Abstract
Opiate addiction has risen substantially during the past decade. New treatments to combat opiate addiction are sorely needed. The current study was conducted to determine the acute individual and interactive effects of bupropion and dextromethorphan in a rat model of opiate self-administration using the short-acting synthetic opioid remifentanil. Both of these drugs have been found to reduce self-administration of nicotine. Bupropion and dextromethorphan and their combination had differential effects depending on whether the rats showed low or high baseline remifentanil self-administration. The rats with higher initial remifentanil self-administration showed a significant decrease in remifentanil self-administration with bupropion and dextromethorphan treatment, compared to the vehicle control condition. This decrease in self-remifentanil administration was most pronounced when combination of the higher doses of bupropion and dextromethorphan were administered. In contrast, the rats with lower baseline remifentanil self-administration showed the opposite effect of drug treatment with an increase in remifentanil self-administration with drug treatment compared to the vehicle control condition. This study shows that combination bupropion and dextromethorphan affects remifentanil self-administration in a complex fashion with differential effects on low and high baseline responders. In subjects with high baseline remifentanil self-administration, bupropion and dextromethorphan treatment significantly reduced self-administration, whereas in subjects with low baseline remifentanil self-administration, bupropion increased remifentanil self-administration and dextromethorphan had no discernible effect. This finding suggests that combination bupropion-dextromethorphan should be tested in humans, with a focus on treating people with high-level opiate use.
Keywords: Bupropion, Dextromethorphan, Remifentanil, Self-administration, Rats, Opioids
Introduction
There is currently an epidemic of opioid abuse in the United States with lethal opioid overdoses increasing 200% from 2000–2014, aggravated by the potent opioid fentanyl (Rudd et al. 2016). Current treatment options for opioid addiction include full μ-opioid agonists and partial agonists with slow onset and long half-lives such as buprenorphine and methadone; however, these treatments tend to be associated with high relapse rates (Stotts et al. 2009). Another treatment is naltrexone, a rapid-onset and short acting μ-opioid antagonist, which is effective as a rescue treatment, not for cessation of further drug taking (Stotts et al. 2009). New pharmacological treatments are greatly needed to decrease opioid use and ultimately decrease the high rates of opiate overdose.
Remifentanil used in the current study is a synthetic opioid with a similar pharmacological profile to other potent μ-opioid agonists in the fentanyl family, and like other μ-opioid agonists, it induces analgesic effects and a state of euphoria in its users (Glass et al. 1993). Remifentanil is an ideal opioid to be used for studies on opiate self-administration because of its short half-life and low risk of overdose. Remifentanil is classified as an anilidopiperidine opioid, along with fentanyl (Beers and Camporesi 2004). However, unlike fentanyl and other anilidopiperidine opioids, remifentanil has long ester chains which are rapidly degraded by esterases in the blood. Remifentanil’s basicity also allows it to transit the blood-brain barrier easily. As a result of these attributes, remifentanil shows a half-life of 3–10 minutes, but also demonstrates high potency. Despite its rapid degradation, remifentanil has been demonstrated to have abuse potential in both human and rat models (Baylon et al. 2000; Levine and Bryson 2010). Having a short half-life, high potency, and similar pharmacodynamics to other mu-agonists makes remifentanil safe, convenient, and relevant for studies on opiate use.
Opioids are self-administered by animals (Becker et al. 2000). There is evidence that μopioid agonists like remifentanil stimulate dopamine release in the nucleus accumbens and cause a reinforcing effect that contributes to the addictive effects of these drugs (Xi et al. 1998). It has also been discovered that the μ-opioid receptor is the most responsive opiate receptor in rats (Devine and Wise 1994). Even rats with lesions to the nucleus accumbens do not show a decrease in self-administration of heroin, another μ-opioid agonist (Pettit et al. 1984), suggesting the role of other sites of action besides nucleus accumbens dopamine receptors.
In the current study the interactions of dextromethorphan and bupropion were studied for reducing remifentanil self-administration in rats. Dextromethorphan has a variety of actions. It is a non-selective serotonin reuptake inhibitor, increasing synaptic levels of serotonin (Schwartz et al. 2007). The metabolite of dextromethorphan, dextrophan, is a NMDA glutamate receptor antagonist, and there is evidence that its inhibitory effect on NMDA receptors results in antidepressant activity in mice (Sakhaee et al. 2017). In fact dextromethorphan shares a similar profile to ketamine, a rapid-acting antidepressant, with actions at NMDA, μ-opioid, serotonin, sigma-1, and muscarinic sites (Krupitsky et al. 2002). It has been demonstrated that ketamine works to improve abstinence rates from heroin in heroin-dependent participants, with positive correlations between ketamine dose and abstinence rates (Krupitsky et al. 2002). It has also been shown that both dextromethorphan and ketamine reduce the physiologic response to opiate withdrawal (Jovaiša et al. 2006; Malek et al. 2013). Dextromethorphan at doses of 3–30 mg/kg has already been demonstrated to reduce nicotine self-administration in rats (Briggs et al. 2016; Glick et al. 2002; Levin et al. 2018). It is therefore plausible that dextromethorphan would work similarly with opioids. Additionally, dextromethorphan has been shown to potentiate the antinociceptive effect of some μ agonists in rats but decrease the antinociceptive effect in others (Chen et al. 2005). It will therefore be interesting to see the effects of dextromethorphan on self-administrations of remifentanil, a μ-opioid agonist.
Dextromethorphan is FDA approved for humans as a cough suppressant. When administered alone, it is rapidly metabolized by the hepatic enzyme CYP2D6 into dextrophan, such that only minimal levels of dextromethorphan remain in serum (Nguyen et al. 2016). However, if dextromethorphan is co-administered with a CYP2D6 inhibitor such as bupropion or quinidine, levels of serum dextromethorphan remain sufficiently elevated, crosses the blood-brain barrier more easily (Kotlyar et al. 2005) and exerts meaningful effects at multiple receptors (NMDA, serotonin, norepinephrine, dopamine, nicotine- acetylcholine, Sigma-1 and μ-opioid) (Nguyen et al. 2016). Combination dextromethorphan and quinidine - “Neudexta,” is FDA approved for treatment of pseudobulbar effect. Combination dextromethorphan-bupropion - “AXS-05,” is being tested by Axsome Therapeutics Inc. in Phase III trials as a treatment for treatment-resistant depression, and in Phase II trials for dementia-related agitation. AXS-05 has also demonstrated significant reduction compared to bupropion in smoking in a study by Duke University and Axsome Therapeutics Inc. (Davis et al. 2019).
Meanwhile, bupropion, aside from inhibiting metabolism of dextromethorphan, is both a dopamine and a norepinephrine reuptake inhibitor (Stahl et al. 2004b). It is also a nicotinic antagonist (Slemmer et al. 2000). It is thus used both for major depressive disorder and for smoking cessation, but unlike standard SSRIs, it has milder side effects, not causing sexual dysfunction, weight loss, or somnolence as often (Fava et al. 2005).. We have shown in the rat model of self-administration that bupropion significantly reduces nicotine self-administration (Hall et al. 2015) in a doses of 25 and 75 mg/kg.
Both bupropion and dextromethorphan have been shown to significantly reduce nicotine self-administration and bupropion attenuates the metabolism of dextromethorphan. Based on these findings, we hypothesized that there would be a dose-related effect of dextromethorphan and bupropion to suppress self-administration of remifentanil. We also hypothesized that the drug combination would have additive or greater effect than either drug alone. To test these hypotheses, we compared ninedifferent combinations of dextromethorphan and bupropion and assessed these in rats with different levels of baseline remifentanil self-administration.
Methods
Subjects
Adult female Sprague-Dawley rats were housed in approved standard laboratory conditions in a Duke University vivarium facility next to the testing room to minimize stress induced by transporting the rats. For the bupropion and dextromethorphan effects on remifentanil self-administration study the rats were singly housed to prevent gnawing on the IV catheters and harnesses. For the bupropion and dextromethorphan effects on food self-administration and locomotor activity the rats were housed in groups of 2–3. A separate set of animals from those used for remifentanil self-administration were used for the food self-administration and locomotor studies because differential self-administration of remifentanil could have had differential residual effects on food-motivated responding or locomotor activity. Within both studies all of the animals received all of the bupropion and dextromethorphan doses in a counterbalanced order twice. The day-night cycle was reversed (7:00 AM to 7:00 PM dark) so that the rats were in their active phase during the behavioral testing. All behavioral tests were carried out during the dark phase of the dark-light cycle between 9:00 AM and 4:00 PM. Rats for the self-administration experiment had ad lib access to water and were fed a standard rat chow once daily throughout the study to maintain approximately 85% ad lib weight, with food amounts adjusted from 8–16 g per day as they grew to provide a lean healthy growth curve. All procedures for testing the animals were approved by the Duke University Animal Care and Use Committee and conformed to the Animal Care Guide by NIH.
Preparation of Drugs
Solutions of remifentanil (NIDA), dextromethorphan (Sigma) and bupropion (NIDA) were prepared in pyrogen-free glassware in sterilized isotonic saline solution and passed through a 0.2 μm filter (Millipore Corp, Billerica, MA, USA) for sterilization. All solutions were kept refrigerated in the dark between experiments and were brought to room temperature before administration.
Experimental Design and Procedure
The effects of acute bupropion and dextromethorphan on remifentanil self-administration were studied (N = 14 for the study) in a repeated measures design in which the order of control injections, doses and combinations were administered to the subjects in a counterbalanced order to prevent any confounding between drug dose and order of administration. After each treatment session there was a one-day non-treatment session to control for possible shifting baselines of self-administration through the course of the study.
Intravenous Remifentanil Self-administration
Chronically indwelling intravenous jugular catheters were implanted i.v. under ketamine (60 mg/kg) and dexdomitor (15 mg/kg) anesthesia and were flushed daily with a 0.3 ml solution containing 100U/ml heparinized saline. After each self-administration session, the remifentanil remaining in each port was withdrawn and a sterile lock consisting of heparinized saline 500U/ml with 0.4 mg Gentamicin was infused. Barbiturate injection tests through the catheter were used to verify the patency of catheters. Only the data from the rats with patent catheters for the duration of the study were used for analysis.
For behavioral training, rats were placed in dual lever operant test chambers (Med Associates, Georgia, VT, USA). Each chamber was equipped with a tone generator, house light, cue light above each lever, and a metal tether to cover the drug delivery line. A computer programmed with MED-PC software managed experimental events and data collection. Each catheter was connected to a Micro Liter Syringe Pump, and tethers made of polyethylene tubing with huber needles for access to ports and catheters. During each self-administration session, the rats wore infusion harnesses to connect them to the tethers.
Initially, the rats were trained daily with tutor sessions, lasting 30 min, to press the levers for food pellet reinforcers for three sessions. Half the animals were rewarded for responding on the right lever and the other half for responding on the left lever. Only the cue light over the correct lever was illuminated while the light over the incorrect lever was off. Pressing on the correct lever was rewarded by immediate delivery of one 45-mg food pellet and activation of the feedback tone for 0.5 sec. There was no timeout period in the tutor sessions.
After the pellet sessions, animals had catheters surgically implanted under ketamine anesthesia to provide access for remifentanil self-administration by IV infusion. A plastic SoloPort was attached intraoperatively to a polyurethane catheter and inserted into a subcutaneous interscapular pocket and sutured to underlying fascia. The day after the surgery, the rats began self-administration sessions with remifentanil (0.3 mg/kg/infusion, i.v.) as the reinforcer.
A lever press on the active side l resulted in the activation of the feedback tone for 0.5 sec, the immediate delivery of one 50-μl infusion of remifentanil in less than 1 sec. Each infusion was immediately followed by a 20-sec timeout in which the house light goes on and cue lights go out and responses are recorded but not reinforced. Inactive lever presses were recorded but resulted in no infusion. There were five sessions of training for remifentanil self-administration before the onset of bupropion and dextromethorphan drug treatment sessions. All of the animals had the same number of training sessions to provide all with equal testing history. The benchmark infusion dose of remifentanil was set at 0.3 mg/kg/infusion, i.v. FR was set at FR1. Each Remifentanil infusion sessions was 1-hour. Animals were tested not more than once/day between 9 AM and 4 PM.
Food-motivated Responding
In a separate set of young adult female Sprague-Dawley rats (N=12) the same doses and combinations of bupropion and dextromethorphan were tested for effects on food motivated responding. The same FR1 schedule and same operant stations were used as with the drug self-administration studies except that the rats were not have IV catheters implanted and instead of drug reinforcement they were reinforced with 45-mg food pellets.
Locomotor Activity
The rats used to assess bupropion and dextromethorphan effects on food-motivated responding were then tested for the effects of these drugs on locomotor activity. Locomotor activity was assessed in an enclosed maze in the shape of a figure-8. The Figure-8 apparatus consisted of a continuous alley that measured 10 cm × 10 cm, with the entire maze measuring 70 cm × 42 cm. Animals were allowed to freely explore and locomotor activity was assessed by the crossing of eight photobeams located at equal points in the alley. Each locomotor test session lasted 1 h, and photobeam breaks were tallied in 5 min blocks across the session.
Statistical Analysis
The data were evaluated with analysis of variance (SuperAnova by SAS, Cary, NC, USA). Analysis was done for between and within subjects factors. The final N was 14 rats that completed the remifentanil study. To assess the effects of the drug treatments in rats with slow or rapid uptake of remifentanil, the rats were split into groups below and above the median for remifentanil self-administration during the first five sessions of access, before the bupropion and dextromethorphan drug treatments began. Low and high responder status was a between subjects factor and bupropion and dextromethorphan were within subjects factors with N=7 in each of these groups for a total. N=14 for the remifentanil self-administration study. Following up significant effects, planned comparisons were made between controls and each of the dose groups. As recommended by Snedecor and Cochran (Snedecor and Cochran 1967) interactions of p<0.10 were followed-up by tests of the simple main effects. Dunnett’s test was used for comparisons of the treated conditions to control. The final threshold for statistical significance was always an alpha of p<0.05 (two-tailed).
Results
Remifentanil Motivated Responding
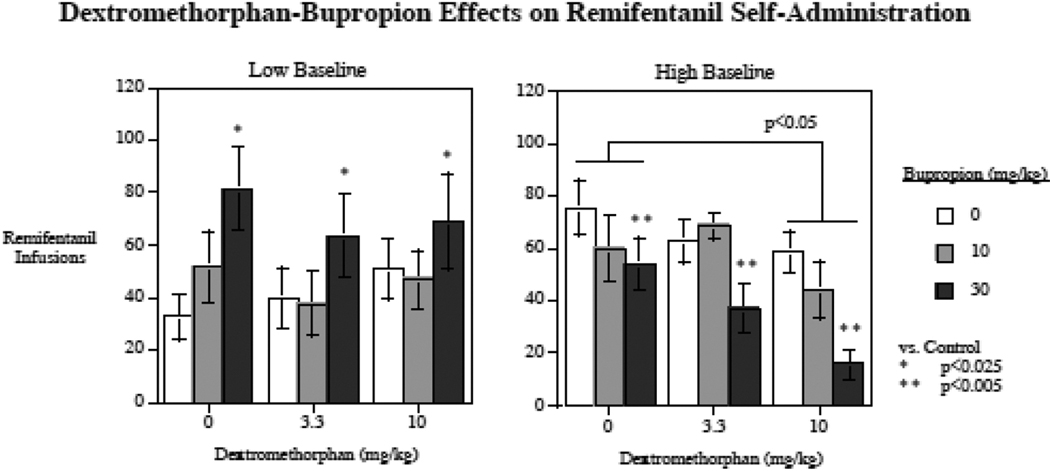
Acute bupropion and dextromethorphan each had significant effects on remifentanil self-administration, but showed differential effects in rats with low compared to high baseline remifentanil self-administration. The interactions of bupropion x low/high response status (F(2,24) = 12.33, p < 0.0005), and dextromethorphan x low/high response status (F(2,24) = 3.15, p < 0.07) triggered follow-up tests of the simple main effects of these drugs in the low and high responding groups. Figure 1 shows the remifentanil self-administration rate after each of the drug treatments, alone or in combination as well as vehicle control. Because interactions were observed for each drug treatment with low and high baseline levels of remifentanil self-administration, data for each of these subgroups is displayed separately. The differential drug effects in low compared to high responders are visually noticeable.
Figure 1.
Acute bupropion and dextromethorphan effects on remifentanil self-administration in low and high responding rats (mean ± sem). (N = 14)
First, under the vehicle control condition, the low vs. high baseline response continued to demonstrate respectively low (32.4 ± 8.7) and high (75.4 ± 10.5) levels of remifentanil infusions per session during the drug treatment part of the study (F(1,12) = 10.00, p < 0.01).
Among rats with low baseline response, there was a significant (F(2,12) = 5.23, p < 0.025) main effect of bupropion, with the 30 mg/kg (p < 0.025) but. Not the 10 mg/kg bupropion dose causing a significant increase in remifentanil self-administration. No significant effect of dextromethorphan on remifentanil self-administration in the low responders was detected.
Among rats with high baseline self-administration, the main effects of both bupropion (F(2,12) = 7.79, p < 0.01) and dextromethorphan (F(2,12) = 7.71, p < 0.01) were significant. The 30 mg/kg bupropion dose (p < 0.005) and the 10 mg/kg dose of dextromethorphan (p < 0.05) significantly decreased remifentanil self-administration in the high responding rats. Neither of the lower doses produced significant effects on remifentanil self-administration. There was no detected interaction between the two drug treatments. An additive effect was seen with the combination of the two high doses of the drugs, with the most pronounced effect being a 79.2 % decrease in remifentanil self-administration relative to performance of the same animals after control injections (Fig. 1).
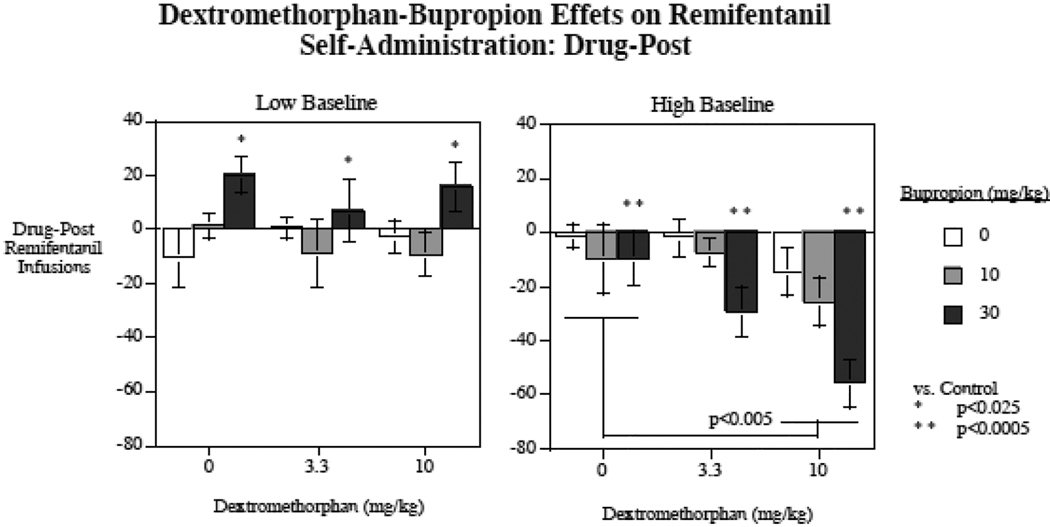
In addition to the remifentanil self-administration sessions run after treatment with bupropion, dextromethorphan, the combination, and the vehicle control, there were treatmentfree sessions run after each drug treatment session. This served a check for residual drug effects. We did not see any signs of persisting drug effects in these drug free sessions with the analysis of variance assessment of these data. These treatment-free sessions also served to assess any shifts in baseline remifentanil self-administration over the course of the study. There was a significant (p < 0.005) linear rising baseline of remifentanil self-administration over the course of the study from 54.4 ± 8.0 remifentanil infusions per session in the initial session to 63.9 ± 7.9 in the last. To accommodate for this rising baseline, the self-administered remifentanil infusions each treatment free session was subtracted from the preceding treatment session. No significant effects of prior dextromethorphan (p = 0.59) or bupropion (p = 0.72) treatment was seen during the drug-free sessions (Table 1). Figure 2 shows the difference scores of acute bupropion and dextromethorphan effects on remifentanil self-administration during drug treatment sessions minus remifentanil self-administration during the treatment-free sessions following each drug treatment session. This analysis of difference scores showed generally the same results as the raw scores, but with somewhat larger differences between drug treatments and control, which was expected with the correction for the rising baseline of self-administration. With the difference score analysis there was a significant main effect of dextromethorphan (F(2,24) = 4.86, p < 0.025), with the 10 mg/kg dextromethorphan dose causing a significant (p < 0.01) overall reduction in remifentanil self-administration. There were significant interactions of bupropion x low vs. high baseline responders (F(2,24) = 14.24, p < 0.0005) and dextromethorphan x low vs. high baseline responders (F(2,24) = 3.99, p < 0.05). As with the analysis of the raw scores, the low baseline responders group showed a significant main effect of bupropion (F(2,12) = 4.72, p < 0.05). The 30 mg/kg dose of bupropion caused a significant (p < 0.025) increase in remifentanil self-administration whereas the lower 10 mg/kg bupropion dose did not produce a significant effect. No significant effect of dextromethorphan was seen with the low responding group. With the high responding group, as with the raw score analysis, both bupropion (F(2,12) = 12.56, p < 0.005) and dextromethorphan (F(2,12) = 7.36, p < 0.01) had significant main effects. The higher doses of bupropion (p < 0.0005) and dextromethorphan (p < 0.005) caused a significant decrease in remifentanil self-administration among high baseline responders. The interaction of bupropion x dextromethorphan was not significant, as a simple additive effect of the two treatments was seen as shown in figure 2. The combination of the higher doses of the two drugs caused the greatest decrease in remifentanil self-administration, a decrease of 55.7 ± 8.7 infusions per session in the high responded group relative to remifentanil self-administration in the following non-treatment sessions.
Table 1.
Rremifentanil self-administration during the treatment-free sessions following each drug treatment session. The data are the number of remifentanil infusions per session (mean ± sem). (N = 14)
| Dextromenthorphan (mg/kg) | |||
|---|---|---|---|
| Bupropion (mg/kg) | 0 | .3.3 | 10 |
| 0 | 59.9±8.6 | 52.1±8.3 | 63.6±7.1 |
| 10 | 60.1±8.5 | 61.2±8.5 | 52.8±6.4 |
| 30 | 62.7±7.1 | 61.6±6.2 | 62.3±7.0 |
Figure 2.
Acute bupropion and dextromethorphan effects on remifentanil self-administration in low and high responding rats adjusted for remifentanil self-administration during the treatment-free sessions following each drug treatment session. The data are the number of remifentanil infusions per session with treatments including vehicle minus the number of remifentanil infusions per session without treatments following each treatment session (mean ± sem). (N = 14)
Food Motivated Responding
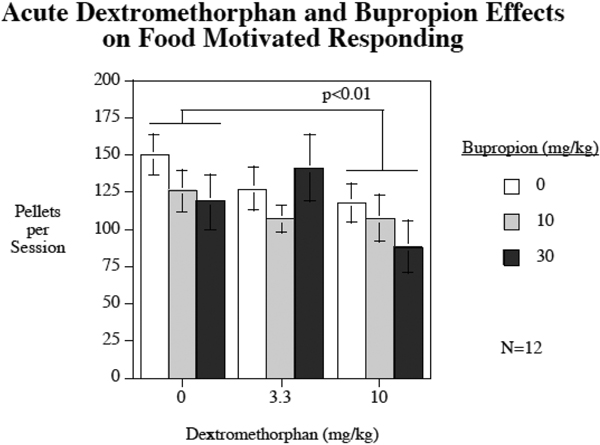
With the tests of bupropion and dextromethorphan separate and combined effects on food motivated responding there was a significant (F(2,22) = 5.18, p < 0.025) main effect of dextromethorphan decreasing food self-administration (Fig. 3). Comparisons of each dose to showed that the higher 10 mg/kg dextromethorphan dose (p < 0.01), but not the lower 3.3 mg/kg dextromethorphan dose (p = 0.49) significantly reduced food self-administration. After vehicle injections the rats self-administered 149.7±13.5 food pellets whereas after 10 mg/kg dextromethorphan injections they self-administered 117.8±13.4 food pellets. No significant main effect of bupropion (p = 0.41) or significant dextromethorphan x bupropion effects (p = 0.27) were seen with food-motivated responding.
Figure 3.
Acute bupropion and dextromethorphan effects on food-motivated responding (mean ± sem).
Locomotor Activity
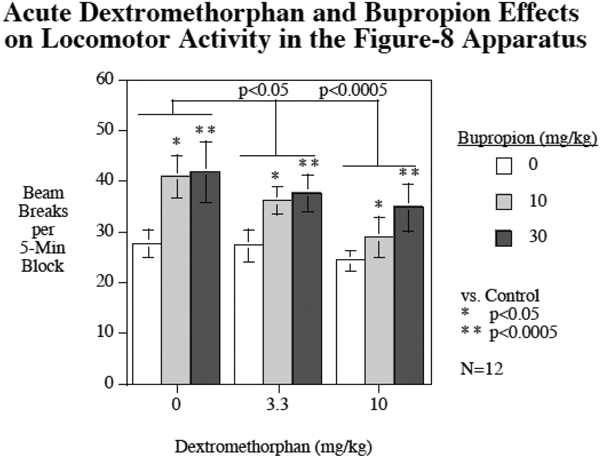
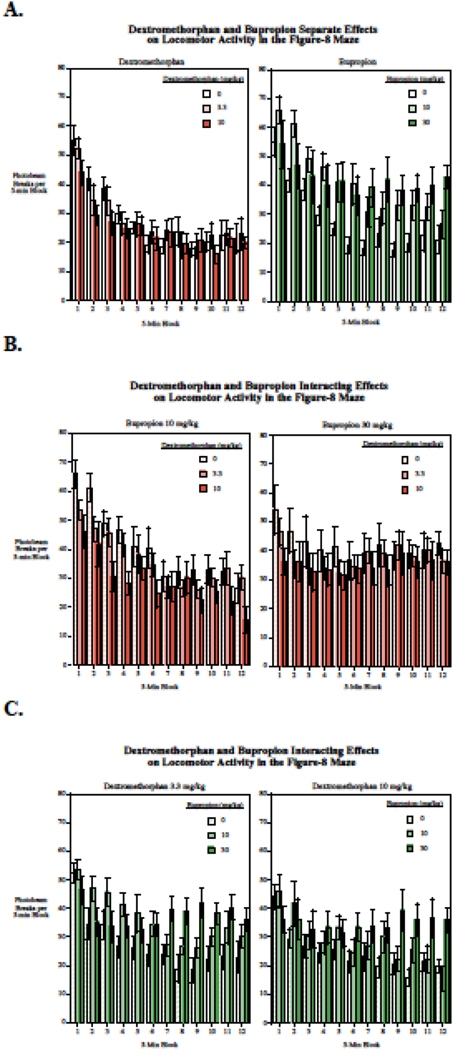
Both bupropion (F(2,22) = 8.86, p < 0.005) and dextromethorphan (F(2.22) = 13.58, p < 0.001) produced significant main effects on locomotor activity in the Figure-8 apparatus with bupropion increasing locomotor activity and dextromethorphan decreasing it (Fig. 4). Comparisons of each of the doses vs. control showed that both the 10 mg/kg (p < 0.05) and higher 30 mg/kg (p < 0.0005) doses of bupropion significantly increased locomotor activity, whereas both the lower 3.3 mg/kg (p < 0.05) and higher 10 mg/kg (p < 0.0005) doses of dextromethorphan significantly decreased locomotor activity. There was a highly significant effect of habituation or slowing response over the course of the hour-long session (F(11,121) = 28.80, p < 0.0005). Both the bupropion x session block (F(22,242) = 7.11, p < 0.0005) and the dextromethorphan x session block (F(22,242) = 2.30, p < 0.005) interactions were significant. Follow-up tests on the linear trends of habituation over the session showed that both bupropion (F(2,22) = 24.59, p < 0.0005) and dextromethorphan (F(2,22) = 7.18, p < 0.005) had significant main effects lowering the linear trend of habituation over the course of the session. Comparisons of each dose to control showed that the higher 30 mg/kg dose of bupropion (p < 0.0005) significantly reduced habituation with a 94% reduction in habituation, but the lower 10 mg.kg bupropion dose (p = 0.49) did not significantly change habituation. Both the 3.3 mg/kg (p < 0.005) and the 10 mg/kg (p < 0.005) doses of dextromethorphan significantly reduced habituation of locomotor activity across the session with 38% and 41% reductions in habituation respectively. The interaction of bupropion x dextromethorphan with regard to habituation of locomotor activity was not significant (p = 0.92). Figure 5 shows the detailed data of dexreomethorphan and bupropion effects individually and in combination across the twelve 5min blocks of the test session.
Figure 4.
Acute bupropion and dextromethorphan effects on locomotor activity, mean beam breaks per five-minute block (mean ± sem) over the 1-h session. (N = 12).
Figure 5.
Acute bupropion and dextromethorphan effects on locomotor activity, for each five-minute block, beam breaks per block (mean ± sem). (N = 12). A. Effects of bupropion and dextromethorphan on locomotor activity when given alone. B. Dextromethorphan actions on bupropion effects. C. Bupropion actions on dextromethorphan effects.
Discussion
These studies demonstrated in a rat model that acute administration of bupropion and dextromethorphan each had significant effects on remifentanil self-administration. Interestingly the effects were opposite in rats with low vs. high baseline remifentanil self-administration. In rats with low baseline opiate self-administration, bupropion significantly increased remifentanil self-administration and dextromethorphan had no discernable effect. In contrast, in rats with high baseline opiate self-administration, each drug individually caused significant decreases in remifentanil self-administration. When the two drugs were combined in high responding rats, there was an additive effect of decreased remifentanil self-administration with the combination of 30 mg/kg of bupropion and 10 mg/kg of dextromethorphan showing a 79.2% decrease in remifentanil self-administration, the largest decrease observed in the study. The doses of dextromethorphan and bupropion that effectively reduced remifentanil self-administration in the high responding subjects were in the dose ranges we had previously found to significantly reduce nicotine self-administration (Briggs et al. 2016; Hall et al. 2015; Briggs et al. 2016; Glick et al. 2002; Levin et al. 2018).
Dextromethorphan when given alone is rapidly metabolized by CYP2D6 into dextrophan which does not cross the blood brain barrier as efficiently as dextromethorphan (Kotlyar et al. 2005). That said, dextrophan dose have great binding affinity and antagonism of NMDA receptors and sigma-1 receptors (Nguyen et al. 2016). Dextromethorphan without a metabolic inhibitor has been used with limited success in the past to reduce opiate use (Weinbroum et al. 2000). Specifically, it has been used to reduce opiate use related to post-operative pain (Choi et al. 2003; King et al. 2016). A combination medication Morphidex (dextromethorphan-morphine) was even tested in phase II and III trials, but failed to show significant reduction in opiate use across the sample (Galer et al. 2005). An important finding in this study is that dextromethorphan had no significant effect on low baseline remifentanil users. On the other hand, high baseline remifentanil users showed a significant reduction of opiate use on dextromethorphan. Dextromethorphan has been shown to decrease symptoms of opiate withdrawal (Raith et al 2004), and it is conceivable that the behavior of animals with higher baseline opiate use would have more severe withdrawal symptoms and would modify behavior in the presence of medication that reduces withdrawal. In any case, the findings in this study may in fact be relevant to future studies – i.e. it might be found to be most effective in individuals with high level baseline opiate use, and it might be found to be more effective when used with metabolic inhibitor. A human trial would be needed to determine whether the effects observed in this study translates to humans.
Bupropion, in a dose range that had previously been found to significantly decrease nicotine self-administration (Hall et al. 2015), was found in the current study to have discordant effects in subjects with low and high remifentanil self-administration levels. Bupropion increased remifentanil use in low baseline self-administrators. Bupropion increases synaptic dopamine and norepinephrine (Stahl et al. 2004a) and it known that norepinephrine activity is integral to opiate reinforcement (Ventura et al. 2005). At a minimum, the finding should lead to caution when considering human studies that might include the use of bupropion in low-level opiate users. Interestingly, in high baseline remifentanil self-administrators, bupropion led to a significant decrease in opiate use. This discordance suggests that high baseline opiate use may create a categorically different set of circumstances in which medication takes effect, such that for example, additional dopamine and norepinephrine becomes therapeutic. These effects would need to be assessed within human trials to see if similar findings emerged.
Food-motivated responding was significantly reduced by the higher (10 mg/kg) dextromethorphan dose, decreasing food self-administration by approximately 21% relative to control. This more general effect of reducing motivated behavior may explain the effect of the 10 mg/kg dextromethorphan dose reducing remifentanil in the high responding group. Relevant to the dextromethorphan-induced decrease in food motivated responding is its documented effect of producing nausea in humans (Carbonaro et al. 2018). However, if this general reduction in motivated behavior was important for remifentanil self-administration, one would have expected that 10 mg/kg of dextromethorphan would also decrease remifentanil self-administration in the low responding group, but it did not. The low self-administrating subjects by definition had lower motivation to take remifentanil. Dextromethorphan may only impact highly motivated behavior, as seen in the current studies with high remifentanil responders during drug self-administration and in highly motivated food-restricted animals during food self-administration. Interestingly, bupropion was not found to significantly affect food motivated responding., yet it had significant effects on remifentanil self-administration. Bupropion effects seem to be more specific to drug-motivated responding. Yet, bupropion did not have a blanket effect of reducing remifentanil self-administration. It only did so in the high remifentanil preferring rats. In the low-preferring rats bupropion significantly increased remifentanil self-administration, suggesting different neural substrates driving remifentanil self-administration in the low and high preferring groups.
Both dextromethorphan and bupropion significantly affected locomotor activity, but in opposite directions. Both doses of bupropion significantly increased locomotor activity and both doses of dextromethorphan significantly decreased locomotor activity. When given alone dextromethorphan did little to affect locomotor activity. Rather, its effect derived from countering the hyperactivity caused by bupropion. Others have also found that acute bupropion causes locomotor hyperactivity in rats (Redolat et al. 2005) and we previously found that dextromethorphan decreases locomotor activity (Levin et al. 2018). Might have the effects of dextromethorphan and bupropion derived from their effects on activity? The two behavioral effects appear to be relative related. The two drugs had opposite effects on locomotor activity but produced similar effects on opiate self-administration. When given in combination dextromethorphan attenuated the bupropion effect on locomotor activity but the drug combination produced greater effects on remifentanil self-administration. Given that the combination of bupropion and dextromethorphan had the largest effect in reducing remifentanil self-administration, effects on locomotor activity did not appear to drive the effect.
Bupropion is a potent CYP2D6 inhibitor that metabolism of dextromethorphan increasing its serum concentration (e.g. 39-fold) elimination time (from 3 hours to over 24 hours), and consequently, increasing its activity within the brain. Exploration of combination dextromethorphan and bupropion was motivated by findings in human trials in which AXS-05 (combination bupropion + dextromethorphan) was found to decrease tobacco smoking in humans (Davis et al. 2019). Previous research has shown that both bupropion and dextromethorphan each significantly decreases nicotine self-administration in rats (Hall et al. 2015; Levin et al. 2018), and bupropion is an effective treatment for tobacco smoking cessation (George et al. 2002). In this study, there was a robust response to combined bupropion-dextromethorphan use in high baseline responders with almost 79% reduction in self administration of remifentanil. This large signal deserves evaluation in human trials, and may be most promising in individuals with higher level opiate use.
In conclusion, this study provides both encouraging results and caution for drug development to combat opiate addiction. Each of treatments, bupropion and dextromethorphan, significantly decreased voluntary intake of the opiate remifentanil in heavy users. In combination the two drugs had an additive effects, producing a substantial reduction in remifentanil self-administration. However, caution must be taken in consideration of the result of this study that with light opiate users, bupropion significantly increased intake and dextromethorphan was ineffective. These treatments appear to show the greatest promise for heavy opiate users. Further experimental animal research could help identify critical mechanisms for the divergent effects in light and heavy opiate users. Given that both bupropion and dextromethorphan are approved for human use, further research to test efficacy in the clinical setting to should be pretty straightforward.
Highlights.
Bupropion and dextromethorphan had differential effects in rats with low or high baseline remifentanil self-administration.
The rats with higher remifentanil self-administration showed a significant decrease with bupropion and dextromethorphan treatment.
The decrease in self-remifentanil administration was most pronounced when combination of of bupropion and dextromethorphan.
Rats with lower baseline remifentanil self-administration showed the opposite effect of drug treatment with an increase in remifentanil self-administration.
This finding suggests that combination bupropion-dextromethorphan should be tested in humans, with a focus on treating people with high-level opiate use.
Acknowledgement
This research was supported by the P50 grant DA027840 from NIDA.
Footnotes
Conflicts of Interest
Dr. Davis receives research funding from Axsome Therapeutics, Inc.
The other authors declare no conflicts of interest regarding the current research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baylon GJ, Kaplan HL, Somer G, Busto UE, Sellers EM (2000) Comparative abuse liability of intravenously administered remifentanil and fentanyl. Journal of Clinical Psychopharmacology 20: 597–606. [DOI] [PubMed] [Google Scholar]
- Becker B, Grecksch G, Brodemann R, Kraus J, Peters B, Schroeder H, Thiemann W, Loh H, ., Hollt V (2000) Morphine self-administration in mu-opioid receptor deficient mice. Naunyn-Schmiedeberg’s Archives of Pharmacology 361: 584–589. [DOI] [PubMed] [Google Scholar]
- Beers R, Camporesi E (2004) Remifentanil update. CNS Drugs 18: 1085–1104. [DOI] [PubMed] [Google Scholar]
- Briggs SA, Wells C, Slade S, Jaskowski P, Morrison M, Hall BJ, Rezvani AH, Rose JE, Levin ED (2016) Dextromethorphan interactions with serotonergic and histaminergic treatments to reduce nicotine self-administration,. Pharmacology, Biochemistry and Behavior 142: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonaro TM, Johnson MW, Hurwitz E, Griffith RR (2018) Double-blind comparison of the two hallucinogens psilocybin and dextromethorphan: similarities and differences in subjective experiences. Psychopharmacology 235: 521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Eagle YH, Lok-Hi C, Pao-Luh T (2005) Dextromethorphan differentially affects opioid antinociception in rats. Br J Pharmacol 144: 400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DMA, Kliffer AP, Douglas MJ (2003) Dextromethorphan and intrathecal morphine for analgesia after Caesarean section under spinal anaesthesia. British Journal of Anaesthesia 90: 653–658. [DOI] [PubMed] [Google Scholar]
- Davis JM, Dennis PA, Levin ED, Rose JE (2019) Combination Dextromethorphan and Bupropion (AXS-05) Reduces Tobacco Smoking More than Bupropion Alone. Nicotine Tob Res under review. [Google Scholar]
- Devine DP, Wise RA (1994) Self-administration of morphine, DAMGO, and DPDPE into the ventral tegmental area of rats. Journal of Neuroscience 14: 1978–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Thase ME, Clayton A, Stahl SM, Pradko JF, Johnston JA (2005) 15 Years of Clinical Experience With Bupropion HCl: From Bupropion to Bupropion SR to Bupropion XL. Primary Care Companion: Journal of Clinical Psychiatry 7: 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galer BS, Lee D, Ma T, Nagle B, Schlagheck TG (2005) MorphiDex (morphine sulfate/dextromethorphan hydrobromide combination) in the treatment of chronic pain: three multicenter, randomized, double-blind, controlled clinical trials fail to demonstrate enhanced opioid analgesia or reduction in tolerance. Pain 115: 284–295. [DOI] [PubMed] [Google Scholar]
- George TP, Vessicchio JC, Termine A, Bregartner TA, Feingold A, Rounsaville BJ, Kosten TR (2002) A placebo controlled trial of bupropion for smoking cessation in schizophrenia. Biol Psychiatry 52: 53–61. [DOI] [PubMed] [Google Scholar]
- Glass PS, Hardman D, Kamiyama Y, Quill TJ, Marton G, Doon KH, Grosse CM, Hermann D (1993) Preliminary pharmacokinetics and pharmacodynamics of an ultra-short-acting opioid: remifentanil (GI87084B). . Anesthesia and Analgesia 77: 1032–1040. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Kitchen BA (2002) Modulation of nicotine self-administration in rats by combination therapy with agents blocking alpha 3 beta 4 nicotinic receptors. Eur J Pharmacol 448: 185–91. [DOI] [PubMed] [Google Scholar]
- Hall BJ, Slade S, Wells C, Rose JE, Levin ED (2015) Bupropion-varenicline interactions and nicotine self-administration behavior in rats. Pharmacology, Biochemistry, and Behavior 130: 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovaiša T, Laurinenas G, Vosylius S, Šipylaite J, Badaras R, Ivaškevičius J (2006) Effects of ketamine on precipitated opiate withdrawal. Medicina 42: 625–634. [PubMed] [Google Scholar]
- King MR, Ladha KS, Gelineau AM, Anderson TA (2016) Perioperative Dextromethorphan as an Adjunct for Postoperative Pain: a Meta-Analysis of Randomized Controlled Trials. Anesthesiology 124: 696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlyar M, Brauer L, Tracy TS, Hatsukami DK, Harris J, Bronars CA, Adson DE (2005) Inhibition of CYP2D6 activity by bupropion. Journal of Clinical Psychopharmacology 25: 226–229. [DOI] [PubMed] [Google Scholar]
- Krupitsky E, Burakov A, Romanoca T, Dunaevsky I, Strassman R, Grinenko A (2002) Ketamine psychotherapy for heroin addiction: immediate effects and two-year follow-up. Journal of Substance Abuse Treatment 23: 273–283. [DOI] [PubMed] [Google Scholar]
- Levin ED, Slade S, Wells C, Rezvani AH (2018) Mutually augmenting interactions of dextromethorphan and sazetidine-A for reducing nicotine self-administration in rats. Pharmacology, Biochemistry and Behavior 166: 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AI, Bryson EO (2010) Intranasal self-administration of remifentanil as the foray into opioid abuse by an anesthesia resident. Anesthesia & Analgesia 110: 524–525. [DOI] [PubMed] [Google Scholar]
- Malek A, Shakrokh A, Bohlool HA (2013) The therapeutic effect of adding dextromethorphan to clonidine for reducing symptoms of opioid withdrawal: a randomized clinical trial. ISRN Psychiatry: 546030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L, Thomas KL, Lucke-Wold BP, Cavendish JZ (2016) Dextromethorphan: An update on its utility for neurological and neuropsychiatric disorders. Pharmacol Ther 159: 1–22. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Ettenberg A, Bloom FE, Koob GF (1984) Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology 84: 167–173. [DOI] [PubMed] [Google Scholar]
- Redolat R, Vidal J, Gomez MC, Carrasco MC (2005) Effects of acute bupropion administration on locomotor activity in adolescent and adult mice. Behavioural Pharmacology 16: 59–62. [DOI] [PubMed] [Google Scholar]
- Rudd RA, Aleshire N, Zibbell JE, Gladden RM (2016) Increases in drug and opioid overdose deaths- United States, 2000–2014. Morbidity and Mortality Weekly Report 64: 13781382. [DOI] [PubMed] [Google Scholar]
- Sakhaee E, Ostadhadi S, Khan MI, Yousefi F, Norouzi-Javidan A, Akbarian R, Chamanara M, ., Zolfaghari S, Dehpour AR (2017) The role of NMDA receptor and nitric oxide/cyclic guanosine monophosphate pathway in the antidepressant-like effect of dextromethorphan in mice forced swimming test and tail suspension test. Biomedical Pharmacotherapy 85: 627–634. [DOI] [PubMed] [Google Scholar]
- Schwartz AR, Pizon AF, Brooks DE (2007) Dextromethorhpan-induced serotonin syndrome. Clinical Toxicology: 771–773. [DOI] [PubMed] [Google Scholar]
- Slemmer JE, Martin BR, ., Damaj MI (2000) Bupropion is a nicotinic antagonist. The Journal of Pharmacology and Exerimental Therapeutics 295: 321–327. [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG (1967) Statistical Methods. Iowa State University Press, Ames, Iowa [Google Scholar]
- Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S (2004a) A Review of the Neuropharmacology of Bupropion, a Dual Norepinephrine and Dopamine Reuptake Inhibitor. Primary Care Companion to the Journal of Clinical Psychiatry 6: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S (2004b) A review of the Neuropharmacology of Bupropion, a Dual Norepinephrine and Dopamine Reuptake Inhibitor. Primary Care Companion: Journal of Clinical Psychiatry 6: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotts AL, Dodrill CL, Kosten TR (2009) Opioid dependence treatment: Options in pharmacotherapy. Expert Opinion on Pharmacotherapy 10: 1727–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Alcaro A, Puglisi-Allegra S (2005) Prefrontal Cortical Norepinephrine Release Is Critical for Morphine-induced Reward, Reinstatement and Dopamine Release in the Nucleus Accumbens Cerebral Cortex 15: 1877–1886. [DOI] [PubMed] [Google Scholar]
- Weinbroum AA, Rudick V, Paret G, Ben-Abraham R (2000) Canadian Journal of Anesthesia 47: 585–596. [DOI] [PubMed] [Google Scholar]
- Xi Z, Fuller SA, Stein EA (1998) Dopamine release in the nucleus accumbens during heroin self-administration is modulated by kappa opioid receptors: an in vivo fast-cyclic voltammetry study. The Journal of Pharmacology and Experimental Therapeutics 284: 151–161. [PubMed] [Google Scholar]