Abstract
The fundamental defect(s) that drives atopic dermatitis (AD) remains controversial. “Outside in” proponents point to the important association of filaggrin gene mutations and other skin barrier defects with AD. The “inside out” proponents derive support from evidence that AD occurs in genetic animal models with overexpression of Type 2 immune pathways in their skin, and humans with gain-of-function mutations in their Type 2 response develop severe AD. The observation that therapeutic biologics, targeting Type 2 immune responses, can reverse AD provides compelling support for the importance of “inside out” mechanisms of AD. In this review, we propose a central role for epithelial cell dysfunction that accounts for the dual role of skin barrier defects and immune pathway activation in AD. The complexity of AD has its roots in the dysfunction of the epithelial barrier that allows the penetration of allergens, irritants, and microbes into a cutaneous milieu which facilitates the induction of Type 2 immune responses. The AD phenotypes and endotypes that result in chronic skin inflammation and barrier dysfunction are modified by genes, innate/adaptive immune responses, and different environmental factors that cause skin barrier dysfunction. There is also compelling evidence that skin barrier dysfunction can alter the course of childhood asthma, food allergy, and allergic rhinosinusitis. Effective management of AD requires a multi-pronged approach, not only restoring cutaneous barrier function, microbial flora, and immune homeostasis, but also enhancement of skin epithelial differentiation.
Keywords: atopic dermatitis, food allergy, skin barrier, peanut allergy, epithelial barrier
INTRODUCTION
Allergic diseases such as atopic dermatitis (AD), food allergy (FA), and asthma affect more than 30% of the general population (1–3). These diseases have significant health and socio-economic effects. Allergic diseases are well documented to be associated with epithelial barrier dysfunction, allowing tissue penetration of allergens, irritants, and microbes. These then lead to the release of epithelial cytokines such as thymic stromal lymphopoietin (TSLP) and IL-33, which play a pivotal role in driving Type 2 immune and inflammatory responses (4,5). Although cytokines such as interferon gamma, IL-17, and IL-22 can modify the course of allergic responses, Type 2 cytokines such as IL-4, IL-13, TSLP, and IL-33 play a central role in the development of allergic diseases (6,7). AD, in particular, is the most atopic of all allergic diseases as environmental antigens and foods, processed in the skin elicit an IgE response. Childhood onset of AD is often associated with FA, asthma, and allergic rhinitis, i.e. the atopic march (8). The skin barrier dysfunction in AD is thought to play a key role in the atopic march. It starts with AD, followed by epicutaneous allergen sensitization and FA. The link from AD to respiratory allergy is more controversial; however, progression of the atopic march is facilitated in patients who develop IgE to foods and inhalant allergens (9), and is strongly associated with filaggrin loss-of-function gene mutations. Childhood asthma onset-specific gene loci have identified the skin as an important target tissue (10). Severity, age of onset, and duration of AD are risk factors for the atopic march (11,12).
In this review, we will discuss the role of cutaneous barrier dysfunction in the pathogenesis of AD, particularly the relationship of AD with FA. The relative importance of these individual abnormalities can be teased out using animal models and therapeutic interventions; however, in most patients, the progression of AD requires both the skin barrier defect and immune pathway activation. For a discussion of the contributions of an excessive Type 2 innate and acquired immune response in AD, the reader is referred to a recent review by Honda and Kabashima (13). We propose that the dualistic role of skin barrier and immune abnormalities has its roots in the lack of terminal keratinocyte differentiation in the skin and propose that durable therapeutic approaches require combination therapy to target both the defective skin barrier and Type 2 immune activation in AD. This has important clinical implications for new approaches to controlling AD and progression of the atopic march.
CUTANEOUS BARRIER DYSFUNCTION IN AD
Skin barrier dysfunction is the hallmark of AD. A strong skin barrier in healthy individuals is needed to repel the invasion of microbes, allergens, and irritants from the environment, thereby preventing engagement of the Type 2 enhancing immune pathway that occurs in the skin. In a birth cohort study, increased transepidermal water loss (TEWL) at day 2 of life, occurred one year prior to the onset of AD and FA (14, 15). Since increased TEWL is a biomarker of skin barrier dysfunction, this study suggests early intervention is required to prevent epicutaneous allergen sensitization. Increased TSLP has also been found in the skin prior to onset of AD suggesting that prevention of the atopic march also requires intervention with Type 2 pathways (16).
Filaggrin deficiency as the paradigm for skin barrier dysfunction (Figure 1)
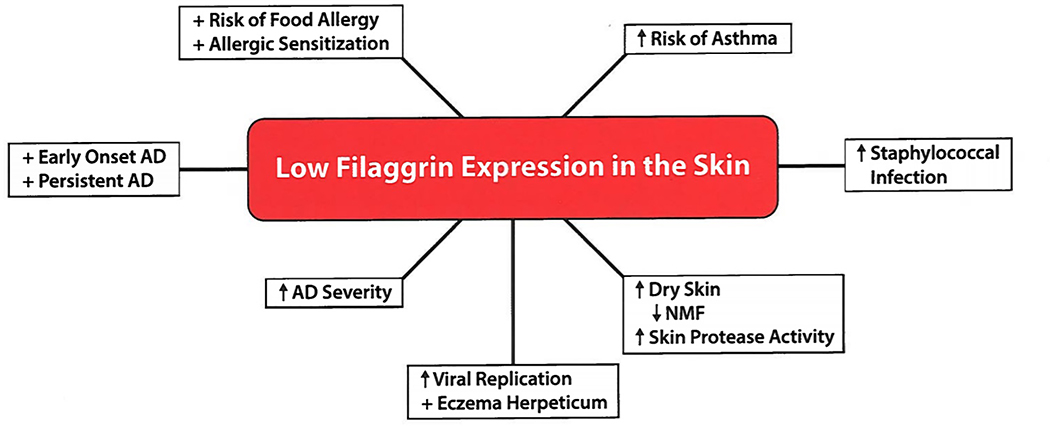
Figure 1.
Consequences of Filaggrin Deficiency on Atopic Dermatitis and Modifying the Course of Allergic Diseases.
Loss-of-function filaggrin (FLG) gene mutations are the strongest known genetic risk factor for AD (17). The presence of FLG mutations increases AD risk more than three-fold compared to the general population. It is also a disease modifier predisposing to earlier AD onset, persistence of AD, and increased disease severity. There are ethnic differences in the types of FLG mutations found in AD. FLG mutations are particularly common in northern Europeans with R501X and 2282del4 as the major FLG mutations. In Asian populations, FLG P478S and C3321delA variants, not commonly found in European populations, are associated with increased risk of AD (18). In African American children, loss-of-function mutations in FLG2 are associated with increased AD risk. Several novel FLG gene mutations, not commonly seen in European Americans, were recently described in African American AD.
Mouse models have demonstrated that FLG gene mutations are associated with enhanced percutaneous microbial and allergen penetration and reduced inflammatory thresholds to irritants and haptens (19,20). Knockdown of FLG expression has been shown to impair keratinocyte differentiation of human keratinocyte organotypic cultures (21). Epithelial damage leads to innate immune activation, including release of pro-inflammatory cytokines and chemokines by keratinocytes (22), and enhanced antigen presentation by Langerhans cells and dermal dendritic cells (23). Reduced levels of acidic filaggrin breakdown products raise skin pH and activate skin proteases (24–26), thereby contributing to skin barrier dysfunction. Protease activated receptor activation has also been shown to induce the pro-Th2 cytokine, TSLP (27).
Beyond FLG null mutations, a number of additional factors play an important role in the regulation of filaggrin expression and the skin barrier in general. For example, Type 2-mediated cutaneous inflammation can result in reduced filaggrin expression in AD skin, even in subjects without FLG mutations (28). The combination of Type 2 cytokines with heterozygote FLG gene mutations can profoundly reduce FLG to almost undetectable levels. Reduced FLG intragenic copy number is an independent risk factor of AD (29). DNA methylation of the CpG site in FLG gene region has also been reported to significantly increase AD risk (30). The FLG gene is only one of approximately 45 genes within the epidermal differentiation complex (EDC) on chromosome 1q21. Many of these genes, including involucrin (IVL) and loricrin (LOR), may also contribute to AD cutaneous barrier dysfunction. The levels of hornerin and other filaggrin-like proteins, including FLG2, are also decreased in the skin of AD patients (31,32), but filaggrin deficiency appears to have the greatest impact on skin barrier structure and function in AD. IL-22 has also been shown to be overexpressed in the skin of severe AD, and it inhibits filaggrin skin expression (33); however, in contrast to Type 2 cytokines, it does not inhibit keratinocyte antimicrobial peptide production (34). Other proinflammatory cytokines, like TNFa (35), IL-25, or IL-33 (36), have also been found to inhibit filaggrin expression.
Failure of terminal keratinocyte differentiation in AD skin (Figure 2)
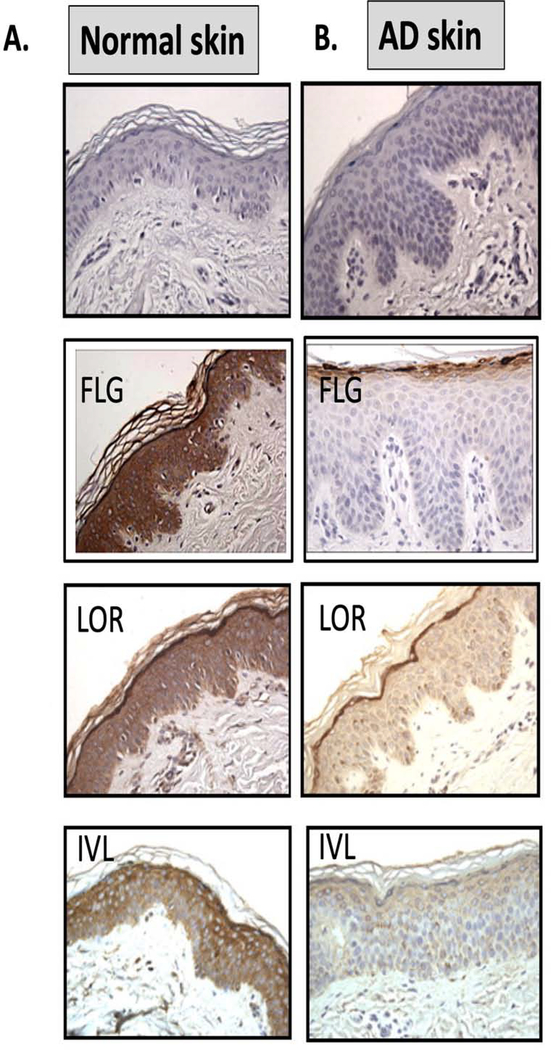
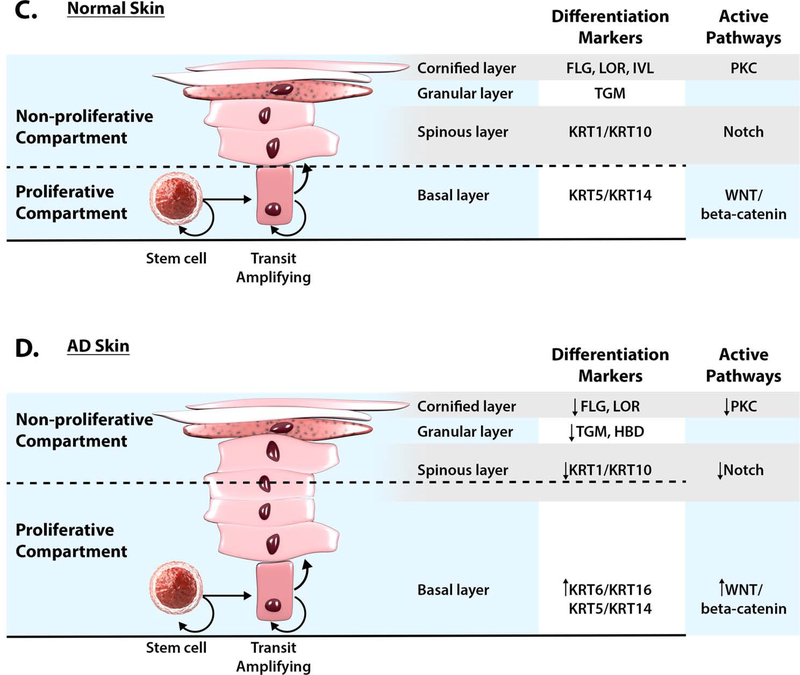
Figure 2. Lack of Keratinocyte Terminal Differentiation in Atopic Dermatitis.
Abnormalities in keratinocyte differentiation in AD skin result in hyperplasia of the basal layer, reduction of spinous layer and inhibition of markers of terminal differentiation (FLG, IVL, LOR) in AD (B) as compared to NA skin (A) (reproduction with permission from reference 4 and reference 134). Schematic of epidermal differentiation pattern in normal (C) and AD skin (D) is shown. The keratinocyte differentiation process is an integrated multi-step program of sequential changes in gene expression and cell structure, as the cells migrate from the proliferative basal layer, through spinous and granular layers, into the cornified layer, which functions as a skin barrier. Cells proliferate in the basal layer of epidermis. In the spinous (suprabasal) layer, cells irreversibly exit the cell cycle and switch from KRT5/KRT14 to KRT1/KRT10 production. Wnt/beta-catenin pathway is active in the proliferating epidermis, while keratinocyte differentiation in the spinous layer is under control of the Notch pathway. Deficient Notch activity alters epidermal differentiation in AD skin, expanding the proliferative compartment and influencing subsequent changes in epidermal differentiation program. Changes in extracellular Ca2+ and lipid metabolism trigger the protein kinase C (PKC) pathway activation and regulates transcription of late differentiation markers in granular layer, FLG, LOR, IVL, HBD and TGM1. Inhibition of terminal differention marker expression is observed in AD skin.
Permissions obtained from reference 28 and reference 134 to publish edited portions of images.
AD epidermis is characterized by broad defects in terminal keratinocyte differentiation (37). This allows enhanced allergen penetration through the epidermis and systemic IgE sensitization. There is an expansion of cells in the stratum basale (SB) layer, with a concomitant reduction in cells of the stratum spinosum and stratum granulosum layers (38). Hyperproliferating epithelium is associated with overexpression of KRT6/KRT16 (38). Consistent with a block in terminal keratinocyte differentiation, AD skin has reduced expression of mature skin barrier proteins including filaggrin, involucrin, Loricrin, antimicrobial peptides, and beta defensins (28,35,40).
Tight junctions in the granular layer form an additional component of the skin barrier, limiting penetration of allergens and pathogenic microbes, facilitating paracellular passage of soluble mediators, and regulating TEWL. Tight junctions are composed of transmembrane proteins such as claudin-1 (CLDN1), which are essential for skin barrier function and control of TEWL regulation (41). CLDN1 is reduced in the skin of AD patients (42). Polymorphisms in the CLDN1 gene have been found in AD, particularly those with a history of eczema herpeticum (43). Knockdown of CLDN1 expression in keratinocytes enhances HSV-1 infectivity.
S100A7, S100A8, and S100S9 proteins are upregulated in AD skin (44, 45). These proteins act as amplifiers of the immune response. As an example, S100A9 activated keratinocytes causes a selective increase in IL-33 production. Th2 cytokines inhibit S100A11 protein expression, which is required for the regulation of skin barrier integrity (46).
Disruption of Keratinocyte Differentiation in AD skin
Evolving research suggests that keratinocyte differentiation is disrupted in AD with hyperproliferation of stem cells in the basal layer (Figure 3). Cutaneous Notch signaling plays a key role in the promotion and maintenance of the keratinocyte differentiated state (47,48), while Wnt activity is essential for stem cell maintenance and supports cell proliferation (49). Inhibition of Notch receptor expression has been shown in AD skin (50,51), while in healthy control patients significant Notch expression was observed in the suprabasal epidermal layers (50). Reduced Notch expression appears to be a selective feature of AD skin, as increased epidermal Notch expression has been found in other inflammatory skin diseases such as psoriasis and lichen planus (52,50).
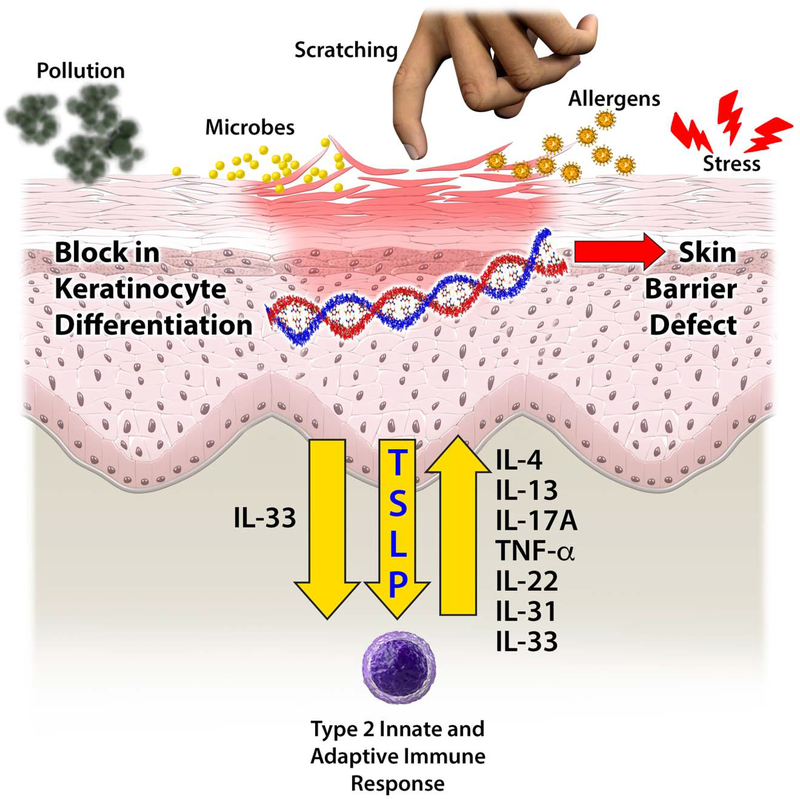
Figure 3. Causes of Cutaneous Barrier Dysfunction in AD and Allergic Diseases.
This includes genetic mutations, environmental influences (including microbes, scratching allergens) and the immune response (including IL4, IL13, IL17A, TNF-α, IL22, TSLP, IL31, IL33).
Dry skin in AD with increased TEWL has been linked to increased aquaporin 3 (AQP3) expression (53,54). Increased AQP3 expression has been found in the stratum basale and stratum spinosum of patients with AD (54). Interestingly, AQP3 has been identified as a transcriptional target of Notch1. Inhibition of Notch signaling increased the expression of AQP3 (55). Thus, decreased Notch signaling in AD may increase AQP3-mediated TEWL leading to dry skin.
Epidermal Notch deficiency in mice induces AD-like skin pathology, with dry skin, acanthosis, spongiosis, hyperkeratosis, and massive dermal infiltration of eosinophils and mast cells (50,56,51,57). Notably, epidermal Notch deficiency is associated with a significant production of TSLP by keratinocytes, with increased numbers of DCs, enhanced expression of IL-4 and IL-13, and increased serum IgE levels (50,56,51,57). Notch deficient murine keratinocytes develop pronounced defects of epidermal barrier integrity and cornified envelope formation (58,56). Keratinocyte-derived TSLP has recently been shown to stimulate cutaneous sensory neurons to promote itch (59). Thus, there may be a direct link between epidermal Notch deficiency and TSLP-induced pruritus in AD.
We propose that Notch deficiency in AD skin is fundamental to the inhibition of epidermal differentiation and skin barrier deficiency. At the same time, Notch insufficiency in the epidermis results in keratinocyte hyperplasia, as cells in the basal layer expand, while maintaining their proliferative, undifferentiated state, thus, reprogramming epidermal differentiation in AD skin. Notch activation is known to inhibit the Wnt pathway (60,61). Reduction in signal strength induced by loss of Notch1 in epithelial cells augments Wnt signaling, and induces a permissive environment for the outgrowth of proliferating cells in murine models (62,63). Evidence for Wnt/beta-catenin pathway activation has been shown in allergic rhinosinusitis (64,65) and psoriasis (66,67), supporting epidermal hyperplasia. Lastly, as stated above, Notch deficiency supports type 2 inflammation.
At the same time, skin scratching, environmental insults and bacterial colonization in AD promote release of alarmins (IL-1alpha, IL-33, IL-18, IL-36), that also promote epidermal hyperplasia and establishment of type 2 inflammation in the skin (68,69). It is noteworthy that epidermal hyperplasia would be expected to prevent cells from terminal differentiation. Of interest, IL-1 family member cytokines have been shown to activate Wnt expression in keratinocytes (66) thus adding to the complexity of inflammatory events in AD.
Microbial Dysbiosis reflects Cutaneous Barrier Dysfunction in AD
AD skin is predisposed to colonization or infection by pathogenic microbes, most notably Staphylococcus aureus (70,71). Work in our laboratory and others have demonstrated that IL-4 and IL-13 promote S. aureus invasion/colonization (40,72–74) due to inhibition of epidermal barrier function (75), induction of S. aureus skin binding sites (e.g. fibronectin), and decreased antimicrobial peptide production in AD skin (40,76,77). The pivotal role of IL-4 and IL-13 in causing S. aureus colonization was recently demonstrated by a report that dupilumab, a therapeutic monoclonal antibody against IL-4 receptor alpha, an antagonist of IL-4 and IL-13 signaling, caused greater reduction in S. aureus colonization than placebo in AD patients (78). Superantigen-producing S. aureus skin colonization has been shown to be potent activators of IL-4, IL-13 and IL-22 production in AD (79), supporting the role of S. aureus in inducing and maintaining AD skin inflammation.
Longitudinal studies have demonstrated that S. aureus colonization emerges during the exacerbation of AD (80,81). Recent studies support a bidirectional dialogue between skin bacteria and host keratinocytes, with commensal microbiota educating host immune responses and, conversely, atopic immune signaling shaping microbial dysbiosis (80,82).
Next-generation sequencing of bacterial DNA collected from AD skin has documented increased S. aureus colonization and decreased bacterial diversity (81,83). Specific S. aureus strains have been associated with AD severity (84). S. aureus clones identified in severe AD patients were enriched for the expression of virulence factors. Murine skin colonization models have demonstrated S. aureus strain-specific differences in elicitation of skin inflammation and immune signatures characteristic of AD patients. Specifically, S. aureus isolates from AD patients with more severe flares induced epidermal thickening and expansion of cutaneous Th2 and Th17 cells, suggesting that functional differences of staphylococcal strains may contribute to the complexity of AD disease (84).
Normal skin microbiota play an important role in the development of innate barrier immunity (85–87), limit of pathogen invasion (88), and control of T regulatory cell function (89). Birth cohort studies have shown that the presence of coagulase-negative Staphylococcus spp. at two months of life might protect infants against later development of AD (90). Early-life skin colonization with S. aureus may also contribute to AD onset in infancy (91). Several virulence factors (including lytic toxins, enterotoxins, proteases, etc.) produced by S. aureus contribute to AD by acting on keratinocytes (cell lysis, proinflammatory cytokine production, inhibition of keratinocyte differentiation program) and immune activation (T cell clonal expansion, production of proinflammatory cytokines) (75,92,93). S. aureus exploits epidermal barrier defects in AD to trigger cytokine expression (94). Activation of serine proteases is essential for S. aureus penetration into the skin (95). Importantly, antimicrobial function of commensal microflora is critical for controlling S. aureus colonization (88).
Cytokine Role in Barrier Dysfunction
Overproduction of IL-4/IL-13 creates a permissive environment for S. aureus growth and attachment to AD skin (96). In addition, AD skin has reduced filaggrin expression, accompanied by reduced levels of FLG breakdown products (PCA, UCA) on the skin. Acidification of the skin by filaggrin breakdown products has been shown to reduce expression of S. aureus secreted and cell wall-associated proteins, including proteins involved in colonization (clumping factor B, fibronectin binding protein A) and immune evasion (protein A) (97). In addition, filaggrin breakdown products inhibit the expression of iron-regulated surface determinant A by S. aureus. In contrast, reduced levels of filaggrin breakdown products support S. aureus colonization. Th2 cytokines can also enhance the effects of staphylococcal products. For instance, keratinocytes in AD, as compared to normal skin, have increased sensitivity to alpha-toxin, a cytolytic toxin produced by S. aureus. Differentiated keratinocytes are protected from cell death, whereas cells treated with IL-4/IL-13 have increased sensitivity to alpha toxin-induced lethality (72). The combination of IL-4/IL-13 induce biochemical changes that decrease levels of acid SMase, an enzyme that cleaves an alpha toxin ligand called sphingomyelin (75). SMase and its enzymatic product, phosphocholine, prevent IL-4/IL-13-mediated increases in alpha toxin-induced cell death (75).
We recently demonstrated an interplay between S. aureus cell wall component lipoteichoic acid (LTA) and IL-4/IL-13 in the inhibition of wound healing in AD skin (98). We found that keratinocytes are highly responsive to LTA (with change in expression of genes involved in regulation of epidermal development), wound responses, keratinocyte proliferation, regulation of cell differentiation, and Notch signaling pathways (99). We have reported that staphylococcal LTA inhibits the expression of the early keratinocyte differentiation markers, keratins (KRT1, KRT10), desmocollin (DSC1), and desmoglein (DSG1), that are essential for skin barrier function and have determined that these events are p63 dependent. Interestingly S. aureus colonization has also been associated with onset of food allergy (100). This may reflect the detrimental effect of S. aureus on the skin barrier and enhanced epicutaneous food allergy sensitization.
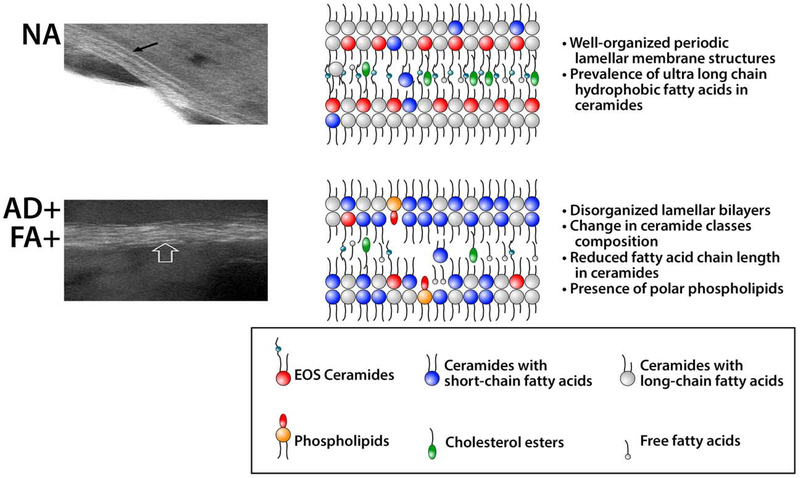
Lipid Abnormalities (Figure 4)
Figure 4. Lipid Barrier Abnormalities in AD Skin.
The intercellular lipids (the “mortar”) are an integral component of the stratum corneum skin barrier. They consist of a heterogeneous mixture of ceramides, free fatty acids, and cholesterol. These lipids are produced in the stratum granulosum and stored in lamellar bodies, and then secreted into extracellular space in the transition to the stratum corneum. In healthy skin nonatopic (NA), lipid lamellae are well organized; EOS Ceramides and ceramides with ultra long-chain fatty acids form a crystalline structure. In AD skin, the increase in ceramides with short-chain fatty acids as well as the presence of polar lipids disrupt lamella structure. Gaps in lipid lamellae structures of AD skin may support penetration of allergens and water loss through skin barrier. Electron microscopy photographs of NA and AD skin are reproduced from previously published work (120).
Permission obtained from reference 120 to publish edited portions of images.
Dr. Christian Cole and colleagues stratified the analysis of AD skin transcriptome based on FLG gene mutations and found that AD with normal FLG genotype have aberrant changes in the expression of enzymes involved in the metabolism and synthesis of lipids (101). These observations suggest the importance of lipid metabolism in AD independent of FLG genotype. Extracellular lipids account for almost 10% of the stratum corneum (SC) structural mass (102–105). Skin lipids can also have anti-inflammatory and antimicrobial properties (106). Free fatty acids and sphingoid bases have documented antimicrobial activity, including activity against S. aureus (107,108). Several free fatty acids serve as natural agonists for peroxisome proliferator-activated receptor transcriptional factors that are essential for the regulation of lipid metabolism enzymes in the skin (109).
Several research groups have demonstrated reduction in skin ceramide levels, in particular in esterified ω-hydroxy fatty acid (EO) sphingosine (S) ceramides (EOS-Ceramides), in AD skin in parallel with a decline in free fatty acid chain length (110,111). These changes in skin lipid composition resulted in abnormal lipid organization and increased TEWL in AD skin. Notably, changes in ceramide levels and free fatty acids chain length distribution did not correlate with FLG genotype, but correlated with AD severity and levels of FLG breakdown products.
Concurrent reduction in ceramide and free fatty acid chain length in the SC of AD patients suggested alterations in the common elongase synthetic pathway for ceramides and free fatty acids. We investigated whether the hyperactivated Type 2 immune response altered AD skin lipid metabolism. To address this question, we analyzed SC lipids from AD subjects and IL-13 skin-specific transgenic mice (112). Mass spectrometric analysis of lesional SC from AD subjects and IL-13 transgenic mice revealed an increased proportion of short-chain (N-14:0–24:0) non-hydroxy fatty acid sphingosine ceramides (NS-ceramides) and 14:0–22:0-lysophosphatidylcholines (LPC) with a simultaneous decline in the proportion of corresponding long-chain species (N-26:0–32:0 sphingolipids and 24:0–30:0-LPC) when compared to healthy controls. An increase in short-chain LPC species was also observed in non-lesional AD skin. Similar changes were observed in IL-4/IL-13-driven responses in Ca2+-differentiated human keratinocytes in vitro, all being blocked by STAT6 silencing with siRNA, a master transcriptional factor regulator of IL-4/IL-13 signaling. RNA sequencing analysis performed on SC of AD as compared to healthy subjects identified decreased expression of the fatty acid elongases, ELOVL3 and ELOVL6, that accounted for the observed changes in AD skin lipids. IL-4/IL-13 also inhibited ELOVL3 and ELOVL6 expression in keratinocyte cultures in a STAT6-dependent manner. Downregulation of ELOVL3/ELOVL6 expression in keratinocytes by siRNA decreased the proportion of long-chain fatty acids globally and in sphingolipids. Thus, our data strongly support the pathogenic role of Type 2 immune activation in AD skin lipid expression (112).
Environmental Factors
Environmental or climatic factors can also adversely impact skin barrier integrity, thereby contributing to AD risk and severity. It has also been documented that mechanical damage, including repetitive scratching, use of detergents, humidity, exogenous proteases, and air pollution, have a negative impact on FLG expression (113).
Cross-talk between the skin and gut has recently been demonstrated in a mouse model of AD and FA. In these experiments, tape stripping mouse skin, used as a surrogate for scratching or mechanical injury, caused expansion and activation of small intestinal mast cells (MCs), increased intestinal permeability, and promoted food anaphylaxis in mouse models (114). The mechanism involved release of IL-33 systemically from keratinocytes after skin tape stripping. IL33 acted on intestinal tuft cells to secrete IL-25 to drive the expansion and activation of intestinal innate lymphoid cells (ILC2s), which provided IL-4 and IL-13 that targeted MCs to expand in the intestine. Mice with skin induced MC expansion had increased IgE-mediated food anaphylaxis in this study. Duodenal mast cells were also increased in biopsies from patients with AD and FA. These studies suggest that, in addition to promoting cutaneous sensitization to food antigens, scratching may play an important role in IgE-mediated food anaphylaxis in AD by expanding and activating intestinal MCs.
CAN EPITHELIAL PROFILING BE USED TO DECIPHER AD PHENOTYPES?
The development of precision medicine approaches to AD require us to develop noninvasive methods to better understand mechanisms underlying the various clinical phenotypes of AD (115). Although AD is often referred to with standard clinical definitions which include pruritus and certain patterns of skin inflammation, this “one-size-fits-all” approach fails to accommodate the complexity of this skin disease. AD patients often present with characteristic phenotypes that transcend local patterns of skin inflammation. These include associations with food allergy and the atopic march, propensity to S. aureus colonization or infection, disseminated viral infection (e.g. eczema herpeticum), different ages of AD onset (e.g. early vs late onset of AD), non-responders to therapy (including corticosteroid insensitivity or dupilumab non-responders), and AD severity (mild vs severe courses of illness). Our current fund of knowledge falls short of accounting for these various AD phenotypes, but evolving advances in technology facilitated by a multi-omics approach are expected to provide new insights that explain these various phenotypes and endotypes.
The strong association of FLG null mutations with AD, FA, and childhood associated asthma suggests skin barrier dysfunction contributes to the atopic march (8). Clinically, this is supported by reports that risk factors for the development of peanut allergy in children include the epicutaneous application of peanut-containing creams or oils, skin infection, and severity of AD (116). Importantly, several clinical studies support the concept that children become allergic to peanut through environmental exposure (117,118). Furthermore, household peanut consumption was reported to highly correlate with peanut levels in house dust (118).
Only one third of AD patients, however, develop FA (119). As part of a NIH/NIAID Atopic Dermatitis Research Network (ADRN) protocol, we used a minimally invasive skin tape strip (STS) measure of the SC in combination with a comprehensive multi-omics approach to determine whether the non-lesional skin of AD with FA (AD+FA+) children have superficial skin biomarkers which distinguish them from AD without FA (AD+FA-) and non-atopic (NA) children (120). Despite similar skin disease severity, the SC integrity and filaggrin content were significantly lower in children who were AD+FA+ as compared to AD+FA-. Lipid profiling of the SC in the AD+FA+ group revealed a relative reduction in the ultra long chained ceramides, esterified ω-hydroxy fatty acid (EO) sphingosine (S) ceramides (CER) required for normal skin barrier function. At the same time, a significant increase in NS CER levels was observed in AD+FA+ skin samples, resulting in a disproportionate decrease in EOS CER in the skin of these patients. Shotgun metagenomic studies revealed that the skin of AD+FA+ children was colonized with an overabundance of S. aureus. Interestingly, STS proteomics revealed an immature keratin profile consistent with keratinocyte hyperproliferation in the SC of AD+FA+ participants. A network analysis demonstrated keratin 5, 14 and 16 expression, and reduced filaggrin breakdown products, were strongly correlated with AD+FA+. Along with increased TEWL, these were the most important predictors of AD+FA+.
Consistent with the skin barrier dysfunction in non-lesional skin, AD+FA+ individuals had high dendritic cell and Type 2 immune activation profiles in their skin tape transcriptome. Interestingly, transcripts for the Type 2 cytokines IL13, CCL17, and CCL22 were elevated in both AD+FA+ and AD+FA- compared to NA. However, transcripts for Type 2 immune receptors, i.e. IL4R, CCR8, and CRLF2 (TSLP receptor), were higher in AD+FA+ as compared to AD+FA- or NA. These data suggest that the nonlesional skin of AD+FA+ children exhibit a unique constellation of skin biomarkers that distinguish them from AD+FA- and NA children. These data support the importance of skin barrier dysfunction in the pathogenesis of epicutaneous sensitization to environmental foods and may contribute to the persistence or severity of FA by chronically stimulating Type 2 immune responses in the skin.
CLINICAL IMPLICATIONS
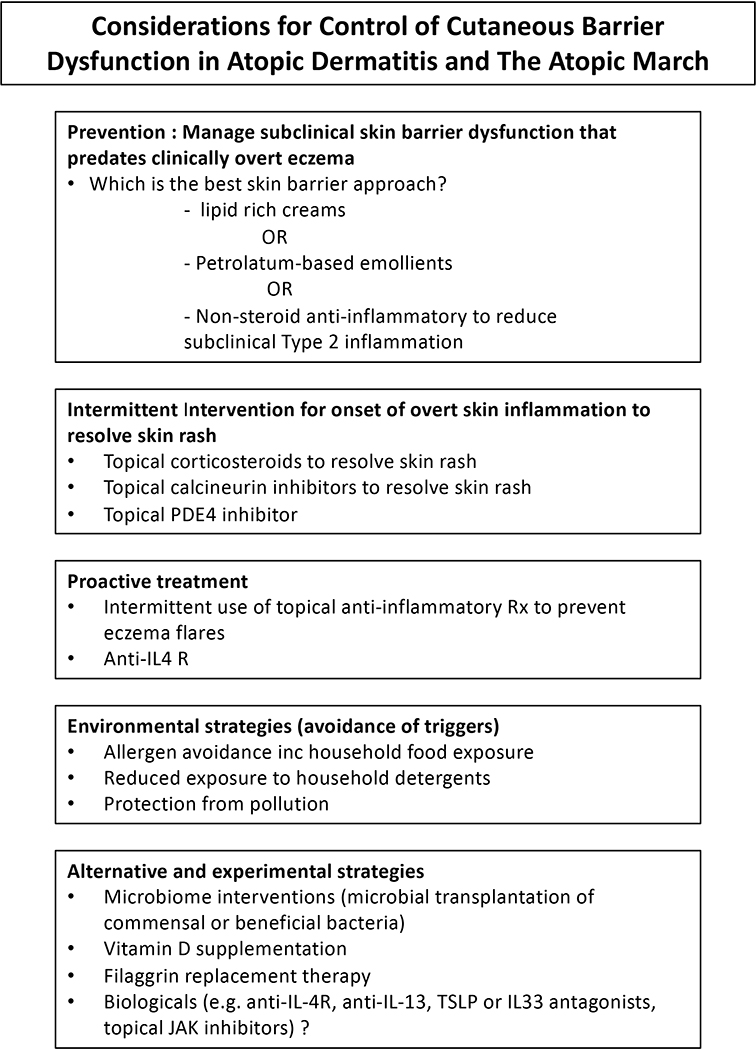
The finding that cutaneous barrier dysfunction occurs in the non-lesional skin of children with AD, FA, and possibly childhood asthma, has important clinical implications (121). This concept suggests the need to institute early skin care even before the onset of clinical eczema and the potential importance of doing total body skin care beyond the inflammatory skin rash observed in AD (122). A multipronged approach for the prevention and management of atopic infants and children should be considered and are summarized in Figure 5.
Figure 5.
Management Strategies for the Prevention and Treatment of AD and The Atopic March
Prevention
Several, but not all, birth cohort studies have reported that the use of emollients to improve the skin barrier can prevent occurrence of eczema (123–125). In the successful studies, skin emollients reduced the occurrence of AD by approximately 50%. The reason for lack of uniform success after application of skin emollients requires further study, but it is important to note that not all emollients have the same effect on the skin and it has been proposed a skin emollient that reflects the lipid composition of the skin is needed in order to obtain maximum benefits (126). Other factors should also be considered, including time of introduction and adherence to therapy.
Proactive Therapy
The concomitant often subclinical allergic inflammation that accompanies atopic skin can also reduce skin barrier function and may drive IgE responses to occur after foods have penetrated the skin. Duration and severity of eczema is associated with occurrence of FA (116). Therefore, prevention of FA may require proactive skin barrier and anti-inflammatory therapy to reduce Type 2 immune responses to epicutaneous allergen sensitization. In this regard, a recent report found that early aggressive treatment with moisteurizer and topical corticosteroids shortened the duration of eczema in infants and resulted in fewer food allergies at 2 years of age (127). Antagonism of Type 2 cytokines such as IL-4, IL-13, TSLP, or IL-33 may enhance epithelial function and reduce allergen sensitization in patients with established AD (128).
Environmental Control
Since low humidity, skin irritants (including detergents, pollutants, and hard water), and environmental allergens (like dust mite derived serine proteases) are known to induce eczema, avoidance of these environmental factors may prevent cutaneous barrier dysfunction. Studies report that household consumption of food allergens and exposure to environmental foods in household dust result in potential epicutaneous sensitization (129,130). The information from these studies lead to questions about how to change or control these environmental factors, such as if household members should restrict their food ingestion around food allergic children or if a skin barrier cream should be used to prevent epicutaneous sensitization. Exploring these questions will lead to understanding what methods work best and what variety of methods may be available to patients and their families.
Microbiome
S. aureus is known to breakdown the skin barrier and inhibit T regulatory cell activity while S. aureus colonization has been found to predate or occur during the development of AD and FA (70,71). Recent studies suggest S. aureus colonization is increased on AD skin due to loss of commensal bacteria that produce antimicrobial peptides (88). Studies in the Atopic Dermatitis Research Network are examining whether targeted transplantation of S. hominis can reduce S. aureus colonization in AD (NCT03151148). An additional clinical study is evaluating whether topical application of the Gram-negative coccobacillus, Roseomonas mucosa, can be used to treat AD (NCT03151148). Another approach is to determine whether swabbing neonates born by caesarian section with mother’s vaginal microbiota will reduce allergen sensitization (NCT03567707).
Alternative Considerations
Vitamin D is critical in regulating skin development, including formation of filaggrin, lipid lamellae formation, and induction of innate immune responses (131). Therefore maintaining the normal vitamin D levels is essential for the generation and maintenance of the skin barrier. Importantly, direct sunlight has been found to be even more beneficial in reducing the incidence of infant eczema than vitamin D supplementation (132). Other treatments that are under development include experimental filaggrin replacement therapies, drugs that skip over loss of function FLG mutations, and topical FLG monomers (133). A comprehensive summary of the approved therapeutics and pharmacological therapies in development for the treatment of AD was provided in a recent review (6).
CONCLUSIONS
The clinical manifestations and skin pathology in AD are driven by cutaneous barrier dysfunction and an overly active Type 2 immune response which is exaggerated by skin injury from environmental factors including scratching, S. aureus colonization, allergen penetration, stress, irritation from pollution, detergents, hard water, etc. Causes of this abnormal skin barrier are complex and driven by a combination of genetic, environmental, and immunologic factors which affect gene expression, structural proteins, and lipid profiles. These factors likely account for the heterogeneity of AD onset, severity, and natural history of this skin disease. FLG gene mutations are the most profound single-gene defects identified in AD, but the most cause common of filaggrin deficiency stems from skin immune activation and environmental factors. Filaggrin deficiency promotes skin inflammation and inflammatory cell infiltration due to increased percutaneous transfer of antigens and chemical haptens, alteration of skin acidification. This in turn supports the activation of skin proteases which further inhibits skin barrier homeostasis and also supports the onset of Type 2 inflammatory response through TSLP and IL-33 activation.
Skin-gut interactions have now been demonstrated and have their origins in skin injury induced release of IL33 from keratinocytes leading to intestinal mast cell hyperplasia (114). Importantly, systemic release of TSLP may contribute to high circulating IgE levels which promote not only FA, but respiratory allergy (12). AD pathobiology evolves from a complex interaction of epidermal barrier disruption, high Type 2 immune response, and an imbalanced skin microbiota which promotes cutaneous barrier dysfunction. The development of noninvasive skin sampling techniques will allow early identification of abnormalities in the skin barrier and facilitate early intervention. These new approaches will play a key role in targeting high risk patient populations for early introduction of cutaneous epithelial repair and therapies which prevent progression of Type 2 immune activation. We believe that the interplay between genetic predisposition, microbial colonization, and Type 2 inflammatory responses are important for the development of epidermal barrier abnormalities and onset of allergic responses. Furthermore, issues of adherence to management programs, timing of intervention, and duration of skin barrier repair are unknown. Beyond prevention is the challenge of effective intervention in a vulnerable population. Onset of lesional AD requires effective control of local and systemic immune activation for optimal management. Early intervention (microbiome, biologics targeting Type 2 immune activation) may improve long term outcomes for AD and reduce the systemic allergen sensitization leading to associated allergic diseases in the gastrointestinal and respiratory tract. An important finding is that AD skin is associated with a hyperproliferative epidermal compartment accompanied by reduced terminal keratinocyte differentiation independent of filaggrin mutations and immune activation. The key challenge now is identification of interventions directed towards the protection of skin barrier function in early infancy to prevent onset of Type 2 inflammatory responses and development of allergy. Accessibility of AD skin tissue to repeated mechanistic sampling is likely to provide a window to our fundamental understanding of allergic disease.
“What Do We Know?”.
Filaggrin is abnormally low in the skin of peanut allergic subjects irrespective of concomitant AD. Low filaggrin results from genetic mutations, environmental factors and immune activation.
The lack of terminal keratinocyte differentiation contributes to cutaneous barrier dysfunction in AD.
Nonlesional skin in AD with FA have features of cutaneous barrier dysfunction that are as abnormal as lesional AD.
Cutaneous barrier dysfunction allows allergens unrestricted entry into the skin to activate release of IL33 and TSLP from keratinocytes and induction of Type 2 immune responses.
S. aureus colonization in AD is the result of excessive Type 2 inflammation and reduction in beneficial commensal bacteria.
“What is Still Unknown?”.
What is the time frame for prevention of AD?
What is the ideal emollient for skin barrier protection?
Do skin emollients have the same effect on non-atopic versus atopic skin?
What are the causes of different AD phenotypes? Can they be distinguished by epithelial profiling?
What predictive tests will inform us for precision medicine approaches in AD?
ACKNOWLEDGEMENTS
The authors acknowledge Nicole Meiklejohn for her editorial assistance in preparing this manuscript.
Funding: The project was funded in part by NIH/NIAMS grant R01 AR41256, NIH/NIAID grant U19 AI117673, NIH/NCRR grant UL1 RR025780 and The Edelstein Family Chair of Pediatric Allergy-Immunology at National Jewish Health.
Conflict of Interest Statement: EG and EB declare no COI. DL receives NIAID/NIH funding from The Atopic Dermatitis Research Network, NIAID/CoFAR, and NIAMS/NIH. He has also consulted for Boehringer-Ingelheim, Janssen Biotech, Regeneron, Sanofi-Genzyme,Genentech, and Incyte.
Abbreviations used
- AD
atopic dermatitis
- CER
ceramide
- CLDN1
claudin-1
- EDC
epidermal differentiation complex
- ELOVL
fatty acid elongase
- FA
food allergy
- EOS
ω-hydroxy fatty acid (EO) sphingosine (S)
- FLG
filaggrin
- IL
interleukin
- IVL
involucrin
- KLK
kallikrein proteases
- KRT
keratin
- LOR
loricrin
- LTA
lipoteichoic acid
- NMF
natural moisturizing factor
- NS
ceramides (non-hydroxy fatty acid sphingosine ceramides)
- PCA
pyrrolidone carboxylic acid
- TEWL
transepidermal water loss
- TGM1
transglutaminase 1
- SEB
staphylococcal enterotoxin B
- SB
stratum basale
- SC
stratum corneum
- SG
stratum granulosum
- SMase
sphingomyelinase
- SPRR
small proline rich protein
- SS
stratum spinosum
- STAT
signal transducer and activator of transcription
- TSLP
thymic stromal lymphopoietin
- UCA
urocanic acid
- ULCFA
ultra long chain fatty acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Martin PE, Koplin JJ, Eckert JK, Lowe AJ, Ponsonby AL, Osborne NJ, et al. The prevalence and socio-demographic risk factors of clinical eczema in infancy: a population-based observational study. Clin Exp Allergy 2013;43(6):642–51. DOI: 10.1111/cea.12092. [DOI] [PubMed] [Google Scholar]
- 2.Holgate ST, Wenzel S, Postma DS, Weiss ST, Renz H, and Sly PD. Asthma. Nat Rev Dis Primers 2015;1:15025 DOI: 10.1038/nrdp.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters RL, Koplin JJ, Gurrin LC, Dharmage SC, Wake M, Ponsonby AL, et al. The prevalence of food allergy and other allergic diseases in early childhood in a population-based study: HealthNuts age 4-year follow-up. J Allergy Clin Immunol 2017;140:145–53. DOI: 10.1016/j.jaci.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Walker MT, Green JE, Ferrie RP, Queener AM, Kaplan MH, Cook-Mills JM. Mechanism for initiation of food allergy: dependence on skin barrier mutations and environmental allergen costimulation. J Allergy Clin Immunol 2018;141(5):1711–25. DOI: 10.1016/j.jaci.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy K, Heimall J, Spergel JM. Advances in atopic dermatitis in 2017. J Allergy Clin Immunol 2018;142(6):1740–7. DOI: 10.1016/j.jaci.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol 2020; 124(1):28–35. DOI: 10.1016/j.anai.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Brunner PM, Israel A, Zhang N, Leonard A, Wen HC, Huynh T, et al. Early-onset pediatric atopic dermatitis is characterized by TH2/TH17/TH22-centered inflammation and lipid alterations. J Allergy Clin Immunol 2018;141(6):20194–106. DOI: 10.1016/j.jaci.2018.02.040. [DOI] [PubMed] [Google Scholar]
- 8.Davidson WF, Leung DYM, Beck LA, Berin CM, Boguniewicz M, Busse WW, et al. Report from the National Institute of Allergy and Infectious Diseases workshop on “Atopic dermatitis and the atopic march: Mechanisms and interventions.” J Allergy Clin Immunol 2019;143(3):894–913. DOI: 10.1016/j.jaci.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran MM, Lefebvre DL, Dharma C, Dai D, Lou WYW, Subbarao, et al. Predicting the atopic march: results from the Canadian Healthy Infant Longitudinal Development study. J Allergy Clin Immunol 2018;141(2):601–7. DOI: 10.1016/j.jaci.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 10.Pividori M, Schoettler N, Nicolae DL, Ober C, Im HK. Shared and distinct genetic risk factors for childhood-onset and adult-onset asthma: genome-wide and transcriptome-wide studies. Lancet Respir Med 2019;7(6):509–22. DOI: 10.1016/S2213-2600(19)30055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin PE, Eckert JK, Koplin JJ, Lowe AJ, Gurrin LC, Dharmage SC, et al. Which infants with eczema are at risk of food allergy? Results from a population-based cohort. Clin Exp Allergy 2015;45(1):255–64. DOI: 10.1111/cea.12406. [DOI] [PubMed] [Google Scholar]
- 12.Hill DA, Spergel JM. The atopic march: critical evidence and clinical relevance. Ann Allergy Asthma Immunol 2018;120(2):131–7. DOI: 10.1016/j.anai.2017.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honda T, Kabashima K. Reconciling innate and acquired immunity in atopic dermatitis. J Allergy Clin Immunol February, 2020. [DOI] [PubMed] [Google Scholar]
- 14.Kelleher M, Dunn-Galvin A, Hourihane JO, Murray D, Campbell LE, McLean WH, et al. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol 2015;135(4):930–5. DOI: 10.1016/j.jaci.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Kelleher MM, Dunn-Galvin A, Gray C, Murray DM, Kiely M, Kenny L, et al. Skin barrier impairment at birth predicts food allergy at 2 years of age. J Allergy Clin Immunol 2016;137(4):1111–6 DOI: 10.1016/j.jaci.2015.12.1312. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Kim BE, Lee J, Han Y, Jun HY, Kim H, et al. Epidermal thymic stromal lymphopoietin predicts the development of atopic dermatitis during infancy. J Allergy Clin Immunol 2016; 137:1282–5. DOI: 10.1016/j.jaci.2015.12.1306. [DOI] [PubMed] [Google Scholar]
- 17.Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365(14):1315–27. DOI: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- 18.Drislane C, Irvine AD. The role of filaggrin in atopic dermatitis and allergic disease. Ann Allergy Asthma Immunol 2020;124(1):36–43. DOI: 10.1016/j.anai.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Scharschmidt TC, Man MQ, Hatano Y, Crumrine D, Gunathilake R, Sundberg JP, et al. Filaggrin deficiency confers a paracellular barrier abnormality that reduces inflammatory thresholds to irritants and haptens. J Allergy Clin Immunol 2009;124(3):496–506. DOI: 10.1016/j.jaci.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet 2009;41(5):602–8. DOI: 10.1038/ng.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pendaries V, Malaisse J, Pellerin L, Le Lamer M, Nachat R, Kezic S, et al. Knockdown of filaggrin in a three-dimensional reconstructed human epidermis impairs keratinocyte differentiation. J Invest Dermatol 2014;134(12):2938–46. DOI: 10.1038/jid.2014.259. [DOI] [PubMed] [Google Scholar]
- 22.Kezic S, O’Regan GM, Lutter R, Jakasa I, Koster ES, Saunders S, et al. Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. J Allergy Clin Immunol 2012;129(4):1031–9. DOI: 10.1016/j.jaci.2011.12.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida K, Kubo A, Fujita H, Yokouchi M, Ishii K, Kawasaki H, et al. Distinct behavior of human Langerhans cells and inflammatory dendritic epidermal cells at tight junctions in patients with atopic dermatitis. J Allergy Clin Immunol 2014;134(4):856–64. DOI: 10.1016/j.jaci.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Elias PM, Wakefield JS. Mechanisms of abnormal lamellar body secretion and the dysfunctional skin barrier in patients with atopic dermatitis. J Allergy Clin Immunol 2014;134(4):781–91. DOI: 10.1016/j.jaci.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vávrová K, Henkes D, Strüver K, Sochorová M, Školová B, Witting MY, et al. Filaggrin deficiency leads to impaired lipid profile and altered acidification pathways in a 3D skin construct. J Invest Dermatol 2014;134(3):746–53. DOI: 10.1038/jid.2013.402. [DOI] [PubMed] [Google Scholar]
- 26.Hachem JP, Man MQ, Crumrine D, Uchida Y, Brown BE, Rogiers V, et al. Sustained serine proteases activity by prolonged increase in pH leads to degradation of lipid processing enzymes and profound alterations of barrier function and stratum corneum integrity. J Invest Dermatol 2005;125(3):510–20. DOI: 10.1111/j.0022-202X.2005.23838.x. [DOI] [PubMed] [Google Scholar]
- 27.Briot A, Deraison C, Lacroix M, Bonnart C, Robin A, Besson C, et al. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med 2009;206(5):1135–47. DOI: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, Debenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol 2007;120(1):150–5. DOI: 10.1016/j.jaci.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown SJ, Kroboth K, Sandilands A, Campbell LE, Pohler E, Kezic S, et al. Intragenic copy number variation within filaggrin contributes to the risk of atopic dermatitis with a dose-dependent effect. J Invest Dermatol 2012;132(1):98–104. DOI: 10.1038/jid.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziyab AH, Karmaus W, Holloway JW, Zhang H, Ewart S, and Arshad SH. DNA methylation of the filaggrin gene adds to the risk of eczema associated with loss-of-function variants. J Eur Acad Dermatol Venereol 2013;27(3):e420–3. DOI: 10.1111/jdv.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pellerin L, Henry J, Hsu CY, Balica S, Jean-Decoster C, Méchin MC, et al. Defects of filaggrin-like proteins in both lesional and nonlesional atopic skin. J Allergy Clin Immunol 2013;131(4):1094–102. DOI: 10.1016/j.jaci.2012.12.1566. [DOI] [PubMed] [Google Scholar]
- 32.Pendaries V, Le Lamer M, Cau L, Hansmann B, Malaisse J, Kezic S, et al. In a three-dimensional reconstructed human epidermis filaggrin-2 is essential for proper cornification. Cell Death Dis 2015;6:e1656 DOI: 10.1038/cddis.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol 2006;36(5):1309–23. DOI: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 34.Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suárez-Fariñas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol 2008;159(5):1092–102. DOI: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim BE, Howell MD, Guttman-Yassky E, Gilleaudeau PM, Cardinale IR, Boguniewicz M, et al. TNF-alpha downregulates filaggrin and loricrin through c-Jun N-terminal kinase: role for TNF-alpha antagonists to improve skin barrier. J Invest Dermatol 2011;131(6):1272–9. DOI: 10.1038/jid.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryu WI, Lee H, Bae HC, Ryu HJ, and Son SW. IL-33 down-regulates filaggrin expression by inducing STAT3 and ERK phosphorylation in human keratinocytes. J Dermatol Sci 2016;82(2):131–4. DOI: 10.1016/j.jdermsci.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Guttman-Yassky E, Suárez-Fariñas M, Chiricozzi A, Nograles KE, Shemer A, Fuentes-Duculan J, et al. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J Allergy Clin Immunol 2009;124(6):1235–44. DOI: 10.1016/j.jaci.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 38.Jensen JM, Fölster-Holst R, Baranowsky A, Schunck M, Winoto-Morbach S, Neumann C, et al. Impaired sphingomyelinase activity and epidermal differentiation in atopic dermatitis. J Invest Dermatol 2004;122(6):1423–31. DOI: 10.1111/j.0022-202X.2004.22621.x. [DOI] [PubMed] [Google Scholar]
- 39.Suárez-Fariñas M, Tintle SJ, Shemer A, Chiricozzi A, Nograles K, Cardinale I, et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol 2011;127(4):954–64. DOI: 10.1016/j.jaci.2010.12.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med 2002;347(15):1151–60. DOI: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 41.Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol 2002;156(6):1099–111. DOI: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C, et al. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol 2011;127(3):773–86. DOI: 10.1016/j.jaci.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Benedetto A, Slifka MK, Rafaels NM, Kuo IH, Georas SN, Boguniewicz M, et al. Reductions in claudin-1 may enhance susceptibility to herpes simplex virus 1 infections in atopic dermatitis. J Allergy Clin Immunol 2011;128(1):242–6. DOI: 10.1016/j.jaci.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin S, Park CO, Shin JU, Noh JY, Lee YS, Lee NR, et al. DAMP molecules S100A9 and S100A8 activated by IL-17A and house-dust mites are increased in atopic dermatitis. Exp Dermatol 2014;23(12):938–41. DOI: 10.1111/exd.12563. [DOI] [PubMed] [Google Scholar]
- 45.Gittler JK, Shemer A, Suárez-Fariñas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol 2012;130(6):1344–54. DOI: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howell MD, Fairchild HR, Kim BE, Bin L, Boguniewicz M, Redzic JS, et al. Th2 cytokines act on S100/A11 to downregulate keratinocyte differentiation. J Invest Dermatol 2008;128(9):2248–58. DOI: 10.1038/jid.2008.74. [DOI] [PubMed] [Google Scholar]
- 47.Nowell C, Radtke F. Cutaneous Notch signaling in health and disease. Cold Spring Harb Perspect Med 2013;3:a017772 DOI: 10.1101/cshperspect.a017772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. Embo j 2001;20:3427–36. DOI: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell 2017;169:985–99. DOI: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 50.Dumortier A, Durham AD, Di Piazza M, Vauclair S, Koch U, Ferrand G, et al. Atopic dermatitis-like disease and associated lethal myeloproliferative disorder arise from loss of Notch signaling in the murine skin. PLoS One 2010;5:e9258 DOI: 10.1371/journal.pone.0009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murthy A, Shao YW, Narala SR, Molyneux SD, Zuniga-Pflucker JC, Khokha R. Notch activation by the metalloproteinase ADAM17 regulates myeloproliferation and atopic barrier immunity by suppressing epithelial cytokine synthesis. Immunity 2012;36:105–19. DOI: 10.1016/j.immuni.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Abdou AG, Maraee AH, Sharaf A, Elnaidany NF. Up-regulation of Notch-1 in psoriasis: an immunohistochemical study. Ann Diagn Pathol 2012;16:177–84. DOI: 10.1016/j.anndiagpath.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 53.Nakahigashi K, Kabashima K, Ikoma A, Verkman AS, Miyachi Y, Hara-Chikuma M. Upregulation of aquaporin-3 is involved in keratinocyte proliferation and epidermal hyperplasia. J Invest Dermatol 2011;131:865–73. DOI: 10.1038/jid.2010.395. [DOI] [PubMed] [Google Scholar]
- 54.Olsson M, Broberg A, Jernas M, Carlsson L, Rudemo M, Suurkula M, et al. Increased expression of aquaporin 3 in atopic eczema. Allergy 2006;61:1132–7. DOI: 10.1111/j.1398-9995.2006.01151.x. [DOI] [PubMed] [Google Scholar]
- 55.Guo L, Chen H, Li Y, Zhou Q, Sui Y. An aquaporin 3-notch1 axis in keratinocyte differentiation and inflammation. PLoS One 2013;8:e80179 DOI: 10.1371/journal.pone.0080179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin HY, Kao CH, Lin KM, Kaartinen V, Yang LT. Notch signaling regulates late-stage epidermal differentiation and maintains postnatal hair cycle homeostasis. PLoS One 2011;6:e15842 DOI: 10.1371/journal.pone.0015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weber S, Niessen MT, Prox J, Lullmann-Rauch R, Schmitz A, Schwanbeck R, et al. The disintegrin/metalloproteinase Adam10 is essential for epidermal integrity and Notch-mediated signaling. Development 2011;138:495–505. DOI: 10.1242/dev.055210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Franzke CW, Cobzaru C, Triantafyllopoulou A, Loffek S, Horiuchi K, Threadgill DW, et al. Epidermal ADAM17 maintains the skin barrier by regulating EGFR ligand-dependent terminal keratinocyte differentiation. J Exp Med 2012; 209:1105–19. DOI: 10.1084/jem.20112258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013;155:285–95. DOI: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Devgan V, Mammucari C, Millar SE, Brisken C, Dotto GP. p21WAF1/Cip1 is a negative transcriptional regulator of Wnt4 expression downstream of Notch1 activation. Genes Dev 2005;19:1485–95. DOI: 10.1101/gad.341405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayward P, Brennan K, Sanders P, Balayo T, DasGupta R, Perrimon N, et al. Notch modulates Wnt signalling by associating with Armadillo/beta-catenin and regulating its transcriptional activity. Development 2005;132:1819–30. DOI: 10.1242/dev.01724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Demehri S, Turkoz A, Manivasagam S, Yockey LJ, Turkoz M, Kopan R. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell 2012;22:494–505. DOI: 10.1016/j.ccr.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Piazza M, Nowell CS, Koch U, Durham AD, Radtke F. Loss of cutaneous TSLP-dependent immune responses skews the balance of inflammation from tumor protective to tumor promoting. Cancer Cell 2012;22:479–93. DOI: 10.1016/j.ccr.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 64.Boscke R, Vladar EK, Konnecke M, Husing B, Linke R, Pries R, et al. Wnt Signaling in Chronic Rhinosinusitis with Nasal Polyps. Am J Respir Cell Mol Biol 2017;56:575–84. DOI: 10.1165/rcmb.2016-0024OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ordovas-Montanes J, Dwyer DF, Nyquist SK, Buchheit KM, Vukovic M, Deb C, et al. Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature 2018;560:649–54. DOI: 10.1038/s41586-018-0449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gudjonsson JE, Johnston A, Stoll SW, Riblett MB, Xing X, Kochkodan JJ, et al. Evidence for altered Wnt signaling in psoriatic skin. J Invest Dermatol 2010;130:1849–59. DOI: 10.1038/jid.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Romanowska M, Evans A, Kellock D, Bray SE, McLean K, Donandt S, et al. Wnt5a exhibits layer-specific expression in adult skin, is upregulated in psoriasis, and synergizes with type 1 interferon. PLoS One 2009;4:e5354 DOI: 10.1371/journal.pone.0005354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Archer NK, Jo JH, Lee SK, Kim D, Smith B, Ortines RV, et al. Injury, dysbiosis, and filaggrin deficiency drive skin inflammation through keratinocyte IL-1alpha release. J Allergy Clin Immunol 2019;143:1426–43.e6. DOI: 10.1016/j.jaci.2018.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu H, Archer NK, Dillen CA, Wang Y, Ashbaugh AG, Ortines RV, et al. Staphylococcus aureus epicutaneous exposure drives skin inflammation via IL-36-Mediated T Cell responses. Cell Host Microbe 2017;22:653–66. DOI: 10.1016/j.chom.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paller AS, Kong HH, Seed P, Naik S, Scharschmidt TC, Gallo RL, et al. The microbiome in patients with atopic dermatitis. J Allergy Clin Immunol 2019;143(1):26–35. DOI: 10.1016/j.jaci.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakatsuji T, Gallo RL. The role of the skin microbiome in atopic dermatitis. Ann Allergy Asthma Immunol 2019;122(3):263–269. DOI: 10.1016/j.anai.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brauweiler AM, Goleva E, Leung DYM. Th2 cytokines Increase Staphylococcus aureus alpha toxin-induced keratinocyte death through the signal transducer and activator of transcription 6 (STAT6). J Invest Dermatol 2014;134(8):2114–21. DOI: 10.1038/jid.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kisich KO, Carspecken CW, Fiéve S, Boguniewicz M, Leung DY. Defective killing of Staphylococcus aureus in atopic dermatitis is associated with reduced mobilization of human beta-defensin-3. J Allergy Clin Immunol 2008;122(1):62–8. DOI: 10.1016/j.jaci.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 74.Kisich KO, Howell MD, Boguniewicz M, Heizer HR, Watson NU, Leung DY. The constitutive capacity of human keratinocytes to kill Staphylococcus aureus is dependent on beta-defensin 3. J Invest Dermatol 2007;127(10):2368–80. DOI: 10.1038/sj.jid.5700861. [DOI] [PubMed] [Google Scholar]
- 75.Brauweiler AM, Bin L, Kim BE, Oyoshi MK, Geha RS, Goleva E, et al. Filaggrin-dependent secretion of sphingomyelinase protects against staphylococcal alpha-toxin-induced keratinocyte death. J Allergy Clin Immunol 2013;131(2):421–7. DOI: 10.1016/j.jaci.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity 2006;24(3):341–8. DOI: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 77.Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol 2003;171(6):3262–9. DOI: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- 78.Callewaert C, Nakatsuji T, Knight R, Kosciolek T, Vrbanac A, Kotol P, et al. IL-4R alpha blockade by dupilumab decreases Staphylococcus aureus colonization and increases microbial diversity in atopic dermatitis. J Invest Dermatol 2020;140(1):191–202. DOI: 10.1016/j.jid.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Niebuhr M, Scharonow H, Gathmann M, Mamerow D, Werfel T. Staphylococcal exotoxins are strong inducers of IL-22: a potential role in atopic dermatitis. J Allergy Clin Immunol 2010;126(6):1176–83. DOI: 10.1016/j.jaci.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 80.Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 2012;22(5):850–9. DOI: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH, et al. Dysbiosis and Staphyloccus aureus colonization drives inflammation in atopic dermatitis. Immunity 2015;42(4):756–66. DOI: 10.1016/j.immuni.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Honda K, Littman DR. The microbiota in adaptive immmune homeostasis and disease. Nature 2016; 535(7610):75–84. DOI: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 83.Oh J, Freeman AF, NICS Comparative Sequencing Program, Park M, Sokolic R, Candotti F, et al. The altered landscape of the human skin microbiome in patients with primary immunodeficiencies. Genome Res 2013;23(12):2103–14. DOI: 10.1101/gr.159467.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Byrd AL, Deming C, Cassidy SKB, Harrison OJ, Ng WI, Conlan S, et al. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci Transl Med 2017;9(397). DOI: 10.1126/scitranslmed.aal4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med 2009;15(12):1377–82. DOI: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Linehan JL, Harrison OJ, Han SJ, Byrd AL, Vujkovic-Cvijin I, Villarino AV, et al. Non-classical immunity controls microbiota impact on skin immunity and tissue repair. Cell 2018;172(4):784–96. DOI: 10.1016/j.cell.2017.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Naik S, Bouladoux N, Linehan JL, Han SJ, Harrison OJ, Wilhelm C, et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 2015;520(7545):104–8. DOI: 10.1038/nature14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. 2017;9(378). DOI: 10.1126/scitranslmed.aah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scharschmidt TC, Vasquez KS, Truong HA, Gearty SV, Pauli ML, Nosbaum A, et al. A wave of regulatory T cells into neonatal skin mediates tolerance to commensal microbes. Immunity 2015;43(5):1011–21. DOI: 10.1016/j.immuni.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kennedy EA, Connolly J, Hourihane JO, Fallon PG, McLean WHI, Murray D, et al. Skin microbiome before development of atopic dermatitis: early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J Allergy Clin Immunol 2017;139(1):166–72. DOI: 10.1016/j.jaci.2016.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meylan P, Lang C, Mermoud S, Johannsen A, Norrenberg S, Hohl D, et al. Skin colonization by Staphylococcus aureus precedes the clinical diagnosis of atopic dermatitis in infancy. J Invest Dermatol 2017;137(12):2497–504. DOI: 10.1016/j.jid.2017.07.834. [DOI] [PubMed] [Google Scholar]
- 92.Spaulding AR, Salgado-Pabón W, Kohler PL, Horswill AR, Leung DY, and Schlievert PM. Staphylococcal and streptococcal superantigen exotoxins. Clin Microbiol Rev 2013;26(3):422–47. DOI: 10.1128/CMR.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Foster TJ, Geoghegan JA, Ganesh VK, Höök M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 2014;12(1):49–62. DOI: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nakatsuji T, Chen TH, Two AM, Chun KA, Narala S, Geha RS, et al. Staphylococcus aureus exploits epidermal barrier defects in atopic dermatitis to trigger cytokine expression. J Invest Dermatol 2016;136(11):2192–200. DOI: 10.1016/j.jid.2016.05.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Williams MR, Nakatsuji T, Sanford JA, Vrbanac AF, and Gallo RL. Staphylococcus aureus induces increased serine protease activity in keratinocytes. J Invest Dermatol 2017;137(2):377–84. DOI: 10.1016/j.jid.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cho SH, Strickland I, Tomkinson A, Fehringer AP, Gelfand EW, Leung DY. Preferential binding of Staphylococcus aureus to skin sites of Th2-mediated inflammation in a murine model. J Invest Dermatol 2001;116(5):658–63. DOI: 10.1046/j.0022-202x.2001.01331.x. [DOI] [PubMed] [Google Scholar]
- 97.Miajlovic H, Fallon PG, Irvine AD, Foster TJ. Effect of filaggrin breakdown products on growth of and protein expression by Staphylococcus aureus. J Allergy Clin Immunol 2010;126(6):1184–90. DOI: 10.1016/j.jaci.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brauweiler AM, Goleva E, Hall CF, and Leung DY. Th2 cytokines suppress lipoteichoic acid induced matrix metalloproteinase expression and keratinocyte migration in response to wounding. J Invest Dermatol 2015;153(10):2550–3. DOI: 10.1038/jid.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brauweiler AM, Hall CF, Goleva E, Leung DYM. Staphylococcus aureus lipoteichoic acid inhibits keratinocyte differentiation through a p63-mediated pathway. J Invest Dermatol 2017;137(9):2030–3. DOI: 10.1016/j.jid.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tsilochristou O, du Toit G, Sayre PH, Roberts G, Lawson K, Sever ML, et al. Association of Staphylococcus aureus colonization with food allergy occurs independently of eczema severity. J Allergy Clin Immunol 2019;144(2):494–503. DOI: 10.1016/j.jaci.2019.04.025. [DOI] [PubMed] [Google Scholar]
- 101.Cole C, Kroboth K, Schurch NJ, Sandilands A, Sherstnev A, O’Regan GM, et al. Filaggrin-stratified transcriptomic analysis of pediatric skin identifies mechanistic pathways in patients with atopic dermatitis. J Allergy Clin Immunol 2014;134(1):82–91. DOI: 10.1016/j.jaci.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Feingold KR, Elias PM. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim Biophys Acta 2014;1841(3):280–94. DOI: 10.1016/j.bbalip.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 103.Sahle FF, Gebre-Mariam T, Dobner B, Wohlrab J, Neubert RH. Skin diseases associated with the depletion of stratum corneum lipids and stratum corneum lipid substitution therapy. Skin Pharmacol Physiol 2015;28(1):42–55. DOI: 10.1159/000360009. [DOI] [PubMed] [Google Scholar]
- 104.van Smeden J, Bouwstra JA. Stratum corneum lipids: their role for the skin barrier function in healthy subjects and atopic dermatitis patients. Curr Probl Dermatol. 2016;49:8–26. DOI: 10.1159/000441540. [DOI] [PubMed] [Google Scholar]
- 105.Drake DR, Brogden KA, Dawson DV, Wertz PW. Thematic review series: skin lipids. Antimicrobial lipids at the skin surface. J Lipid Res 2008;49(1):4–11. DOI: 10.1194/jlr.R700016-JLR200. [DOI] [PubMed] [Google Scholar]
- 106.Arikawa J, Ishibashi M, Kawashima M, Takagi Y, Ichikawa Y, Imokawa G. Decreased levels of sphingosine, a natural antimicrobial agent, may be associated with vulnerability of the stratum corneum from patients with atopic dermatitis to colonization by Staphylococcus aureus. J Invest Dermatol 2002;119(2):433–9. DOI: 10.1046/j.1523-1747.2002.01846.x. [DOI] [PubMed] [Google Scholar]
- 107.Takigawa H, Nakagawa H, Kuzukawa M, Mori H, Imokawa G. Deficient production of hexadecenoic acid in the skin is associated in part with the vulnerability of atopic dermatitis patients to colonization by Staphylococcus aureus. Dermatology 2005;211(3):240–8. DOI: 10.1159/000087018. [DOI] [PubMed] [Google Scholar]
- 108.Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci U S A 1997;94(9):4318–23. DOI: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schmuth M, Jiang YJ, Dubrac S, Elias PM, Feingold KR. Thematic review series: skin lipids. Peroxisome proliferator-activated receptors and liver X receptors in epidermal biology. J Lipid Res 2008;49(3):499–509. DOI: 10.1194/jlr.R800001-JLR200. [DOI] [PubMed] [Google Scholar]
- 110.Ishikawa J, Narita H, Kondo N, Hotta M, Takagi Y, Masukawa Y, et al. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol 2010;130(10):2511–4. DOI: 10.1038/jid.2010.161. [DOI] [PubMed] [Google Scholar]
- 111.van Smeden J, Janssens M, Kaye EC, Caspers PJ, Lavrijsen AP, Vreeken RJ, et al. The importance of free fatty acid chain length for the skin barrier function in atopic eczema patients. Exp Dermatol 2014;23(1):45–52. DOI: 10.1111/exd.12293. [DOI] [PubMed] [Google Scholar]
- 112.Berdyshev E, Goleva E, Bronova I, Dyjack N, Rios C, Jung J, et al. Lipid abnormalities in atopic skin are driven by Type 2 cytokines. JCI Insight 2018;3(4). DOI: 10.1172/jci.insight.98006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thyssen JP, Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol 2014;134(4):792–9. DOI: 10.1016/j.jaci.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 114.Leyva-Castillo JM, Galand C, Kam C, Burton O, Guish M, Musser MA, et al. Mechanical skin injury promotes food anaphylaxis by driving intestinal mast cell expansion. Immunity 2019;50(5):1262–75. DOI: 10.1016/j.immuni.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cabanillas B, Brehler AC, Novak N. Atopic dermatitis phenotypes and the need for personalized medicine. Curr Opin Allergy Clin Immunol 2017;17(4):309–315. DOI: 10.1097/ACI.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lack G, Fox D, Northstone K, Golding J, Avon Longitudinal Study of Parents and Children Study Team. Factors associated with the development of peanut allergy in childhood. N Engl J Med 2003;348(11):977–85. DOI: 10.1056/NEJMoa013536. [DOI] [PubMed] [Google Scholar]
- 117.Brough HA, Simpson A, Makinson K, Hankinson J, Brown S, Douiri A, et al. Peanut allergy: effect of environmental peanut exposure in children with filaggrin loss-of-function mutations. J Allergy Clin Immunol 2014;134(4):867–75. DOI: 10.1016/j.jaci.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brough HA, Liu AH, Sicherer S, Makinson K, Douiri A, Brown SJ, et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J Allergy Clin Immunol 2015;135(1):164–70. DOI: 10.1016/j.jaci.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eigenmann PA, Scherer SH, Borkowski TA, Cohen BA, Sampson HA. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics 1998;101(3):e8 DOI: 10.1542/peds.101.3.e8. [DOI] [PubMed] [Google Scholar]
- 120.Leung DYM, Calatroni A, Zaramela LS, LeBeau PK, Dyjack N, Brar K, et al. The nonlesional skin surface distinguishes atopic dermatitis with food allergy as a unique endotype. Sci Transl Med 2019;11(480). DOI: 10.1126/scitranslmed.aav2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goleva E, Berdyshev E, Leung DY. Epithelial barrier repair and prevention of allergy. J Clin Invest 2019;129(4):1463–74. DOI: 10.1172/JCI124608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lowe AJ, Leung DYM, Tang MLK, Su JC, Allen KJ. The skin as a target for prevention of the atopic march. Ann Allergy Asthma Immunol 2018;120(2):145–51. DOI: 10.1016/j.anai.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 123.Simpson EL, Chalmers JR, Hanifin JM, Thomas KS, Cork MJ, McLean WH, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol 2014;134(4):818–23. DOI: 10.1016/j.jaci.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Horimukai K, Morita K, Narita M, Kondo M, Kitazawa H, Nozaki M, et al. Application of moisturizer to neonates prevents development of atopic dermatitis. J Allergy Clin Immunol 2014;134(4):824–30. DOI: 10.1016/j.jaci.2014.07.060. [DOI] [PubMed] [Google Scholar]
- 125.Lowe AJ, Su JC, Allen KJ, Abramson MJ, Cranswick N, Robertson CF, et al. A randomized trial of a barrier lipid replacement strategy for the prevention of atopic dermatitis and allergic sensitization: the PEBBLES pilot study. Br J Dermatol 2018;178(1):e19–e21. DOI: 10.1111/bjd.15747. [DOI] [PubMed] [Google Scholar]
- 126.Elias PM, Sugarman J. Does moisturizing the skin equate with barrier repair therapy? Ann Allergy Asthma Immunol 2018;121(6):653–6. DOI: 10.1016/j.anai.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Miyaji Y, Yang L, Yamamoto-Hanada K, Narita M, Saito H, Ohya Y. Earlier aggressive treatment to shorten the duration of eczema in infants resulted in fewer food allergies at 2 years of age. Allergy Clin Immunol Pract 2019;piiS2213–2198(19)31018–9. DOI: 10.1016/j.jaip.2019.11.036. [DOI] [PubMed] [Google Scholar]
- 128.Sicherer SH, Wood RA, Perry TT, Jones SM, Leung DYM, Henning AK, et al. Clinical factors associated with peanut allergy in a high‐risk infant cohort. Allergy 2019;74(11):2199–2211. DOI: 10.1111/all.13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fox AT, Sasieni P, du Toit G, Syed H, Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. J Allergy Clin Immunol 2009;123(2):417–23. DOI: 10.1016/j.jaci.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 130.Tsakok T, Woolf R, Smith CH, Weidinger S, Flohr C. Atopic dermatitis: the skin barrier and beyond. Br J Dermatol 2019;180(3):464–74. DOI: 10.1111/bjd.16934. [DOI] [PubMed] [Google Scholar]
- 131.Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Choi EH, et al. 25 Hydroxyvitamin D 1 alpha-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J Invest Dermatol 2004;122(4):984–92. DOI: 10.1111/j.0022-202X.2004.22424.x. [DOI] [PubMed] [Google Scholar]
- 132.Rueter K, Jones AP, Siafarikas A, Lim EM, Bear N, Noakes PS, et al. Direct infant UV light exposure is associated with eczema and immune development. J Allergy Clin Immunol 2019;143(3):1012–20. DOI: 10.1016/j.jaci.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 133.Egawa G, Kabashima K. Barrier dysfunction in the skin allergy. Allergol Int 2018;67(1):3–11. DOI: 10.1016/j.alit.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 134.Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol 2008;126:332–7. DOI: 10.1016/j.clim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]