
| Gene | Accession number | Sequence (5’-3’) |
|---|---|---|
| Agrp | NM_001271806.1 | F: TGACCAAATCCACCCCCTCC R: TTCCTGTAGCCAGGGCATGA |
| Npy | NM_023456.3 | F: TAACAAGCGAATGGGGCTGT R: TTCAAGCCTTGTTCTGGGGG |
| Pomc | NM_001278581.1 | F: GGCGACGGAAGAGAAAAGAGG R: TGTTCAGTCTCCTGCCTGTCG |
| Cart | NM_013732.7 | F: TGGATGATGCGTCCCATG R: TACTTCTTCTCATAGATCGGAAT |
| Orexin | NM_010410.2 | F: TTCCTTCTACAAAGGTTCCCTGG R: CGTAGAGACGGCAGGAACAC |
| Adipoq | NM_009605.5 | F: TGACGACACCAAAAGGGCTC R: CACAAGTTCCCTTGGGTGGA |
| Lep | NM_008493.3 | F: CACACACGCAGTCGGTATCC R: ACTCAGAATGGGGTGAAGCC |
| Lipe | NM_010719.5 | F: GGGTGACTCTAACGCGACTC R: CCTTTAATGGGTGGGGCTGA |
| Acaca | NM_133360.2 | F: GAGAGGGGTCAAGTCCTTCC R: CTGCTGCCGTCATAAGACAA |
| Fasn | NM_007988.3 | F: TGCTCCCAGCTGCAGGC R: GCCCGGTAGCTCTGGGTGTA |
| Ppara | NM_011144.6 | F: ATGAAGAGGGCTGAGCGTAG R: AAACGCAACGTAGAGTGCTGT |
| Cpt1a | NM_013495.2 | F: CAGCACCTGTACCGCCTCGC R: GCCGTCATCAGCAACCGGCC |
| Ucp1 | NM_009463.3 | F: ACTGCCACACCTCCAGTCATT R: CTTTGCCTCACTCAGGATTGG |
| Sirt3 | NM_022433.2 | F: TTTCTTTCACAACCCCAAGC R: ACAGACCGTGCATGTAGCTG |
| Pgc1a | NM_008904.2 | F: TGCCATTGTTAAGACCGA R: GGTCATTTGGTGACTCTGG |
| Hprt1 | NM_013556.2 | F: CACAGGACTAGAACACCTGC R: GCTGGTGAAAAGGACCTCT |
| Pgk1 | NM_008828.3 | F: CTGACTTTGGACAAGCTGGACG R: GCAGCCTTGATCCTTTGGTTG |
| Atp5e | NM_025983.3 | F: TGGCGACAGGCTGGACTCAG R: GCTGCCCGAAGTCTTCTCAGCG |
Results
Treatment of C57BL6/J female mice with olanzapine resulted in significant increase in food intake (Fig. 1A) and body weight gain (Fig. 1B). In contrast, treatment of C57BL6/J male mice with olanzapine did not result in increased food intake or weight gain compared with control treated mice (Fig 1C–D).
Figure 1. Olanzapine-induced food intake and weight gain in female C57BL/6 mice.
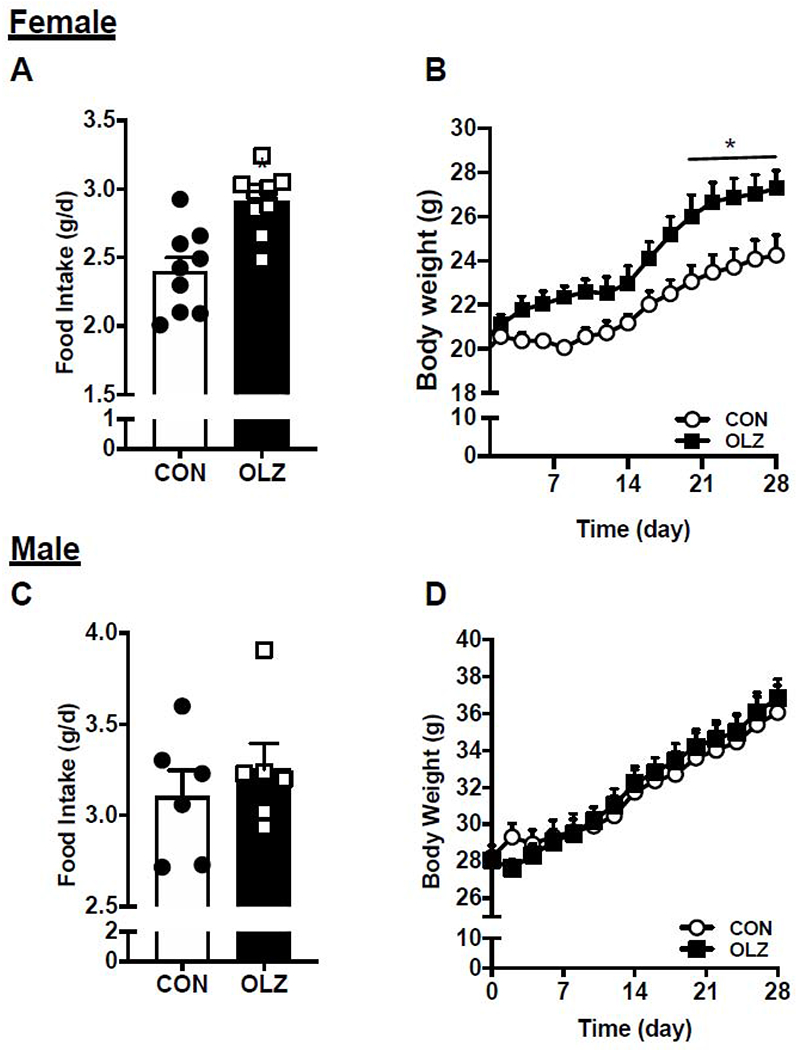
(A) Food intake and (B) body weight in female C57BL/6 mice. (C) Food intake and (D) body weight in male C57BL/6 mice. Average daily food intake was analyzed using student t-test while body weight was analyzed using two-way ANOVA with Sidak’s multiple comparisons test. Values are expressed as mean ± SEM, *p < 0.05, n = 8 per group.
A strain survey of response to olanzapine revealed AKR/J (Fig. 2A), CBA/J (Fig. 2B), C3H/HeJ (Fig. 2C), DBA/1J (Fig. 2D), SJL/J (Fig. 2E) and 129X1/SvJ (Fig. 2F) male mice did not gain additional weight in response to olanzapine. In addition, olanzapine did not significantly change food intake in these strains (Fig. 2A–F). Interestingly, we observed that olanzapine treatment of male Balb/cJ mice resulted in significant increase in body weight and weight gain (Fig. 3A–B, supplemental Fig.1A) that became more pronounced after ~14-28 days of treatment. However, this weight gain does not appear to be driven by increased food intake (Fig. 3C, supplemental Fig. 1B) and in agreement with this no significant differences in appetite regulating hypothalamic neuropeptides, including Agouti-related protein (Agrp), Neuropeptide Y (Npy), Orexin (Hcrt), Cocaine- and amphetamine-regulated transcript (Cart) and Pro-opiomelanocortin (Pomc), were observed between olanzapine and control treated mice (Fig. 3D). Olanzapine treatment did not significantly change expression of lipogenic genes Acetyl-CoA Carboxylase alpha (Acaca) and Fatty acid synthase (Fasn), lipolytic genes carnitine palmitolytransferase (Cpt1a) Hormone-sensitive lipase Lipe or metabolic genes leptin (Lep) and adiponectin (Adipoq) in the gonadal white adipose tissue (Fig. 3E). However, olanzapine treatment resulted in reduction in expression of genes implicated in energy metabolism41 including peroxisome proliferator-activated receptor gamma coactivator 1-alpha (Ppargc1a) and peroxisome proliferator activated receptor alpha (Ppara) in brown adipose tissue while no significant differences were observed in Fasn, Sirtuin 3 (Sirt3) and uncoupling protein 1 (Ucp1) (Fig. 3F). Female Balb/cJ treated with olanzapine gained a similar amount of weight (Fig. 3G) and had no differences in food intake (Fig. 3H) compared with control treated mice.
Figure 2. Effect of olanzapine on weight gain and food intake in different male mouse strains.
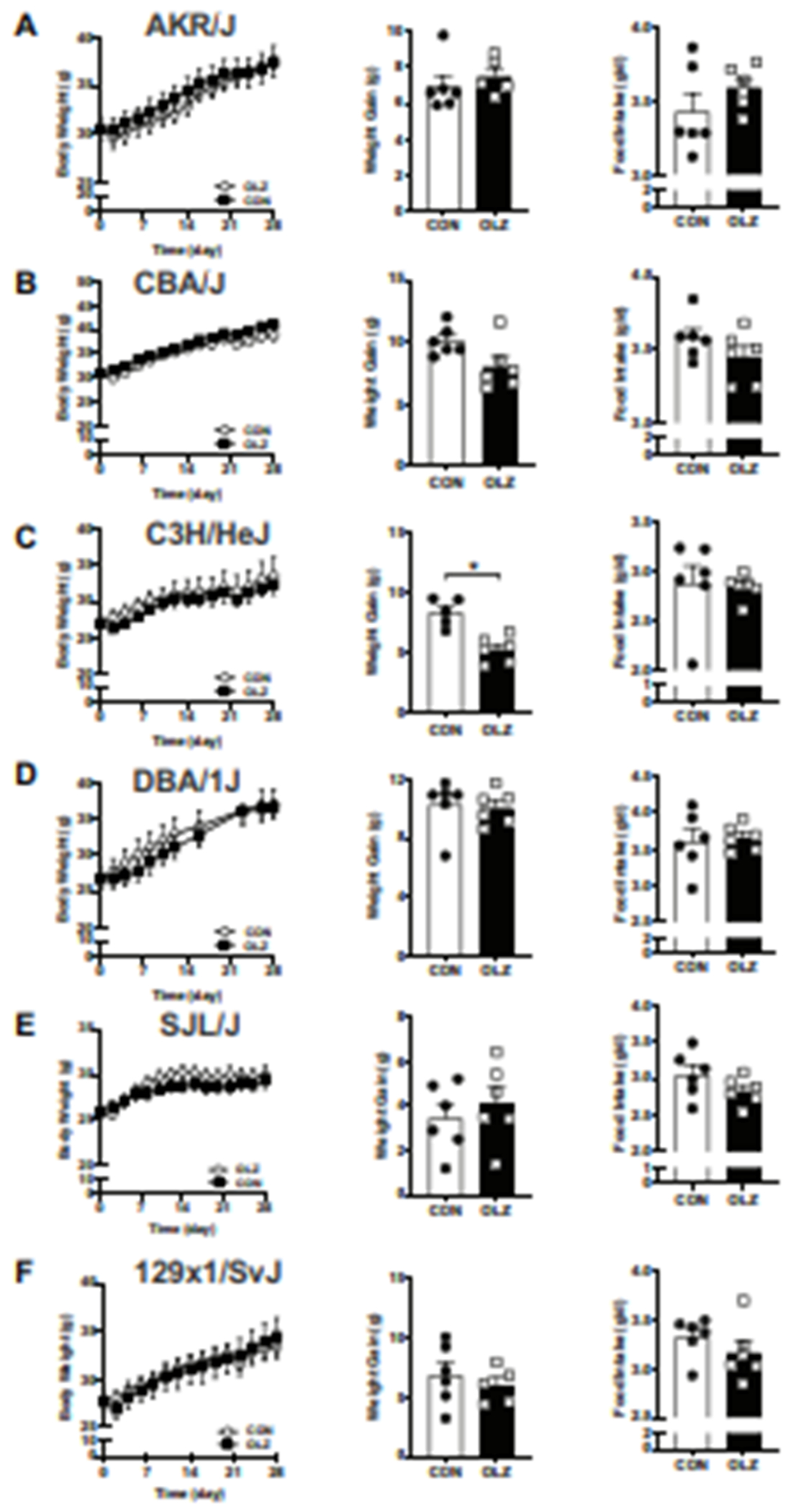
(A) AKR/J, (B) CBA/J, (C) C3H/HeJ, (D) DBA/1J, (E) SJL/J, (F) 129X1/SvJ. Body weight was analyzed using two-way ANOVA with Sidak’s multiple comparisons test. Weight gain and average daily food intake were analyzed using student t-test. Values are expressed as mean ± SEM, n = 6 per group.
Figure 3. Effect of olanzapine on food intake and weight gain in Balb/c mice.
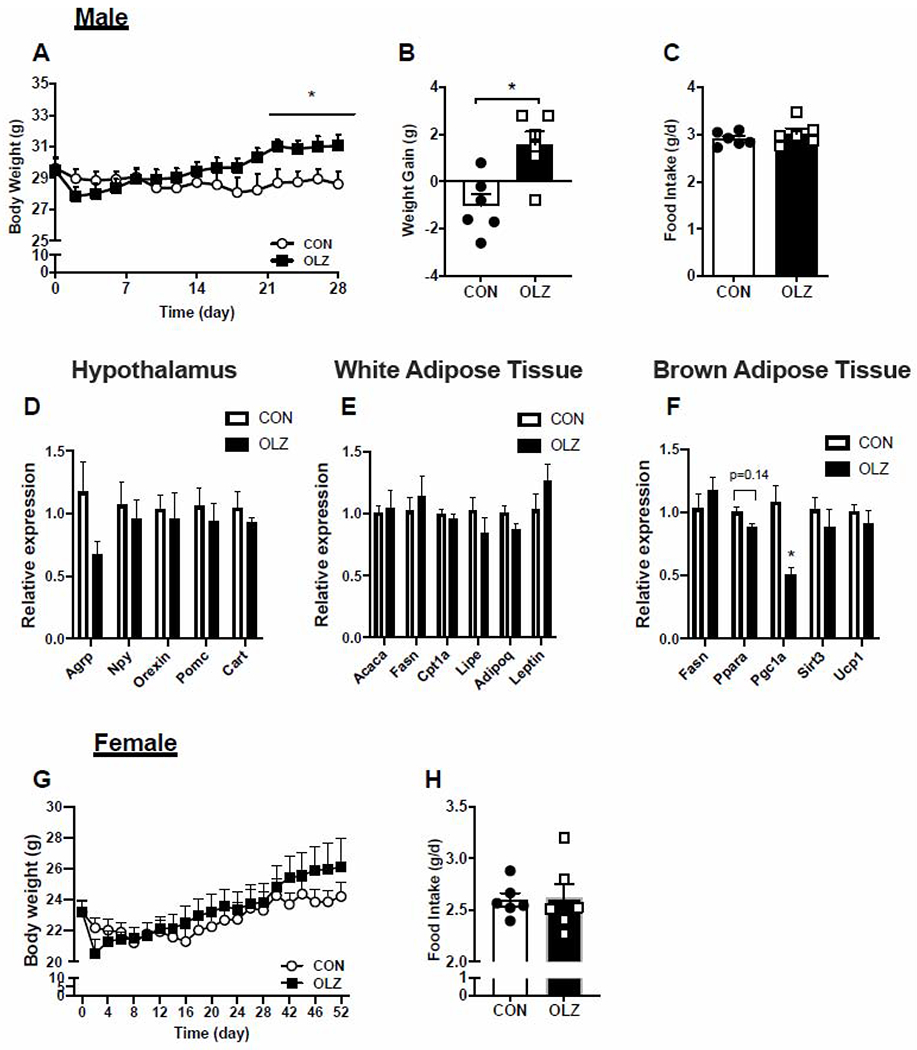
(A) Body weight, (B) weight gain, (C) food intake, gene expression in the (D) hypothalamus (E) gonadal white adipose tissue and (F) interscapular brown adipose tissue in male Balb/c mice in response to olanzapine treatment. (G) Body weight and (H) food intake in female Balb/c mice treated with olanzapine. Body weight and food intake were analyzed using two-way ANOVA with Sidak’s multiple comparisons test. qPCR data were analyzed by students t-test and corrected for multiple comparisons using the Holm-Sidak method, with alpha = 0.05. Values are expressed as mean ± SEM, *p < 0.05, n = 6 per group.
Discussion
AP drugs have the unwanted side effect of significant weight gain7 that often leads to the development of metabolic disease. While both men and women gain weight in response to APs, rodent studies have found only females gain significant weight 29,30,40,42,43, while male rodents appear to be resistant to AP-induced weight gain 43. Because genetic background plays a significant role in the susceptibiliy of mice to a wide array of metabolic traits 37–39, we explored the effect of AP treatment on weight gain in seven strains of male mice.
In these studies, we determined that male Balb/c mice developed late onset susceptibility to olanzapine-induced weight gain. Balb/c male mice are protected from high-fat diet induced obesity 39, and as expected, we observed a similar effect in our control HFD fed mice in our study. However, olanzapine treatment of male Balb/c mice resulted in significant weight gain compared with control treated mice. No significant differences in food intake were observed, suggesting that these late onset weight differences may be mediated through olanzapine-induced changes in energy expenditure. While we did not directly measure energy expenditure, we observed decreased gene expression of Ppargc1a and Ppara in the brown adipose tissue associated with lower energy expenditure 44 AP-induced weight gain in rodents and humans is initially driven by increased food intake 45,46, but later in the course of treatment, body weight gain persists 47,48 even when changes in food intake are no longer present 46,49. This suggests that changes in energy expenditure may play a significant role in AP-induced weight gain in the later course of treatment 50. The small number of studies and isolated observations on human patients treated with APs, where energy expenditure has been measured, have, to date, yielded equivocal results 8,51–54 Our studies suggest male Balb/c mice may provide a model of the late onset changes in energy expenditure observed after chronic AP treatment. However, olanzapine treatment in female Balb/c did not significantly affect weight gain as noted in other studies 55. Therefore, these studies suggest a further sexual dimorphic effect of APs on energy expenditure in Balb/c mice. Future studies will be needed to delineate the effect of APs on energy expenditure in male and female Balb/c mice.
Amphetamine-induced hyperlocomotion is a standard test used to test the preclinical efficacy of AP drugs whereby amphetamine treatment results in hyperactivity that is blunted by AP treatment56. Interestingly, both Balb/c 57,58, and C57BL6 mice are commonly used in these tests 29,59 suggesting multiple strains of mice can model the therapeutic efficacy of APs in humans. However, the metabolic side effects of APs are strain and sex dependent in mice suggesting they are not directly linked to the therapeutic effect of APs.
While both men and women are both susceptible to gain weight in response to APs, there is a growing body of evidence that females have a higher risk of weight gain 60–62, 63–66. The most compelling evidence comes from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) where women were more likely than men to have metabolic syndrome compared with the general male population 67. Therefore, while male rodents are more resistant to AP-induced weight gain, this may reflect aspects of body weight observations in clinical studies after AP treatment.
In summary, there is a lack of understanding of the molecular mechanisms underlying the sexual dimorphism of AP-induced weight gain in rodent and human studies 68–70. While we have identified male Balb/c mice are susceptible to late onset weight gain in response to APs, further studies are needed to identify a robust model of AP-induced food intake and weight gain that more closely models the effect of APs in humans. This is an important area of research as patients often take APs for many years and understanding AP-mediated changes in energy expenditure and body weight regulation warrants further investigation.
Supplementary Material
Acknowledgements:
This work was supported by the National Institutes of Health grant R01DK117872 awarded to O.O and the Larry L Hillblom foundation fellowship awarded to RCZ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moore TJ & Mattison DR Adult Utilization of Psychiatric Drugs and Differences by Sex, Age, and Race. JAMA internal medicine 177, 274–275, doi: 10.1001/jamainternmed.2016.7507 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Painter JT et al. Analysis of the Appropriateness of Off-Label Antipsychotic Use for Mental Health Indications in a Veteran Population. Pharmacotherapy 37, 438–446, doi: 10.1002/phar.1910 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Wofford MR, King DS & Harrell TK Drug-induced metabolic syndrome. Journal of clinical hypertension 8, 114–119 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comer JS, Mojtabai R & Olfson M National trends in the antipsychotic treatment of psychiatric outpatients with anxiety disorders. The American journal of psychiatry 168, 1057–1065, doi: 10.1176/appi.ajp.2011.11010087 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Nesvag R et al. The incidence, psychiatric co-morbidity and pharmacological treatment of severe mental disorders in children and adolescents. European psychiatry : the journal of the Association of European Psychiatrists 49, 16–22, doi: 10.1016/j.eurpsy.2017.12.009 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Verdoux H, Tournier M & Begaud B Antipsychotic prescribing trends: a review of pharmaco-epidemiological studies. Acta psychiatrica Scandinavica 121,4–10, doi: 10.1111/j.1600-0447.2009.01425.x (2010). [DOI] [PubMed] [Google Scholar]
- 7.Bak M, Fransen A, Janssen J, van Os J & Drukker M Almost all antipsychotics result in weight gain: a meta-analysis. PloS one 9, e94112, doi: 10.1371/journal.pone.0094112 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fountaine RJ et al. Increased food intake and energy expenditure following administration of olanzapine to healthy men. Obesity 18, 1646–1651, doi: 10.1038/oby.2010.6 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Gothelf D et al. Weight gain associated with increased food intake and low habitual activity levels in male adolescent schizophrenic inpatients treated with olanzapine. The American journal of psychiatry 159, 1055–1057, doi: 10.1176/appi.ajp.159.6.1055 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Jensen GL Drug-induced hyperphagia: what can we learn from psychiatric medications? JPEN. Journal of parenteral and enteral nutrition 32, 578–581, doi: 10.1177/0148607108321708 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Kluge M et al. Clozapine and olanzapine are associated with food craving and binge eating: results from a randomized double-blind study. Journal of clinical psychopharmacology 27, 662–666, doi: 10.1097/jcp.0b013e31815a8872 (2007). [DOI] [PubMed] [Google Scholar]
- 12.De Hert M, Detraux J, van Winkel R, Yu W & Correll CU Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nature reviews. Endocrinology 8, 114–126, doi: 10.1038/nrendo.2011.156 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Attux C, Quintana MI & Chaves AC Weight gain, dyslipidemia and altered parameters for metabolic syndrome on first episode psychotic patients after six-month follow-up. Braz J Psychiatry 29, 346–349, doi: 10.1590/s1516-44462006005000061 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Lee E, Leung CM & Wong E Atypical antipsychotics and weight gain in Chinese patients: a comparison of olanzapine and risperidone. The Journal of clinical psychiatry 65, 864–866, doi: 10.4088/jcp.v65n0620 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Lipkovich I et al. Early predictors of weight gain risk during treatment with olanzapine: analysis of pooled data from 58 clinical trials. Psychopharmacology bulletin 42, 23–39 (2009). [PubMed] [Google Scholar]
- 16.Taylor JH, Jakubovski E, Gabriel D & Bloch MH Predictors and Moderators of Antipsychotic-Related Weight Gain in the Treatment of Early-Onset Schizophrenia Spectrum Disorders Study. J Child Adolesc Psychopharmacol 28, 474–484, doi: 10.1089/cap.2017.0147 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albaugh VL et al. Hormonal and metabolic effects of olanzapine and clozapine related to body weight in rodents. Obesity 14, 36–51, doi: 10.1038/oby.2006.6 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arjona AA, Zhang SX, Adamson B & Wurtman RJ An animal model of antipsychotic-induced weight gain. Behavioural brain research 152, 121–127, doi: 10.1016/j.bbr.2003.09.040 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Cooper GD, Pickavance LC, Wilding JP, Halford JC & Goudie AJ A parametric analysis of olanzapine-induced weight gain in female rats. Psychopharmacology 181, 80–89, doi: 10.1007/s00213-005-2224-4 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Ersland KM et al. One-Year Treatment with Olanzapine Depot in Female Rats: Metabolic Effects. The international journal of neuropsychopharmacology 22, 358–369, doi: 10.1093/ijnp/pyz012 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goudie AJ, Smith JA & Halford JC Characterization of olanzapine-induced weight gain in rats. Journal of psychopharmacology 16, 291–296, doi: 10.1177/026988110201600402 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Lykkegaard K et al. The once-daily human GLP-1 analog, liraglutide, reduces olanzapine-induced weight gain and glucose intolerance. Schizophrenia research 103, 94–103, doi: 10.1016/j.schres.2008.05.011 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Mann S et al. Chronic olanzapine administration in rats: effect of route of administration on weight, food intake and body composition. Pharmacology, biochemistry, and behavior 103, 717–722, doi: 10.1016/j.pbb.2012.12.002 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Pouzet B, Mow T, Kreilgaard M & Velschow S Chronic treatment with antipsychotics in rats as a model for antipsychotic-induced weight gain in human. Pharmacology, biochemistry, and behavior 75, 133–140, doi: 10.1016/s0091-3057(03)00042-x (2003). [DOI] [PubMed] [Google Scholar]
- 25.Skrede S et al. Olanzapine depot formulation in rat: a step forward in modelling antipsychotic-induced metabolic adverse effects. The international journal of neuropsychopharmacology 17, 91–104, doi: 10.1017/S1461145713000862 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Skrede S et al. Olanzapine, but not aripiprazole, weight-independently elevates serum triglycerides and activates lipogenic gene expression in female rats. The international journal of neuropsychopharmacology 15, 163–179, doi: 10.1017/S1461145711001271 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Skrede S et al. Lack of Ovarian Secretions Reverts the Anabolic Action of Olanzapine in Female Rats. The international journal of neuropsychopharmacology 20, 1005–1012, doi: 10.1093/ijnp/pyx073 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper GD et al. Effects of olanzapine in male rats: enhanced adiposity in the absence of hyperphagia, weight gain or metabolic abnormalities. Journal of psychopharmacology 21, 405–413, doi: 10.1177/0269881106069637 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Perez-Gomez A et al. A phenotypic Caenorhabditis elegans screen identifies a selective suppressor of antipsychotic-induced hyperphagia. Nature communications 9, 5272, doi: 10.1038/s41467-018-07684-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lord CC et al. The atypical antipsychotic olanzapine causes weight gain by targeting serotonin receptor 2C. The Journal of clinical investigation 127, 3402–3406, doi: 10.1172/JCI93362 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ader M et al. Metabolic dysregulation with atypical antipsychotics occurs in the absence of underlying disease: a placebo-controlled study of olanzapine and risperidone in dogs. Diabetes 54, 862–871, doi: 10.2337/diabetes.54.3.862 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Albaugh VL et al. Olanzapine promotes fat accumulation in male rats by decreasing physical activity, repartitioning energy and increasing adipose tissue lipogenesis while impairing lipolysis. Molecular psychiatry 16, 569–581, doi: 10.1038/mp.2010.33 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davey KJ et al. Gender-dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology 221, 155–169, doi: 10.1007/s00213-011-2555-2 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Aichhorn W, Whitworth AB, Weiss EM & Marksteiner J Second-generation antipsychotics: is there evidence for sex differences in pharmacokinetic and adverse effect profiles? Drug safety 29, 587–598 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Cope MB et al. Antipsychotic drug-induced weight gain: development of an animal model. International journal of obesity 29, 607–614, doi: 10.1038/sj.ijo.0802928 (2005). [DOI] [PubMed] [Google Scholar]
- 36.West DB, Boozer CN, Moody DL & Atkinson RL Dietary obesity in nine inbred mouse strains. The American journal of physiology 262, R1025–1032, doi: 10.1152/ajpregu.1992.262.6.R1025 (1992). [DOI] [PubMed] [Google Scholar]
- 37.Kahle M et al. Phenotypic comparison of common mouse strains developing high-fat diet-induced hepatosteatosis. Molecular metabolism 2, 435–446, doi: 10.1016/j.molmet.2013.07.009 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norheim F et al. Gene-by-Sex Interactions in Mitochondrial Functions and Cardio-Metabolic Traits. Cell metabolism 29, 932–949 e934, doi: 10.1016/j.cmet.2018.12.013 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montgomery MK et al. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia 56, 1129–1139, doi: 10.1007/s00125-013-2846-8 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Morgan AP et al. The antipsychotic olanzapine interacts with the gut microbiome to cause weight gain in mouse. PloS one 9, e115225, doi: 10.1371/journal.pone.0115225 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guazzoni G et al. Long term experience with the prostatic spiral for urinary retention due to benign prostatic hyperplasia. Scand J Urol Nephrol 25, 21–24, doi: 10.3109/00365599109024523 (1991). [DOI] [PubMed] [Google Scholar]
- 42.Boyda HN, Tse L, Procyshyn RM, Honer WG & Barr AM Preclinical models of antipsychotic drug-induced metabolic side effects. Trends in pharmacological sciences 31, 484–497, doi: 10.1016/j.tips.2010.07.002 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Castellani LN et al. Preclinical and Clinical Sex Differences in Antipsychotic-Induced Metabolic Disturbances: A Narrative Review of Adiposity and Glucose Metabolism. J Psychiatr Brain Sci 4, doi: 10.20900/jpbs.20190013 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma BK, Patil M & Satyanarayana A Negative regulators of brown adipose tissue (BAT)-mediated thermogenesis. J Cell Physiol 229, 1901–1907, doi: 10.1002/jcp.24664 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Q et al. Hypothalamic ghrelin signalling mediates olanzapine-induced hyperphagia and weight gain in female rats. The international journal of neuropsychopharmacology 17, 807–818, doi: 10.1017/S1461145713001697 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Huang XF, Han M, Huang X, Zavitsanou K & Deng C Olanzapine differentially affects 5-HT2Aand2C receptor mRNA expression in the rat brain. Behavioural brain research 171, 355–362, doi: 10.1016/j.bbr.2006.03.040 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Deng C Effects of antipsychotic medications on appetite, weight, and insulin resistance. Endocrinology and metabolism clinics of North America 42, 545–563, doi: 10.1016/j.ecl.2013.05.006 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Pai N, Deng C, Vella SL, Castle D & Huang XF Are there different neural mechanisms responsible for three stages of weight gain development in anti-psychotic therapy: temporally based hypothesis. Asian journal of psychiatry 5, 315–318, doi: 10.1016/j.ajp.2012.05.005 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Zhang Q et al. Olanzapine reduced brown adipose tissue thermogenesis and locomotor activity in female rats. Progress in neuro-psychopharmacology & biological psychiatry 51, 172–180, doi: 10.1016/j.pnpbp.2014.02.003 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Cuerda C, Velasco C, Merchan-Naranjo J, Garcia-Peris P & Arango C The effects of second-generation antipsychotics on food intake, resting energy expenditure and physical activity. Eur J Clin Nutr 68, 146–152, doi: 10.1038/ejcn.2013.253 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Graham KA et al. Effect of olanzapine on body composition and energy expenditure in adults with first-episode psychosis. The American journal of psychiatry 162, 118–123, doi: 10.1176/appi.ajp.162.1.118 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Sharpe JK, Stedman TJ, Byrne NM, Wishart C & Hills AP Energy expenditure and physical activity in clozapine use: implications for weight management. Aust N Z J Psychiatry 40, 810–814, doi: 10.1080/j.1440-1614.2006.01888.x (2006). [DOI] [PubMed] [Google Scholar]
- 53.Stefanidis A et al. The role of thermogenesis in antipsychotic drug-induced weight gain. Obesity 17, 16–24, doi: 10.1038/oby.2008.468 (2009). [DOI] [PubMed] [Google Scholar]
- 54.Virkkunen M, Wahlbeck K, Rissanen A, Naukkarinen H & Franssila-Kallunki A Decrease of energy expenditure causes weight increase in olanzapine treatment - a case study. Pharmacopsychiatry 35, 124–126, doi: 10.1055/s-2002-31521 (2002). [DOI] [PubMed] [Google Scholar]
- 55.Li H et al. Chronic Olanzapine Treatment Induces Disorders of Plasma Fatty Acid Profile in Balb/c Mice: A Potential Mechanism for Olanzapine-Induced Insulin Resistance. PloS one 11, e0167930, doi: 10.1371/journal.pone.0167930 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gil-Ad I et al. A novel analog of olanzapine linked to sarcosinyl moiety (PGW5) demonstrates high efficacy and good safety profile in mouse models of schizophrenia. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 24, 425–436, doi: 10.1016/j.euroneuro.2013.11.009 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Bradford AM, Savage KM, Jones DN & Kalinichev M Validation and pharmacological characterisation of MK-801-induced locomotor hyperactivity in BALB/C mice as an assay for detection of novel antipsychotics. Psychopharmacology 212, 155–170, doi: 10.1007/s00213-010-1938-0 (2010). [DOI] [PubMed] [Google Scholar]
- 58.Gogos A, Bogeski M & van den Buuse M Role of serotonin-1A receptors in the action of antipsychotic drugs: comparison of prepulse inhibition studies in mice and rats and relevance for human pharmacology. Behav Pharmacol 19, 548–561, doi: 10.1097/FBP.0b013e32830cd822 (2008). [DOI] [PubMed] [Google Scholar]
- 59.Van Swearingen AE, Walker QD & Kuhn CM Sex differences in novelty- and psychostimulant-induced behaviors of C57BL/6 mice. Psychopharmacology 225, 707–718, doi: 10.1007/s00213-012-2860-4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Basson BR et al. Factors influencing acute weight change in patients with schizophrenia treated with olanzapine, haloperidol, or risperidone. The Journal of clinical psychiatry 62, 231–238 (2001). [DOI] [PubMed] [Google Scholar]
- 61.Bobes J et al. Weight gain in patients with schizophrenia treated with risperidone, olanzapine, quetiapine or haloperidol: results of the EIRE study. Schizophrenia research 62, 77–88 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Iversen TSJ et al. Side effect burden of antipsychotic drugs in real life - Impact of gender and polypharmacy. Progress in neuro-psychopharmacology & biological psychiatry 82, 263–271, doi: 10.1016/j.pnpbp.2017.11.004 (2018). [DOI] [PubMed] [Google Scholar]
- 63.Covell NH, Weissman EM & Essock SM Weight gain with clozapine compared to first generation antipsychotic medications. Schizophrenia bulletin 30, 229–240, doi: 10.1093/oxfordjournals.schbul.a007074 (2004). [DOI] [PubMed] [Google Scholar]
- 64.Haack S, Seeringer A, Thurmann PA, Becker T & Kirchheiner J Sex-specific differences in side effects of psychotropic drugs: genes or gender? Pharmacogenomics 10, 1511–1526, doi: 10.2217/pgs.09.102 (2009). [DOI] [PubMed] [Google Scholar]
- 65.Hakko H et al. Are females at special risk of obesity if they become psychotic? The longitudinal Northern Finland 1966 Birth Cohort Study. Schizophrenia research 84, 15–19, doi: 10.1016/j.schres.2006.03.020 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Lau SL et al. Predicting Weight Gain in Patients Treated With Clozapine: The Role of Sex, Body Mass Index, and Smoking. Journal of clinical psychopharmacology 36, 120–124, doi: 10.1097/JCP.0000000000000476 (2016). [DOI] [PubMed] [Google Scholar]
- 67.McEvoy JP et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophrenia research 80, 19–32, doi: 10.1016/j.schres.2005.07.014 (2005). [DOI] [PubMed] [Google Scholar]
- 68.Miller CL On the mechanism of action of antipsychotic drugs: a chemical reaction not receptor blockade. Current drug discovery technologies 10, 195–208 (2013). [DOI] [PubMed] [Google Scholar]
- 69.Panariello F, Polsinelli G, Borlido C, Monda M & De Luca V The role of leptin in antipsychotic-induced weight gain: genetic and non-genetic factors. Journal of obesity 2012, 572848, doi: 10.1155/2012/572848 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shams TA & Muller DJ Antipsychotic induced weight gain: genetics, epigenetics, and biomarkers reviewed. Current psychiatry reports 16, 473, doi: 10.1007/s11920-014-0473-9 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


