Abstract
The mammalian Golgi apparatus is a highly dynamic organelle, which is normally localized in the juxtanuclear space and plays an essential role in the regulation of cellular homeostasis. While posttranslational modification of cargo is mediated by the resident enzymes (glycosyltransferases, glycosidases, and kinases), the ribbon structure of Golgi and its cisternal stacking mostly rely on the cooperation of coiled-coil matrix golgins. Among them, giantin, GM130, and GRASPs are unique, because they form a tripartite complex and serve as Golgi docking sites for cargo delivered from the endoplasmic reticulum (ER). Golgi undergoes significant disorganization in many pathologies associated with a block of the ER-to-Golgi or intra-Golgi transport, including cancer, different neurological diseases, alcoholic liver damage, ischemic stress, viral infections, etc. In addition, Golgi fragments during apoptosis and mitosis. Here, we summarize and analyze clinically relevant observations indicating that Golgi fragmentation is associated with the selective loss of Golgi residency for some enzymes and, conversely, with the relocation of some cytoplasmic proteins to the Golgi. The central concept is that ER and Golgi stresses impair giantin docking site but have no impact on the GM130–GRASP65 complex, thus inducing mislocalization of giantin-sensitive enzymes only. This cardinally changes the processing of proteins by eliminating the pathways controlled by the missing enzymes and by activating the processes now driven by the GM130–GRASP65-dependent proteins. This type of Golgi disorganization is different from the one induced by the cytoskeleton alteration, which despite Golgi de-centralization, neither impairs function of golgins nor alters trafficking.
Keywords: Golgi morphology, golgins, giantin, GM130, GRASP65, Golgi-resident enzymes, ER stress, cancer
Over the past three decades, the structure and function of the Golgi apparatus have attracted ever-increasing attention in the biomedical research. The Golgi is the central sorting and transportation station for the posttranslational modification of cargo molecules, which is provided by resident proteins represented by different families of glycosyltransferases, glycosidases, and kinases. Under normal physiological conditions, the Golgi localizes within the juxtanuclear area and forms a ribbon-like, convoluted structure composed of a stack of cisterns and associated vesicles. However, it has become clear that, besides processing of proteins, Golgi is also involved in various integrated cellular processes and responses, such as mitosis, apoptosis, stress, autophagy, and carcinogenesis [1-5]. Many of these intracellular events are associated with the rearrangements of Golgi morphology, from mild enlargement to severe fragmentation and unstacking. For example, as we described previously [6], treatment of VA-13 cells (HepG2 cells expressing alcohol dehydrogenase) with ethanol results in the expansion of Golgi membranes after 24 h and Golgi conversion to the fragmented phenotype after 72 h (Fig. 1). The determination of the extent of Golgi fragmentation is based on simple calculations, which we have employed previously [7] and used here to analyze the data presented by other groups. Briefly, the entire cell was divided into three areas: nuclear (A), perinuclear (B), and cytoplasmic (C). After subtraction of the nuclear region, the cytoplasm is considered as two-third of (B + C), while one-third of this sum is denoted as the perinuclear area, where the Golgi is located. We consider the Golgi fragmented if separate Golgi membranes are detected beyond the perinuclear area. This type of Golgi disorganization is described by some authors as a peripheral fragmentation, contrary to the central Golgi fragmentation when membranes lose stacking but still concentrate in the perinuclear area [8, 9]. In this review, we will mostly highlight the evidence of abnormal distribution of cytoplasmic and Golgi resident proteins in cells, whose Golgi is experiencing peripheral fragmentation.
Fig. 1.
Types of Golgi morphology in VA-13 cells (staining for giantin): compact Golgi in the control cells is converted to expanded and fragmented states after 24 and 72 h of treatment with 35 mM ethanol, accordingly; bars, 10 μm. Note that the expanded Golgi, contrary to the fragmented one, still demonstrates the connectivity of its membranes.
GOLGINS: MORE THAN A SCAFFOLD
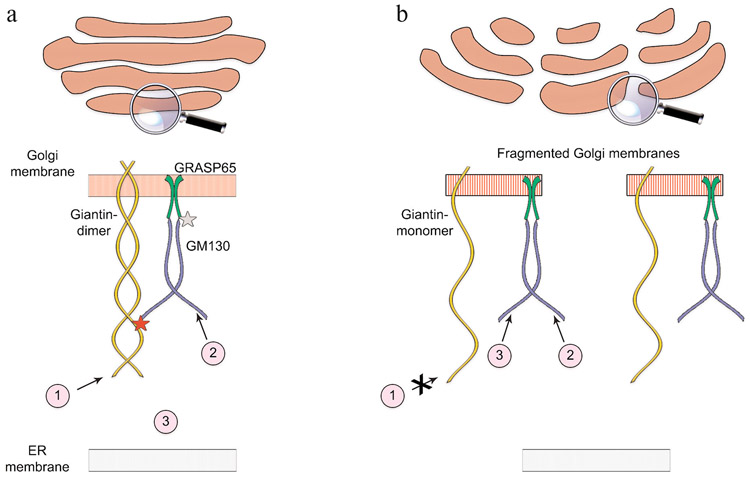
Several Golgi matrix proteins, referred to as golgins, form the structural scaffold of the Golgi. Most of these proteins are predicted to form coiled-coil dimers, whose C-terminals mediate attachment to Golgi membranes, and N-terminals project into the cytoplasm for up to 400 nm [10]. The function of golgins is coordinated by the Golgi reassembly stacking proteins (GRASPs) [11]. Among these proteins, giantin, GM130, and GRASP65 have been the most studied. Giantin is the highest-molecular-weight (376 kDa) Golgi matrix dimeric protein with a large (⩾350 kDa) A-terminal cytoplasmic region [12]. It is distributed throughout the Golgi stack, with predominant positioning in the medial cisternae [13, 14]. GM130 is a cis-Golgi-localized, segmented coiled-coil dimer that binds to Golgi membranes through its C-terminal region via preferential interactions with GRASP65 [15, 16] (Fig. 2a). The functioning of both golgins and GRASPs is tightly linked to the fidelity of glycan processing [16-20]; however, the detailed molecular processes are not well understood. The clue comes from the mounting evidence indicating that golgins serve as the docking sites for different types of Golgi-targeting vesicles [21-24], implying that transportation of the Golgi resident enzymes to their location sites relies on their cooperation with Golgi matrix proteins. This concept has been gaining momentum since the early observation by the Warren group, indicating that isolated rat liver Golgi matrix contains both α-mannosidase II (Man-II) and mannosyl-(α-1,3)-glycoprotein β-1,2-N-acetylglucosaminyltransferase (MGAT1) in the remnants of Golgi cisternae, which can be removed by the treatment with low salt. Washout of salt restores the binding of these enzymes to the membranes; however, re-binding is abolished in the presence of proteinase K, signifying that the protein complexes between the cisternae represent critical docking sites for Golgi enzymes [25].
Fig. 2.
Differential Golgi targeting mechanisms. a) Under normal conditions, Golgi complex is a compact ribbon-like structure, and dimeric giantin, GM130, and GRASP65 form the tripartite tether, which determines docking of different Golgi resident enzymes: 1) giantin-dependent; 2) GM130-dependent; 3) cytoplasmic proteins, whose GM130-specific Golgi docking site is presumably masked by giantin. b) In fragmented Golgi membranes, giantin is predominantly presented as a monomer, and the interaction between giantin and GM130 is disrupted, allowing docking of cytoplasmic enzymes at the accessible GM130 site (3). Notably, the docking of giantin-sensitive enzymes is altered (1), while Golgi targeting for the GM130-sensitive enzymes remains intact (2).
GIANTIN AND GM130–GRASP65: TOGETHER OR SEPARATE?
Despite the obvious importance of golgins for Golgi architecture, individual silencing of related genes does not substantially change visible positioning of this organelle. Indeed, depletion of cis-Golgi proteins (GPP130, GRASP65, GM130, or golgin-160 [16, 18, 26, 27]), medial-Golgi proteins (golgin-84 and giantin [16, 28]), and trans-Golgi proteins (golgin-97, p230, GCC185, and TMF [29-32]) may affect the Golgi ribbon and induce central Golgi fragmentation, but it has no significant effect on the perinuclear location of Golgi. Moreover, in the mouse model of GRASP65 or giantin knockouts, Golgi still appeared juxtanuclear, and no significant alterations in the development of these animals have been reported [33, 34]. The most probable explanation of this phenomenon is the exchangeability of golgins, where the lack of one matrix protein can be compensated by the overexpression of others. For example, the knockdown of GRASP65 gene induces an increase in the GM130 and giantin content [16]. Meanwhile, knockdown of GRASP65 does not affect the intra-Golgi position of GM130 [16, 18, 20], since GM130 can be tethered to the Golgi via giantin [16]. In advanced prostate cancer (PCa) cells, Golgi undergoes fragmentation, which is associated with a reduction of giantin levels and its de-dimerization [20] (Fig. 2b). In these cells, depletion of GRASP65 reduces the GM130 levels and GM130 association with Golgi [20], confirming the importance of both giantin dimer and GRASP65 for the GM130 tethering to Golgi.
One may assume that polarization of Golgi is primarily determined by the cytoskeleton [35]. Certainly, treatment with cytochalasin D (actin destabilization agent) [36] or nocodazole (microtubule depolymerization agent) [37] results in extensive Golgi fragmentation. However, concurrent lack of different golgins can itself cause significant alterations in the Golgi structure. For instance, during apoptotic Golgi disorganization, caspase-mediated cleavage of several golgins preceded major changes in the organization of both actin and tubulin cytoskeletons [38-41]. In our observations [42], codepletion of giantin and GM130 was sufficient to induce peripheral fragmentation of Golgi.
It has been postulated that giantin cooperates with GM130 via interactions with the vesicle docking protein p115, suggesting a common contribution to the trafficking of coatomer protein complex I (COPI) vesicles [43]. However, this model fails to explain intra-Golgi transportation of COPI in intra-medial- and medial-trans-Golgi directions because, contrary to giantin, distribution of GM130–GRASP65 is restricted to cis-Golgi only [44]. This implies that giantin may interfere with intra-Golgi trafficking independently from GM130–GRASP65. Also, further studies revealed that the response of giantin to different cellular perturbations is distinct from that of GM130 and GRASP65. It is known that the dysfunction of GTPases involved in endoplasmic reticulum (ER)-to-Golgi or intra-Golgi trafficking results in ER stress, loss of Golgi integrity, and collapse of its membranes into the ER [45]. For instance, in cells overexpressing a GTP-restricted mutant of Sarlp (H79G) or treated with different inhibitors of ARF1, brefeldin A (BFA) and AMF-26, most of Golgi resident proteins (including golgin-45, GRASP55, and giantin) relocate partially to the ER. However, both GM130 and GRASP65 are completely retained in the punctate cytoplasmic structures, namely Golgi remnants [46, 47]. A similar effect was observed in cells treated with an inhibitor of GBF1 (guanine nucleotide exchange factor for ARF1), golgicide A [45]. Next, Golgi membranes undergo fusion with ER elements in BFA-treated cells [48]; however, co-treatment with BFA and 50 μM H-89, the protein kinase inhibitor, results in the appearance of only tubular structures toward the ER, where GM130-, GRASP65-, GRASP55-, and p115-positive elements are segregated from giantin-specific tubules [49]. Finally, depletion of GM130 disrupts the association of GRASP65 with the Golgi and reduces its expression; however, it is dispensable for giantin localization and has no impact on cargo transport [16, 18, 50]. Further, the work from our laboratory indicates that the specific vesicular complexes carrying Golgi-resident glycosyltransferases are COPI- and COPII-independent and employ two separate recruitment sites at the Golgi: giantin and the complex of GM130 and GRASP65 [16] (Fig. 2, a and b).
Most of the Golgi-resident proteins are type II membrane proteins consisting of a short cytoplasmic tail at the N-terminus, which is followed by a transmembrane domain, a short stem, and a large catalytic region [51]. The cytoplasmic tail of glycosyltransferases is involved in every intracellular trafficking step, including ER exit [52], Golgi targeting [16], Golgi retention [53-58], and recycling [59]. Different research groups, including ours, have clearly demonstrated that Golgi localization of several resident enzymes requires giantin [16, 20, 34, 60, 61]. On the other hand, other Golgi proteins appear to be GM 130-sensitive [16, 42, 62-65]. Our screening of known giantin-sensitive enzymes revealed the dibasic KK, RR, or KR motifs in their cytoplasmic domain (Fig. 3, left, bold/underlined); however, the N-terminal tail of the GM130–GRASP65-dependent enzymes primarily contains different combinations of hydrophobic motifs (Fig. 3, right, bold/underlined). The only exception was found in α-1,2-mannosidase I (Man-I), whose cytoplasmic domain contains both a strong hydrophobic region and one RR motif; however, giantin depletion has no significant effect on the intra-Golgi position of Man-I [42]. Interestingly, direct interaction with both GRASP65 and GRASP55 was essential for Golgi docking of several members of the p24 family. Importantly, p24a has two C-terminal valine residues that are required for this interaction [66], again confirming the importance of hydrophobic interactions for docking to either GRASPs or GM130 and implying that this phenomenon is applicable not only for Golgi resident enzymes but also for other proteins.
Fig. 3.
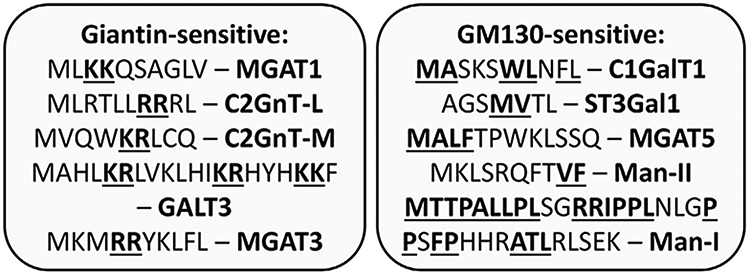
Amino acid sequences of the cytoplasmic domain of Golgi resident enzymes. Dibasic KK, KR and RR motifs in giantin-sensitive enzymes and hydrophobic regions in GM130-sensitive enzymes are bolded/underlined.
MISLOCALIZATION OF CELLULAR ENZYMES OR “THERE AND BACK AGAIN”
In summary here, it appears that the combination of giantin, GM130, and GRASP65 is critical for Golgi homeostasis, and dysfunction of either protein inevitably results in selective mislocalization of resident enzymes, depending on their specificity. In hepatic cells treated with ethanol, the loss of dimeric giantin is associated with the redistribution of MGAT1 to the cytoplasm [42]. For the same reason, in advanced PCa cells, core 2 N-acetyl-glucosaminyltransferase-L (C2GnT-L) was found to be mislocalized, which results in the domination of alternative “pro-metastatic” O-glycosylation via β-galactoside-α-2,3-sialyltransferase-1 (ST3Gal1), which uses GM130–GRASP65 as the Golgi docking site [20]. Furthermore, both in vitro and in vivo data indicate that ethanol-induced fragmentation of Golgi in PCa cells is accompanied by mislocalization of an “anti-metastatic” Golgi enzyme, N-acetylglucosaminyltransferase-III (MGAT3), thus inducing activation of a “pro-oncogenic” Golgi glycosyltransferase, N-acetylglucosaminyltransferase-V (MGAT5), which still remains in the fragmented Golgi membranes [67]. In recent years, multiple observations describe the redistribution of Golgi proteins to different intracellular structures. Nevertheless, the underlying translocation mechanisms are not yet understood, and the Golgi-docking partners for most of the resident enzymes still need to be identified. For example, neutralization of pH in the Golgi results only in the enlargement of the stack, but it does not affect its perinuclear position. Still, it causes a redistribution of different Golgi glycosyltransferases to the ER [68, 69]. Disialoganglioside 3 (GD3) synthase converts ceramide to the ganglioside in the Golgi. This enzyme has been shown to translocate to the mitochondria during apoptosis, where it triggers the swelling of mitochondria, as well as the release of cytochrome c, apoptosis-inducing factor, and caspase 9 [70]. Trip230, a coactivator of the thyroid hormone receptor, is localized predominantly to the vicinity of the Golgi; however, during the cell cycle, it translocates to the nucleus [71]. Conversely, some proteins can be shifted in the opposite, ER-to-Golgi direction. CerS1, one of a family of enzymes responsible for the de novo synthesis of ceramide, is a typical ER-resident that translocates to the fragmented Golgi upon UV light-or dithiothreitol-induced stress [72].
Similar behavior was observed for the Golgi-localized kinases. In PCa cells, ethanol-induced Golgi disorganization induces a shift of giantin-sensitive glycogen synthase kinase 3β (GSK3β) from Golgi to the cytoplasm [61], which results in the downstream activation of cytoplasmic histone deacetylase 6 (HDAC6) and subsequent transactivation of androgen receptor (AR), the driver of prostate tumor cell proliferation. Additionally, under normal conditions, cAMP-dependent protein kinase (cAMP-dPK II) resides in the Golgi, but stimulation of adenylate cyclase by forskolin leads to Golgi disorganization and redistribution of this enzyme to the nucleus. Interestingly, forskolin removal reverses Golgi morphology and restores the intra-Golgi positioning of cAMP-dPK II [73]. Similarly, SOK1, a Ste20 protein kinase of the germinal center kinase (GCK) family, is localized to the Golgi, where it functions in a signaling pathway required for cell migration and polarization. SOK1 drives the apoptotic response to reactive oxygen species (ROS) and chemical anoxia, a model of ischemia characterized by marked ROS production and severe ATP depletion. Importantly, this activation is associated, again, with Golgi disorganization and translocation of SOK1 to the nucleus [74]. Treatment of colon cancer cells with the bile-specific deoxycholic acid alters Golgi structure, induces partial translocation of protein kinase Cη (PKCη) from Golgi to the cytoplasm, and reduces protein secretion [75]. The extracellular signal-regulated kinase 8 (ERK8) localizes to the Golgi but, upon growth factor stimulation, it is segregated from the enlarged Golgi membranes [76]. The same study indicates that depletion of phosphatidylinositol (PI) 4-kinase (PI4KA) results in cytoplasmic Golgi and redistribution of GalNAc-transferase from Golgi to the ER. Similarly, serine/threonine-protein kinase 16 (STK16) resides in the Golgi and regulates actin dynamics, but the depletion or inhibition of STK16 causes Golgi fragmentation [77].
Therefore, kinases may directly interfere with Golgi positioning and localization of various resident proteins. Translocation of the βγ subunit of G-protein-coupled receptors from the plasma membrane to the Golgi is associated with Golgi disorganization and is triggered by protein kinase D (PKD) [78]. This finding is reminiscent of the effects of butanol on Golgi, which are characterized by fragmentation and loss of βIII spectrin in the trans-Golgi, as well as its phosphorylation, presumably by the c-Src kinase [79, 80]. Activation of Src kinase per se has a profound effect on Golgi morphology [81], with the same study showing that pancreatic, tumor-derived cells have a fragmented Golgi phenotype that can be reversed via inhibition of Src. Finally, oxidative stress can induce Golgi scattering [1] and cause phosphorylation of the calcium-dependent phospholipid-binding protein annexin II by the Lyn, a member of the Src kinases, leading to the translocation of annexin II from the Golgi to the ER [82].
On the other hand, growing evidence indicates that different cytoplasmic kinases may dislocate to the Golgi upon disturbance of its ribbon organization. During mitosis-specific Golgi fragmentation, cyclin-dependent kinase 1 (Cdk1–cyclin B) phosphorylates GRASP65, which, in turn, serves as a docking site for the Polo-like kinase 1 (Plk1) [83]. Additionally, GRASP65 can be phosphorylated by ERK, which results in the loss of GRASP65 oligomerization and Golgi cisternal unstacking [84]. Similarly, the timing of the appearance of activated mitogen-activated protein kinase kinase 1 (MEK1) in the Golgi also coincides with the initiation of its mitotic fragmentation [85]. Treatment of cells with the platelet-activating factor stimulates translocation of protein kinase Ca (PKCa) to the Golgi; however, this was not the case in cells with Golgi collapsed by pre-treatment with BFA [86].
GOLGI RESIDENT PROTEINS: ARE THEY THE POWER BEHIND THE THRONE?
At this time, an important question has arisen: what influence do other resident enzymes have on Golgi morphology? In some instances, the silencing of genes for Golgi proteins has little effect on the Golgi morphology. For example, out of 70 human Rab GTPases, 20 proteins showed Golgi localization [87] but, to the best of our knowledge, only Rab1, Rab6a, Rab18, and Rab41 are required for compact and perinuclear Golgi [20, 88, 89]. However, many independent studies have shown that depletion of different Golgi-associated proteins drastically affects Golgi organization, resulting in the loss of ribbon formation and scattering of Golgi elements throughout the cell [90-92]. Nevertheless, these data need to be interpreted tentatively, as not every case of Golgi disorganization induced by the downregulation of its resident proteins can be attributed to their direct contribution to the Golgi architecture. For instance, knockdown of conserved oligomeric Golgi (COG) proteins Cog 2, 4, 6, and 8 compromises the morphological integrity of the Golgi; however, COG complex-deficient cells were not defective in Golgi re-assembly after BFA washout [93].
In many cases, depletion of individual Golgi proteins impairs intra-Golgi trafficking of cargo, followed by stalled protein export to and from the Golgi, resulting in slow ER-to-Golgi trafficking, development of ER stress, and subsequent unfolded protein response (UPR) [94, 95]. Ultimately, sub-lethal ER stress inevitably results in Golgi disorganization because of the impaired trafficking and processing of golgins [6, 96, 97]. Alternatively, ER stress can be initiated by mutations in proteins and their subsequent misfolding and aggregation in the ER, which causes fragmentation of the Golgi and disrupts ER-to-Golgi traffic [98]. For example, depletion of MGAT1 does not impair Golgi positioning and morphology [93]; however, mutated MGAT1, which retains in the ER, results in Golgi disorganization and subsequent selective relocation of other Golgi enzymes to the ER, including Man-II and β1,4-galactosyltransferase 1 (GalT) [99]. Broadly speaking, there is no ER stress without Golgi stress, and vice versa.
Thus, we may postulate that only ER- or Golgi-stress related Golgi disorganization may result in mislocalization of Golgi enzymes and abnormal N- or O-glycan processing [4, 100]. Now, it is becoming clear why neither nocodazole nor cytochalasin D are able to alter glycosylation of cargo, despite destabilization of microtubule or actin network and extensive Golgi de-centralization [7, 101, 102]. Indeed, intra-Golgi transport still can occur in these cells (reviewed by Mironov and Beznoussenko [8]). However, we observed that in the cells treated with these drugs, the giantin-sensitive Golgi enzyme C2GnT-M is still situated in the Golgi [7], which implies that this type of Golgi fragmentation is likely not associated with abrogation of the ER-to-Golgi trafficking and alterations of the golgin network. Remarkably, Golgi may undergo physiological short-time de-centralization similar to that induced by microtubule-disrupting drugs. For instance, this has been observed during differentiation of myoblasts into myotubes [103, 104], or upon delivery of uroplakins to the apical plasma membrane of superficial uroepithelial cells of the urinary bladder [104]. But again, all these types of Golgi remodeling were associated with the impairment of the microtubule organization and redistribution of intermediate filaments rather than alteration of the Golgi scaffold. Quite contrary, BFA-induced Golgi collapse and subsequent ER stress [105] are accompanied by: (i) degradation of most Golgi matrix proteins, including giantin [106], (ii) giantin de-dimerization (our observation, manuscript in preparation), (iii) translocation of Golgi-resident enzymes into the ER, and (iv) abnormal glycosylation [7, 8].
GM130-GRASP65 COMPLEX: A “CRISIS MANAGER”
As we mentioned above, under stress conditions, most of the golgins are located very close to the ER cisternae [22]. Some of them can even be detected in the ER, such as GRASP55 and giantin, which also have been found to lose their dimeric structure; nevertheless, GM130–GRASP65 are detected only in these fragmented Golgi membranes [6, 46, 47]. For example, ethanol-induced Golgi disorganization does induce monomerization of giantin but has no significant impact on the cooperation between GM130 and GRASP65 [6, 61]. In addition, we have shown that giantin, but not GM130 or GRASP65, is required for post-alcohol recovery of compact and perinuclear Golgi, which, in turn, is a prerequisite for successful processing and trafficking of hepatic glycoproteins to the cell surface [107]. Recently, we detected a large fraction of AKT1 kinase in the Golgi of ethanol-treated PCa cells, which, however, was not observed in the cells depleted by GM130 (manuscript in preparation). It seems logical to hypothesize that the Golgi disorganization, the lack of giantin dimer, and the loss of cooperation between giantin and GM130 may stimulate the opening of new GM130-specific docking sites that have been previously masked by giantin (Fig. 2b).
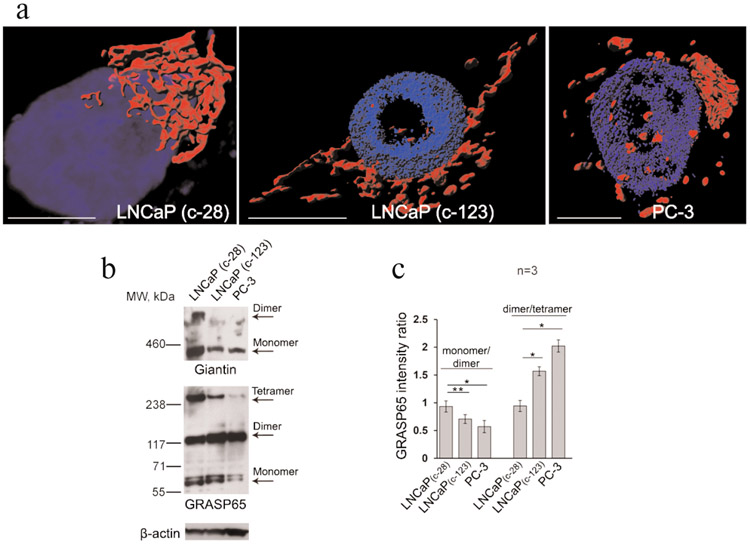
We were mindful of the newly emergent paradigm that GM130 is the main post-ER stress Golgi tether, and observations from other labs validate this concept. Indeed, cells infected with coxsackievirus B3 (CVB3), a causative agent of viral myocarditis, pancreatitis, and meningitis in humans, demonstrate disorganization of the Golgi, which is associated with the complex between coxsackievirus B3 (CVB3) and GM130 [108]. Additionally, Golgi undergoes significant reorganization in the cortical neurons of hibernating animals, which is associated with an increase in the GM130 expression [109]. Another clinically important example of GM130’s interference with Golgi remodeling is found in cancer. The link between carcinogenesis and alterations in Golgi structure is well documented. In different cultured cells lines and tumor tissue sections, Golgi fragmentation was associated with atypical glycosylation, relocation of different Golgi enzymes to the ER, and enhanced metastatic potential of the cells [110-114]. Furthermore, in a mouse model of lung cancer, downregulation of GM130 reduces angiogenesis and cancer cell invasion, as well as induces autophagy, thus promoting cell death [115]. In androgennegative PCa cells, which demonstrate a fragmented Golgi phenotype, the activity of GM130 is directly linked to the formation of pro-metastatic glycan epitopes [20]. Remarkably, despite massive disorganization, stacking of Golgi membranes in these cells is preserved, implying that GRASPs are important for the cancer-specific Golgi remodeling. Indeed, contrary to the androgen-sensitive low-passage LNCaP cells, their high-passage counter-parts and PC-3 cells, which represent advanced androgen-refractory PCa cells, demonstrate fragmented Golgi and reduced giantin dimers [20] (Fig. 4, a and b, top panel). Surprisingly, the levels of GRASP65 tetramers were reduced in both high-passage LNCaP and PC-3 cells and, conversely, the amount of GRASP65 dimer was enhanced (Fig. 4b, lower panel), implying that GRASP65 oligomerization, but not its dimerization, is impaired (Fig. 4, b and c). The precise mechanism of these conformational changes still needs to be determined, and future studies in different cancer cells should reveal whether GRASP55 and other golgins are involved in the maintenance of this Golgi phenotype.
Fig. 4.
a) 3D reconstruction of structured illumination microscopy of Golgi (stained by giantin) in androgen-sensitive low-passage LNCaP (c-28), androgen-refractory high passage LNCaP (c-123), and PC-3 cells; bars, 10 μm. b) Giantin and GRASP65 Western blotting of cell lysates from panel (a); β-actin was a loading control. Oligomerization of GRASP65 and giantin was validated by the sucrose sedimentation analysis. c) Quantification of the intensity of bands corresponding to the GRASP65 monomer, dimer, and tetramer presented as a densitometric ratio to β-actin. Calculations performed within the same exposure, and data represent mean ± SD from three independent experiments; *p < 0.01, **p < 0.001.
THE VICIOUS CIRCLE OF POST-ER STRESS TRAFFIC
The next question involves the impact of Golgi disorganization on secretion. It has been shown that knockdown of GRASPs, alone or combined, can accelerate anterograde protein trafficking through the Golgi membranes, even though it also has negative effects on protein glycosylation and sorting [102]. Similarly, cells treated with the Golgi stress inducer golgicide A demonstrate an enhancement of cargo trafficking despite Golgi fragmentation [45]. Furthermore, oversecretion of matriptase and integrins, the drivers of tumor metastasis, is documented in different types of cancer, whose cells exhibit disorganized Golgi [116, 117]. Additionally, tau protein can be released by neurons, an event linked to the propagation of tau pathology in Alzheimer’s disease. Increased neuronal activity results in Golgi fragmentation, which is mediated by calmodulin-dependent protein kinase (CaMK) and positively correlates with tau release [118]. Surprisingly, upon Golgi disorganization, some Golgi matrix proteins are secreted out of the cells. In hepatocellular carcinoma (HCC) tissues, Golgi is fragmented [113], and Golgi protein 73 (GOLPH2, GP73) is detectable in the serum of these patients. Furthermore, the content of GP73 decreased following surgical resection of HCC lesions but increased with tumor recurrence [119]. Different autoimmune diseases, especially Sjögren’s syndrome, systemic lupus erythematosus, rheumatoid arthritis, and hepatitis B, are characterized by the expression of Golgi autoantigens in the serum [120]. To date, well-characterized Golgi autoantigens include giantin, p230, golgin-160, GM130, p115, golgin-97, and golgin-67 (reviewed by Hong et al. [120]). The nature of this phenomenon remains unknown and could be a subject of future studies that could also address the question whether golgins are involved in extracellular signaling.
Fragmentation of the Golgi apparatus is also observed in other different neurodegenerative diseases, which, in contrast, are characterized by the impaired axonal transport and accumulation of protein aggregates in the cell [121, 122]. This echoes other observations, demonstrating that impairment of Golgi morphology may slow down the intracellular trafficking [91]. Therefore, two alternative scenarios can be envisaged: 1) some proteins are rapidly secreted from the cells, bypassing Golgi [123], or 2) trafficking of other cargo through the fragmented Golgi stacks is accelerated by the tight contacts between ER and Golgi membranes, which otherwise seems non-realistic in cells with perinuclear and compact Golgi due to the segregation of ER and Golgi membranes. From the logical point of view, the latter seems rational, as the fusion of ER and Golgi could rescue intracellular trafficking and processing of aggregated proteins. However, prompt ER-to-Golgi trafficking does not necessarily correlate with the acceleration of subsequent transportation and secretion. Certainly, due to a lack of appropriate maturation and processing, some proteins, especially large cargo like collagen, are stacked in the fragmented Golgi membranes, thus reciprocally augmenting ER stress [6, 124, 125] (Fig. 5). This situation can be described using the chess term “zugzwang”, where a player sees only moves that will damage his position, and yet does not have the option of passing.
Fig. 5.
The “vicious cycle” of Golgi disorganization and ER stress.
Therefore, either elimination of the ER-to-Golgi trafficking or its acceleration, should be considered only as a transient adaptive mechanism, since persistent or severe ER stress inevitably results in the apoptotic cell death [126]. But this is not the case in cancer cells! ER stress and subsequent UPR are known to be pro-survival mechanisms in cancer [127] and we were able to detect tight ER–Golgi contact sites in advanced PCa cells (our unpublished observations). However, it remains to be determined as to how cancer cells maintain ER stress at the sub-lethal level. Perhaps, the answer will come from future studies of the anti-apoptotic mechanisms related to the changes in Golgi architecture. Similarly, despite significant progress in the understanding of the mechanisms that govern Golgi disorganization [7, 20, 82, 128-130], the dynamic localization of proteins in the fragmented Golgi, as well as the trafficking between separate ministacks, still require more attention. While we would not insist that “all roads lead to Golgi”, the unlocking of Golgi paradigms and resolving the complexity of its diversified signals has the potential to open up unprecedented possibilities for new therapeutic approaches for many serious pathologies.
Acknowledgments
Funding. Work from the author’s lab cited here was supported by the K01AA022979-01 and 1R01AA027242-01A1 awards from the National Institute on Alcohol and Alcohol Abuse and the Nebraska Center for Integrated Biomolecular Communication Systems Biology Core (NIGMS P20-GM113126).
Abbreviations:
- BFA
brefeldin A
- COPI (COPII)
coatomer protein complex I (II)
- ER stress
endoplasmic reticulum stress
- GRASPs
Golgi reassembly stacking proteins
- Man-I
α-1,2-mannosidase I
- Man-II
α-mannosidase II
- MGAT1
mannosyl-(α-1,3)-glycoprotein β-1,2-N-acetylglucosaminyltransferase
- PCa
prostate cancer (cells)
Footnotes
Conflict of interest statement. The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Compliance with ethical standards. This article does not contain any studies with human participants or animals performed by the author.
REFERENCES
- 1.Hicks SW, and Machamer CE (2005) Golgi structure in stress sensing and apoptosis, Biochim. Biophys. Acta, 1744, 406–414. [DOI] [PubMed] [Google Scholar]
- 2.Geng J, and Klionsky DJ (2010) The Golgi as a potential membrane source for autophagy, Autophagy, 6, 950–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Micaroni M, Stanley AC, Khromykh T, Venturato J, Wong CX, Lim JP, Marsh BJ, Storrie B, Gleeson PA, and Stow JL (2013) Rab6a/a’ are important Golgi regulators of pro-inflammatory TNF secretion in macrophages, PLoS One , 8, e57034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrosyan A (2015) Onco-Golgi: is fragmentation a gate to cancer progression? Biochem. Mol. Biol. J , 1, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrosyan A, and Cheng PW (2014) Golgi fragmentation induced by heat shock or inhibition of heat shock proteins is mediated by non-muscle myosin IIA via its interaction with glycosyltransferases, Cell Stress Chaperones, 19, 241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrosyan A, Cheng PW, Clemens DL, and Casey CA (2015) Downregulation of the small GTPase SAR1A: a key event underlying alcohol-induced Golgi fragmentation in hepatocytes, Sci. Rep, 5, 17127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrosyan A, and Cheng PW (2013) A non-enzymatic function of Golgi glycosyltransferases: mediation of Golgi fragmentation by interaction with non-muscle myosin IIA, Glycobiology, 23, 690–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mironov AA, and Beznoussenko GV (2011) Molecular mechanisms responsible for formation of Golgi ribbon, Histol. Histopathol, 26, 117–133. [DOI] [PubMed] [Google Scholar]
- 9.Mironov AA, Sesorova IS, Seliverstova EV, and Beznoussenko GV (2017) Different Golgi ultrastructure across species and tissues: implications under functional and pathological conditions, and an attempt at classification, Tissue Cell, 49, 186–201. [DOI] [PubMed] [Google Scholar]
- 10.Munro S (2011) The golgin coiled-coil proteins of the Golgi apparatus, Cold Spring Harb. Perspect. Biol, 3, a005256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin CY, Madsen ML, Yarm FR, Jang YJ, Liu X, and Erikson RL (2000) Peripheral Golgi protein GRASP65 is a target of mitotic Polo-like kinase (Plk) and Cdc2, Proc. Natl. Acad. Sci. USA, 97, 12589–12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linstedt AD, and Hauri HP (1993) Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa, Mol. Biol. Cell, 4, 679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Menarguez JA, Prekeris R, Oorschot VM, Scheller R, Slot JW, Geuze HJ, and Klumperman J (2001) Peri-Golgi vesicles contain retrograde but not anterograde proteins consistent with the cisternal progression model of intra-Golgi transport, J. Cell. Biol, 155, 1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szul T, and Sztul E (2011) COPII and COPI traffic at the ER–Golgi interface, Physiology (Bethesda), 26, 348–364. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura N, Lowe M, Levine TP, Rabouille C, and Warren G (1997) The vesicle docking protein p115 binds GM130, a cis-Golgi matrix protein, in a mitotically regulated manner, Cell, 89, 445–455. [DOI] [PubMed] [Google Scholar]
- 16.Petrosyan A, Ali MF, and Cheng PW (2012) Glycosyltransferase-specific Golgi-targeting mechanisms, J. Biol. Chem, 287, 37621–37627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshi G, Chi Y, Huang Z, and Wang Y (2014) Abeta-induced Golgi fragmentation in Alzheimer’s disease enhances Abeta production, Proc. Natl. Acad. Sci. USA, 111, E1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puthenveedu MA, Bachert C, Puri S, Lanni F, and Linstedt AD (2006) GM130 and GRASP65-dependent lateral cisternal fusion allows uniform Golgi-enzyme distribution, Nat. Cell Biol, 8, 238–248. [DOI] [PubMed] [Google Scholar]
- 19.Jarvela T, and Linstedt AD (2014) Isoform-specific tethering links the Golgi ribbon to maintain compartmentalization, Mol. Biol. Cell, 25, 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrosyan A, Holzapfel MS, Muirhead DE, and Cheng PW (2014) Restoration of compact Golgi morphology in advanced prostate cancer enhances susceptibility to galectin-1-induced apoptosis by modifying mucin O-glycan synthesis, Mol. Cancer Res, 12, 1704–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfeffer SR (2001) Constructing a Golgi complex, J. Cell Biol, 155, 873–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward TH, Polishchuk RS, Caplan S, Hirschberg K, and Lippincott-Schwartz J (2001) Maintenance of Golgi structure and function depends on the integrity of ER export, J. Cell Biol, 155, 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barr FA, and Short B (2003) Golgins in the structure and dynamics of the Golgi apparatus, Curr. Opin. Cell Biol, 15, 405–413. [DOI] [PubMed] [Google Scholar]
- 24.Appenzeller-Herzog C, and Hauri HP (2006) The ER–Golgi intermediate compartment (ERGIC): in search of its identity and function, J. Cell Sci, 119, 2173–2183. [DOI] [PubMed] [Google Scholar]
- 25.Slusarewicz P, Nilsson T, Hui N, Watson R, and Warren G (1994) Isolation of a matrix that binds medial Golgi enzymes, J. Cell Biol, 124, 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hicks SW, Horn TA, McCaffery JM, Zuckerman DM, and Machamer CE (2006) Golgin-160 promotes cell surface expression of the beta-1 adrenergic receptor, Traffic, 7, 1666–1677. [DOI] [PubMed] [Google Scholar]
- 27.Natarajan R, and Linstedt AD (2004) A cycling cis-Golgi protein mediates endosome-to-Golgi traffic, Mol. Biol. Cell, 15, 4798–4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sohda M, Misumi Y, Yamamoto A, Nakamura N, Ogata S, Sakisaka S, Hirose S, Ikehara Y, and Oda K (2010) Interaction of golgin-84 with the COG complex mediates the intra-Golgi retrograde transport, Traffic, 11, 1552–1566. [DOI] [PubMed] [Google Scholar]
- 29.Lieu ZZ, Lock JG, Hammond LA, La Gruta NL, Stow JL, and Gleeson PA (2008) A trans-Golgi network golgin is required for the regulated secretion of TNF in activated macrophages in vivo , Proc. Natl. Acad. Sci. USA, 105, 3351–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taneja TK, Ma D, Kim BY, and Welling PA (2018) Golgin-97 targets ectopically expressed inward rectifying potassium channel, kir2.1, to the trans-Golgi network in COS-7 cells, Front. Physiol, 9, 1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reddy JV, Burguete AS, Sridevi K, Ganley IG, Nottingham RM, and Pfeffer SR (2006) A functional role for the GCC185 golgin in mannose 6-phosphate receptor recycling, Mol. Biol. Cell, 17, 4353–4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamane J, Kubo A, Nakayama K, Yuba-Kubo A, Katsuno T, Tsukita S, and Tsukita S (2007) Functional involvement of TMF/ARA160 in Rab6-dependent retrograde membrane traffic, Exp. Cell. Res, 313, 3472–3485. [DOI] [PubMed] [Google Scholar]
- 33.Veenendaal T, Jarvela T, Grieve AG, van Es JH, Linstedt AD, and Rabouille C (2014) GRASP65 controls the cis-Golgi integrity in vivo, Biol. Open, 3, 431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lan Y, Zhang N, Liu H, Xu J, and Jiang R (2016) Golgb1 regulates protein glycosylation and is crucial for mammalian palate development, Development, 143, 2344–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thyberg J, and Moskalewski S (1999) Role of micro-tubules in the organization of the Golgi complex, Exp. Cell Res, 246, 263–279. [DOI] [PubMed] [Google Scholar]
- 36.May JA, Ratan H, Glenn JR, Losche W, Spangenberg P, and Heptinstall S (1998) GPIIb-IIIa antagonists cause rapid disaggregation of platelets pretreated with cytochalasin D. Evidence that the stability of platelet aggregates depends on normal cytoskeletal assembly, Platelets, 9, 227–232. [DOI] [PubMed] [Google Scholar]
- 37.Turner JR, and Tartakoff AM (1989) The response of the Golgi complex to microtubule alterations: the roles of metabolic energy and membrane traffic in Golgi complex organization, J. Cell Biol, 109, 2081–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherjee S, Chiu R, Leung SM, and Shields D (2007) Fragmentation of the Golgi apparatus: an early apoptotic event independent of the cytoskeleton, Traffic, 8, 369–378. [DOI] [PubMed] [Google Scholar]
- 39.Lowe M, Lane JD, Woodman PG, and Allan VJ (2004) Caspase-mediated cleavage of syntaxin 5 and giantin accompanies inhibition of secretory traffic during apoptosis, J. Cell Sci, 117, 1139–1150. [DOI] [PubMed] [Google Scholar]
- 40.Cheng JP, Betin VM, Weir H, Shelmani GM, Moss DK, and Lane JD (2010) Caspase cleavage of the Golgi stacking factor GRASP65 is required for Fas/CD95-mediated apoptosis, Cell Death Dis., 1, e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker A, Ward C, Sheldrake TA, Dransfield I, Rossi AG, Pryde JG, and Haslett C (2004) Golgi fragmentation during Fas-mediated apoptosis is associated with the rapid loss of GM130, Biochem. Biophys. Res. Commun, 316, 6–11. [DOI] [PubMed] [Google Scholar]
- 42.Casey CA, Bhat G, Holzapfel MS, and Petrosyan A (2016) Study of ethanol-induced Golgi disorganization reveals the potential mechanism of alcohol-impaired N-glycosylation, Alcohol Clin. Exp. Res, 40, 2573–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sonnichsen B, Lowe M, Levine T, Jamsa E, Dirac-Svejstrup B, and Warren G (1998) A role for giantin in docking COPI vesicles to Golgi membranes, J. Cell Biol, 140, 1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakamura N (2010) Emerging new roles of GM130, a cis-Golgi matrix protein, in higher order cell functions, J. Pharmacol. Sci, 112, 255–264. [DOI] [PubMed] [Google Scholar]
- 45.Ignashkova TI, Gendarme M, Peschk K, Eggenweiler HM, Lindemann RK, and Reiling JH (2017) Cell survival and protein secretion associated with Golgi integrity in response to Golgi stress-inducing agents, Traffic, 18, 530–544. [DOI] [PubMed] [Google Scholar]
- 46.Yoshimura S, Yamamoto A, Misumi Y, Sohda M, Barr FA, Fujii G, Shakoori A, Ohno H, Mihara K, and Nakamura N (2004) Dynamics of Golgi matrix proteins after the blockage of ER to Golgi transport, J. Biochem, 135, 201–216. [DOI] [PubMed] [Google Scholar]
- 47.Kim J, Noh SH, Piao H, Kim DH, Kim K, Cha JS, Chung WY, Cho HS, Kim JY, and Lee MG (2016) Monomerization and ER relocalization of GRASP is a requisite for unconventional secretion of CFTR, Traffic, 17, 733–753. [DOI] [PubMed] [Google Scholar]
- 48.Klausner RD, Donaldson JG, and Lippincott-Schwartz J (1992) Brefeldin A: insights into the control of membrane traffic and organelle structure, J. Cell Biol, 116, 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tenorio MJ, Luchsinger C, and Mardones GA (2015) Protein kinase A activity is necessary for fission and fusion of Golgi to endoplasmic reticulum retrograde tubules, PLoS One, 10, e0135260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kodani A, and Sutterlin C (2008) The Golgi protein GM130 regulates centrosome morphology and function, Mol. Biol. Cell, 19, 745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colley KJ (1997) Golgi localization of glycosyltransferases: more questions than answers, Glycobiology, 7, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giraudo CG, and Maccioni HJ (2003) Endoplasmic reticulum export of glycosyltransferases depends on interaction of a cytoplasmic dibasic motif with Sar1, Mol. Biol. Cell, 14, 3753–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osman N, McKenzie IF, Mouhtouris E, and Sandrin MS (1996) Switching amino-terminal cytoplasmic domains of α(1,2)fucosyltransferase and α(1,3)galactosyltransferase alters the expression of H substance and Galα(1,3)Gal, J. Biol. Chem, 271, 33105–33109. [DOI] [PubMed] [Google Scholar]
- 54.Quintero CA, Valdez-Taubas J, Ferrari ML, Haedo SD, and Maccioni HJ (2008) Calsenilin and CALP interact with the cytoplasmic tail of UDP-Gal:GA2/GM2/GD2 β-1,3-galactosyltransferase, Biochem. J, 412, 19–26. [DOI] [PubMed] [Google Scholar]
- 55.Wassler MJ, Foote CI, Gelman IH, and Shur BD (2001) Functional interaction between the SSeCKS scaf-folding protein and the cytoplasmic domain of β1,4-galactosyltransferase, J. Cell Sci, 114, 2291–2300. [DOI] [PubMed] [Google Scholar]
- 56.Schmitz KR, Liu J, Li S, Setty TG, Wood CS, Burd CG, and Ferguson KM (2008) Golgi localization of glycosyltransferases requires a Vps74p oligomer, Dev. Cell, 14, 523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ali MF, Chachadi VB, Petrosyan A, and Cheng PW (2012) Golgi phosphoprotein 3 determines cell binding properties under dynamic flow by controlling Golgi localization of core 2 N-acetylglucosaminyltransferase 1, J. Biol. Chem, 287, 39564–39577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petrosyan A, Ali MF, and Cheng PW (2015) Keratin 1 plays a critical role in Golgi localization of core 2 N-acetylglucosaminyltransferase M via interaction with its cytoplasmic tail, J. Biol. Chem, 290, 6256–6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okamoto M, Yoko-o T, Miyakawa T, and Jigami Y (2008) The cytoplasmic region of α-1,6-mannosyltransferase Mnn9p is crucial for retrograde transport from the Golgi apparatus to the endoplasmic reticulum in Saccharomyces cerevisiae , Eukaryot. Cell, 7, 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stevenson NL, Bergen DJM, Skinner REH, Kague E, Martin-Silverstone E, Robson Brown KA, Hammond CL, and Stephens DJ (2017) Giantin-knockout models reveal a feedback loop between Golgi function and glycosyltransferase expression, J. Cell Sci, 130, 4132–4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manca S, Frisbie CP, LaGrange CA, Casey CA, Riethoven JM, and Petrosyan A (2019) The role of alcohol-induced Golgi fragmentation for androgen receptor signaling in prostate cancer, Mol. Cancer Res, 17, 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Preisinger C, Short B, De Corte V, Bruyneel E, Haas A, Kopajtich R, Gettemans J, and Barr FA (2004) YSK1 is activated by the Golgi matrix protein GM130 and plays a role in cell migration through its substrate 14-3-3ζ, J. Cell Biol, 164, 1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rivero S, Cardenas J, Bornens M, and Rios RM (2009) Microtubule nucleation at the cis-side of the Golgi apparatus requires AKAP450 and GM130, EMBO J., 28, 1016–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song K, Gras C, Capin G, Gimber N, Lehmann M, Mohd S, Puchkov D, Rodiger M, Wilhelmi I, Daumke O, Schmoranzer J, Schurmann A, and Krauss M (2019) A SEPT1-based scaffold is required for Golgi integrity and function, J. Cell Sci, 132, jcs225557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baschieri F, Confalonieri S, Bertalot G, Di Fiore PP, Dietmaier W, Leist M, Crespo P, Macara IG, and Farhan H (2014) Spatial control of Cdc42 signalling by a GM130–RasGRF complex regulates polarity and tumorigenesis, Nat. Commun, 5, 4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barr FA, Preisinger C, Kopajtich R, and Korner R (2001) Golgi matrix proteins interact with p24 cargo receptors and aid their efficient retention in the Golgi apparatus, J. Cell Biol, 155, 885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kubyshkin AV, Fomochkina II, and Petrosyan AM (2018) The impact of alcohol on pro-metastatic N-glycosylation in prostate cancer, Krym. Zh. Eksp. Klin. Med, 8, 11–20. [PMC free article] [PubMed] [Google Scholar]
- 68.Axelsson MA, Karlsson NG, Steel DM, Ouwendijk J, Nilsson T, and Hansson GC (2001) Neutralization of pH in the Golgi apparatus causes redistribution of glycosyltransferases and changes in the O-glycosylation of mucins, Glycobiology, 11, 633–644. [DOI] [PubMed] [Google Scholar]
- 69.Rivinoja A, Hassinen A, Kokkonen N, Kauppila A, and Kellokumpu S (2009) Elevated Golgi pH impairs terminal N-glycosylation by inducing mislocalization of Golgi glycosyltransferases, J. Cell Physiol, 220, 144–154. [DOI] [PubMed] [Google Scholar]
- 70.Rippo MR, Malisan F, Ravagnan L, Tomassini B, Condo I, Costantini P, Susin SA, Rufini A, Todaro M, Kroemer G, and Testi R (2000) GD3 ganglioside directly targets mitochondria in a Bcl-2-controlled fashion, FASEB J., 14, 2047–2054. [DOI] [PubMed] [Google Scholar]
- 71.Chen Y, Chen PL, Chen CF, Sharp ZD, and Lee W H. (1999) Thyroid hormone, T3-dependent phosphorylation and translocation of Trip230 from the Golgi complex to the nucleus, Proc. Natl. Acad. Sci. USA, 96, 4443–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sridevi P, Alexander H, Laviad EL, Min J, Mesika A, Hannink M, Futerman AH, and Alexander S (2010) Stress-induced ER to Golgi translocation of ceramide synthase 1 is dependent on proteasomal processing, Exp. Cell. Res, 316, 78–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nigg EA, Hilz H, Eppenberger HM, and Dutly F (1985) Rapid and reversible translocation of the catalytic subunit of cAMP-dependent protein kinase type II from the Golgi complex to the nucleus, EMBO J. , 4, 2801–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nogueira E, Fidalgo M, Molnar A, Kyriakis J, Force T, Zalvide J, and Pombo CM (2008) SOK1 translocates from the Golgi to the nucleus upon chemical anoxia and induces apoptotic cell death, J. Biol. Chem, 283, 16248–16258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Byme AM, Foran E, Sharma R, Davies A, Mahon C, O’Sullivan J, O’Donoghue D, Kelleher D, and Long A (2010) Bile acids modulate the Golgi membrane fission process via a protein kinase Cη-/protein kinase D-dependent pathway in colonic epithelial cells, Carcinogenesis, 31, 737–744. [DOI] [PubMed] [Google Scholar]
- 76.Chia J, Tham KM, Gill DJ, Bard-Chapeau EA, and Bard FA (2014) ERK8 is a negative regulator of O-GalNAc glycosylation and cell migration, Elife, 3, e01828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu J, Yang X, Li B, Wang J, Wang W, Liu J, Liu Q, and Zhang X (2017) STK16 regulates actin dynamics to control Golgi organization and cell cycle, Sci. Rep, 7, 44607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saini DK, Karunarathne WK, Angaswamy N, Saini, , Cho JH, Kalyanaraman V, and Gautam N (2010) Regulation of Golgi structure and secretion by receptor-induced G protein βγ complex translocation, Proc. Natl. Acad. Sci. USA, 107, 11417–11422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Siddhanta A, Radulescu A, Stankewich MC, Morrow JS, and Shields D (2003) Fragmentation of the Golgi apparatus. A role for βIII spectrin and synthesis of phosphatidylinositol 4,5-bisphosphate, J. Biol. Chem, 278, 1957–1965. [DOI] [PubMed] [Google Scholar]
- 80.Nedrelow JH, Cianci CD, and Morrow JS (2003) c-Src binds αΙΙ spectrin’s Src homology 3 (SH3) domain and blocks calpain susceptibility by phosphorylating Tyr1176, J. Biol. Chem, 278, 7735–7741. [DOI] [PubMed] [Google Scholar]
- 81.Weller SG, Capitani M, Cao H, Micaroni M, Luini A, Sallese M, and McNiven MA (2010) Src kinase regulates the integrity and function of the Golgi apparatus via activation of dynamin 2, Proc. Natl. Acad. Sci. USA, 107, 5863–5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsuda D, Nakayama Y, Horimoto S, Kuga T, fteda K, Kasahara K, and Yamaguchi N (2006) Involvement of Golgi-associated Lyn tyrosine kinase in the translocation of annexin II to the endoplasmic reticulum under oxidative stress, Exp. Cell. Res, 312, 1205–1217. [DOI] [PubMed] [Google Scholar]
- 83.Preisinger C, Korner R, Wind M, Lehmann WD, Kopajtich R, and Barr FA (2005) Plk1 docking to GRASP65 phosphorylated by Cdk1 suggests a mechanism for Golgi checkpoint signalling, EMBO J., 24, 753–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wei JH, and Seemann J (2009) Remodeling of the Golgi structure by ERK signaling, Commun. Integr. Biol, 2, 35–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Colanzi A, Sutterlin C, and Malhotra V (2003) RAF1-activated MEK1 is found on the Golgi apparatus in late prophase and is required for Golgi complex fragmentation in mitosis, J. Cell Biol, 161, 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Faghiri Z, and Bazan NG (2006) Selective relocalization and proteasomal downregulation of PKCa induced by platelet-activating factor in retinal pigment epithelium, Invest. Ophthalmol. Vis. Sci, 47, 397–404. [DOI] [PubMed] [Google Scholar]
- 87.Liu S, and Storrie B (2012) Are Rab proteins the link between Golgi organization and membrane trafficking? Cell. Mol. Life Sci, 69, 4093–4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bard F, Casano L, Mallabiabarrena A, Wallace E, Saito K, Kitayama H, Guizzunti G, Hu Y, Wendler F, Dasgupta R, Perrimon N, and Malhotra V (2006) Functional genomics reveals genes involved in protein secretion and Golgi organization, Nature, 439, 604–607. [DOI] [PubMed] [Google Scholar]
- 89.Dejgaard SY, Murshid A, Erman A, Kizilay O, Verbich D, Lodge R, Dejgaard K, Ly-Hartig TB , Pepperkok R, Simpson JC, and Presley JF (2008) Rab18 and Rab43 have key roles in ER–Golgi trafficking, J. Cell Sci, 121, 2768–2781. [DOI] [PubMed] [Google Scholar]
- 90.Rybakin V, Gounko NV, Spate K, Honing S, Majoul ΙV, Duden R, and Noegel AA (2006) Crn7 interacts with AP-1 and is required for the maintenance of Golgi morphology and protein export from the Golgi, J. Biol. Chem, 281, 31070–31078. [DOI] [PubMed] [Google Scholar]
- 91.Lee JE, Yang YM, Liang FX, Gough DJ, Levy DE, and Sehgal PB (2012) Nongenomic STAT5-dependent effects on Golgi apparatus and endoplasmic reticulum structure and function, Am. J. Physiol. Cell Physiol, 302, C804–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aksnes H, Van Damme P, Goris M, Starheim KK, Marie M, Stove SI, Hoel C, Kalvik TV, Hole K, Glomnes N, Furnes C, Ljostveit S, Ziegler M, Niere M, Gevaert K, and Arnesen T (2015) An organellar Na-acetyltransferase, Naa60, acetylates cytosolic N-termini of transmembrane proteins and maintains Golgi integrity, Cell Rep., 10, 1362–1374. [DOI] [PubMed] [Google Scholar]
- 93.Pokrovskaya ΙD, Willett R, Smith RD, Morelle W, Kudlyk T, and Lupashin VV (2011) Conserved oligomeric Golgi complex specifically regulates the maintenance of Golgi glycosylation machinery, Glycobiology, 21, 1554–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu YX, Liu L, Caffaro CE, and Hirschberg CB (2010) !nhibition of Golgi apparatus glycosylation causes endoplasmic reticulum stress and decreased protein synthesis, J. Biol. Chem, 285, 24600–24608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hong SH, Chang SH, Cho KC, Kim S, Park S, Lee AY, Jiang HL, Kim HJ, Lee S, Yu KN, Seo HW, Chae C, Kim KP, Park J, and Cho MH (2016) Endoplasmic reticulum–Golgi intermediate compartment protein 3 knockdown suppresses lung cancer through endoplasmic reticulum stress-induced autophagy, Oncotarget, 7, 65335–65347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu W, Xu R, Zhang G, Jin J, Szulc ZM, Bielawski J, Hannun YA, Obeid LM, and Mao C (2005) Golgi fragmentation is associated with ceramide-induced cellular effects, Mol. Biol. Cell, 16, 1555–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stieber A, Chen Y, Wei S, Mourelatos Z, Gonatas J, Okamoto K, and Gonatas NK (1998) The fragmented neuronal Golgi apparatus in amyotrophic lateral sclerosis includes the trans-Golgi-network: functional implications, Acta Neuropathol., 95, 245–253. [DOI] [PubMed] [Google Scholar]
- 98.Graves TK, Patel S, Dannies PS, and Hinkle PM (2001) Misfolded growth hormone causes fragmentation of the Golgi apparatus and disrupts endoplasmic reticulum-to-Golgi traffic, J. Cell Sci, 114, 3685–3694. [DOI] [PubMed] [Google Scholar]
- 99.Nilsson T, Hoe MH, Slusarewicz P, Rabouille C, Watson R, Hunte F, Watzele G, Berger EG, and Warren G (1994) Kin recognition between medial Golgi enzymes in HeLa cells, EMBO J., 13, 562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang X, and Wang Y (2016) Glycosylation quality control by the Golgi structure, J. Mol. Biol, 428, 3183–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stallcup KC, Raine CS, and Fields BN (1983) Cytochalasin B inhibits the maturation of measles virus, Virology, 124, 59–74. [DOI] [PubMed] [Google Scholar]
- 102.Xiang Y, Zhang X, Nix DB, Katoh T, Aoki K, Tiemeyer M, and Wang Y (2013) Regulation of protein glycosylation and sorting by the Golgi matrix proteins GRASP55/65, Nat. Commun, 4, 1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lu Z, Joseph D, Bugnard E, Zaal KJ, and Ralston E (2001) Golgi complex reorganization during muscle differentiation: visualization in living cells and mechanism, Mol. Biol. Cell, 12, 795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kreft ME, Di Giandomenico D, Beznoussenko GV, Resnik N, Mironov AA, and Jezernik K (2010) Golgi apparatus fragmentation as a mechanism responsible for uniform delivery of uroplakins to the apical plasma membrane of uroepithelial cells, Biol. Cell, 102, 593–607. [DOI] [PubMed] [Google Scholar]
- 105.Moon JL, Kim SY, Shin SW, and Park JW (2012) Regulation of brefeldin A-induced ER stress and apoptosis by mitochondrial NADP(+)-dependent isocitrate dehydrogenase, Biochem. Biophys. Res. Commun, 417, 760–764. [DOI] [PubMed] [Google Scholar]
- 106.Puri S, Telfer H, Velliste M, Murphy RF, and Linstedt AD (2004) Dispersal of Golgi matrix proteins during mitotic Golgi disassembly, J. Cell Sci, 117, 451–456. [DOI] [PubMed] [Google Scholar]
- 107.Casey CA, Thomes P, Manca S, and Petrosyan A (2018) Giantin is required for post-alcohol recovery of Golgi in liver cells, Biomolecules, 8, E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hawley DA, Schaefer JF, Schulz DM, and Muller J (1983) Cytomegalovirus encephalitis in acquired immunodeficiency syndrome, Am. J. Clin. Pathol, 80, 874–877. [DOI] [PubMed] [Google Scholar]
- 109.Anton-Fernandez A, Leon-Espinosa G, DeFelipe J, and Munoz A (2015) Changes in the Golgi apparatus of neocortical and hippocampal neurons in the hibernating hamster, Front. Neuroanat, 9, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kellokumpu S, Sormunen R, and Kellokumpu I (2002) Abnormal glycosylation and altered Golgi structure in colorectal cancer: dependence on intra-Golgi pH, FEBS Lett, 516, 217–224. [DOI] [PubMed] [Google Scholar]
- 111.Halberg N, Sengelaub CA, Navrazhina K, Molina H, Uryu K, and Tavazoie SF (2016) PITPNC1 recruits RAB1B to the Golgi network to drive malignant secretion, Cancer Cell, 29, 339–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mao P, Nakao K, and Angrist A (1966) Human prostatic carcinoma: an electron microscope study, Cancer Res. , 26, 955–973. [PubMed] [Google Scholar]
- 113.Ghadially FN, and Parry EW (1966) Ultrastructure of a human hepatocellular carcinoma and surrounding non-neoplastic liver, Cancer, 19, 1989–2004. [DOI] [PubMed] [Google Scholar]
- 114.Egea G, Franci C, Gambus G, Lesuffleur T, Zweibaum A, and Real FX (1993) cis-Golgi resident proteins and O-glycans are abnormally compartmentalized in the RER of colon cancer cells, J. Cell Sci, 105 (Pt. 3), 819–830. [DOI] [PubMed] [Google Scholar]
- 115.Chang SH, Hong SH, Jiang HL, Minai-Tehrani A, Yu KN, Lee JH, Kim JE, Shin JY, Kang B, Park S, Han K, Chae C, and Cho MH (2012) GOLGA2/GM130, cis-Golgi matrix protein, is a novel target of anticancer gene therapy, Mol. Ther, 20, 2052–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cooper CR, Chay CH, and Pienta KJ (2002) The role of αvβ3 in prostate cancer progression, Neoplasia, 4, 191–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.List K, Szabo R, Molinolo A, Sriuranpong V, Redeye V, Murdock T, Burke B, Nielsen BS, Gutkind JS, and Bugge TH (2005) Deregulated matriptase causes ras-independent multistage carcinogenesis and promotes ras-mediated malignant transformation, Genes Dev, 19, 1934–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thayer DA, Jan YN, and Jan LY (2013) Increased neuronal activity fragments the Golgi complex, Proc. Natl. Acad. Sci. USA, 110, 1482–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mao Y, Yang H, Xu H, Lu X, Sang X, Du S, Zhao H, Chen W, Xu Y, Chi T, Yang Z, Cai J, Li H, Chen J, Zhong S, Mohanti SR, Lopez-Soler R, Millis JM, Huang J, and Zhang H (2010) Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma, Gut, 59, 1687–1693. [DOI] [PubMed] [Google Scholar]
- 120.Hong HS, Chung WH, Hung SI, Chen MJ, Lee SH, and Yang LC (2004) Clinical association of anti-Golgi autoantibodies and their autoantigens, Scand. J. Immunol, 59, 79–87. [DOI] [PubMed] [Google Scholar]
- 121.Fan J, Hu Z, Zeng L, Lu W, Tang X, Zhang J, and Li T (2008) Golgi apparatus and neurodegenerative diseases, Int. J. Dev. Neurosci, 26, 523–534. [DOI] [PubMed] [Google Scholar]
- 122.Gosavi N, Lee HJ, Lee JS, Patel S, and Lee SJ (2002) Golgi fragmentation occurs in the cells with prefibrillar α-synuclein aggregates and precedes the formation of fibrillar inclusion, J. Biol. Chem, 277, 48984–48992. [DOI] [PubMed] [Google Scholar]
- 123.Grieve AG, and Rabouille C (2011) Golgi bypass: skirting around the heart of classical secretion, Cold Spring Harb. Perspect. Biol, 3, a005298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wolins N, Bosshart H, Kuster H, and Bonifacino JS (1997) Aggregation as a determinant of protein fate in post-Golgi compartments: role of the luminal domain of furin in lysosomal targeting, J. Cell Biol, 139, 1735–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mala JG, and Rose C (2010) Interactions of heat shock protein 47 with collagen and the stress response: an unconventional chaperone model? Life Sci., 87, 579–586. [DOI] [PubMed] [Google Scholar]
- 126.Rao RV, Castro-Obregon S, Frankowski H, Schuler M, Stoka V, del Rio G, Bredesen DE, and Ellerby HM (2002) Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway, J. Biol. Chem, 277, 21836–21842. [DOI] [PubMed] [Google Scholar]
- 127.Madden E, Logue SE, Healy SJ, Manie S, and Samali A (2019) The role of the unfolded protein response in cancer progression: from oncogenesis to chemoresistance, Biol. Cell, 111, 1–17. [DOI] [PubMed] [Google Scholar]
- 128.Petrosyan A, Casey CA, and Cheng PW (2016) The role of Rab6a and phosphorylation of non-muscle myosin IIA tailpiece in alcohol-induced Golgi disorganization, Sci. Rep, 6, 31962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Allan VJ, Thompson HM, and McNiven MA (2002) Motoring around the Golgi, Nat. Cell Biol, 4, E236–242. [DOI] [PubMed] [Google Scholar]
- 130.Petrosyan A, Ali MF, Verma SK, Cheng H, and Cheng PW (2012) Non-muscle myosin IIA transports a Golgi glycosyltransferase to the endoplasmic reticulum by binding to its cytoplasmic tail, Int. J. Biochem. Cell Biol, 44, 1153–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]