Abstract
Introduction
Smoking during pregnancy poses serious risks to baby and mother. Few disseminable programs exist to help pregnant women quit or reduce their smoking. We hypothesized that an SMS text-delivered scheduled gradual reduction (SGR) program plus support texts would outperform SMS support messages alone.
Methods
We recruited 314 pregnant women from 14 prenatal clinics. Half of the women received theory-based support messages throughout their pregnancy to promote cessation and prevent relapse. The other half received the support messages plus alert texts that gradually reduced their smoking more than 3–5 weeks. We conducted surveys at baseline, end of pregnancy, and 3 months postpartum. Our primary outcome was biochemically validated 7-day point prevalence abstinence at late pregnancy. Our secondary outcome was reduction in cigarettes per day.
Results
Adherence to the SGR was adequate with 70% responding to alert texts to smoke within 60 minutes. Women in both arms quit smoking at the same rate (9%–12%). Women also significantly reduced their smoking from baseline to the end of pregnancy from nine cigarettes to four; we found no arm differences in reduction.
Conclusions
Support text messages alone produced significant quit rates above naturally occurring quitting. SGR did not add significantly to helping women quit or reduce. Sending support messages can reach many women and is low-cost. More obstetric providers might consider having patients who smoke sign up for free texting programs to help them quit.
Implications
A disseminable texting program helped some pregnant women quit smoking.
Clinical Trial Registration number: NCT01995097.
Introduction
Smoking during pregnancy is associated with a plethora of negative effects on the fetus, including preterm birth, low birth weight, fetal growth restriction, sudden infant death syndrome, and attention deficit disorder.1–6 Despite known evidence of the harms, only half of women quit when they learn they are pregnant; most others cannot quit on their own.7 Most who continue to smoke do reduce their cigarette consumption, however, which has been shown to improve fetal health.6,8,9 Many have designed interventions to help pregnant women quit; these interventions either are not easily disseminated or have small effect sizes.10–14
People who smoke often smoke in response to internal and external cues. Scheduled gradual reduction (SGR) might help reduce cue-dependent smoking among pregnant women15 because they smoke their cigarettes based on a predetermined schedule, not when they are cued to smoke.15 SGR has been shown to promote cessation in nonpregnant populations16,17 with quit rates up to 44%. Given most pregnant women's strong desire to quit smoking and preferences for cutting down to quit over abrupt cessation,18 an intervention that uses scheduled gradual reduction could help pregnant women quit.6,19,20
Previous SGR studies have used instructions and paper diaries or hand-held electronic devices such as Palm pilots.16,21–25 Methods tested previously have been subject to difficulties with implementation and adherence in that they required too much of participants. Using SMS texting to deliver the SGR intervention has the potential to systematically deliver the reduction intervention and enhance assessment of adherence. Smokers can receive SMS “alert texts” when they are asked to smoke and then text back within a limited time frame that they have or have not smoked.
SGR helps unlink smoking with cues. People who smoke also need help with the psychological and behavioral aspects of quitting.15,16,21–26 None has tested whether SGR plus a behavioral intervention outperforms a behavioral intervention alone. Interventions based on Social Cognitive Theory27 have addressed motivation, self-efficacy, outcome expectations, barriers, and skills. Traditionally, the behavior intervention occurs face-to-face, via telephone, or a combination of the two. In the last two decades, SMS texting has successfully been used to promote many behavior changes, including smoking cessation, weight loss, and glycemic control.14,28–39 SMS texting interventions cost less than other medium and should be explored as a way to help pregnant smokers quit.
This randomized controlled trial aimed to test whether an SMS text-based scheduled gradual reduction and counseling intervention helped more pregnant women quit than an SMS counseling intervention only. A secondary aim was to test the effect of the SGR intervention compared to support messages alone on smoking reduction.
Methods
Sample
We recruited women who were between 10 and 30 weeks pregnant, had smoked at least 100 cigarettes in their lifetime, smoked three or more cigarettes every day in the prior 7 days, were current smokers, were not using NRT in the prior 7 days, willing to try to quit smoking, were enrolled in prenatal care, were aged 18 or older, and who spoke English. We excluded women with evidence of cognitive or mental health problems who could not reliably provide informed consent.
Recruitment
We recruited women from 14 prenatal clinics in Central North Carolina from March 2014 to December 2017. We used two different methods. For some clinics, we reviewed all new obstetric patient charts weekly. We sent women with a history of tobacco use a letter from their provider informing them of the study and asking them to call a toll-free number if they prefer not to be contacted about study participation. We called women who did not opt-out and screened them. At the other clinics, nurses assessed smoking status for all new obstetric patients with the one-item measure developed by Mullen and colleagues to improve disclosure of smoking among women.40 Nurses faxed screener forms weekly. We called women who indicated they are currently smoking and permitted us to contact them. A staff member assessed eligibility and willingness to participate.
Study staff met potential participants at a convenient location to obtain written consent and administer the baseline survey, which was programmed onto research laptops for direct data entry. Staff provided all ineligible women (eg, those who smoked less than 3 cigarettes per day) with self-help cessation materials and a list of local smoking cessation programs. They randomly assigned consented participants to one of the two study arms (support messages only [SM] vs. support messages + SGR [SM + SGR]). We stratified randomization on the number of cigarettes before pregnancy, prior preterm birth, and prenatal care site (private vs. public).
Interventions
Common Elements Among Both Arms: Cell Phones, Support Messages, and Self-help Materials
Staff offered cell phones with unlimited texting plans to women who did not own their cell phone or had unreliable service. Staff also gave all women an educational pamphlet (“It's Time to Quit Smoking”), a patient aid developed by the American College of Obstetricians and Gynecologists (ACOG) to provide current information about the benefits of quitting smoking.41 Our automated system sent support messages starting at enrollment and continuing up until 35 weeks gestation. We developed and pilot-tested the support messages using those from Smokefree.gov. We met with 10 women who smoked during pregnancy, did cognitive testing of each message to refine the wording, and modified them based on women's feedback.
The support messages addressed key elements from Social Cognitive Theory, such as self-efficacy and outcome expectations. We also included problem-solving tips to prepare participants for their quit date and deal with stressors that might induce relapse (see Table 1 for sample texts). Finally, we included facts about the impact of smoking on the pregnancy as well as messages dealing with the emotional aspects of guilt and shame. In the first 7 weeks, messages primarily focused on motivation, outcome expectations, and problem-solving. In the later weeks, messages focused on self-efficacy and problem-solving. The messages in the late third trimester focused on known predictors of postpartum relapse, including partner support and self-efficacy. The support message library contained 160 messages, enough to send all women messages through 35 weeks gestation (range 96–160 texts sent). During weeks 1–6, we sent women 1–2 text messages per day. During week 8 through 35 weeks gestation, we sent only three texts per week. Women in the support messages only arm were asked to set a quit date within 2–3 weeks. Women in the SGR arm had their quit date set for them (please see below).
Table 1.
Summary of Support Text Message Library and Sample Messages
| Construct | Number of messages | Examples |
|---|---|---|
| Greetings | 2 | Thank you for completing the Baby Steps program. We hope the rest of your pregnancy goes well and that you can welcome your newborn baby as a non-smoker |
| Attributions | 2 | Message 1 of 2: How you talk to yourself is important. On days you have smoked more than you wanted to, notice what you say to yourself. Message 2 of 2: Instead of saying, “I'm awful because I smoked so much” say, “Everyone has bad days. Tomorrow I will do better.” |
| Guilt/shame | 6 | People might be making you feel ashamed that you're smoking. You're doing your best for your baby. you're a good mom for being in the program and trying |
| Information | 16 | Smoking fact: Women who smoke less or quit are more likely to keep their baby full term. Full term babies are healthier. Not smoking helps after you have your baby too. Babies not around smoke r less likely to have breathing problems like asthma or ear infections |
| Motivation | 16 | Say to yourself all the reasons you're quitting. The more you say it, the more real it will be for you Quitting is not just good foryouand your baby. It's good for everyone around u. Quitting is a way you can protect all those you care about |
| Problem solving | 38 | Message 1 or 2: For lots of people, smoking goes hand in hand with parts of the day. Think about what habits are tied to your smoking. Message 2 of 2: What can you do instead of smoke when you do those habits Example: if you smoke after meals, try going for a walk, brushing your teeth, or washing the dishes |
| Self-efficacy | 50 | Think about other hard things you have done in your life. You have the strength to try to quit just like you have done other hard things. Quitting smoking is a process that includes ups and downs. Don't let the downs stop you. Learn from bumps and keep moving towards your goal |
| Outcome expectations | 15 | Every cigarette you do not smoke is more oxygen your baby gets. Congrats on working to make your baby healthy and strong. Quitting smoking helps your baby's health right away. Quitting in the next few weeks will make a difference. |
| Stress | 7 | You might feel that you have too much going on in your life to quit. Take things one day at a time. Every cigarette you don't smoke helps your baby. |
| Support | 8 | If friends and family are doing things that make it harder not to smoke, let them know what they can do that is more helpful. |
SGR Arm Only: SGR Alerts
Staff explained the following to women randomized to the SGR arm: (1) the purpose of the SGR is to gradually reduce from the cigarettes they smoked at baseline to 0 cigarettes by the first day of the 4th or 5th week of their enrollment in the study. (2) They will receive two different types of texts: one at the times they are scheduled to smoke and one that provides them with supportive messages. (3) During the start-up week (week 1), they would be asked to text the letter “s” every time they smoked to assess their smoking pattern. We calculated the average number of cigarettes per day at the end of week 1.
Using the algorithm used by Cinciripini et al. and in our pilot study,16 we calculated the number of cigarettes per day (CPD) women should smoke when they are reducing. The algorithm reduces the number of cigarettes smoked per day by 1/3 each week. We adjusted the algorithm so women smoking 7 or fewer CPD had a 3-week reduction period, and women smoking 8 or more had a 4-week reduction period. We asked women to text back within 60 minutes of receiving an alert and text “s” indicating she smoked or “ns” if she did not. Finally, after the first week, we texted all women to assess their perceived difficulty of reduction. Women who reported that it was difficult were given the option to extend the reduction period by 1 week.
Measures
We paid participants for surveys: $20 for baseline, $25 for end of pregnancy, and $30 for postpartum plus a $20 bonus for all surveys. At baseline, we assessed demographics, pregnancy history, and smoking history variables (Table 2).42–45
Table 2.
Participant Characteristics
| Total | Support messages (SM) | Smoking gradual reduction + SM | |
|---|---|---|---|
| Characteristics | (N = 314) | (N = 163) | (N = 154) |
| Age (M, SD) | 28 (5) | 28 (6) | 27 (5) |
| Partnered (%) | 55 | 54 | 57 |
| Education (%) | |||
| Less than high school | 26 | 26 | 26 |
| High school/GED | 31 | 33 | 28 |
| Vocational school | 6 | 4 | 7 |
| Some college | 32 | 30 | 34 |
| College graduate or higher | 6 | 7 | 5 |
| Race (%) | |||
| White | 39 | 38 | 40 |
| Black | 53 | 54 | 52 |
| Other (14 reported more than one race) | 8 | 8 | 8 |
| Employment (%) | |||
| Employed | 34 | 28 | 40 |
| Not employed | 66 | 72 | 60 |
| Financial security (%) (missing n = 10) | |||
| Enough money for special things | 19 | 20 | 18 |
| Little spare money for extra things | 40 | 31 | 51 |
| Have money only to pay bills | 16 | 18 | 13 |
| Difficulty paying the bills | 22 | 28 | 16 |
| Site (%) | |||
| Durham | 85 | 85 | 85 |
| Fayetteville | 15 | 15 | 15 |
| Took study cell phone | 46 | 54 | 38 |
| First pregnancy (%) | 12 | 11 | 14 |
| Wantedness of this pregnancy (missing n = 3) | |||
| Wanted to be pregnant now | 32 | 31 | 33 |
| Wanted to be pregnant later | 46 | 47 | 44 |
| Not sure if wanted to be pregnant | 6 | 4 | 8 |
| Did not want to be pregnant | 16 | 17 | 14 |
| Partner smokes (%) | 73 | 71 | 75 |
| Number of other household smokers (Median, IQR) (range 0–10) (missing n = 2) | 1 (2) | 1 (2) | 1 (1) |
| Cigarettes per day before this pregnancy (M, SD) | 17 (9) | 17 (10) | 17 (7) |
| Cigarettes per day in this pregnancy (M, SD) | 9 (5) | 9 (5) | 9 (5) |
| Nicotine dependence (FTND) (0–9) (M, SD) | 4 (2) | 4 (2) | 4 (2) |
| Withdrawal symptoms (M, SD) | 2 (1) | 2 (1) | 2 (1) |
Adherence
We calculated adherence in two ways. First, we measured the time to respond to the SGR message. Second, we created a categorical variable (0 = respond in more than 60 minutes, 1 = respond in ≤60 minutes). We told women they could win a raffle for a $25 gift card each week in which they responded to 80% or more alert texts. To assess the fidelity of the SGR program, we asked women at their postpartum survey their agreement with this statement, “I smoked when I wanted, not in response to the alert texts” (1 = completely disagree to 7 = completely agree) and “How often did you get a text but were unable to smoke and you just smoked later” (1 = never, 2 = a few times, 3 = sometimes, 4 = a lot of times). We assured them that their honesty is important to our ability to develop new programs in the future.
General Process Measures
At the end of pregnancy and postpartum surveys, we asked women to rate the helpfulness of the SGR program and the support messages separately (1 = not at all helpful to 5 = very helpful).
Primary Outcome—Smoking Status
At all time-points, we assessed 7-day point-prevalence abstinence and biochemically validated the results via saliva samples. We sent saliva samples to Dr Jed Rose's lab at Duke for analysis. They analyzed the saliva samples for the presence of cotinine using radio-immunoassay (RIA).46–48 We used a cut-point of 10 ng/mL48 for saliva cotinine to discriminate abstainers and smokers (Table 3).
Table 3.
Seven-Day Point Prevalence Abstinence Rates With 95% Confidence Intervals (CI) by Arma
| Intent to treat (N = 314) | Observed (N = 254)b | |||
|---|---|---|---|---|
| SM only (N = 160) | SM + SGR (N = 154) | SM only (N = 132/119) | SM + SGR (N = 122) | |
| Assessment | Percent (95% CI) | Percent (95% CI) | Percent (95% CI) | Percent (95% CI) |
| End of pregnancy | 9 (5 to 13) | 9 (5 to 14) | 11 (6 to 16) | 12 (7 to 17) |
| 3-month postpartum | 5 (2 to 8) | 5 (2 to 8) | 7 (2 to 8) | 6 (2 to 8) |
aPercents (95% CI controlled for baseline education and partner smoking status).
b N = 254 at end of pregnancy and N = 234 at 3 months postpartum.
Secondary Outcome—Smoking Reduction
To assess reduction, we assessed the absolute difference in the number of cigarettes smoked from baseline to end of pregnancy, 50% reduction, which is consistent with other SGR studies,49 and the percent of women who smoke five or fewer cigarettes per day.
Process Measures
We assessed women's perceptions of the interventions by asking how helpful they found the support messages and SGR messages (SGR arm only) (1 = not at all helpful to 5 = extremely helpful) as well as the likelihood they would recommend the program to a friend (1 = definitely would not recommend to 5 = definitely would recommend).
We used chi-square test and logistic regression to test for a difference between arms on smoking cessation rate at end of pregnancy (1-sided alpha of 0.025), controlling for two of the strongest covariates known to be related to smoking during pregnancy: education and partner smoking50,51 to remove any by-chance variance that randomization missed. All covariates remained in the model, regardless of their statistical significance. We included lost-to-follow-up participants in the analysis by assuming that they were still smoking. We present cessation rates within each arm and the covariate-adjusted rates with 95% confidence intervals. To assess the impact of the lost-to-follow-up participants in the analysis, only those participants who provided follow-up data were analyzed and compared with results with imputation. We also compared the two arms on the percentage of women lost-to-follow-up and describe the potential effect of any obtained differences on the generalizability of our results. We were powered at 80% to detect arm differences of 0.10 versus 0.185.16,24,52
Results
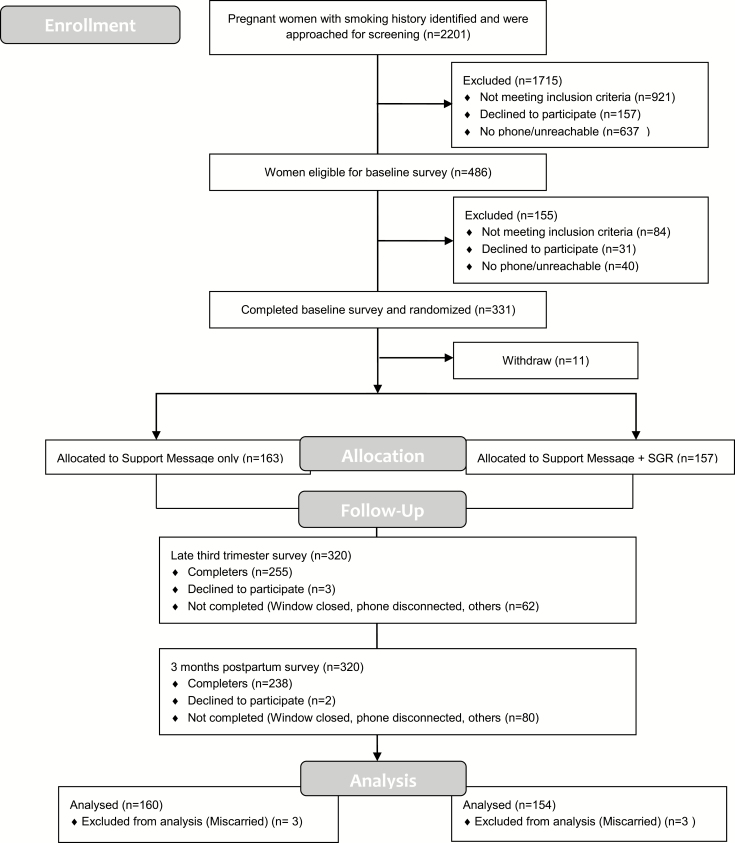
See the CONSORT diagram (Figure 1). No arm differences existed on attrition rates at end of pregnancy (SM: 18% vs. SM + SGR: 21%, p = .46) or 3-months postpartum (SM: 26% vs. 25%, p = .95). Of our sample, 53% were African American, 44% had more than high school education, 55% were married or living with a partner, and 66% were not employed for pay (Table 2). Only 46% chose to receive a study phone. Women in the support messages alone were more likely to be unemployed (p = .03) and have difficulty paying the bills (p = .0003) than women in the scheduled gradual reduction arm.
Figure 1.
Study flow diagram. SGR = SMS text-based scheduled gradual reduction and counseling intervention; support only = SMS text-based counseling intervention only.
Primary Analyses
Adherence
In the first week when women were asked to text when they smoked each cigarette, 95% (145/154) sent their data. Women in that first week reported fewer cigarettes (average 7 cigarettes per day) than they did at baseline (average 9 cigarettes per day) and confirmed at the end of the week that the number was accurate. Thus, women were reducing their cigarettes before the SGR program began. When calculating data for SGR adherence, we removed data when women responded to less than half of smoking prompts in one day (occurred for 28% of days). Once the SGR program started in the second week and women received alert texts to smoke, they responded within the 60-minute window 70% of the time. Of those responses, 88% responded with “S” indicating they smoked. Most (72%) did not report that they smoked when they wanted instead of in response to an alert. Further, few (17%) reported that there were “a lot of times” that they received an alert text and did not smoke but smoked later.
Cessation
Women in both arms had a similar quit rate: intent-to treat (Support messages only: 9%, CI: 5%–13% vs. SM + SGR: 9%, CI: 4% to 14% p = .94); observed data (Support messages only: 11%, CI: 6% to 16% vs. SM + SGR: 12%, CI: 7% to 17% p = .85).
Reduction
We found no arm differences on any of the endpoints or in the reduction of smoking (Tables 3 and 4). Similar absolute differences of cigarettes smoked were reported (SM: 5.09, CI: 4.24- 5.94 vs. SM + SGR: 5.00, CI: 4.08–5.94, p = .90). At the end of pregnancy, more than half reported reduction by at least 50%: (SM: 0.57, CI: 0.48–0.67 vs. SM + SGR: 0.58, CI: 0.48–0.67, p = ..93) At the end of pregnancy, 68% of women reported smoking five or fewer cigarettes with no arm differences: (SM: 0.64, CI: 0.55–0.73 vs. SM + SGR: 0.73, CI: 0.64–0.82, p = ..13).
Process Measures
Women did not differ in their perceptions of the intervention in terms of helpfulness (SM: M = 4.83, SD = 1.80 vs. SM + SGR: M = 5.10, SD = 1.93) or in whether they would recommend the program to a friend (SM: M = 6.45, SD = 1.17 vs. SM + SGR: M = 6.65, SD = 0.89).
Discussion
This is one of the first studies to test scheduled gradual reduction via texting to promote cessation in pregnancy. We compared support messages only program to a more intensive program where women were asked to follow a program of gradually reducing their cigarettes while receiving support text messages. We expected women who received the scheduled gradual reduction intervention to have higher quit rates and higher reduction rates than women receiving the support messages only. We did not find an added benefit of the scheduled gradual reduction on either outcome.
Despite the null trial results, this study provides an important contribution to the development of effective cessation support for pregnant women. First, women in both arms had clinically significant quit rates compared with the 2%–5% of women who can quit on their own if they continue to smoke after learning of their pregnancy.7,53 Thus, helping 9%–12% of pregnant women quit smoking through a low-intensity, scalable intervention shows promise and is consistent with other programs that have used SMS texting to promote cessation among pregnant women.14,28 Previous research suggests that pregnant smokers prefer text-based programs compared with face-to-face counseling and a subgroup of women may prefer programs designed to reduce to quit rather than abrupt cessation.18 Further, this study differs from previous SMS texting studies as we recruited women proactively from OB clinics, and thus, might be more generalizable than studies in which only women who were interested in quitting smoking proactively enrolled.
There are several reasons why the scheduled gradual reduction did not add above support messages. First, it might have been that women did not truly adhere tightly to the schedule. Even though 70% of women texted back that they smoked in response to the alert text, we cannot verify their actual smoking. The recent development of mobile devices that measure CO would be an excellent addition to text-based cessation studies and enable real-time assessment and validation of smoking.54,55 Second, delivery of real-time SMS texting programs have many logistical challenges. For example, there might have been times when women could not respond to our texts due to limited access to a cell phone during work or school. Further, cell phone service and reception were limited in some areas that resulted in missed texts. Finally, most women had already reduced their cigarettes to less than 10 cigarettes a day by the time we started reducing them further. Indeed most pregnant women who continue to smoke have already reduced their smoking once they learn they are pregnant.49 Further, many women in the SGR group reduced even further in the first week of data collection, perhaps due to self-monitoring. Thus, the SGR might not have been as effective with women who have already experienced two phases of reduction in their smoking before the SGR program began. Although we do not have data on heavier smokers, we believe this method might work better for heavier smokers who have not yet reduced. Given the clinically significant quit rate in the SGR group, we recommend further exploration and refinement of SGR interventions as a method to help pregnant smokers quit.
One promising finding was that just sending support messages encouraged 9%–12% of women to quit. Of those still smoking, more than half reduced their smoking by at least 50%, cutting down to four cigarettes per day on average. This is significant given women who smoke ≤5 cigarettes per day have similar birth outcomes to those who do not smoke.6 This intervention is scalable, easy to follow, and can have a great impact on pregnant women who smoke.
This study has limitations. Because women enrolled in a trial, they might not represent all pregnant smokers. Further, we collected data from women in person. Even though we biochemically validated their smoking status, they might have felt accountable to the data collectors. Also, we do not have a true control arm to which to compare our interventions.
This study has broad implications for helping pregnant women quit. Simply texting support messages throughout their pregnancy seems to help some quit and almost all to reduce significantly. Future steps include learning ways to facilitate enrollment to the texting program.
Table 4.
Rate and 95% CI for Cigarettes Per Day and 50% Reduction Among Smokers at Late Pregnancy
| Intent to treat (N = 283) | Observed (N = 222) | |||
|---|---|---|---|---|
| SM only (n = 144) | SM + SGR (N = 139) | SM only (n = 115) | SM + SGR (N = 107) | |
| Smoking rate | Rate (95% CI) | Rate (95% CI) | Rate (95% CI) | Rate (95% CI) |
| 50% reduction cigs/day (%) | 46 (38 to 54) | 45 (36 to 53) | 57 (48 to 67) | 58 (48 to 67) |
| Smokes ≤5 cigs/daya (%) | ||||
| 64 (55 to 73) | 73 (64 to 82) | |||
aFor observed data only.
Funding
This work was supported by the National Cancer Institute (R01CA166149).
Declaration of Interests
None declared.
Supplementary Material
References
- 1. Goldenberg RL, Davis RO, Cliver SP, et al. Maternal risk factors and their influence on fetal anthropometric measurements. Am J Obstet Gynecol. 1993;168(4):1197–1203; discussion 1203. [DOI] [PubMed] [Google Scholar]
- 2. Chapin J, Root W; American College of Obstetricians and Gynecologists Improving obstetrician-gynecologist implementation of smoking cessation guidelines for pregnant women: an interim report of the American College of Obstetricians and Gynecologists. Nicotine Tob Res. 2004;6(suppl 2):S253–S257. [DOI] [PubMed] [Google Scholar]
- 3. Castles A, Adams EK, Melvin CL, Kelsch C, Boulton ML. Effects of smoking during pregnancy. Five meta-analyses. Am J Prev Med. 1999;16(3):208–215. [DOI] [PubMed] [Google Scholar]
- 4. Salihu HM, Aliyu MH, Pierre-Louis BJ, Alexander GR. Levels of excess infant deaths attributable to maternal smoking during pregnancy in the United States. Matern Child Health J. 2003;7(4):219–227. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. Smoking during pregnancy—United States, 1990–2002. MMWR Morb Mortal Wkly Rep. 2004;53(39):911–915. [PubMed] [Google Scholar]
- 6. Kataoka MC, Carvalheira APP, Ferrari AP, Malta MB, de Barros Leite Carvalhaes MA, de Lima Parada CMG. Smoking during pregnancy and harm reduction in birth weight: a cross-sectional study. BMC Pregnancy Childbirth. 2018;18(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tong VT, Dietz PM, Morrow B, et al. ; Centers for Disease Control and Prevention (CDC). Trends in smoking before, during, and after pregnancy—Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000–2010. MMWR Surveill Summ. 2013;62(6):1–19. [PubMed] [Google Scholar]
- 8. Li CQ, Windsor RA, Perkins L, Goldenberg RL, Lowe JB. The impact on infant birth weight and gestational age of cotinine-validated smoking reduction during pregnancy. JAMA. 1993;269(12):1519–1524. [PubMed] [Google Scholar]
- 9. Tong VT, England LJ, Dietz PM, Asare LA. Smoking patterns and use of cessation interventions during pregnancy. Am J Prev Med. 2008;35(4):327–333. [DOI] [PubMed] [Google Scholar]
- 10. Windsor R, Woodby L, Miller T, Hardin M. Effectiveness of Smoking Cessation and Reduction in Pregnancy Treatment (SCRIPT) methods in Medicaid-supported prenatal care: Trial III. Health Educ Behav. 2011;38(4):412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Vries H, Bakker M, Mullen PD, van Breukelen G. The effects of smoking cessation counseling by midwives on Dutch pregnant women and their partners. Patient Educ Couns. 2006;63(1-2):177–187. [DOI] [PubMed] [Google Scholar]
- 12. Akhu-Zaheya LM, Shiyab WY. The effect of short message system (SMS) reminder on adherence to a healthy diet, medication, and cessation of smoking among adult patients with cardiovascular diseases. Int J Med Inform. 2017;98:65–75. [DOI] [PubMed] [Google Scholar]
- 13. Naughton F, Cooper S, Foster K, et al. Large multi-centre pilot randomized controlled trial testing a low-cost, tailored, self-help smoking cessation text message intervention for pregnant smokers (MiQuit). Addiction. 2017;112(7):1238–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abroms LC, Johnson PR, Leavitt LE, et al. A randomized trial of text messaging for smoking cessation in pregnant women. Am J Prev Med. 2017;53(6):781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cinciripini PM, Wetter DW, McClure JB. Scheduled reduced smoking: effects on smoking abstinence and potential mechanisms of action. Addict Behav. 1997;22(6):759–767. [DOI] [PubMed] [Google Scholar]
- 16. Cinciripini PM, Lapitsky L, Seay S, Wallfisch A, Kitchens K, Van Vunakis H. The effects of smoking schedules on cessation outcome: can we improve on common methods of gradual and abrupt nicotine withdrawal? J Consult Clin Psychol. 1995;63(3):388–399. [DOI] [PubMed] [Google Scholar]
- 17. Lindson N, Aveyard P, Hughes JR. Reduction versus abrupt cessation in smokers who want to quit. Cochrane Database Syst Rev. 2010:CD008033. [DOI] [PubMed] [Google Scholar]
- 18. Sloan M, Hopewell S, Coleman T, Cooper S, Naughton F. Smoking cessation support by text message during pregnancy: a qualitative study of views and experiences of the MiQuit intervention. Nicotine Tob Res. 2017;19(5):572–577. doi:10.1093/ntr/ntw241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. England LJ, Kendrick JS, Wilson HG, Merritt RK, Gargiullo PM, Zahniser SC. Effects of smoking reduction during pregnancy on the birth weight of term infants. Am J Epidemiol. 2001;154(8):694–701. [DOI] [PubMed] [Google Scholar]
- 20. McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18(2):156–170. [DOI] [PubMed] [Google Scholar]
- 21. Ostroff JS, Burkhalter JE, Cinciripini PM, et al. Randomized trial of a presurgical scheduled reduced smoking intervention for patients newly diagnosed with cancer. Health Psychol. 2014;33(7):737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Riley W, Jerome A, Behar A, Weil J. Computer and manual self-help behavioral strategies for smoking reduction: initial feasibility and one-year follow-up. Nicotine Tob Res. 2002;4(suppl 2):S183–S188. [DOI] [PubMed] [Google Scholar]
- 23. Riggs RL, Hughes JR, Pillitteri JL. Two behavioral treatments for smoking reduction: a pilot study. Nicotine Tob Res. 2001;3(1):71–76. [DOI] [PubMed] [Google Scholar]
- 24. Etter JF, Huguelet P, Perneger TV, Cornuz J. Nicotine gum treatment before smoking cessation: a randomized trial. Arch Intern Med. 2009;169(11):1028–1034. [DOI] [PubMed] [Google Scholar]
- 25. Cummings KM, Emont SL, Jaén C, Sciandra R. Format and quitting instructions as factors influencing the impact of a self-administered quit smoking program. Health Educ Q. 1988;15(2):199–216. [DOI] [PubMed] [Google Scholar]
- 26. Berkman ET, Dickenson J, Falk EB, Lieberman MD. Using SMS text messaging to assess moderators of smoking reduction: validating a new tool for ecological measurement of health behaviors. Health Psychol. 2011;30(2):186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall, Inc.; 1986. [Google Scholar]
- 28. Naughton F, Prevost AT, Gilbert H, Sutton S. Randomized controlled trial evaluation of a tailored leaflet and SMS text message self-help intervention for pregnant smokers (MiQuit). Nicotine Tob Res. 2012;14(5):569–577. [DOI] [PubMed] [Google Scholar]
- 29. Patrick K, Raab F, Adams MA, et al. A text message-based intervention for weight loss: randomized controlled trial. J Med Internet Res. 2009;11(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodgers A, Corbett T, Bramley D, et al. Do u smoke after txt? Results of a randomised trial of smoking cessation using mobile phone text messaging. Tob Control. 2005;14(4):255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Franklin VL, Waller A, Pagliari C, Greene SA. A randomized controlled trial of Sweet Talk, a text-messaging system to support young people with diabetes. Diabet Med. 2006;23(12):1332–1338. [DOI] [PubMed] [Google Scholar]
- 32. Haapala I, Barengo NC, Biggs S, Surakka L, Manninen P. Weight loss by mobile phone: a 1-year effectiveness study. Public Health Nutr. 2009;12(12):2382–2391. [DOI] [PubMed] [Google Scholar]
- 33. Brendryen H, Kraft P. Happy ending: a randomized controlled trial of a digital multi-media smoking cessation intervention. Addiction. 2008;103(3):478–484; discussion 485. [DOI] [PubMed] [Google Scholar]
- 34. Obermayer JL, Riley WT, Asif O, Jean-Mary J. College smoking-cessation using cell phone text messaging. J Am Coll Health. 2004;53(2):71–78. [DOI] [PubMed] [Google Scholar]
- 35. Spohr SA, Nandy R, Gandhiraj D, Vemulapalli A, Anne S, Walters ST. Efficacy of SMS text message interventions for smoking cessation: a meta-analysis. J Subst Abuse Treat. 2015;56:1–10. [DOI] [PubMed] [Google Scholar]
- 36. Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev. 2016;4:CD006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Head KJ, Noar SM, Iannarino NT, Grant Harrington N. Efficacy of text messaging-based interventions for health promotion: a meta-analysis. Soc Sci Med. 2013;97:41–48. [DOI] [PubMed] [Google Scholar]
- 38. Cole-Lewis H, Kershaw T. Text messaging as a tool for behavior change in disease prevention and management. Epidemiol Rev. 2010;32:56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fjeldsoe BS, Marshall AL, Miller YD. Behavior change interventions delivered by mobile telephone short-message service. Am J Prev Med. 2009;36(2):165–173. [DOI] [PubMed] [Google Scholar]
- 40. Mullen PD, Ito JR, Carbonari JP, DiClemente CC. Assessing the congruence between physician behavior and expert opinion in smoking cessation counseling. Addict Behav. 1991;16(5):203–210. [DOI] [PubMed] [Google Scholar]
- 41. The American College of Obstetricians and Gynecologists. It's Time to Quit Smoking. Washington, DC: The American College of Obstetricians and Gynecologists; 2014. [Google Scholar]
- 42. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 43. Kozlowski LT, Porter CQ, Orleans CT, Pope MA, Heatherton T. Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug Alcohol Depend. 1994;34(3):211–216. [DOI] [PubMed] [Google Scholar]
- 44. Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF. Reliability of the Fagerström Tolerance Questionnaire and the Fagerström test for nicotine dependence. Addict Behav. 1994;19(1):33–39. [DOI] [PubMed] [Google Scholar]
- 45. Tate JC, Schmitz JM. A proposed revision of the Fagerström Tolerance Questionnaire. Addict Behav. 1993;18(2):135–143. [DOI] [PubMed] [Google Scholar]
- 46. Langone JJ, Gjika HB, Van Vunakis H. Nicotine and its metabolites. Radioimmunoassays for nicotine and cotinine. Biochemistry. 1973;12(24):5025–5030. [DOI] [PubMed] [Google Scholar]
- 47. Sepkovic DW, Haley NJ. Biomedical applications of cotinine quantitation in smoking related research. Am J Public Health. 1985;75(6):663–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Haley NJ, Axelrad CM, Tilton KA. Validation of self-reported smoking behavior: biochemical analyses of cotinine and thiocyanate. Am J Public Health. 1983;73(10):1204–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lindson N. Cochrane reviews—in their own words. Reduction versus abrupt cessation in smokers who want to quit. J Evid Based Med. 2010;3(2):133. [DOI] [PubMed] [Google Scholar]
- 50. Swamy GK, Roelands JJ, Peterson BL, et al. Predictors of adverse events among pregnant smokers exposed in a nicotine replacement therapy trial. Am J Obstet Gynecol. 2009;201(4):354.e1–354.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Riaz M, Lewis S, Naughton F, Ussher M. Predictors of smoking cessation during pregnancy: a systematic review and meta-analysis. Addiction. 2018;113(4):610–622. [DOI] [PubMed] [Google Scholar]
- 52. Hughes JR, Solomon LJ, Livingston AE, Callas PW, Peters EN. A randomized, controlled trial of NRT-aided gradual vs. abrupt cessation in smokers actively trying to quit. Drug Alcohol Depend. 2010;111(1–2):105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Crozier SR, Robinson SM, Borland SE, Godfrey KM, Cooper C, Inskip HM; SWS Study Group Do women change their health behaviours in pregnancy? Findings from the Southampton Women's Survey. Paediatr Perinat Epidemiol. 2009;23(5):446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dar R. Effect of real-time monitoring and notification of smoking episodes on smoking reduction: a Pilot Study of a Novel Smoking Cessation App. Nicotine Tob Res. 2018;20(12):1515–1518. doi:10.1093/ntr/ntx223. [DOI] [PubMed] [Google Scholar]
- 55. Meredith SE, Robinson A, Erb P, et al. A mobile-phone-based breath carbon monoxide meter to detect cigarette smoking. Nicotine Tob Res. 2014;16(6):766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.