Abstract
The interrelationships between atrial fibrillation (AF) and heart failure (HF) are complex and poorly understood, yet the number of patients with AF and HF continues to increase worldwide. Thus, there is a need for initiatives that prioritize research on the intersection between AF and HF. This report summarizes the proceedings of a virtual workshop convened by the National Heart, Lung, and Blood Institute to identify important research opportunities in AF and HF. Key knowledge gaps were reviewed and research priorities were proposed for characterizing the pathophysiological overlap and deleterious interactions between AF and HF; preventing HF in persons with AF; preventing AF in individuals with HF; and addressing symptom burden and health status outcomes in AF and HF. These research priorities will hopefully help inform, encourage, and stimulate innovative, cost-efficient, and transformative studies to enhance the outcomes of patients with AF and HF.
Keywords: atrial fibrillation, heart failure, research
Atrial Fibrillation (AF) and Heart Failure (HF)
Atrial fibrillation (AF) and heart failure (HF) are highly prevalent clinical conditions that frequently co-exist.1–3 It is well known that patients with HF are at increased risk of AF.1–3 Of the estimated 5.8 million Americans with HF with reduced ejection fraction (HFrEF) or preserved EF (HFpEF), up to 40% develop AF.1, 4 AF itself can cause HF via different mechanisms.2, 3 Risk factors are similar for both AF and HF, including advancing age, male sex, tobacco use, alcohol consumption, physical inactivity, sleep disorders, obesity, hypertension, diabetes mellitus, coronary heart disease, and valvular heart disease. In addition, there are underlying genetic predispositions for both conditions.2, 3 Many have called for better understanding of mechanisms predisposing to AF in HF patients and to HF in AF patients, identifying high risk subgroups of patients with AF or HF for screening and prevention, and detecting and treating asymptomatic or paroxysmal AF early on as a means to prevent AF and HF progression. Many have also called for improved understanding of symptom burden in AF versus HF and the best approaches to utilizing and refining patient-reported outcomes, improving monitoring, and tailoring treatment to patient-specific benefit in order to optimize the quality of care. Therefore, there is a need for a platform that allows discussion and consideration of research priorities that will help address these gaps in knowledge.
Recognizing the importance of AF research, in 2008 the National Heart, Lung, and Blood Institute (NHLBI) convened an expert panel to identify gaps and recommend research strategies focused on improving AF prevention.5 To build on this prior work, the NHLBI recently launched a series of webinar-based workshops covering different areas in AF. The first virtual workshop in the series focused on catheter ablation of AF.6 The second workshop, held on August 14, 2019, had an overall theme of advancing research on the complex interrelationship between AF and HF. The webinar provided a platform for the identification of research priorities by covering the following four specific topics in AF and HF: 1) pathophysiological overlap between AF and HF, 2) prevention of HF in individuals with AF, 3) prevention of AF in individuals with HF, and 4) symptom burden in AF and HF. This report summarizes the content of the webinar. In addition, upon the report’s publication, the topic frameworks and recorded webinar will be posted on the NHLBI website.7
The Overlapping Pathophysiology Between AF and HF
It is well known that AF can lead to cardiomyopathy and HF via different mechanisms including persistent tachycardia, abnormalities in calcium handling, changes in ion channel expression, irregular ventricular response leading to abnormal excitation-contraction coupling, and neurohormonal activation.1, 8, 9 Studies have shown that left atrial fibrosis, stretch, and denervation, as well as the downregulation of natriuretic peptides that occur in AF can aggravate both HFrEF and HFpEF.9–13 However, other causal links between HF and AF likely differ between HFrEF and HFpEF and, as such, should be evaluated differently for these conditions. Neurohormonal activation is more intense with HFrEF and may be further aggravated by the fast heart rate and irregularity of AF.11–13 In contrast, inflammation that may predispose to AF may initially be more relevant to the metabolic milieu of HFpEF, but immune activation increases with severity of disease in both HFrEF and HFpEF.14–16
Tachycardiomyopathy is a type of cardiomyopathy that develops as a result of rapidly conducted AF. Development and resolution of tachycardiomyopathy caused by AF are defined for HFrEF by changes in left ventricular (LV) EF, but a parallel indicator does not exist for HFpEF.1 HFpEF definitions and staging are further complicated by distinct phenotypes relating to presence or absence of obesity and baseline venous congestion, which worsen diastolic function, exertional dyspnea, and hospitalizations that characterize clinical HFpEF.1
AF, obstructive sleep apnea (OSA), and HF cluster together.17, 18 These three conditions share not only similar risk factors but are influenced by and have effects on neurohormonal activation.11, 17 Tachycardiomyopathy from AF may reflect not only the direct effect of persistently elevated heart rates but also the adverse effects of sympathetic stimulation. The rapid activation rate and irregularity of ventricular response in AF cause intermittent reduction in diastolic pressure, which, in turn, further activates the sympathetic nerves.11 Microneurography studies show that muscle sympathetic nerve activity as well as circulating plasma norepinephrine are increased in patients with OSA.19, 20 Continuous positive airway pressure therapy can attenuate the increased sympathetic tone.20 It can also improve LVEF in patients with HFrEF.21 These findings suggest that OSA is a modifiable risk factor for both AF and HF. In addition to beta-blocker therapy, neuromodulation methods that further reduce sympathetic output might provide additional therapeutic benefit in patients with AF and HF with or without OSA.22–24 Whereas baroreflex activation therapy has been approved for selected patients with HF, recent randomized clinical trials of vagal nerve stimulation in HFrEF did not include patients with chronic AF.25–27 It remains possible that patients with AF, OSA, and HF may benefit from neuromodulation methods that reduce sympathetic nerve activity.
Current indications and selection of interventions for rhythm control of AF have appropriately mostly focused on improvement of symptoms, which is a top priority.28–32 However, the potential for “cure” of tachycardiomyopathy elevates the urgency of identification and aggressive treatment of AF in at risk patients, even without compelling current symptoms. Toward that end, the following knowledge gaps were identified:
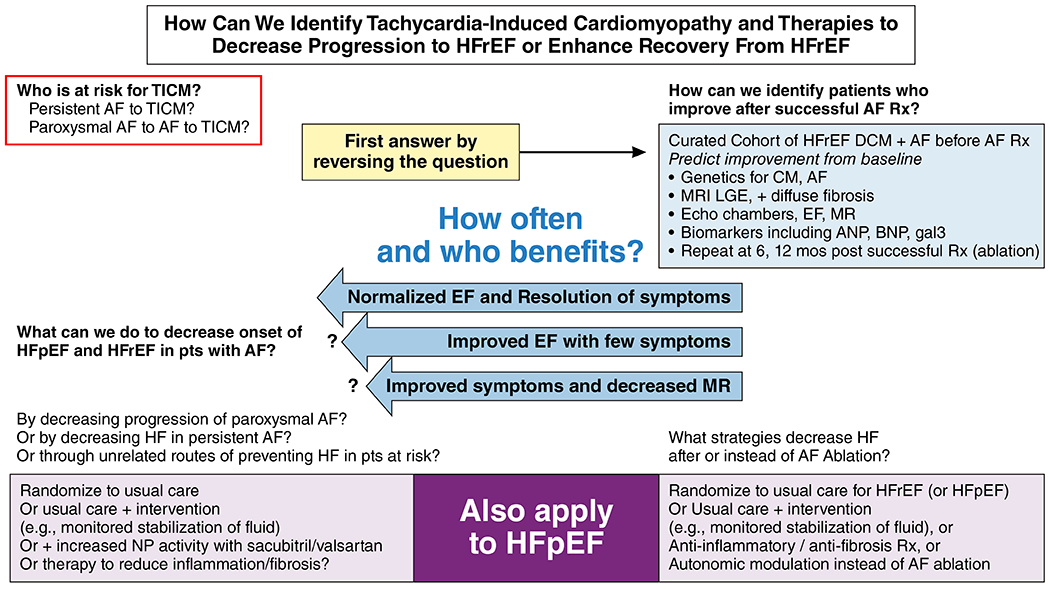
A crucial knowledge gap is the risk profile and prevalence of tachycardiomyopathy identified retrospectively by normalization of a reduced EF. This condition appears to be most common in patients without coronary heart disease, particularly those in whom HF appears synchronously or after the onset of AF, and those with little or no ventricular fibrosis detected by late gadolinium enhancement on cardiac magnetic resonance imaging (MRI).32 The best way to address this gap is to curate a carefully phenotyped cohort of patients undergoing definitive therapy for AF, including baseline cardiac MRI and echo imaging, genetic testing, and serial evaluation to follow changes in LV function/fibrosis and symptom status.
Further studies should investigate treatment regimens, including pharmacologic and direct neurohormonal modulation. These interventions should be tested for their role in enhancing recovery from and preventing tachycardiomyopathy in patients at high risk for progression due to a monitored high burden of paroxysmal AF (Figure 1).
An important priority is the development of medications for treating AF that either do not lead to adverse HF outcomes or can improve HF outcomes.
Another knowledge gap relates to whether vigilant maintenance of volume balance and optimal left atrial pressures can reduce progression from paroxysmal to persistent AF and improve the outcomes of AF ablation and other rhythm control strategies.
Figure 1:
Identifying tachycardia-induced cardiomyopathy and effective therapies for it
The following prioritized research opportunities were identified:
1. To establish the risk profiles and prevalence of tachycardiomyopathy with complete and partial reversibility of LV dysfunction. This may be best accomplished through a curated cohort of patients with AF and HF in whom the following are characterized: biomarkers, fibrosis on cardiac MRI, cardiac structure on cardiac MRI and echocardiography, genomic (methylation, transcriptomic, proteomic, metabolomic, etc.) and genetic profiles of cardiomyopathy and AF, peak VO2 testing, and patient-reported outcomes before and at 6 and 12 months after ablation, to determine frequency and predictors of meaningful improvement in LV function and outcomes.
2. To conduct a randomized trial of intensive maintenance of volume status vs. usual care to reduce progression of HF and progression of paroxysmal to persistent AF as well as following AF ablation in adults with either HFrEF or HFpEF. Outcomes in both HFrEF and HFpEF would include diastolic function, left atrial volume, and patient-reported symptoms and function, and in HFrEF, LVEF and LV dimensions.
3. To conduct randomized clinical trials of catheter ablation, antiarrhythmic drugs, and prevention in patients with AF and HF. To enhance the feasibility of such trials, pragmatic and other innovative trial designs should be leveraged.
Research to Prevent HF in Individuals with AF
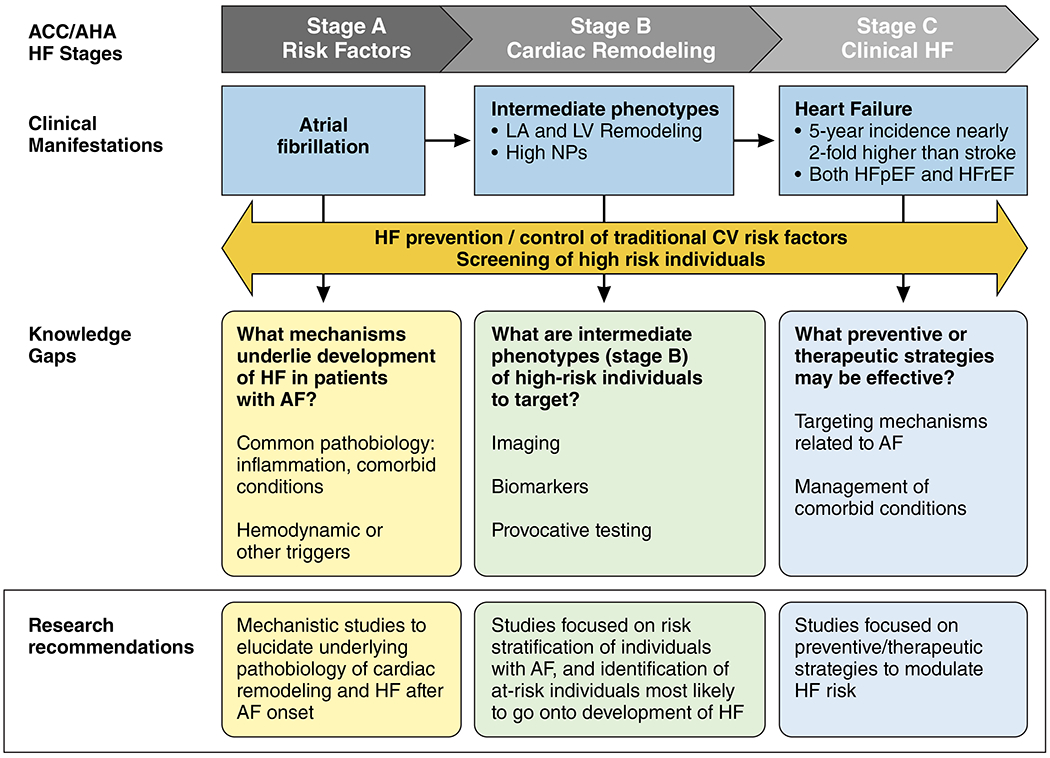
As noted above, AF and HF are closely intertwined, with each condition predisposing to the other.1–4, 33, 34 The risk of both HFrEF and HFpEF is elevated in patients with AF.1–4, 33, 34 Notably, the 5-year incidence of HF is nearly twice that of incident stroke after AF diagnosis, yet the clinical focus has been squarely on stroke prevention after AF, whereas little is known about HF prevention in this growing population.35 There are a number of randomized clinical trials not focused on individuals with AF demonstrating that HF can be prevented among high-risk individuals.36–38 For example, selected antihypertensive treatments (ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) and HYVET (Hypertension in the Very Elderly Trial)) may prevent the development of HF.37, 38 Whether such HF preventive strategies are generalizable to individuals with AF has not been established.
Observational studies suggest that traditional modifiable risk factors account for more than half of the population’s attributable risk of developing HF among persons with AF, and these may be even more prevalent in HFpEF.39, 40 This suggests that AF may provide an opportunity to focus preventive efforts with careful attention on known cardiovascular risk factors. For example, initial studies evaluating weight loss and intensive risk factor control in AF patients have reported favorable effects on cardiac structure and function that may reduce incident HF.41 However, clinical and therapeutic strategies to prevent HF among patients with AF remain largely understudied, and randomized controlled trials should investigate the efficacy of such strategies (Figure 2).
Figure 2:
Preventing HFpEF and HFrEF in individuals with AF
With the recognition that incident HF is common among patients with AF, and that HF can be prevented in the context of prior clinical trials in broader populations, the following knowledge gaps were identified:
There is limited understanding as to whether HF after AF occurs due to shared underlying mechanisms, with a common pathobiology of AF and HF. In contrast, there may also be hemodynamic and other triggers for cardiac remodeling that are specifically driven by AF that make the progression to HFrEF or HFpEF more likely.
Strategies to identify individuals with AF at highest risk for progression to HF are needed. Whether biomarker or imaging modalities may help risk-stratify individuals in a clinically meaningful way and whether screening will lead to improved outcomes are largely unknown.
In individuals with AF, the role of intensive cardiovascular risk factor control, such as aggressive hypertension treatment, weight loss strategies, or more targeted therapies in preventing progression to HF has not been well studied.
The following research opportunities were proposed:
1. Mechanistic studies are needed to elucidate the underlying pathobiology of cardiac remodeling, HFpEF, and HFrEF after AF onset.
2. Studies should focus on risk stratification of individuals with AF, and identification of at-risk individuals most likely to develop HFrEF or HFpEF, leveraging clinical, biochemical, imaging, or genomic/genetic data. Through detection of atrial and ventricular fibrosis and accurate measurement of hemodynamics, cardiac MRI specifically may be important in elucidating factors responsible for the development and progression of HF in AF patients.
3. Studies should focus on identifying preventive/therapeutic strategies to effectively reduce the risk of developing HFpEF and HFrEF in AF patients.
Research to Prevent AF in Individuals with HF
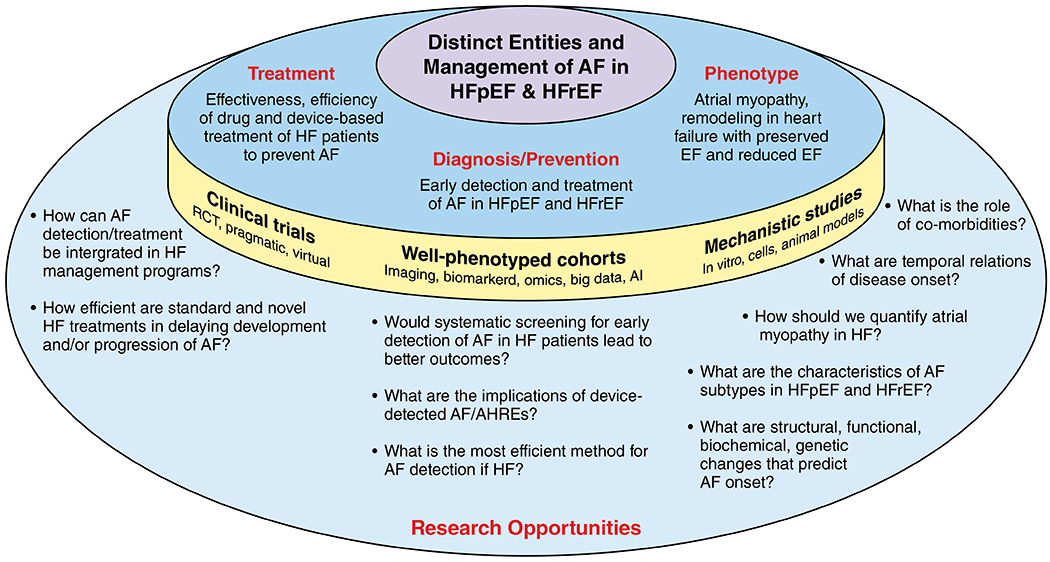
Remodeling in HF and the resultant atrial myopathy with impaired left atrial hemodynamics predispose patients with HF to developing AF.42 AF often develops in patients with HF, possibly with an increasing prevalence from HFrEF to HF with mid-range EF (HFmrEF) to HFpEF.2, 33, 43–46. Because of the worse clinical outcomes of patients with HF who develop AF,2, 33, 47 comprehensive early management of upstream and possibly downstream risk factors may potentially improve mild-to-moderate HF and prevent or delay the onset of AF.48–51 Restoration of sinus rhythm by ablation therapy in symptomatic paroxysmal or persistent AF and HFrEF may improve outcomes,29, 30 whereas anti-arrhythmic drugs have more pronounced adverse side effects in HF patients (Figure 3).
Figure 3:
Preventing AF in patients with HF
While AF onset in HF patients is a discrete event, it could also be an indicator or a trigger of HF deterioration with further impairment of cardiac output and hemodynamics.52 Patient characteristics significantly differ by HF type; HFpEF patients are generally older, are more likely to be women, and often have heterogeneous comorbidities including hypertension, obesity and diabetes, whereas HFrEF patients are relatively young, and have a higher prevalence of coronary heart disease. This renders the definition of an exact AF-HF phenotype very difficult. Compared with HFrEF, HFpEF is associated with different remodeling and biomarker profiles in AF.42, 53, 54 In HFrEF, AF appears to be a sign of advanced disease with a more homogeneous elevation of biomarkers indicative of severe cardiac disease; whereas in HFpEF the biomarker pattern is less predictable and reproducible.53 It is noteworthy that establishing the diagnosis of HFpEF can be extremely difficult in patients with AF given that the 2 conditions have overlapping symptomatology and both can lead to an elevated NT-pro-brain natriuretic peptide (NT-pro-BNP) and echocardiographic markers of diastolic dysfunction (e.g. atrial enlargement).15
HF patients tend to have frequent medical encounters, so asymptomatic paroxysmal AF may be detected earlier during routine follow-up compared to patients without HF. Cardiac implantable electronic devices, in particular in patients with HFrEF, may permit early detection of AF, especially of short and asymptomatic episodes.55 The prognostic significance of short episodes of AF is unclear; however, many clinicians and patients want to know whether an early rhythm control strategy in such patients would help prevent development of clinical AF and progression of HF. Indeed, in patients with a pacemaker or defibrillator enrolled in the ASSERT (Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial), progression of shorter to longer episodes of subclinical AF was strongly associated with HF hospitalization.56 Many patients with HFpEF do not have implantable devices given a lack of clinical indication, and as a result, detection of AF may be delayed. Studies should examine the role of various screening strategies, including both non-invasive and invasive strategies in patients with HFpEF, and at least one such study using implantable loop recorders is underway.57
The following knowledge gaps were identified for the prevention of AF in individuals with HF:
Determination of efficient methods for AF screening (mode, frequency, and duration) in HF patients, including device-detected AF. In randomized controlled trials, it would be important to test whether treating early detected asymptomatic AF in closely followed HF patients can improve event-free survival (HF deterioration/hospitalization, stroke/systemic embolism, dementia/cognitive decline, and mortality).
Characterization of predictors, ideally modifiable, of AF in HFrEF and HFpEF patients. It is important to focus on different AF subtypes in HFpEF because of the expected increase in prevalence, less knowledge, and high heterogeneity of HFpEF. Defining the role of atrial myopathy in HFpEF and AF is also important.
Understanding the prognostic significance of brief episodes of subclinical AF and the potential benefit of early interventions.
Development of animal models of HFpEF with incident AF to investigate causal pathways.
In-depth phenotyping of HF cohorts with improved non-invasive imaging for atrial structure and function, and atrioventricular interaction. Efforts should better exploit existing and new information from biomarkers, genomics, and genetics,58 including from atrial tissue specimens. It will be important to integrate data across multiple–omics to simultaneously assess their biological meaning to stratify HF subtypes in relation to AF risk. Machine learning analytic methods should be applied to understand the role of individual comorbid conditions and comorbidity burden in the HF-AF relationship, including clinically rich information from electronic health records.59 Once these factors are better identified, it will be important to link them with clinically meaningful outcomes.
Extension of integrated care concepts for HF patients towards prevention, detection, and treatment of AF to improve quality of life and other outcomes. In particular, there is great variability in current management of these comorbid disorders, especially given the lack of evidence in the field, and this variability should be exploited in large, clinically-rich observational registries to link alternative management strategies, adjusting for patient risk, to clinically-important outcomes.
To prevent AF, HFpEF research in this area should be prioritized, given that the knowledge gaps appear to be much larger in HFpEF than in HFrEF, and as HFpEF prevalence is increasing in an aging population with a high prevalence of obesity and hypertension.
Relevant suggested studies on mechanistic background and clinical questions are outlined in Figure 3.
The following prioritized research opportunities were proposed:
1. In randomized controlled trials, test whether treating early detected AF can improve event-free survival (stroke/systemic embolism, heart failure deterioration/hospitalization, mortality, dementia/cognitive decline) and patient-centered outcomes (quality of life, functional status, frailty). Also, the best treatment for early detected AF should be investigated and may include more aggressive rhythm control with available or novel antiarrhythmic drugs, catheter ablation, or device therapies.
2. Explore existing and deeply-phenotyped HF cohorts to define HF subtypes with a high risk of AF and adverse, clinically-important outcomes based on multi-level information in order to highlight pathophysiological pathways for experimental work-up, improve screening efficiency, and identify targets for prevention. Characterize AF phenotypes that are specific to HFpEF versus HFrEF. .
3. Conduct randomized controlled trials comparing the effectiveness in preventing AF of standard and novel HF treatments (e.g., beta-blockers, cardiac resynchronization therapy) in HFrEF and HFpEF patients.
Research on Symptom Burden in AF versus HF
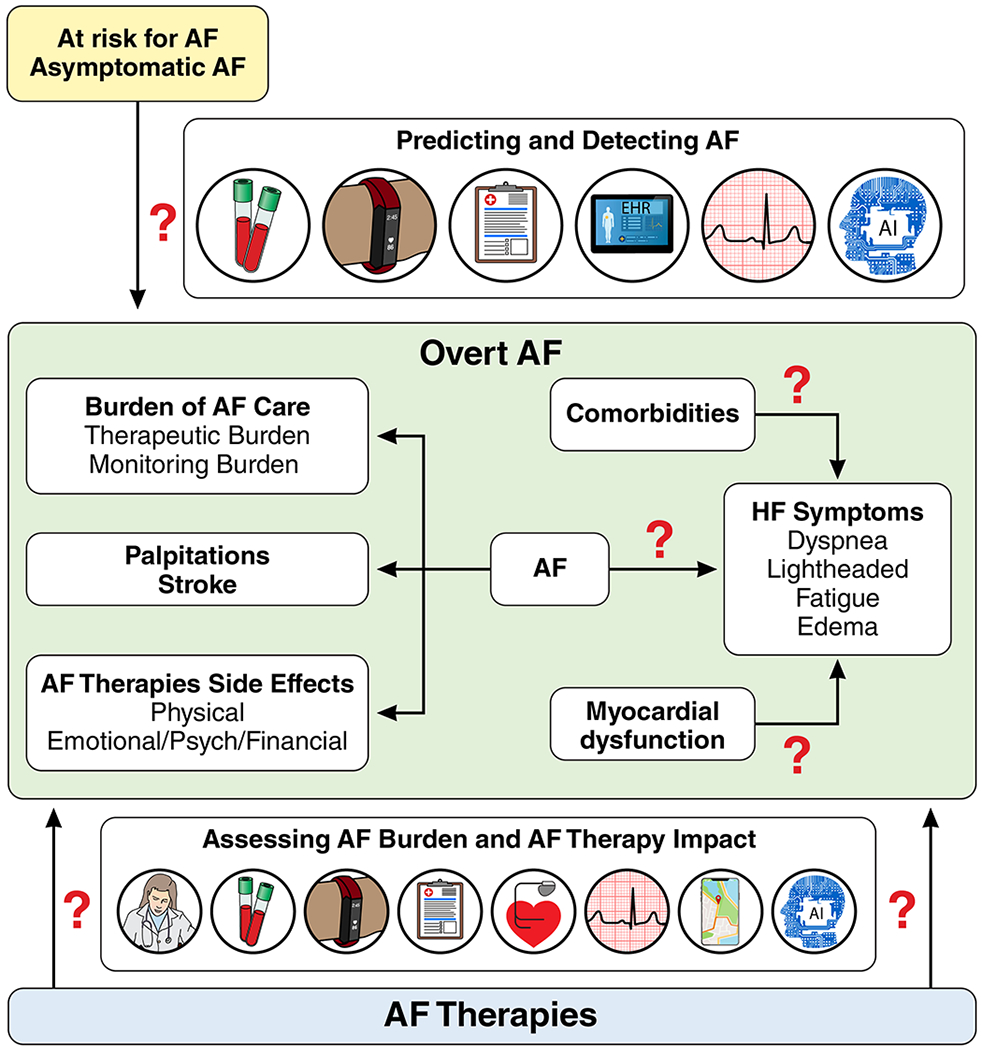
HF and AF symptoms have substantial overlap, including, shortness of breath, dyspnea on exertion, impaired exercise tolerance, and fatigue. There are also symptoms that are more common in one than the other (e.g. palpitations in AF or edema in HF). AF may also be asymptomatic, and yet it can still result in poor outcomes such as HF and stroke.60 HF-like symptoms in AF may reflect physiologic effects of AF in an otherwise normal heart, may indicate occult HFpEF,61 or may represent the interplay of AF and non-cardiac comorbid conditions, which also produce HF-like symptoms.60 Occult HFpEF or various comorbid conditions may affect the impact of AF treatment on symptoms and quality of life.60
Generic health status measures are designed to assess the totality of health in relation to patients’ symptoms, function, and quality of life, whereas disease-specific measures seek to more sensitively capture the impact of a given disease on patients’ symptoms, function, and quality of life. Whereas there are several disease-specific measures for both AF and HF,62–64 the interaction of these diseases with disease-specific measures and the impact of treatment on patients’ health status need further study to better define the impact of new-onset HF on AF patients’ health status and the impact of new-onset AF on HF patients’ health status.65–68 It is important for clinicians to understand what outcomes matter to patients. In addition to “hard” clinical outcomes, patients care about the effects of a given intervention on ability to work, exercise tolerance, cognitive function, and the risk of depression.69, 70
Circulating cardiovascular biomarkers, including NT-pro-BNP, have not been helpful in discriminating pure AF-related from HF-related symptoms, predicting outcomes in AF, or predicting success of AF therapies.71–73 There is a need for better biomarkers that can discriminate HF from AF. New technologies and alternate “biomarkers” including artificial intelligence74 assisted analysis of ECG or images, and wearable and implantable physiologic monitors may provide the means to predict, detect, and monitor AF, evaluate the impact of AF on physiologic parameters reflective of quality of life, as well as shed light on the pathophysiology of HF and AF.75 Such technologies may enable better understanding of the trajectory of health status over time, provide insights into potential future interventions, and allow novel clinical trial designs.75, 76
The following knowledge gaps were identified:
Better definition of the impact of AF and AF burden on patient-reported outcomes in HF and vice versa.
How can we cost-effectively and systematically detect undiagnosed AF in the population in order to determine its impact on quality of life?
How can we discriminate between symptoms due to AF and symptoms due to occult myocardial dysfunction or comorbidities that may persist/progress after AF onset and therapies and limit the impact of AF therapy on quality of life?
What novel physiologic biomarkers will enhance assessment of the burden of AF and the impact of AF therapies at the patient and population levels, or are they needed if patient-reported outcomes can adequately measure the burden?
What combinations of patient-reported outcomes are optimal in monitoring the health status of patients with both AF and HF?
What roles should patient-reported health status measures have in guiding therapeutic interventions, and can care protocols be developed to better assess the application of emerging treatments to patients?
How do race, ethnicity, sex, and age impact symptom burden and quality of life in AF and HF?
What is the variability in symptom control and quality of life across clinical practices in patients with AF and HF and what practice characteristics are associated with the best health status?
Does symptom burden in AF versus HF vary by geographical location?
The following prioritized research opportunities were proposed (see Figure 4):
Figure 4:
Assessing disease burden in AF.
1. Determine if disease-specific, patient-reported outcome measures best determine the impact of AF and AF therapy on quality of life in order to define the best endpoints in AF and HF clinical trials, the most appropriate measures of clinical AF care quality, and the most accurate predictors of AF disease trajectory.
2. Study the effects of AF on cardiovascular function and symptoms in a spectrum of AF patients to determine how to discriminate between symptoms due to occult myocardial dysfunction or comorbidities versus AF.
3. Define clinically-important differences in disease-specific patient-reported outcome measures and their associations with age, sex, and race/ethnicity and the variability in health status across practices determining the proportion of this variability that is due to patient (e.g. socio-demographic, socio-economic, clinical comorbidities and disease severity) and practice characteristics (e.g. treatment).
Conclusions:
As the number of patients with AF and HF continues to rise, it is no longer appropriate to only treat these conditions when they are fully manifest. Research efforts should focus on prevention that extends beyond tachycardiomyopathy and target more effective approaches to AF prevention/treatment in HF patients and HF prevention/treatment in AF patients. To that end, developing a better understanding of the mechanisms underlying predisposition to AF in HF patients and to HF in AF patients, and its relationship to clinically-meaningful outcomes is of paramount importance. This understanding applies to both HFpEF and HFrEF, each of which may relate differently to AF. Such understanding should be coupled with identifying high-risk subgroups of patients with AF or HF for screening and prevention and the best modalities for early detection of these conditions. In addition, efforts should enhance understanding of symptom burden in AF versus HF and define the best approach to utilizing patient-reported outcomes clinically and in research. Addressing the knowledge gaps identified in this report will be critically important. Prioritized research opportunities to help address many of the identified knowledge gaps were proposed (Table). It is hoped that this report will propel investigators to conduct research in the area of AF and HF that will provide definitive information and lead to transformative, lasting, and meaningful improvement in clinical care and patient outcomes.
Table.
Prioritized Research Opportunities for AF and HF
| The Overlapping Pathophysiology Between AF and HF |
|---|
| 1. To establish the risk profiles and prevalence of tachycardiomyopathy with lesser degrees of reversible LV dysfunction. This may be best accomplished through a curated cohort of patients with AF and non-ischemic HF in whom the following are characterized: biomarkers, fibrosis on cardiac MRI, cardiac structure on cardiac MRI and echo, genomic (methylation, transcriptomic, proteomic, etc.), and genetic profiles of cardiomyopathy and AF, peak VO2 testing, and patient-reported outcomes before and at 6 and 12 months after ablation to determine frequency and characteristics predicting meaningful improvement. |
| 2. To conduct a randomized trial of intensive maintenance of volume status vs. usual care to reduce progression of HF and progression of paroxysmal to persistent AF as well as following AF ablation in adults with either HFrEF or HFpEF. Outcomes would include LVEF and LV dimensions in HFrEF, diastolic function and left atrial volume and patient-reported symptoms and function in both HFrEF and HFpEF. |
| 3. To conduct randomized clinical trials of catheter ablation, antiarrhythmic drugs, and prevention in patients with AF and HF. To enhance the feasibility of such trials, pragmatic and other innovative trial designs should be leveraged. |
| Research to prevent HF in individuals with AF |
| 1. Mechanistic studies are needed to elucidate the underlying pathobiology of cardiac remodeling, HFpEF, and HFrEF after AF onset. |
| 2. Studies should focus on risk stratification of individuals with AF, and identification of at-risk individuals most likely to develop HFrEF or HFpEF, leveraging clinical, biochemical, imaging, or genomic/genetic data. Through detection of atrial and ventricular fibrosis and accurate measurement of hemodynamics, cardiac MRI specifically may be important in elucidating factors responsible for the development and progression of HF in AF patients. |
| 3. Studies should focus on identifying preventive/therapeutic strategies to effectively reduce the risk of developing HFpEF and HFrEF in AF patients. |
| Research to prevent AF in individuals with HF |
| 1. In randomized controlled trials, test whether treating early detected AF can improve event-free survival (stroke/systemic embolism, heart failure deterioration/hospitalization, mortality, dementia/cognitive decline) and patient-centered outcomes (quality of life, functional status, frailty). Also, the best treatment for early detected AF should be investigated and may include more aggressive rhythm control with available or novel antiarrhythmic drugs, catheter ablation, or device therapies. |
| 2. Explore existing and new deeply-phenotyped HF cohorts to define HF subtypes with a high risk of AF and adverse outcomes based on multi-level information in order to highlight pathophysiological pathways for experimental work-up, improve screening efficiency, and identify targets for prevention. Characterize AF phenotypes that may be unique in HFpEF versus HFrEF. |
| 3. Conduct randomized controlled trials comparing the effectiveness in preventing AF of standard and novel HF treatments (e.g., beta-blockers, cardiac resynchronization therapy) in HFrEF and HFpEF patients. |
| Research on symptom burden in AF versus HF |
| 1. Determine if disease-specific, patient-reported outcome measures best determine the impact of AF and AF therapy on quality of life in order to define the best endpoints in AF and HF clinical trials, the most appropriate measures of clinical AF care quality, and the most accurate predictors of AF disease trajectory. |
| 2. Study the effects of AF on cardiovascular function and symptoms in a spectrum of AF patients to determine how to discriminate between symptoms due to occult myocardial dysfunction or comorbidities versus AF. |
| 3. Define clinically-important differences in disease-specific patient-reported outcome measures and their associations with age, sex, and race/ethnicity and the variability in health status across practices determining the proportion of this variability that is due to patient (e.g. socio-demographic, socio-economic, clinical comorbidities and disease severity) and practice characteristics (e.g. treatment). |
Acknowledgment:
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Funding Sources: None.
Disclosures: Dr. Al-Khatib receives consulting fees from Milestone Pharmaceuticals, speaking and consulting fees from Medtronic and speaking fees from Bristol-Myers Squibb/Pfizer.
Dr. Benjamin reports receiving research funding from NIH, NHLBI grants R01HL092577, 1R01HL128914, and American Heart Association grant 18SFRN34110082.
Dr. Albert reports receiving research funding from St. Jude Medical, Abbott, Roche Diagnostics, and NIH and consultancy fees from Roche Diagnostics.
Dr. Chen reports having received research equipment for his laboratory from Medtronic Inc.
Dr. Curtis receives fees for speaking and serving on a data monitoring board from Medtronic; fees for speaking and serving on an advisory board from Abbott; fees for serving on an advisory board from Novartis; fees for serving on an advisory board from Sanofi Aventis and Janssen Pharmaceuticals, and fees for speaking from Milestone Pharmaceuticals.
Dr. Lam is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic, and Vifor Pharma; has served as consultant or on the Advisory Board/ Steering Committee/ Executive Committee for Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic, Vifor Pharma, Novartis, Amgen, Merck, Janssen Research & Development LLC, Menarini, Boehringer Ingelheim, Novo Nordisk, Abbott Diagnostics, Corvia, Stealth BioTherapeutics, JanaCare, Biofourmis, Darma, Applied Therapeutics, MyoKardia, WebMD Global LLC, Radcliffe Group Ltd and Corpus.
Dr. Schnabel reports receiving speaking fees from Bristol-Myers Squibb/Pfizer.
Dr. Spertus receives consulting fees from Bayer, AstraZeneca, Janssen, Amgen, Merck; has equity in Outcomes Instruments; serves on the Scientific Advisory Board for United Healthcare and Board of Directors for Blue Cross Blue Shield of Kansas City. He has copyright to KCCQ, SAQ, PAQ.
Dr. Stevenson is an unpaid consultant to Abbott and Biotronik and unpaid Chair of Data Safety and Monitoring Board for Livanova.
Dr. Voors receives consultancy fees and/or research grants from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Novartis, and Roche Diagnostics.
Dr. Go reports having received a research grant through his institution from iRhythm Technologies.
Drs. Alonso, Desvigne-Nickens, Ho, Link, Patton, Redfield, Rienstra, Rosenberg and Cooper, Ms. True-Hills and Ms. Chauhan report no disclosures.
Non-standard Abbreviations and Acronyms:
- HFrEF
heart failure with reduced ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- OSA
obstructive sleep apnea
- ALLHAT
Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial
- HYVET
Hypertension in the Very Elderly Trial
- NT-pro-BNP
NT-pro-brain natriuretic peptide
- HFmrEF
heart failure with mid-range ejection fraction
- ASSERT
Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., , Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ and Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 2.Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, Wang TJ, Levy D, Benjamin EJ and Ho JE. Atrial Fibrillation Begets Heart Failure and Vice Versa: Temporal Associations and Differences in Preserved Versus Reduced Ejection Fraction. Circulation. 2016;133:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlisle MA, Fudim M, DeVore AD and Piccini JP. Heart Failure and Atrial Fibrillation, Like Fire and Fury. JACC Heart Fail. 2019;7:447–456. [DOI] [PubMed] [Google Scholar]
- 4.Roger VL. Epidemiology of heart failure. Circ Res. 2013;113:646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, Ellinor PT, Go AS, Goldschlager NF, Heckbert SR, Jalife J, Kerr CR, Levy D, Lloyd-Jones DM, Massie BM, Nattel S, Olgin JE, Packer DL, Po SS, Tsang TS, Van Wagoner DR, Waldo AL and Wyse DG. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009;119:606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Khatib SM, Benjamin EJ, Buxton AE, Calkins H, Chung MK, Curtis AB, Desvigne-Nickens P, Jais P, Packer DL, Piccini JP, Rosenberg Y, Russo AM, Wang PJ, Cooper LS, Go AS and Workshop C. Research Needs and Priorities for Catheter Ablation of Atrial Fibrillation: A Report From a National Heart, Lung, and Blood Institute Virtual Workshop. Circulation. 2020;141:482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heart National, Lungm and Blood Institute. Webinar Series on Research Priorities in Atrial Fibrillation to Advance Population, Clinical, and Basic Research: Ablation. March 12, 2019. (https://www.nhlbi.nih.gov/events/2019/webinar-series-research-priorities-atrial-fibrillation-ablation). Accessed January 31, 2020.
- 8.Grogan M, Smith HC, Gersh BJ and Wood DL. Left ventricular dysfunction due to atrial fibrillation in patients initially believed to have idiopathic dilated cardiomyopathy. Am J Cardiol. 1992;69:1570–1573. [DOI] [PubMed] [Google Scholar]
- 9.Triposkiadis F, Pieske B, Butler J, Parissis J, Giamouzis G, Skoularigis J, Brutsaert D and Boudoulas H. Global left atrial failure in heart failure. Eur J Heart Fail. 2016;18:1307–1320. [DOI] [PubMed] [Google Scholar]
- 10.Goldberger JJ, Arora R, Green D, Greenland P, Lee DC, Lloyd-Jones DM, Markl M, Ng J and Shah SJ. Evaluating the Atrial Myopathy Underlying Atrial Fibrillation: Identifying the Arrhythmogenic and Thrombogenic Substrate. Circulation. 2015;132:278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda T, Murai H, Kaneko S, Usui S, Kobayashi D, Nakano M, Ikeda K, Takashima S, Kato T, Okajima M, Furusho H and Takamura M. Augmented single-unit muscle sympathetic nerve activity in heart failure with chronic atrial fibrillation. J Physiol. 2012;590:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maslov PZ, Breskovic T, Brewer DN, Shoemaker JK and Dujic Z. Recruitment pattern of sympathetic muscle neurons during premature ventricular contractions in heart failure patients and controls. Am J Physiol Regul Integr Comp Physiol. 2012;303:R1157–R1164. [DOI] [PubMed] [Google Scholar]
- 13.Smith ML, Hamdan MH, Wasmund SL, Kneip CF, Joglar JA and Page RL. High-frequency ventricular ectopy can increase sympathetic neural activity in humans. Heart Rhythm. 2010;7:497–503. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Bauersachs J and Langer HF. Immune mechanisms in heart failure. Eur J Heart Fail. 2017;19:1379–1389. [DOI] [PubMed] [Google Scholar]
- 15.Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA and Rienstra M. Heart Failure With Preserved Ejection Fraction and Atrial Fibrillation: Vicious Twins. J Am Coll Cardiol. 2016;68:2217–2228. [DOI] [PubMed] [Google Scholar]
- 16.van den Berg MP, Mulder BA, Klaassen SHC, Maass AH, van Veldhuisen DJ, van der Meer P, Nienhuis HLA, Hazenberg BPC and Rienstra M. Heart failure with preserved ejection fraction, atrial fibrillation, and the role of senile amyloidosis. Eur Heart J. 2019;40:1287–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasai T and Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol. 2011;57:119–127. [DOI] [PubMed] [Google Scholar]
- 18.Baguet JP, Barone-Rochette G, Tamisier R, Levy P and Pepin JL. Mechanisms of cardiac dysfunction in obstructive sleep apnea. Nat Rev Cardiol. 2012;9:679–688. [DOI] [PubMed] [Google Scholar]
- 19.Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J and Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103:1763–1768. [DOI] [PubMed] [Google Scholar]
- 20.Somers VK, Dyken ME, Clary MP and Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aggarwal S, Nadeem R, Loomba RS, Nida M and Vieira D. The effects of continuous positive airways pressure therapy on cardiovascular end points in patients with sleep-disordered breathing and heart failure: a meta-analysis of randomized controlled trials. Clin Cardiol. 2014;37:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stavrakis S, Humphrey MB, Scherlag BJ, Hu Y, Jackman WM, Nakagawa H, Lockwood D, Lazzara R and Po SS. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol. 2015;65:867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Popovic ZB, Kusunose K and Mazgalev TN. Therapeutic effects of selective atrioventricular node vagal stimulation in atrial fibrillation and heart failure. J Cardiovasc Electrophysiol. 2013;24:86–91. [DOI] [PubMed] [Google Scholar]
- 24.Yuan Y, Liu X, Wan J, Wong J, Bedwell AA, Persohn SA, Shen C, Fishbein MC, Chen LS, Chen Z, Everett THt, Territo PR and Chen PS. Subcutaneous nerve stimulation for rate control in ambulatory dogs with persistent atrial fibrillation. Heart Rhythm. 2019;16:1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gold MR, Van Veldhuisen DJ, Hauptman PJ, Borggrefe M, Kubo SH, Lieberman RA, Milasinovic G, Berman BJ, Djordjevic S, Neelagaru S, Schwartz PJ, Starling RC and Mann DL. Vagus Nerve Stimulation for the Treatment of Heart Failure: The INOVATE-HF Trial. J Am Coll Cardiol. 2016;68:149–158. [DOI] [PubMed] [Google Scholar]
- 26.Premchand RK, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I, DiCarlo LA, Ardell JL, Rector TS, Amurthur B, KenKnight BH and Anand IS. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial. J Card Fail. 2014;20:808–816. [DOI] [PubMed] [Google Scholar]
- 27.Zannad F, De Ferrari GM, Tuinenburg AE, Wright D, Brugada J, Butter C, Klein H, Stolen C, Meyer S, Stein KM, Ramuzat A, Schubert B, Daum D, Neuzil P, Botman C, Caste MA, D’Onofrio A, Solomon SD, Wold N and Ruble SB. Chronic vagal stimulation for the treatment of low ejection fraction heart failure: results of the neural cardiac therapy for heart failure (NECTAR-HF) randomized controlled trial. Eur Heart J. 2014;36:425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, Chen PS, Chen SA, Chung MK, Nielsen JC, Curtis AB, Davies DW, Day JD, d’Avila A, de Groot N, Di Biase L, Duytschaever M, Edgerton JR, Ellenbogen KA, Ellinor PT, Ernst S, Fenelon G, Gerstenfeld EP, Haines DE, Haissaguerre M, Helm RH, Hylek E, Jackman WM, Jalife J, Kalman JM, Kautzner J, Kottkamp H, Kuck KH, Kumagai K, Lee R, Lewalter T, Lindsay BD, Macle L, Mansour M, Marchlinski FE, Michaud GF, Nakagawa H, Natale A, Nattel S, Okumura K, Packer D, Pokushalov E, Reynolds MR, Sanders P, Scanavacca M, Schilling R, Tondo C, Tsao HM, Verma A, Wilber DJ and Yamane T. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N, Mitchell LB, Flaker GC, Pokushalov E, Romanov A, Bunch TJ, Noelker G, Ardashev A, Revishvili A, Wilber DJ, Cappato R, Kuck KH, Hindricks G, Davies DW, Kowey PR, Naccarelli GV, Reiffel JA, Piccini JP, Silverstein AP, Al-Khalidi HR, Lee KL and Investigators C. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA. 2019;321:1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, Schunkert H, Christ H, Vogt J, Bansch D and Investigators C- A. Catheter Ablation for Atrial Fibrillation with Heart Failure. N Engl J Med. 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 31.Verma A, Kalman JM and Callans DJ. Treatment of Patients With Atrial Fibrillation and Heart Failure With Reduced Ejection Fraction. Circulation. 2017;135:1547–1563. [DOI] [PubMed] [Google Scholar]
- 32.Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan AJA, Voskoboinik A, Sugumar H, Lockwood SM, Stokes MB, Pathik B, Nalliah CJ, Wong GR, Azzopardi SM, Gutman SJ, Lee G, Layland J, Mariani JA, Ling LH, Kalman JM and Kistler PM. Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction: The CAMERA-MRI Study. J Am Coll Cardiol. 2017;70:1949–1961. [DOI] [PubMed] [Google Scholar]
- 33.Zakeri R, Chamberlain AM, Roger VL and Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation. 2013;128:1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D’Agostino RB, Murabito JM, Kannel WB and Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. [DOI] [PubMed] [Google Scholar]
- 35.Piccini JP, Hammill BG, Sinner MF, Hernandez AF, Walkey AJ, Benjamin EJ, Curtis LH and Heckbert SR. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J. 2014;35:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ledwidge M, Gallagher J, Conlon C, Tallon E, O’Connell E, Dawkins I, Watson C, O’Hanlon R, Bermingham M, Patle A, Badabhagni MR, Murtagh G, Voon V, Tilson L, Barry M, McDonald L, Maurer B and McDonald K. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310:66–74. [DOI] [PubMed] [Google Scholar]
- 37.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ and Group HS. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. [DOI] [PubMed] [Google Scholar]
- 38.Davis BR, Kostis JB, Simpson LM, Black HR, Cushman WC, Einhorn PT, Farber MA, Ford CE, Levy D, Massie BM, Nawaz S and Group ACR. Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation. 2008;118:2259–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schnabel RB, Rienstra M, Sullivan LM, Sun JX, Moser CB, Levy D, Pencina MJ, Fontes JD, Magnani JW, McManus DD, Lubitz SA, Tadros TM, Wang TJ, Ellinor PT, Vasan RS and Benjamin EJ. Risk assessment for incident heart failure in individuals with atrial fibrillation. Eur J Heart Fail. 2013;15:843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chatterjee NA, Chae CU, Kim E, Moorthy MV, Conen D, Sandhu RK, Cook NR, Lee IM and Albert CM. Modifiable Risk Factors for Incident Heart Failure in Atrial Fibrillation. JACC Heart Fail. 2017;5:552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, Twomey D, Elliott AD, Kalman JM, Abhayaratna WP, Lau DH and Sanders P. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY). J Am Coll Cardiol. 2015;65:2159–2169. [DOI] [PubMed] [Google Scholar]
- 42.Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G and Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8:295–303. [DOI] [PubMed] [Google Scholar]
- 43.Kotecha D, Chudasama R, Lane DA, Kirchhof P and Lip GY. Atrial fibrillation and heart failure due to reduced versus preserved ejection fraction: A systematic review and meta-analysis of death and adverse outcomes. Int J Cardiol. 2016;203:660–666. [DOI] [PubMed] [Google Scholar]
- 44.Eapen ZJ, Greiner MA, Fonarow GC, Yuan Z, Mills RM, Hernandez AF and Curtis LH. Associations between atrial fibrillation and early outcomes of patients with heart failure and reduced or preserved ejection fraction. Am Heart J. 2014;167:369–375 e2. [DOI] [PubMed] [Google Scholar]
- 45.Sartipy U, Dahlstrom U, Fu M and Lund LH. Atrial Fibrillation in Heart Failure With Preserved, Mid-Range, and Reduced Ejection Fraction. JACC Heart Fail. 2017;5:565–574. [DOI] [PubMed] [Google Scholar]
- 46.Zafrir B, Lund LH, Laroche C, Ruschitzka F, Crespo-Leiro MG, Coats AJS, Anker SD, Filippatos G, Seferovic PM, Maggioni AP, De Mora Martin M, Polonski L, Silva-Cardoso J, Amir O and Investigators E-HHL- TR. Prognostic implications of atrial fibrillation in heart failure with reduced, mid-range, and preserved ejection fraction: a report from 14 964 patients in the European Society of Cardiology Heart Failure Long-Term Registry. Eur Heart J. 2018;39:4277–4284. [DOI] [PubMed] [Google Scholar]
- 47.Goyal P, Almarzooq ZI, Cheung J, Kamel H, Krishnan U, Feldman DN, Horn EM and Kim LK. Atrial fibrillation and heart failure with preserved ejection fraction: Insights on a unique clinical phenotype from a nationally-representative United States cohort. Int J Cardiol. 2018;266:112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rienstra M, Hobbelt AH, Alings M, Tijssen JGP, Smit MD, Brugemann J, Geelhoed B, Tieleman RG, Hillege HL, Tukkie R, Van Veldhuisen DJ, Crijns H, Van Gelder IC and Investigators R. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur Heart J. 2018;39:2987–2996. [DOI] [PubMed] [Google Scholar]
- 49.Khatib R, Joseph P, Briel M, Yusuf S and Healey J. Blockade of the renin-angiotensin-aldosterone system (RAAS) for primary prevention of non-valvular atrial fibrillation: a systematic review and meta analysis of randomized controlled trials. Int J Cardiol. 2013;165:17–24. [DOI] [PubMed] [Google Scholar]
- 50.Li D, Shinagawa K, Pang L, Leung TK, Cardin S, Wang Z and Nattel S. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation. 2001;104:2608–2614. [DOI] [PubMed] [Google Scholar]
- 51.Ducharme A, Swedberg K, Pfeffer MA, Cohen-Solal A, Granger CB, Maggioni AP, Michelson EL, McMurray JJ, Olsson L, Rouleau JL, Young JB and Yusuf S. Prevention of atrial fibrillation in patients with symptomatic chronic heart failure by candesartan in the Candesartan in Heart failure: assessment of Reduction in Mortality and morbidity (CHARM) program. Am Heart J. 2006;151:985–991. [DOI] [PubMed] [Google Scholar]
- 52.Zakeri R, Borlaug BA, McNulty SE, Mohammed SF, Lewis GD, Semigran MJ, Deswal A, LeWinter M, Hernandez AF, Braunwald E and Redfield MM. Impact of atrial fibrillation on exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ Heart Fail. 2014;7:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santema BT, Kloosterman M, Van Gelder IC, Mordi I, Lang CC, Lam CSP, Anker SD, Cleland JG, Dickstein K, Filippatos G, Van der Harst P, Hillege HL, Ter Maaten JM, Metra M, Ng LL, Ponikowski P, Samani NJ, Van Veldhuisen DJ, Zwinderman AH, Zannad F, Damman K, Van der Meer P, Rienstra M and Voors AA. Comparing biomarker profiles of patients with heart failure: atrial fibrillation vs. sinus rhythm and reduced vs. preserved ejection fraction. Eur Heart J. 2018;39:3867–3875. [DOI] [PubMed] [Google Scholar]
- 54.O’Neal WT, Sandesara P, Patel N, Venkatesh S, Samman-Tahhan A, Hammadah M, Kelli HM and Soliman EZ. Echocardiographic predictors of atrial fibrillation in patients with heart failure with preserved ejection fraction. Eur Heart J Cardiovasc Imaging. 2017;18:725–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perino AC, Fan J, Askari M, Heidenreich PA, Keung E, Raitt MH, Piccini JP, Ziegler PD and Turakhia MP. Practice Variation in Anticoagulation Prescription and Outcomes After Device-Detected Atrial Fibrillation. Circulation. 2019;139:2502–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong JA, Conen D, Van Gelder IC, McIntyre WF, Crijns HJ, Wang J, Gold MR, Hohnloser SH, Lau CP, Capucci A, Botto G, Gronefeld G, Israel CW, Connolly SJ and Healey JS. Progression of Device-Detected Subclinical Atrial Fibrillation and the Risk of Heart Failure. J Am Coll Cardiol. 2018;71:2603–2611. [DOI] [PubMed] [Google Scholar]
- 57.U.S. National Library of Medicine. ClinicalTrials.gov Ventricular Tachyarrhythmia Detection by Implantable Loop Recording in Patients With Heart Failure and Preserved Ejection Fraction (VIP-HF). September 25, 2019. https://clinicaltrials.gov/ct2/show/NCT01989299?term=implantable+loop+recorders&cond=heart+failure+with+a+preserved+ejection+fraction&rank=1. Accessed January 31, 2020. [DOI] [PMC free article] [PubMed]
- 58.Aleong RG, Sauer WH, Davis G, Murphy GA, Port JD, Anand IS, Fiuzat M, O’Connor CM, Abraham WT, Liggett SB and Bristow MR. Prevention of atrial fibrillation by bucindolol is dependent on the beta(1)389 Arg/Gly adrenergic receptor polymorphism. JACC Heart Fail. 2013;1:338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tugwell P and Knottnerus JA. Multimorbidity and Comorbidity are now separate MESH headings. J Clin Epidemiol. 2019;105:vi–viii. [DOI] [PubMed] [Google Scholar]
- 60.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr., , Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM and Yancy CW. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 61.Reddy YNV, Obokata M, Gersh BJ and Borlaug BA. High Prevalence of Occult Heart Failure With Preserved Ejection Fraction Among Patients With Atrial Fibrillation and Dyspnea. Circulation. 2018;137:534–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spertus J, Dorian P, Bubien R, Lewis S, Godejohn D, Reynolds MR, Lakkireddy DR, Wimmer AP, Bhandari A and Burk C. Development and validation of the Atrial Fibrillation Effect on QualiTy-of-Life (AFEQT) Questionnaire in patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:15–25. [DOI] [PubMed] [Google Scholar]
- 63.Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS and Cardiovascular Outcomes Research C. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–715. [DOI] [PubMed] [Google Scholar]
- 64.Spertus JA and Jones PG. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Freeman JV, Simon DN, Go AS, Spertus J, Fonarow GC, Gersh BJ, Hylek EM, Kowey PR, Mahaffey KW, Thomas LE, Chang P, Peterson ED, Piccini JP, Outcomes Registry for Better Informed Treatment of Atrial Fibrillation I and Patients. Association Between Atrial Fibrillation Symptoms, Quality of Life, and Patient Outcomes: Results From the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circ Cardiovasc Qual Outcomes. 2015;8:393–402. [DOI] [PubMed] [Google Scholar]
- 66.Holmes DN, Piccini JP, Allen LA, Fonarow GC, Gersh BJ, Kowey PR, O’Brien EC, Reiffel JA, Naccarelli GV, Ezekowitz MD, Chan PS, Singer DE, Spertus JA, Peterson ED and Thomas L. Defining Clinically Important Difference in the Atrial Fibrillation Effect on Quality-of-Life Score. Circ Cardiovasc Qual Outcomes. 2019;12:e005358. [DOI] [PubMed] [Google Scholar]
- 67.Randolph TC, Simon DN, Thomas L, Allen LA, Fonarow GC, Gersh BJ, Kowey PR, Reiffel JA, Naccarelli GV, Chan PS, Spertus JA, Peterson ED, Piccini JP, Investigators OA and Patients. Patient factors associated with quality of life in atrial fibrillation. Am Heart J. 2016;182:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, Daniels MR, Bahnson TD, Poole JE, Rosenberg Y, Lee KL, Packer DL and Investigators C. Effect of Catheter Ablation vs Medical Therapy on Quality of Life Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA. 2019;321:1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steinberg BA, Dorian P, Anstrom KJ, Hess R, Mark DB, Noseworthy PA, Spertus JA and Piccini JP. Patient-Reported Outcomes in Atrial Fibrillation Research: Results of a Clinicaltrials.gov Analysis. JACC Clin Electrophysiol. 2019;5:599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seligman WH, Das-Gupta Z, Jobi-Odeneye AO, Arbelo E, Banerjee A, Bollmann A, Caffrey-Armstrong B, Cehic DA, Corbalan R, Collins M, Dandamudi G, Dorairaj P, Fay M, Van Gelder IC, Goto S, Granger CB, Gyorgy B, Healey JS, Hendriks JM, Hills MT, Hobbs FDR, Huisman MV, Koplan KE, Lane DA, Lewis WR, Lobban T, Steinberg BA, McLeod CJ, Moseley S, Timmis A, Yutao G and Camm AJ. Development of an international standard set of outcome measures for patients with atrial fibrillation: a report of the International Consortium for Health Outcomes Measurement (ICHOM) atrial fibrillation working group. Eur Heart J. 2020; 41:1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chang KW, Hsu JC, Toomu A, Fox S and Maisel AS. Clinical Applications of Biomarkers in Atrial Fibrillation. Am J Med. 2017;130:1351–1357. [DOI] [PubMed] [Google Scholar]
- 72.Richards M, Di Somma S, Mueller C, Nowak R, Peacock WF, Ponikowski P, Mockel M, Hogan C, Wu AH, Clopton P, Filippatos GS, Anand I, Ng L, Daniels LB, Neath SX, Shah K, Christenson R, Hartmann O, Anker SD and Maisel A. Atrial fibrillation impairs the diagnostic performance of cardiac natriuretic peptides in dyspneic patients: results from the BACH Study (Biomarkers in ACute Heart Failure). JACC Heart Fail. 2013;1:192–199. [DOI] [PubMed] [Google Scholar]
- 73.Richards AM. N-Terminal B-type Natriuretic Peptide in Heart Failure. Heart Fail Clin. 2018;14:27–39. [DOI] [PubMed] [Google Scholar]
- 74.Johnson KW, Torres Soto J, Glicksberg BS, Shameer K, Miotto R, Ali M, Ashley E and Dudley JT. Artificial Intelligence in Cardiology. J Am Coll Cardiol. 2018;71:2668–2679. [DOI] [PubMed] [Google Scholar]
- 75.Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ, Carter RE, Yao X, Rabinstein AA, Erickson BJ, Kapa S and Friedman PA. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet. 2019;394:861–867. [DOI] [PubMed] [Google Scholar]
- 76.Turakhia MP, Desai M, Hedlin H, Rajmane A, Talati N, Ferris T, Desai S, Nag D, Patel M, Kowey P, Rumsfeld JS, Russo AM, Hills MT, Granger CB, Mahaffey KW and Perez MV. Rationale and design of a large-scale, app-based study to identify cardiac arrhythmias using a smartwatch: The Apple Heart Study. Am Heart J. 2019;207:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]


