Abstract
Introduction:
Heart failure patients with reduced ejection fraction (HFrEF) exhibit abnormal locomotor group III/IV afferent feedback during exercise; however, the underlying mechanisms are unclear. Therefore, the purpose of this study was to determine 1) metabo and mechanoreceptor expression in HFrEF and controls and 2) relationships between receptor expression and changes in cardiopulmonary responses with afferent inhibition.
Methods:
Ten controls and 6 HFrEF performed 5 min of cycling exercise at 65% peak workload with lumbar intrathecal fentanyl (FENT) or placebo (PLA). Arterial blood pressure (BP) and catecholamines were measured via radial artery catheter. A vastus lateralis muscle biopsy was performed to quantify cyclooxygenase-2 (COX-2), purinergic 2X3 (P2X3), transient receptor potential vanilloid type 1 (TRPV1), acid-sensing ion channel 3 (ASIC3), Piezo 1, and Piezo 2 protein expression.
Results:
TRPV1 and COX-2 protein expression were greater in HFrEF than controls (both, p<0.04), while P2X3, ASIC3, and Piezo 1 and 2 were not different between groups (all, p>0.16). In all participants, COX-2 protein expression was related to the % change in ventilation (r= −0.66) and MAP (r= −0.82) (both, p<0.01) with FENT (relative to PLA) during exercise. In controls, TRPV1 protein expression was related to the % change in SBP (r=−0.77, p=0.02) and MAP (r=−0.72, p=0.03) with FENT (relative to PLA) during exercise.
Conclusion:
TRPV1 and COX-2 protein levels are elevated in HFrEF compared to controls. These findings suggest that the elevated TRPV1 and COX-2 expression may contribute to the exaggerated locomotor muscle afferent feedback during cycling exercise in HFrEF.
Keywords: blood pressure, exercise pressor reflex, fentanyl, group III/IV muscle afferents, heart failure with reduced ejection fraction
Introduction
Cardiopulmonary and autonomic adjustments to exercise, which include increases in blood pressure, ventilation (V̇E), and heart rate, are mediated by central command and the exercise pressor reflex, while modulated by the arterial baroreceptors (Fisher et al., 2015; Smith et al., 2019). The afferent arm of the exercise pressor reflex is comprised of group III (predominantly mechanically sensitive) and group IV (predominantly metabolically sensitive) afferents located within the interstitum of the contracting muscle (McCloskey & Mitchell, 1972; Adreani et al., 1997). Importantly, inhibition of locomotor muscle group III/IV afferents via intrathecal fentanyl (FENT) results in attenuated increases in V̇E, cardiac output, and blood pressure during exercise in healthy young adults (Amann et al., 2010; Amann et al., 2011b) indicating that locomotor muscle afferent feedback significantly contributes to the cardiopulmonary and autonomic adjustments to exercise.
Exercise intolerance is a hallmark symptom of heart failure with reduced ejection fraction (HFrEF). One of the primary mechanisms responsible for the deterioration of exercise tolerance is exaggerated locomotor muscle neural afferent feedback (Sinoway & Li, 2005; Smith et al., 2006; Piepoli et al., 2008). In fact, inhibition of locomotor muscle group III/IV afferents via FENT results in greater ventilatory efficiency (i.e. ventilatory equivalent for carbon dioxide slope (VE/VCO2 slope)), oxygen uptake kinetics, and locomotor muscle blood flow during exercise in HFrEF (Amann et al., 2014; Olson et al., 2014; Van Iterson et al., 2017). One of the underlying mechanisms responsible for the enhanced locomotor muscle neural feedback in HFrEF is differential expression of receptors associated with the metabolic component of the exercise pressor reflex (i.e. metaboreflex). Specifically, previous studies in the rat-infarct model of HF have investigated expression of receptors associated with the metaboreflex and found greater expression of cyclooxygenase-2 (COX-2) and purinergic 2X3 (P2X3), and lower expression of transient receptor potential vanilloid type 1 (TRPV1) and acid-sensing ion channel 3 (ASIC3) compared to controls (Smith et al., 2005; Wang et al., 2010a; Morales et al., 2012; Wang et al., 2012; Xing et al., 2015; Xing & Li, 2016). However, it is unclear if these findings in animals translate to humans and whether the differential protein expression is associated with cardiopulmonary and neural responses during exercise with locomotor muscle group III/IV afferent inhibition.
Therefore, the purpose of this study was to determine if differences exist in the protein expression of TRPV1, COX-2, P2X3, and ASIC3 between healthy humans and patients with HFrEF. Further, we wanted to determine if differences existed in Piezo 1 and 2, mechano-gated channels associated with mechanoreflex sensitivity (Copp et al., 2016), between groups. Lastly, we sought to determine if relationships were present between protein expression and changes in cardiopulmonary and neural responses with locomotor muscle group III/IV afferent inhibition (via FENT) during cycling exercise. Based on animal literature, we hypothesized that patients with HFrEF would exhibit 1) greater protein expression of COX-2 and P2X3 and lower protein expression of TRPV1 and ASIC3 compared to age-matched controls and 2) significant relationships between increased protein expression and changes (i.e. decreases) in blood pressure and V̇E during exercise with locomotor muscle afferent feedback inhibition.
Methods
Ethical approval:
All aspects of this study were approved by the Mayo Clinic Institutional Review Board (approval no. 09–000032) and conformed to the standards set forth by the latest revision of the Declaration of Helsinki except for registration in a database. All participants were informed about the experimental procedures and potential risk involved, and provided written and verbal informed consent.
Participants:
Six patients with HFrEF and 10 healthy matched control participants (CTL) were recruited for this study and provided written informed consent. The patients with HFrEF were recruited from the Mayo Clinic Heart Failure Service and the Cardiovascular Health Clinic. Inclusion criteria for the patients with HFrEF included diagnosis of ischemic or dilated cardiomyopathy with duration of >1 year of symptoms, stable HF symptoms (>3 months), left ventricular ejection fraction ≤35%, body mass index <35 kg/m2, non-smokers with a smoking history of <15 pack-years, and no diagnosis of coexisting pulmonary disease. Patients with HFrEF performed all testing, while remaining on standard pharmacological therapy. CTL participants were matched for sex, age, and height to the patients with HFrEF and free of cardiovascular, pulmonary, and muscular diseases.
Experimental protocol:
For this single-blind case-control study, participants performed all protocols and measurements during four study visits. On the first study visit, participants were familiarized with all experimental measurements and protocols and then performed an incremental exercise test to volitional fatigue to determine peak oxygen uptake (V̇O2peak). On the second and third study visits, participants were randomized to lower lumber intrathecal injection of fentanyl (FENT) or placebo (PLA) and then performed constant workload submaximal exercise at 65% of peak workload. At rest and during exercise, ventilatory and metabolic variables and arterial blood pressure were measured and arterial blood sampling occurred. On the fourth study visit, a skeletal muscle biopsy was performed of the vastus lateralis for quantification of protein expression levels of TRPV1, COX-2, P2X3, ASIC3, Piezo 1 and Piezo 2.
V̇O2peak:
Participants performed an incremental cycling test to volitional fatigue to determine V̇O2peak using an electronically braked upright cycle ergometer (Lode Corival, Groningen, the Netherlands). The incremental step test consisted of increasing workloads of 20 and 40 W increments for HFrEF and CTL, respectively every 3 min. During the incremental test, participants maintained a pedal frequency of 60 rpm and remained seated. Ventilatory and gas exchange variables were collected during the incremental cycling test (CPX/D, MGC Diagnostics, St. Paul, MN) and averaged over 30 s. Peak workload was determined as the highest workload achieved at V̇O2peak.
Intra-arterial blood pressure and blood sampling:
Following local anesthesia (1% lidocaine), a 20 gauge Teflon catheter (FA-04020; Arrow International Inc., Reading, PA) was placed in the non-dominant radial artery for blood sampling and arterial pressure measurement. Arterial pressure recordings from the pressure transducer (PX-MK099; Edwards Lifesciences, Irvine, CA) were exported to a digital oscilloscope for offline analysis of systolic (SBP), diastolic (DBP), and mean arterial pressure (MAP). At rest and during the last min of exercise, arterial blood was drawn anaerobically over 10–15 s. Epinephrine (Epi) and norepinephrine (NE) plasma concentrations were analyzed via high performance liquid chromatography. Arterial partial pressure of carbon dioxide (PaCO2) was measured in duplicate, averaged, and temperature corrected at a temperature of 37˚C (ABL825 Flex Blood Gas/CO-ox analyzer, Radiometer America Inc. Westlake, OH, USA).
Fentanyl lower lumbar intrathecal injection:
As previously described (Olson et al., 2014), participants were seated in an upright flexed position and the skin and subcutaneous tissues were anaesthetized at the L3-L4 vertebral interspace with 2–4 mL of 1% lidocaine under aseptic technique. During the FENT study visit, a 22g Whitaker needle was advanced to the subarachnoid space, with placement confirmed by visualization of free-flowing cerebrospinal fluid. A small amount of free-flowing cerebrospinal fluid was aspirated and 1 mL of fentanyl (0.05 mg/mL) was injected. The participants remained in the seated position to minimize the cephalad migration of fentanyl. The PLA study visit was identical to the FENT study visit except the advancement of the needed to the subarachnoid space was simulated after subcutaneous local anesthesia.
Submaximal cycling exercise:
Within 2–3 min of placement of the radial intra-arterial catheter and the intrathecal injection (described above), resting data was collected for 5 min. Then, the participants exercised at 65% of peak power for 5 min followed by 5 min of recovery. Immediately following recovery, central chemosensitivity was assessed via CO2 rebreathing. At rest and during the last min of exercise, arterial blood was sampled for PaCO2, Epi, and NE. Ventilatory and gas exchange variables were collected at rest and during exercise using the same methodology used during the incremental test with the average of the last min of rest and exercise reported. V̇E/ V̇CO2 slope was calculated using resting data and the last min of submaximal exercise (Olson et al., 2014; Smith & Olson, 2019).
CO2 rebreathing testing:
Following the submaximal cycling exercise, central chemosensitivity was assessed via CO2 rebreathing testing as previously described (Olson et al., 2014). Briefly, the participants breathed on a mouthpiece connected to a pneumatic switching valve and 6 L rebreathing bag (5% CO2 and 95% O2). Participants breathed room air for 2 min and were then switched to the rebreathing bag for 4 min or until one of the stopping criteria was reached. Stopping criteria included PETCO2 of 65 mmHg, PETO2 of 160 mmHg, V̇E of 100 L/min, or the participants desire to stop. The slope of V̇E versus PETCO2 was used an index of central CO2 chemosensitivity (Olson et al., 2014). The test was performed 2–3 times (with the averaged reported) with 3–5 min between allowing for cardiopulmonary variables to return to baseline levels.
Skeletal muscle biopsy:
Under aseptic technique and following local anesthesia (2% lidocaine), muscle biopsy samples (~50–100 mg) were obtained from the superficial portion of the left vastus lateralis by the percutaneous muscle biopsy technique (Han et al., 2001). The skeletal muscle biopsy samples were cleaned of visible adipose and connective tissue then immediately frozen in liquid nitrogen and stored in a freezer at −80°C for subsequent analysis.
Western blotting:
Frozen muscle samples were homogenized in cold RIPA lysis buffer and protease inhibitor cocktail and the homogenates were centrifuged and supernatants were isolated. Proteins (68 μg) were electrophoretically separated using a 10% Bis Tris NuPage precast gel using NuPAGE SDS Running Buffer for 40 min at 200 V (Criterion Cell (Bio-Rad Laboratories, Hercules, CA) and then transferred to a nitrocellulose membrane (Thermo Scientific, Waltham, MA) for 75 min at 17 V. The membrane was blocked with 5% dried milk for 60 min and then incubated with 1) polyclonal anti-TRPV1 antibody diluted 1:500 (Sigma-Aldrich, Cat# SAB3501027, RRID: AB_2810269), 2) monoclonal anti-COX-2 antibody diluted 1:500 (Cayman Chemical, Cat# 160112, RRID: AB_10078980), 3) monoclonal anti-P2X3 antibody diluted 1:500 (Santa Cruz Biotechnology, Cat# sc-390572, RRID: AB_2810268) and 4) polyclonal anti-ASIC3 antibody diluted 1:200 (Abcam, Cat# ab190638, RRID: AB_2810270) in 10x Tris-buffered saline (TBS), 0.1% Tween-20 (TBS-T) with 0.5% dried milk at 4 C overnight. Membranes were washed and then incubated for 60 min at room temperature with either horseradish peroxidase (for TRPV1 and ASIC3 diluted 1:5,000 and 1:1,000, respectively; ThermoFisher Scientific, Waltham, MA) or IRDye (for COX-2 and P2X3 diluted 1:10,000; LiCor, Lincoln, NE) secondary antibodies in 10x Tris-buffered saline (TBS), 0.1% Tween-20 (TBS-T) with 0.5% dried milk. The same membranes were then stripped and used to determine glyceraldeyde-3-phosphate dehydrogenase (GAPDH) protein expression as an internal control (1:5,000 dilution; ab9485; Abcam, Cambridge, UK) and the TRPV1, COX-2, P2X3, and ASIC3 protein expression reported herein is relative to GAPDH protein expression. Digital images were captured with chemiluminescence for TRPV1 and ASIC3 and infrared fluorescence for COX-2 and P2X3 (Odyssey FC, LiCor, Lincoln, NE). To control for intra-assay variability, the HFrEF and CTL samples were alternated on the blot.
Piezo 1 and 2 total protein expression were performed with the Wes System (Protein Simple, San Jose, CA). The protein samples, primary and secondary antibodies, blocking reagent, wash buffer, and chemiluminescent substrate were loaded on the provided microplate according to the manufacturer’s instructions. Specifically, skeletal muscle samples were diluted to 29 ng/μL in sample buffer (100x diluted ‘10x Sample Buffer’), then mixed with Fluorescent Master Mix and heated at 95°C for 5 min. All reagents: samples, blocking reagent (antibody diluent), primary antibodies (i.e. 1) polyclonal anti-Piezo 1 antibody diluted 1:160 (Novus Biologicals, Cat# NBP1–78537, RRID: AB_11003149) and 2) anti-Piezo 2 antibody diluted 1:100 (a gift from Dr. Ardem Patapoutian of The Scripps Research Institute, La Jolla, CA) (Woo et al., 2014)), HRP-conjugated secondary antibodies and chemiluminescent substrate were pipetted into a plate. Instrument default settings were used (e.g. separation time, temperature). The following criteria are routinely used to distinguish between signal and background: the peak signal-to-noise (S/N) ratio given by the software must be ≥10, and the peak height/baseline ratio (calculated manually from the peak height and baseline values given by the software) must be ≥3. To control for differences in signal between experiments, a 5-point calibration curve of a CTL muscle tissue was included in all runs. Each calibration curve must display a linearity of r2>0.90. Data analysis was performed with Compass software (Protein Simple, San Jose, CA).
Statistical analyses:
Values are reported as mean±standard deviation (SD). Statistical analyses were performed using SigmaStat 2.0 (Jandel Scientific, San Rafael, CA). Normality and equal variance were assessed using the Shapiro-Wilk and Levene tests, respectively and non-parametric tests were used when appropriate. Participant characteristics, protein expression, and % change in cardiopulmonary variables with FENT (relative to PLA) were compared using unpaired t-tests. Cardiopulmonary variables as well as PaCO2, Epi, and NE were compared within (PLA vs. FENT) and between (CTL vs. HFrEF) groups using mixed factorial analysis of variance and Tukey’s post-hoc test when appropriate. Relationships were determined via linear regression. An influential outlier was detected in Figure 3 via Cook’s distance, thus the data point was shown for transparency, but not included in the correlation. Statistical significance was set at p<0.05.
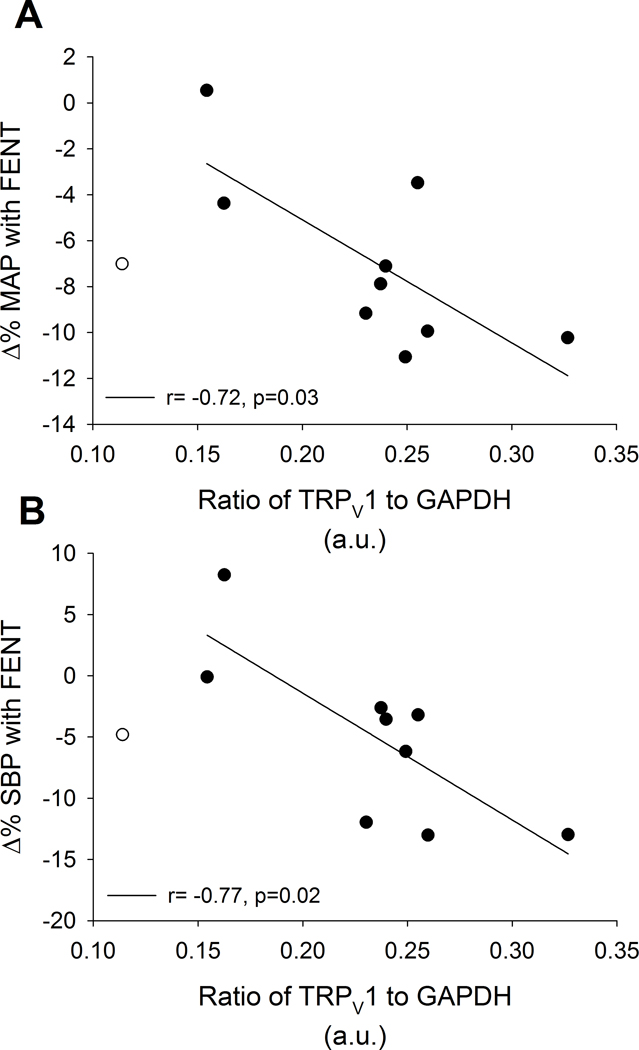
Figure 3: Relationships between TRPV1 and % change in blood pressure with FENT in CTL during exercise.
In CTL during exercise, TRPV1 protein expression was negatively related to the % change with FENT in MAP (A; r= −0.72, p=0.03) and SBP (B; r= −0.77, p=0.02). An influential outlier was detected and shown for transparency (open circle), but not included in the correlation (see Methods).
Results
Participant characteristics:
Age, height, hemoglobin, peak workload, and submaximal workload were not different between HFrEF and CTL (all, p>0.12) (Table 1). HFrEF had a greater BMI and lower V̇O2peak compared to CTL (both, p<0.02).
Table 1:
participant characteristics
| CTL | HFrEF | |
|---|---|---|
| n | 10 | 6 |
| Age (years) | 63 ± 8 | 60 ± 4 |
| Sex (men/women) | 8/2 | 5/1 |
| Height (cm) | 175 ± 9 | 179 ± 5 |
| Weight (kg) | 79 ± 12 | 100 ± 11* |
| Body mass index (kg•m2) | 26 ± 3 | 31 ± 5* |
| Haemoglobin (g•dL−1) | 14.1 ± 1.5 | 14.1 ± 1.1 |
| VO2peak (mL•kg−1•min1) | 27.2 ± 5 | 19.0 ± 3.1* |
| Peak workload (W) | 176 ± 55 | 137 ± 34 |
| Submaximal workload (W) | 116 ± 38 | 87 ± 13 |
| LV ejection fraction (%) | 27 ± 6 | |
| NYHA class: I/II/III | 3/2/1 | |
| ACE I or ARBs | 0 (0) | 6 (100) |
| β-blocker | 0 (0) | 6 (100) |
| Aspirin | 0 (0) | 3 (50) |
| Diuretics | 0 (0) | 5 (83) |
Mean±SD. VO2, oxygen uptake; W, watts; LV, left ventricular; NYHA, New York Heart Association; ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blockers.
significantly different than CTL.
Protein expression:
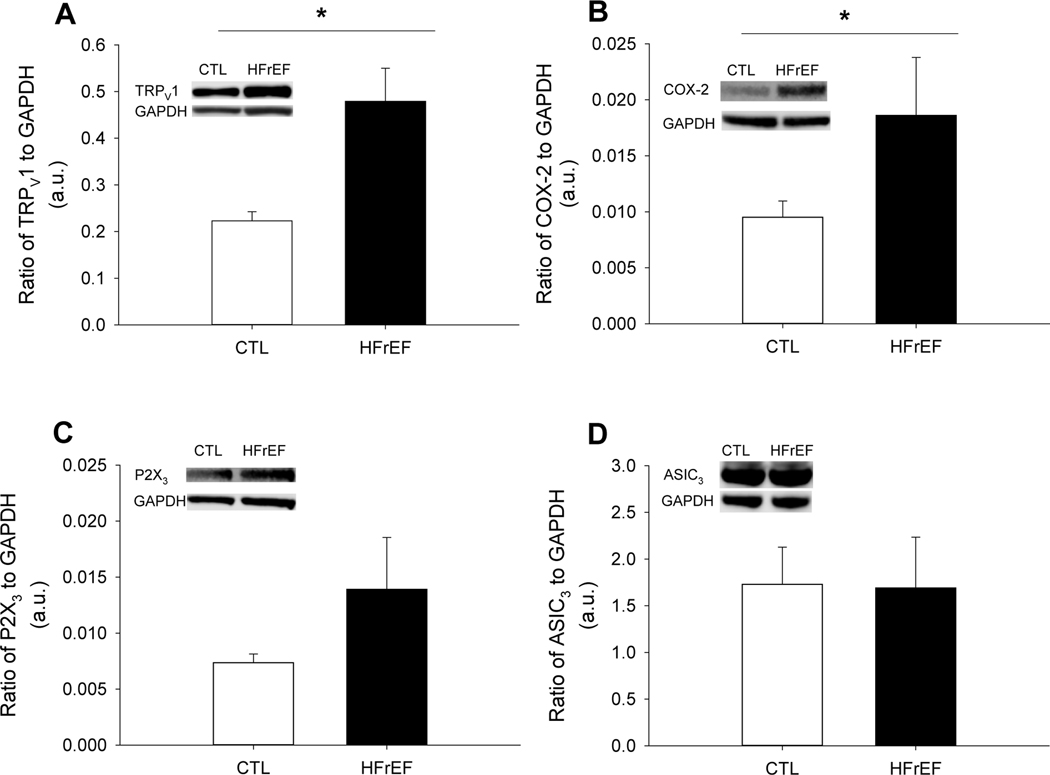
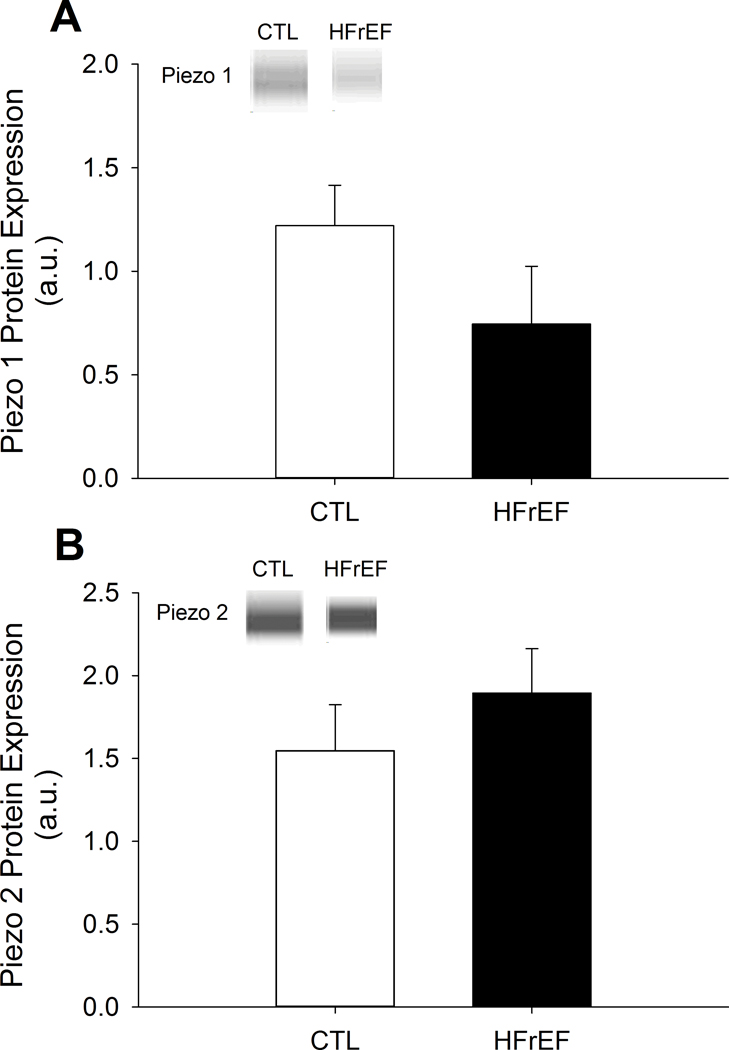
Protein expression of TRPV1 and COX-2 were greater for HFrEF compared to CTL (both, p<0.04), while P2X3 and ASIC3 were not different between groups (p>0.21) (Figure 1). Further, protein expression of Piezo 1 and 2 were not different between HFrEF and CTL (both p>0.16) (Figure 2).
Figure 1: Metaboreceptor protein expression in CTL and HFrEF.
TRPV1 (A), COX-2 (B), P2X3 (C), and ASIC3 (D) protein expression normalized to GAPDH in CTL and HFrEF. TRPV1 and COX-2 protein expression were greater in HFrEF compared to CTL (p<0.04), while P2X3 and ASIC3 were not different (both, p>0.21). Data reported as mean±SD.
Figure 2: Mechanoreceptor protein expression in CTL and HFrEF.
Piezo 1 (A) and 2 (B) protein expression in CTL and HFrEF. There were no differences in Piezo 1 and 2 protein expression between CTL and HFrEF (both, p>0.16). Data reported as mean±SD.
Rest and submaximal exercise responses:
At rest with PLA, HFrEF had greater V̇E and lower SBP and MAP than CTL (all, p<0.05). With FENT compared to PLA, arterial NE was greater in CTL at rest (p<0.01). With FENT compared to PLA, PaCO2 was greater in HFrEF at rest (p<0.01). In addition, HFrEF had greater % decreases in V̇E and fB as well as increase in PaCO2 than CTL with FENT at rest (all, p<0.05).
During exercise with PLA, HFrEF had lower RER, HR, SBP, DBP, and MAP than CTL (all, p<0.05), but higher arterial NE (p=0.03) (Table 2). With FENT compared to PLA, HR, SBP, DBP, and MAP were lower during exercise for CTL (all, p<0.04). With FENT compared to PLA, V̇E, fB, VE/VCO2 slope, SBP, DBP, and MAP were lower and PaCO2 was higher during exercise for HFrEF (all, p<0.02). Further, HFrEF had greater % decreases in V̇E, fB, and VE/VCO2 slope with FENT during exercise than CTL (all, p<0.03), but a greater % increase in PaCO2 (p=0.01).
Table 2:
Resting cardiopulmonary data with PLA and FENT
| CLT | HFrEF | CLT | HFrEF | |||
|---|---|---|---|---|---|---|
| PLA | FENT | PLA | FENT | Δ% | Δ% | |
| V̇O2 (L•min−1) | 0.34 ± 0.03 | 0.33 ± 0.07 | 0.36 ± 0.04 | 0.39 ± 0.05 | −1.9 ± 14.1 | 6.8 ± 9.7 |
| V̇CO2 (L•min−1) | 0.30 ± 0.03 | 0.29 ± 0.05 | 0.33 ± 0.03 | 0.35 ± 0.05* | −1.8 ± 13.7 | 6.7 ± 13.2 |
| V̇E (L•min−1) | 11 ± 2 | 12 ± 2 | 14 ± 2* | 13 ± 2 | 5.6 ± 13.9 | −7.1 ± 11.9* |
| VT(L) | 0.8 ± 0.1 | 0.7 ± 0.2 | 0.8 ± 0.1 | 0.8 ± 0.1 | −1.3 ± 19.3 | 1.2 ± 8.4 |
| fB (breaths•min−1) | 16 ± 4 | 17 ± 4 | 19 ± 2 | 18 ± 2 | 9.3 ± 19.8 | −8.3 ± 8.1* |
| HR (beats•min−1) | 72 ± 12 | 71 ± 11 | 67 ± 11 | 67 ± 8 | 0.2 ±11.5 | 0.6 ± 6.5 |
| SBP (mmHg) | 143 ± 11 | 141 ± 14 | 119 ± 11* | 114 ± 10* | −1.2 ± 8.5 | −4.0 ± 6.8 |
| DBP (mmHg) | 68 ± 10 | 64 ± 8 | 60 ± 8 | 59 ± 8 | −4.8 ± 8.8 | −1.1 ± 7.8 |
| MAP (mmHg) | 93 ± 9 | 90 ± 9 | 80 ± 8* | 77 ± 8* | −3.0 ± 8.3 | −2.7 ± 6.4 |
| PaCO2 (mmHg) | 38 ± 3 | 39 ± 4 | 39 ± 3 | 42 ± 5† | 2.0 ± 5.0 | 7.3 ± 3.6* |
| Arterial Epi (pg•mL−1) | 99 ± 43 | 159 ± 189 | 132 ± 95 | 127 ± 58 | 75.0 ± 207.5 | 16.4 ± 66.0 |
| Arterial NE (pg•mL−1) | 498 ± 149 | 675 ± 249† | 658 ± 161 | 785 ± 203 | 38.0 ± 31.6 | 21.3 ± 28.9 |
Mean±SD. VO2, oxygen uptake; VCO2, carbon dioxide production; RER, respiratory exchange ratio; VE, ventilation; VT, tidal volume; fB, breathing frequency; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PaCO2, arterial carbon dioxide pressure; Epi, epinephrine; NE, norepinephrine.
signficantly different than CTL.
significantly different than PLA.
Relationships:
At rest, the % change in MAP and V̇E from PLA to FENT were negatively related to TRPV1 protein expression in CTL (r= −0.72, p=0.02 and r= −0.80, p<0.01, respectively). Further, the % change in MAP was negatively related to ASIC3 protein expression (r= −0.65, p=0.04) in CTL at rest. No other relationships were present between protein expression and % change in cardiopulmonary variables from PLA to FENT in CTL or HFrEF at rest (p>0.05).
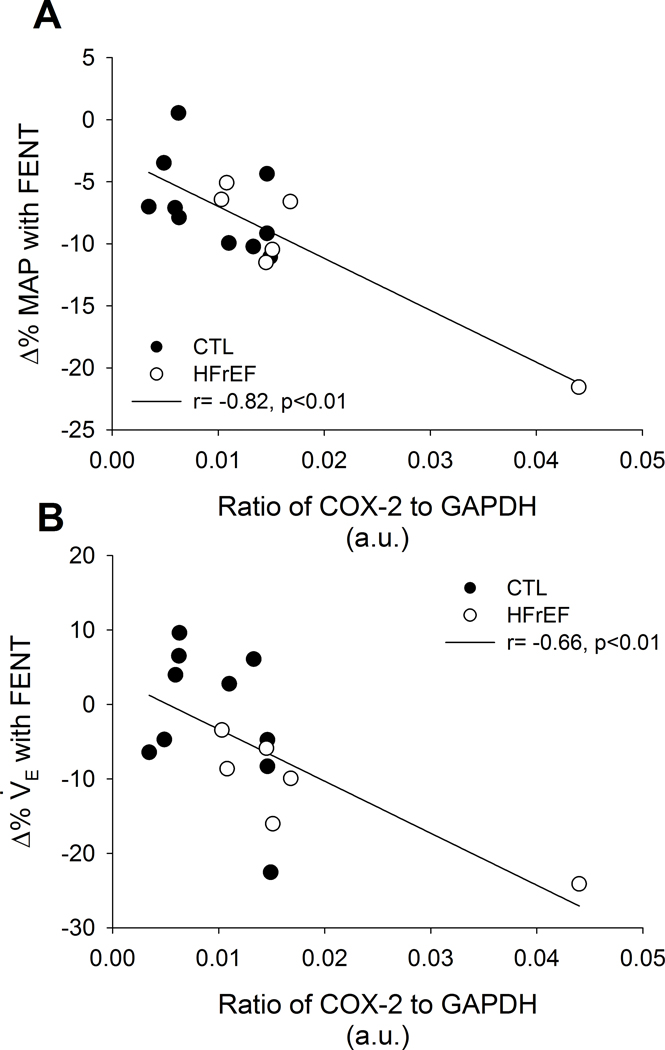
During submaximal exercise, the % change in MAP (r= −0.72, p=0.03) and SBP (r= −0.77, p=0.02) from PLA to FENT were negatively associated with TRPV1 protein expression in CTL (Figure 3). Further, V̇E/V̇CO2 slope from PLA to FENT was positively related to TRPV1 protein expression in CTL (r= 0.81, p<0.01). In all participants, the % changes in MAP (r= −0.82, p<0.01) and V̇E (r= −0.66, p<0.01) from PLA to FENT were negatively associated with COX-2 protein expression (Figure 4). Further, these negative relationships between the % changes in MAP and V̇E from PLA to FENT with COX-2 protein expression remained with the removal of the HFrEF patient with the greatest COX-2 protein expression (r= −0.51, p=0.05 and r= −0.53, p=0.04, respectively). Lastly, the % change in PaCO2 from PLA to FENT was positively associated with TRPV1 protein expression in all participants (r= 0.60, p=0.02). No other relationships were present during submaximal exercise between protein expression and % change in cardiopulmonary variables from PLA to FENT in CTL or HFrEF (p>0.05).
Figure 4: Relationships between COX-2 and % change in blood pressure and ventilation with FENT in CTL and HFrEF during exercise.
In all participants during submaximal exercise, COX-2 protein expression was negatively related to the % change with FENT in MAP (A; r= −0.82, p<0.01) and V̇E (B; r = −0.66, p<0.01). CTLs and HFrEF are presented in closed and open circles, respectively.
Central chemosensitivity:
There were no changes in the VE/PETCO2 slope between PLA and FENT in CTL (PLA: 2.3±0.8 vs. FENT: 2.1±0.9 L/min/mmHg, p=0.15) or HFrEF (PLA: 2.4±0.7 vs. FENT: 2.4±0.6 L/min/mmHg, p=0.69)
Discussion
The major novel findings of the present study are threefold. First, patients with HFrEF exhibited greater protein expression of TRPV1 and COX-2 than CTL, while no differences were present in protein expression of P2X3, ASIC3, Piezo 1, or Piezo 2 between groups. Second, the changes in MAP and V̇E with FENT (relative to PLA) during submaximal exercise were associated with COX-2 protein expression in all participants. Third, the changes in MAP and SBP with FENT (relative to PLA) during submaximal exercise were associated with TRPV1 protein expression in CTL. These findings suggest that HFrEF-induced increases in metaboreceptors influence the cardiopulmonary and neural responses during locomotor muscle exercise.
Protein expression in HFrEF:
In the present study, we found that TRPV1 and COX-2 protein expression levels were elevated in HFrEF compared to CTL, while P2X3 and ASIC3 were not different between groups. To date, there have been minimal studies in humans quantifying protein expression levels of receptors associated with the metabolic and mechanical components of the exercise pressor reflex. Specifically, Antunes-Correa et al found that COX-2 protein expression decreased from pre- to post-exercise training in patients with HFrEF (Antunes-Correa et al., 2014). However, since no control group was included in the study, it is unclear if these protein expression levels were initially elevated in HFrEF compared to healthy individuals. Previous studies using the rat-infarct model of HF have found differential protein expression of TRPV1, COX-2, P2X3, and ASIC3 when compared to healthy rats (Smith et al., 2005; Wang et al., 2010a; Morales et al., 2012; Wang et al., 2012; Xing et al., 2015; Xing & Li, 2016). For example, TRPV1 and ASIC3 have been found to be lower, while COX-2 and P2X3 have been found to be greater in the rat-infarct model of HF compared to control rats (Smith et al., 2005; Wang et al., 2010a; Morales et al., 2012; Wang et al., 2012; Xing et al., 2015; Xing & Li, 2016). The TRPV1, P2X3, and ASIC3 findings presented herein were surprising as previous animal models have shown that hindlimb intra-arterial infusion of capsaicin and lactic acid resulted in blunted increases in group IV afferent activity and blood pressure (Smith et al., 2005; Wang et al., 2010a; Wang et al., 2010b; Xing et al., 2015), while hindlimb intra-arterial infusion of α,β-methylene ATP increased the blood pressure response in HF compared to control rats (Gao et al., 2007). Likely explanations for the discrepant findings in TRPV1 and P2X3 include species differences, length of disease, age (i.e. generally young rats are used), and/or medication use. Further, obesity has been shown to influence the underlying mechanisms of the metaboreflex (Negrao et al., 2001; Milia et al., 2015). Future studies are necessary to determine the independent effect of obesity on protein expression of receptors associated with the metaboreflex. It is important to note that other receptors (not investigated in the present study) associated with the metaboreflex have also been reported to be differentially expressed between HFrEF and CTL (e.g., kinin 2 receptors) (Xing & Li, 2016). For the first time, we also quantified Piezo 1 and 2, mechano-gated channels associated with the mechanoreflex (Copp et al., 2016), protein expression levels in HFrEF and CTL and found no differences between groups. Taken together, these preliminary findings indicate that pathophysiologic mechanisms associated with HFrEF elevate TRPV1 and COX-2 protein expression in humans.
Relationships between cardiopulmonary responses and protein expression in HFrEF:
Neural afferent feedback arising from the locomotor muscles significantly contributes to blood pressure and ventilatory regulation during exercise in humans (Amann et al., 2010, 2011a). To this point, previous studies have demonstrated attenuated increases in blood pressure and V̇E during exercise in HFrEF and CTL participants by inhibition of locomotor muscle group III/IV afferents via intrathecal fentanyl injection (Amann et al., 2010; Amann et al., 2011b; Amann et al., 2014; Olson et al., 2014). Consistent with these previous studies, the present study found that V̇E was reduced at rest and during exercise in patients with HFrEF and blood pressure was reduced during exercise in both groups with FENT.
A secondary purpose of the present study was to determine if relationships were present between the cardiopulmonary variables with FENT (relative to PLA) and metabo- and mechanoreceptor protein expression. We found that the % change in MAP and V̇E with FENT during exercise were associated with COX-2 protein expression levels in all participants. These findings suggest that as COX-2 protein expression increases in patients with HFrEF, there is greater locomotor muscle group III/IV afferent feedback during cycling exercise. Consistent with these relationships, previous studies have found that HFrEF patients have greater prostaglandin production during handgrip exercise and isolated metaboreflex activation compared to age-matched controls (Scott et al., 2002; Scott et al., 2004). Further, previous studies have found that COX inhibition attenuates the exaggerated ventilatory response during isolated metaboreflex activation as well as the blood pressure and sympathetic activity responses to mechanoreflex activation and mechanoreflex sensitization in HFrEF patients and animal models (Scott et al., 2004; Middlekauff et al., 2008; Morales et al., 2012).
We found that the % changes in SBP and MAP during exercise with FENT were associated with TRPV1 protein expression in CTL. Consistent with our findings, recent studies have found that TRPV1 contributes to the blood pressure response during handgrip exercise in healthy adults via the metabolically-sensitive component of the exercise pressor reflex (Dawson et al., 2004; Notay et al., 2018; Vianna et al., 2018). For example, Vianna et al found that topical application of capsaicin-based analgesic balm in healthy men resulted in attenuated increases in blood pressure and muscle sympathetic nerve activity via TRPV1 desensitization when the skeletal muscle metaboreflex was activated (Vianna et al., 2018). Future studies using an interventional approach are necessary to determine the impact of these receptors on the integrative response to whole-body exercise in humans.
Methodological considerations:
There are several methodologic considerations that may have influenced our results. First, while this is the first study in humans comparing a clinical population with matched healthy control participants, we acknowledge the relatively small sample size of the HFrEF group, which may have limited our ability to determine differences in some outcomes (e.g., P2X3 protein expression). Second, the protein expression of TRPV1, COX-2, P2X3, ASIC3, Piezo 1, and Piezo 2 were determined from skeletal muscle biopsy samples, which included neuronal, vascular, muscular, and connective tissue. Third, the relationships between changes in cardiopulmonary and neural responses with FENT presented herein provide essential insight as to the underlying mechanisms contributing to the locomotor muscle group III/IV afferent feedback during large muscle (i.e. whole-body) exercise in humans. However, future interventional studies are necessary to confirm the findings of the present study.
Conclusions:
Patients with HFrEF exhibit greater expression of TRPV1 and COX-2 compared to CTL. Future studies using an interventional approach are necessary to determine if changes in locomotor muscle TRPV1 and COX-2 expression helps to minimize the exaggerated locomotor muscle group III/IV afferent feedback during exercise in HFrEF patients.
Table 3:
Cardiopulmonary responses with PLA and FENT during exercise
| CTL | HFrEF | CTL | HFrEF | |||
|---|---|---|---|---|---|---|
| PLA | FENT | PLA | FENT | Δ% | Δ% | |
| V̇O2 (L•min−1) | 1.6 ± 0.4 | 1.7 ± 0.4 | 1.6 ± 0.3 | 1.7 ± 0.3 | 4.8 ± 9.9 | 1.6 ± 5.5 |
| V̇CO2 (L•min−1) | 1.9 ± 0.5 | 1.9 ± 0.5 | 1.7 ± 0.3 | 1.7 ± 0.3 | 3.1 ± 8.2 | 3.1 ± 10.5 |
| V̇E (L•min−1) | 62 ± 16 | 61 ± 18 | 54 ± 9 | 48 ± 10† | −1.8 ± 9.6 | −11.4 ± 7.6* |
| VT(L) | 2.1 ± 0.5 | 2.1 ± 0.5 | 1.8 ± 0.2 | 1.8 ± 0.3 | −1.7 ± 6.1 | −0.9 ± 9.2 |
| fB (breaths•min−1) | 30 ± 5 | 30 ± 5 | 30 ± 6 | 27 ± 6† | 0.0 ± 6.6 | −10.3 ± 4.8* |
| V̇ENCO2 slope | 31 ± 3 | 29 ± 4 | 31 ± 4 | 26 ± 2† | −2.6 ± 5.7 | −15.2 ±10.3* |
| HR (beats/min) | 129 ± 16 | 123 ± 20† | 102 ± 23* | 96 ± 18* | −4.5 ± 6.7 | −4.5 ± 6.5 |
| SBP (mmHg) | 243 ± 16 | 232 ± 27† | 165 ± 30* | 148 ± 28*† | −5.0 ± 6.6 | −10.3 ± 8.8 |
| DBP (mmHg) | 76 ± 9 | 69 ± 6† | 63 ± 10* | 57 ± 11*† | −9.8 ± 7.3 | −10.2 ± 5.8 |
| MAP (mmHg) | 132 ± 6 | 123 ± 7† | 97 ± 14* | 87 ± 15*† | −10.3 ± 6.1 | −7.0 ± 3.6 |
| PaCO2 (mmHg) | 38 ± 4 | 39 ± 4 | 37 ± 3 | 43 ± 4† | 4.0 ± 5.9 | 14.7 ± 9.0* |
| Arterial Epi (pg•mL−1) | 208 ± 115 | 206 ± 134 | 206 ± 67 | 253 ± 66 | 4.0 ± 36.2 | 33.8 ± 56.4 |
| Arterial NE (pg•mL−1) | 1506 ± 365 | 1670 ± 517 | 2127 ± 853* | 2154 ± 748 | 10.2 ± 33.7 | 5.4 ± 27.1 |
Mean±SD. VO2, oxygen uptake; VCO2, carbon dioxide production; RER, respiratory exchange ratio; VE, ventilation; VT, tidal volume; fB, breathing frequency; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PaCO2, arterial carbon dioxide pressure; Epi, epinephrine; NE, norepinephrine.
signficantly different than CTL.
significantly different than PLA.
New Findings.
What is the central question of this study?
The goal of this study was to compare locomotor muscle metabo and mechanoreceptor expression in heart failure patients and controls. Further, we investigated if relationships existed between the protein expression and cardiopulmonary responses during exercise with locomotor muscle neural afferent feedback inhibition.
What is the main finding and its importance?
The novel findings were that heart failure patients exhibited greater protein expression of TRPV1 and COX-2 than controls. These findings are important as they identify receptors that may underlie the augmented locomotor muscle neural afferent feedback in heart failure.
Acknowledgements:
We are grateful to Dr. Ardem Patapoutian (The Scripps Research Institute, La Jolla, CA) for the generous gift of the Piezo 2 antibody. We also appreciate the participants who completed these studies.
Funding: This work was supported by the National Institutes of Health [HL126638 to TPO, HL139854 to MJJ, AG054454 to IRL] and American Heart Association [18POST3990251 to JRS].
Footnotes
Competing interests: The authors have no conflicts of interest to report for this manuscript.
References
- Adreani CM, Hill JM & Kaufman MP (1997). Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol (1985) 82, 1811–1817. [DOI] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF & Dempsey JA (2010). Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol (1985) 109, 966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF & Dempsey JA (2011a). Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J Physiol 589, 5299–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR & Richardson RS (2011b). On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589, 3855–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Venturelli M, Ives SJ, Morgan DE, Gmelch B, Witman MA, Jonathan Groot H, Walter Wray D, Stehlik J & Richardson RS (2014). Group III/IV muscle afferents impair limb blood in patients with chronic heart failure. Int J Cardiol 174, 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes-Correa LM, Nobre TS, Groehs RV, Alves MJ, Fernandes T, Couto GK, Rondon MU, Oliveira P, Lima M, Mathias W, Brum PC, Mady C, Almeida DR, Rossoni LV, Oliveira EM, Middlekauff HR & Negrao CE (2014). Molecular basis for the improvement in muscle metaboreflex and mechanoreflex control in exercise-trained humans with chronic heart failure. Am J Physiol Heart Circ Physiol 307, H1655–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp SW, Kim JS, Ruiz-Velasco V & Kaufman MP (2016). The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in decerebrate rats. J Physiol 594, 641–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson AN, Walser B, Jafarzadeh M & Stebbins CL (2004). Topical analgesics and blood pressure during static contraction in humans. Med Sci Sports Exerc 36, 632–638. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Young CN & Fadel PJ (2015). Autonomic adjustments to exercise in humans. Compr Physiol 5, 475–512. [DOI] [PubMed] [Google Scholar]
- Gao Z, Xing J, Sinoway L & Li J (2007). P2X receptor-mediated muscle pressor reflex in myocardial infarction. Am J Physiol Heart Circ Physiol 292, H939–945. [DOI] [PubMed] [Google Scholar]
- Han YS, Proctor DN, Geiger PC & Sieck GC (2001). Reserve capacity for ATP consumption during isometric contraction in human skeletal muscle fibers. J Appl Physiol (1985) 90, 657–664. [DOI] [PubMed] [Google Scholar]
- McCloskey DI & Mitchell JH (1972). Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, Maclellan WR, Hage A, Moriguchi J & Patel J (2008). Cyclooxygenase products sensitize muscle mechanoreceptors in humans with heart failure. Am J Physiol Heart Circ Physiol 294, H1956–1962. [DOI] [PubMed] [Google Scholar]
- Milia R, Velluzzi F, Roberto S, Palazzolo G, Sanna I, Sainas G, Pusceddu M, Mulliri G, Loviselli A & Crisafulli A (2015). Differences in hemodynamic response to metaboreflex activation between obese patients with metabolic syndrome and healthy subjects with obese phenotype. Am J Physiol Heart Circ Physiol 309, H779–789. [DOI] [PubMed] [Google Scholar]
- Morales A, Gao W, Lu J, Xing J & Li J (2012). Muscle cyclo-oxygenase-2 pathway contributes to the exaggerated muscle mechanoreflex in rats with congestive heart failure. Exp Physiol 97, 943–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrao CE, Trombetta IC, Batalha LT, Ribeiro MM, Rondon MU, Tinucci T, Forjaz CL, Barretto AC, Halpern A & Villares SM (2001). Muscle metaboreflex control is diminished in normotensive obese women. Am J Physiol Heart Circ Physiol 281, H469–475. [DOI] [PubMed] [Google Scholar]
- Notay K, Klingel SL, Lee JB, Doherty CJ, Seed JD, Swiatczak M, Mutch DM & Millar PJ (2018). TRPV1 and BDKRB2 receptor polymorphisms can influence the exercise pressor reflex. J Physiol 596, 5135–5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson TP, Joyner MJ, Eisenach JH, Curry TB & Johnson BD (2014). Influence of locomotor muscle afferent inhibition on the ventilatory response to exercise in heart failure. Exp Physiol 99, 414–426. [DOI] [PubMed] [Google Scholar]
- Piepoli MF, Dimopoulos K, Concu A & Crisafulli A (2008). Cardiovascular and ventilatory control during exercise in chronic heart failure: role of muscle reflexes. Int J Cardiol 130, 3–10. [DOI] [PubMed] [Google Scholar]
- Scott AC, Wensel R, Davos CH, Georgiadou P, Ceri Davies L, Coats AJ, Francis DP & Piepoli MF (2004). Putative contribution of prostaglandin and bradykinin to muscle reflex hyperactivity in patients on Ace-inhibitor therapy for chronic heart failure. Eur Heart J 25, 1806–1813. [DOI] [PubMed] [Google Scholar]
- Scott AC, Wensel R, Davos CH, Kemp M, Kaczmarek A, Hooper J, Coats AJ & Piepoli MF (2002). Chemical mediators of the muscle ergoreflex in chronic heart failure: a putative role for prostaglandins in reflex ventilatory control. Circulation 106, 214–220. [DOI] [PubMed] [Google Scholar]
- Sinoway LI & Li J (2005). A perspective on the muscle reflex: implications for congestive heart failure. J Appl Physiol (1985) 99, 5–22. [DOI] [PubMed] [Google Scholar]
- Smith JR, Koepp KE, Berg JD, Akinsanya JG & Olson TP (2019). Influence of Sex, Menstrual Cycle, and Menopause Status on the Exercise Pressor Reflex. Med Sci Sports Exerc 51, 874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR & Olson TP (2019). Ventilatory constraints influence physiological dead space in heart failure. Exp Physiol 104, 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH & Garry MG (2006). The mammalian exercise pressor reflex in health and disease. Exp Physiol 91, 89–102. [DOI] [PubMed] [Google Scholar]
- Smith SA, Williams MA, Mitchell JH, Mammen PP & Garry MG (2005). The capsaicin-sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation 111, 2056–2065. [DOI] [PubMed] [Google Scholar]
- Van Iterson EH, Johnson BD, Joyner MJ, Curry TB & Olson TP (2017). Vo2 kinetics associated with moderate-intensity exercise in heart failure: impact of intrathecal fentanyl inhibition of group III/IV locomotor muscle afferents. Am J Physiol Heart Circ Physiol 313, H114–H124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna LC, Fernandes IA, Barbosa TC, Teixeira AL & Nobrega ACL (2018). Capsaicin-based analgesic balm attenuates the skeletal muscle metaboreflex in healthy humans. J Appl Physiol (1985) 125, 362–368. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Li YL, Gao L, Zucker IH & Wang W (2010a). Alteration in skeletal muscle afferents in rats with chronic heart failure. J Physiol 588, 5033–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Li YL, Zucker IH & Wang W (2012). Exercise training prevents skeletal muscle afferent sensitization in rats with chronic heart failure. Am J Physiol Regul Integr Comp Physiol 302, R1260–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Pan YX, Wang WZ, Gao L, Zimmerman MC, Zucker IH & Wang W (2010b). Exercise training prevents the exaggerated exercise pressor reflex in rats with chronic heart failure. J Appl Physiol (1985) 108, 1365–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL & Patapoutian A (2014). Piezo2 is required for Merkel-cell mechanotransduction. Nature 509, 622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J & Li J (2016). Bradykinin Contributes to Sympathetic and Pressor Responses Evoked by Activation of Skeletal Muscle Afferents P2X in Heart Failure. Cell Physiol Biochem 39, 2101–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Lu J & Li J (2015). ASIC3 contributes to the blunted muscle metaboreflex in heart failure. Med Sci Sports Exerc 47, 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]