Abstract
Introduction
We describe the development and pilot testing of the experimental tobacco and nicotine product marketplace (ETM)—a method for studying tobacco and nicotine product (TNP) choices and use behavior in a standardized way.
Aims and Methods
The ETM resembles an online store populated with TNPs. Surveillance activities and data from a US representative survey and consumer reports were used to determine the most popular TNPs for inclusion in the ETM. Standardized information and videos demonstrating how to use the TNPs were provided. To test the feasibility of using the ETM, smokers (n = 119) underwent monitoring of usual brand cigarette smoking and other TNP use (Baseline Phase) followed by access to the ETM (ETM Phase) that included their usual brand cigarettes, e-cigarettes, moist snuff, snus, and nicotine replacement therapy. During the ETM Phase, participants were provided points based on their baseline TNP consumption to exchange for TNPs in the ETM. Participants were advised to exchange points for enough TNPs to last until their next visit and to refrain from using TNPs not obtained in the ETM. A subset of the participants (n = 62) completed a survey on their experience with the ETM.
Results
The majority of the participants stated they were comfortable with navigating the ETM (97%), it was easy to determine product characteristics (89%), and they were satisfied with the products included in the marketplace (85%).
Conclusions
The ETM was well received by the vast majority of the participants and can be utilized by researchers to investigate a variety of TNP policy and regulatory science research questions.
Implications
Patterns of TNP use are complex due to greater availability, marketing, and promotion of a diverse array of TNPs. Innovative methods are needed to experimentally study TNP choices and patterns. Through describing the development of the ETM, we provide researchers with a tool that can be readily adapted to studying a variety of phenomena challenging public health.
Introduction
Patterns of tobacco and nicotine product (TNP) use are complex due to greater availability, marketing, and promotion of a diverse array of TNPs. During 2013–2014, 40% of US tobacco users reported using multiple TNPs, and among multiple TNP users, there were more than 300 different product combinations.1 Given the complexity of the TNP landscape, innovative methods have been developed to experimentally study TNP choices and use patterns. One such method is the experimental tobacco and nicotine marketplace (ETM), an online “store” that provides TNPs and offers the ability to readily manipulate content.2–7 The ETM was developed to evaluate which TNPs serve as cigarette substitutes when prices are manipulated and has since been used to study substitution of other TNPs (e.g., electronic nicotine delivery systems [ENDS]).2–7 To provide guidance to researchers who are interested in utilizing the ETM in their own studies, we provide a detailed description of the setup of an ETM developed for a clinical trial that provides cigarettes and other TNPs at prices reflective of the real-world marketplace to adult cigarette smokers (ClinicalTrials.gov Identifier: NCT03272685). We also present data from pilot testing the ETM, including participant reactions to navigating the ETM, to inform researchers on the feasibility of the ETM.
ETM Development
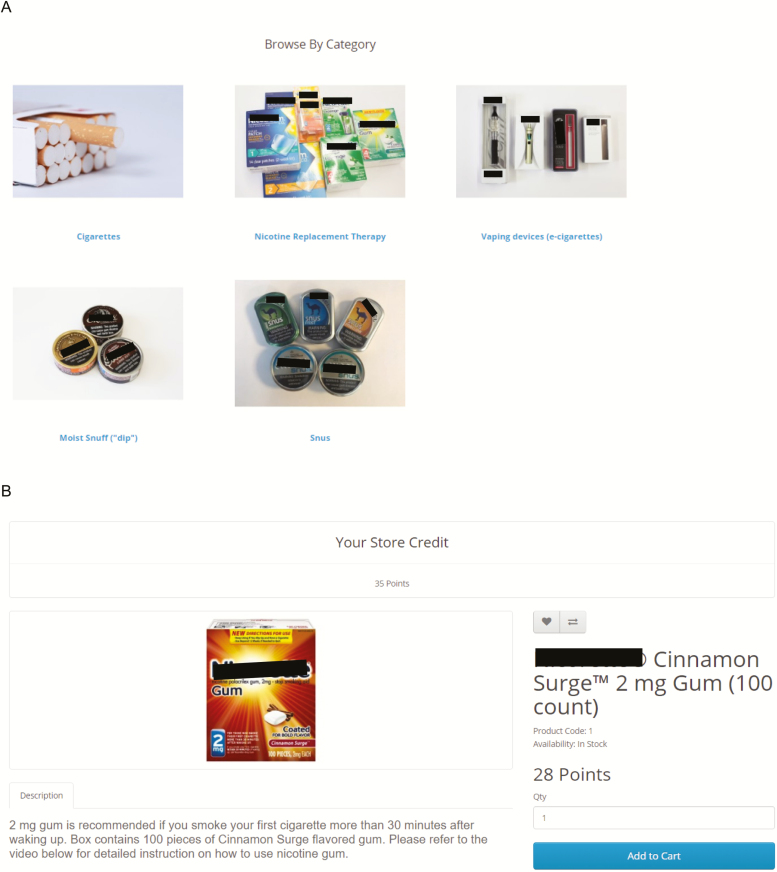
As shown in Figure 1A, the ETM has the appearance of an online store with several TNP categories. As shown in Figure 1B, each TNP in the ETM has standardized information including a photo and description of the product and the points needed to exchange for the product (described in section titled “Participant Points”). This is the first ETM to include a video for each TNP (except for cigarettes) that demonstrates how to use the TNP based on the manufacturer’s recommendations. Similar to a previous version of the ETM,6 OpenCart serves as the platform for the ETM which is a free open source platform for online merchants. Additional information on OpenCart can be found at http://docs.opencart.com/en-gb/introduction/.
Figure 1.
Homepage of the experimental marketplace (A) and example of standardized product (B).
Product Selection
For the purpose of the clinical trial, participant’s usual brand cigarettes and the most popular varieties of the following products were included in the ETM: ENDS, snus, moist snuff, and nicotine replacement therapy (NRT).
Cigarettes
Each participant’s usual brand/subtype of cigarettes (e.g., Newport Menthol 100s) was included in the ETM. A participant’s usual brand of cigarettes was determined by the following question: “What is your usual brand/subtype of manufactured cigarettes?”. Given the array of cigarette brands/subtypes, a photo of a generic cigarette pack was included in the ETM. Based on feedback from the pilot testing (described in section titled “Piloting Testing of the ETM”) that it was unclear whether the participant would receive their usual brand, we included the following statement “The cigarettes you usually smoke” in the description of the product.
Moist Snuff and Snus
Based on a comparison of the most recent PATH data8 and Nielsen Company consumer reports,9 we identified popular brands and flavors of moist snuff (i.e., Copenhagen Long-Cut, Copenhagen Long-Cut Wintergreen, Copenhagen Snuff, Grizzly Fine-Cut Wintergreen, Grizzly Long-Cut Wintergreen) and snus (Camel Snus Frost, Camel Snus Winterchill, Camel Snus Mellow, Skoal Snus Mint, Skoal Snus Smooth Mint).
Electronic Nicotine Delivery Systems
ENDS represent a rapidly diversifying TNP category.10 To understand the varieties of ENDS, study staff visited vape shops and interviewed employees using a structured questionnaire. Questions regarding which devices were most popular, device specifications, and whether the device would be recommended to an inexperienced ENDS user were included. ENDS devices identified included cartridge, pod, pen-style, all-in-one (AIO), and mod/tank systems. The majority of the mods/tank systems were not recommended for inexperienced ENDS users, whereas the others were recommended for inexperienced ENDS users. For the purpose of the clinical trial, we opted to include only ENDS that would likely be used by current smokers who are inexperienced ENDS users. Thus, we selected ENDS representative of the cartridge (Vuse Solo), pod (JUUL), pen-style (Halo Triton), and AIO (Joyetech eGo AIO) systems. Both the cartridge and pod systems selected for the ETM had prefilled pods/cartridges with the following flavors: tobacco, fruit, cream/vanilla, and mint/menthol. Thus, we included a flavor option from each of these categories for the pod and cartridge devices. To be able to compare flavor preferences across ENDS included in the ETM, we selected e-liquid flavors for the pen-style and AIO device that were representative of the same four flavor categories. Regarding nicotine concentrations, the cartridge and pod devices had only one option at the time of surveillance. For the pen-style and AIO devices, we selected nicotine concentrations based on the vape shop employee’s recommendation. For example, the majority of the vape shop employees recommended e-liquid between 3- and 6-mg nicotine/ml for the AIO device.
Nicotine Replacement Therapy
We included nicotine patch, gum, and lozenge. Specifically, Nicoderm CQ Patch in 21-mg nicotine, 14-mg nicotine, and 7-mg nicotine were included in the ETM, as well as Nicorette gum in 2- and 4-mg nicotine options and the flavors Winter Ice Mint, Fruit Chill, and Cinnamon Surge and Nicorette mini lozenge mint flavor in the 2- and 4-mg nicotine options.
Product Pricing and Discounting
Unlike prior ETMs that involved manipulating TNP prices to estimate demand curves and degree of substitution,2–7 each TNP in the ETM was priced at an amount that reflected its price in the real-world marketplace at each study site. Because identifying the price for every potential cigarette brand/subtype would be too burdensome, the price of an average pack of cigarettes at each site was used. We developed a survey to assess the prices of the most popular cigarette brands (i.e., Marlboro, Newport, Camel, Pall Mall, and American Spirit8). The same questionnaire was used to assess the prices of the moist snuff and snus selected to be included in the ETM. Research assistants at each site went to retail outlets to complete the survey. At each site, prices from the different retail outlets were averaged to determine the price displayed in the ETM. Because both NRT and ENDS were available online, market prices of these products were set according to their online price and thus did not vary across the sites. Last, to encourage use of only the ETM, all products were priced in the ETM at 66% of their real-world price.
Participant Points
In lieu of using US currency, participants were allocated points that could be exchanged for products in the ETM. The number of points was determined based on their typical TNP consumption, as determined during the Baseline Phase (described in section titled “Piloting Testing of the ETM”). Point allocation was sufficient such that participants could cover their baseline TNP use per day; we also allocated an additional point per day to allow for experimentation in the ETM. To discourage needless exchange of points for products, points not used were converted to cash at the study end. Participants also received a single-use coupon for ENDS or NRT (i.e., up to 20 points off) because these products were the most expensive and because many retailers provide coupons, discounted starter kits, or specialized entry product pricing.
Piloting Testing of the ETM
This study was reviewed and approved by the University of Minnesota, University of California, San Francisco, and Duke University IRBs. Adult daily cigarette smokers from each site were recruited to test the feasibility of using the ETM. Inclusion criteria were ≥18 years (≥21 at San Francisco site); smoked 5–40 cigarettes per day (CPD); and urinary cotinine level > 1000 ng/ml. Exclusion criteria were expired breath alcohol level > 0.01 g/210 l; breastfeeding, pregnant, or planning to become pregnant; unstable physical or mental health conditions; and positive urinalysis for illicit drugs (excluding cannabis).
Participants attended a screening visit and then underwent 2 weeks’ monitoring of usual brand cigarette smoking and other TNP use (Baseline Phase) followed by two visits, each approximately 1 week apart, with access to the ETM (ETM Phase). Participants attended a follow-up visit where products were returned. Participants used an IVR system to log daily TNP use (i.e., number of cigarettes smoked, ENDS puffs, snus pouches, snuff dips, and pieces of nicotine gum/lozenges). During the ETM Phase, participants were provided points to exchange for products and instructed to exchange points for enough product to last until their next visit. After participants navigated the ETM and finalized their TNP selection, the research assistant provided the TNP(s) to the participant. Participants completed a questionnaire on their ETM experience. Participants were compensated no more than $190 for their transportation ($10 per visit) and time ($20 per visit), and for completing daily IVR calls (up to $40).
Results of Pilot Testing
A total of 119 participants were included in the pilot testing of the ETM. Average participant age was 44.0 (SD = 11.7) years and slightly more than half were male gender (54.6%). The majority of participants were either non-Hispanic Black (49.6%) or non-Hispanic White (35.3%). The average age at which participants initiated smoking daily was 18.9 (SD = 5.1) years. The most popular cigarette brands were Newport (34.4%), Marlboro (26.9%), Camel (11.2%), and American Spirit (6.7%).
During the ETM Phase, all of the participants selected their usual brand of cigarettes. Forty-eight of the participants (40.3%) also selected ENDS making ENDS the most popular product choice after cigarettes. Few participants selected NRT (gum, n = 7; patch, n = 6; lozenge, n = 3), snus (n = 1), or snuff (n = 4). The average CPD during the Baseline Phase (mean = 15.9; SD = 7.0) and the ETM Phase (mean = 15.4; SD = 8.8) did not differ (p = .415). Compared with the Baseline Phase, ENDS use increased during the ETM phase, but use was still minimal Specifically, ENDS use increased from an average of less than one to six puffs per day (p < .001) per participant. There were no significant differences across the two phases in mean use per day of any of the other products (Supplementary Table 1).
Table 1 describes participants’ reactions to the ETM among a subset (n = 62) of the pilot participants who completed the ETM evaluation survey. The majority of the participants stated they were comfortable with navigating the ETM (97%) it was easy to determine product characteristics (89%), and they were satisfied with the products included in the marketplace (85%). One participant indicated that they were “very uncomfortable” with navigating the marketplace also indicated that it was “very difficult” to determine the product characteristics. When asked to explain how to improve the ETM, this participant stated that finding that cigarettes were available was confusing because a generic photo of cigarettes was provided. Based on this feedback, we updated the product description in the ETM next to the generic cigarette photo to state “The cigarettes you usually smoke.” Approximately 10% of participants reported that there were products that they were hoping to see in the ETM, but did not. When asked to explain which products, all reported hoping to see a greater array of ENDS devices and/or flavor/nicotine options.
Table 1.
Results of the experimental tobacco and nicotine marketplace evaluation survey (n = 62)
| Very comfortable | Somewhat comfortable | Neutral | Somewhat uncomfortable | Very uncomfortable | |
|---|---|---|---|---|---|
| How comfortable were you with navigating the online marketplace? | 46 (74%) | 14 (23%) | 0 | 1 (2%) | 1 (2%) |
| How comfortable were you with finding instructional videos on how to use the products? | 43 (69%) | 5 (8%) | 13 (21%) | 1 (2%) | 0 |
| How comfortable were you with determining the number of products that you needed? | 39 (63%) | 15 (24%) | 5 (8%) | 3 (5%) | 0 |
| Very easy | Somewhat easy | Neutral | Somewhat difficult | Very difficult | |
| Was it easy to determine the product characteristics? | 40 (65%) | 15 (24%) | 5 (8%) | 1 (2%) | 1 (2%) |
| Was it easy to determine the points needed for each product? | 51 (82%) | 8 (13%) | 3 (5%) | 0 | 0 |
| Was it easy to determine which products were available in the marketplace? | 56 (90%) | 4 (6%) | 1 (2%) | 1 (2%) | 0 |
| Very satisfied | Somewhat satisfied | Neutral | Somewhat unsatisfied | Very unsatisfied | |
| How satisfied were you with the products included in the marketplace? | 48 (77%) | 5 (8%) | 7 (11%) | 2 (3%) | 0 |
| How satisfied were you with the number of points that you had to exchange for products? | 41 (66%) | 8 (13%) | 10 (16%) | 3 (5%) | 0 |
Conclusions
This article provides guidance to other researchers who are interested in utilizing the ETM in their own research studies. The ETM described herein included participants’ usual brand of cigarettes and a variety of other TNPs priced at 66% of the real-world market value, used points instead of currency, provided instructional videos, and included a coupon for ENDS and NRT. Results from the pilot testing of this ETM indicate that most participants found the ETM easy to navigate and were satisfied with product choices. We also demonstrated that participants’ use behavior did not change after accessing the ETM. Specifically, participants maintained similar CPD, and although they selected ENDS, NRT, and moist snuff from the ETM, they reported using these products minimally. This was expected given that participants were current smokers instructed to obtain enough product to last until their next visit and given access to their usual brand of cigarettes without any intervention, price manipulation, or instructions on which products to choose. Future studies, particularly those that introduce more complexity to the ETM such as through an intervention and/or price manipulation, are recommended to include an evaluation of the participants’ experience with the ETM. For information on the ETM beyond what was provided here, please visit the following resource provided by SRNT University at https://www.pathlms.com/srnt-u/courses/12082.
Although the ETM described herein was standardized across all participants for the purposes of the pilot testing, the OpenCart platform allows for researchers to create multiple “customer groups,” which results in the ability to adapt content for a segment of participants. FDA research priorities,11 including the impact of changes in tobacco product characteristics such as product design, packaging, and flavors, can be evaluated through use of more than one customer group. For example, researchers could examine the impact of the introduction of novel TNPs, modified risk statements, flavor bans, or banning classes of TNPs in one customer group compared with a control setting in another customer group. Researchers could also use this platform to test how to effectively communicate to smokers about the health risks of ENDS and other TNPs by exposing one group to educational material via text or a video and comparing choices and use behavior to a control group. In addition, researchers could use this platform to understand how population subgroups (e.g., race/ethnic minorities) differ in their response to these manipulations. To further mimic the real world, the experimental marketplace could be populated with nontobacco products such as sodas and snacks, and researchers could evaluate how the addition of these products alters choices and behaviors. In addition, although participants in the pilot study were provided with the products that they selected in ETM, researchers may choose a hypothetical scenario where participants, such as youth tobacco users, are not provided products. Last, although the pilot participants accessed the ETM during in-person clinic visits, participants could be provided with a link to the online ETM, such as in a prior study using the ETM,4 and thus open the door to participants from around the world.
Supplementary Material
Supplementary Material
Supplementary data are available at Nicotine and Tobacco Research online.
Funding
This study was funded by National Institute on Drug Abuse grant U54DA031659 and the National Cancer Institute grant P01 CA217806. Support was also provided by the National Institutes of Health (NIH), National Research Service Award T32 DA007097 from the National Institute on Drug Abuse. REDCap (Research Electronic Data Capture) services were provided by grant UL1TR000114 from the National Center for Advancing Translational Sciences (grant UL1TR000114) of the National Institutes of Health. Research reported in this publication was also supported by NIH grant P30 CA77598 utilizing the Biostatistics and Bioinformatics Core–shared resource of the Masonic Cancer Center, University of Minnesota. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Declaration of Interests
None declared.
References
- 1. Kasza KA, Ambrose BK, Conway KP, et al. Tobacco-product use by adults and youths in the United States in 2013 and 2014. N Engl J Med. 2017;376(4):342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Quisenberry AJ, Koffarnus MN, Hatz LE, Epstein LH, Bickel WK. The Experimental Tobacco Marketplace I: substitutability as a function of the price of conventional cigarettes. Nicotine Tob Res. 2016;18(7):1642–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Quisenberry AJ, Koffarnus MN, Epstein LH, Bickel WK. The Experimental Tobacco Marketplace II: substistutability and sex effects in dual electronic cigarette and conventional cigarette users. Drug Alcohol Depend. 2017;178:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heckman BW, Cummings KM, Hirsch AA, et al. A novel method for evaluating the acceptability of substitutes for cigarettes: the experimental tobacco marketplace. Tob Reg Sci. 2017;3(3):266–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bickel WK, Pope DA, Kaplan BA, DeHart WB, Koffarnus MN, Stein JS. Electronic cigarette substitution in the experimental tobacco marketplace: a review. Prev Med. 2018. Dec;117:98–106. Epub 2018 Apr 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pope DA, Poe L, Stein JS, et al. Experimental tobacco marketplace: substitutability of e-cigarette liquid for cigarettes as a function of nicotine strength. Tob Control. 2019;28(2):206–211.s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DeHart WB, Kaplan BA, Pope DA, Mellis AM, Bickel WK. The experimental tobacco marketplace: narrative influence on electronic cigarette substitution. Exp Clin Psychopharmacol. 2019;27(2):115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. United States Department of Health and Human Services. National Institutes of Health. National Institute on Drug Abuse, and United States Department of Health and Human Services. Food and Drug Administration. Center for Tobacco Products. Population Assessment of Tobacco and Health (PATH) Study [United States] Public-Use Files. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor]; 2018. doi:10.3886/ICPSR36498.v8 [Google Scholar]
- 9. CSP. Tobacco: Smokeless 2016. Smokeless Tobacco Convenience-Store Sales Data from Year-End 2015 2016; https://www.cspdailynews.com/category-data/cmh/tobacco/tobacco-smokeless-2016. Accessed September 2018.
- 10. Hsu G, Sun JY, Zhu SH. Evolution of electronic cigarette brands from 2013–2014 to 2016–2017: analysis of brand websites. J Med Internet Res. 2018;20(3):e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Institutes of Health. FDA Center for Tobacco Products Research Interest Areas https://prevention.nih.gov/tobacco-regulatory-science-program/research-priorities. Accessed May 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.