Abstract
Coronavirus disease 2019 (COVID-19) has presented substantial challenges to patient care and impacted health care delivery, including cardiac electrophysiology practice throughout the globe. Based upon the undetermined course and regional variability of the pandemic, there is uncertainty as to how and when to resume and deliver electrophysiology services for arrhythmia patients. This joint document from representatives of the Heart Rhythm Society, American Heart Association, and American College of Cardiology seeks to provide guidance for clinicians and institutions reestablishing safe electrophysiological care. To achieve this aim, we address regional and local COVID-19 disease status, the role of viral screening and serologic testing, return-to-work considerations for exposed or infected health care workers, risk stratification and management strategies based on COVID-19 disease burden, institutional preparedness for resumption of elective procedures, patient preparation and communication, prioritization of procedures, and development of outpatient and periprocedural care pathways.
Keywords: Arrhythmia management, Cardiac electrophysiology, COVID-19
Abbreviations: CIED, cardiac implantable electronic device; ECG, electrocardiography; EP, electrophysiology; HCW, health care workers; ICD, implantable cardioverter defibrillator; ICU, intensive care unit; PCR, polymerase chain reaction; PPE, personal protective equipment; PUI, person under investigation; TEE, transesophageal echocardiography
Background
Coronavirus disease 2019 (COVID-19) has presented an unprecedented challenge to the world, impacting everyday living, resulting in widespread international restrictions to combat the global pandemic. Restrictions on travel, schools, businesses, and social gatherings, including lockdowns, were imposed with a singular aim of reducing the spread of this dangerous viral illness. Health care services have been severely impacted, posing challenges to delivery of care as well as to preservation of resources and personal protective equipment (PPE). The need to limit exposure of patients and health care workers (HCWs) has led hospitals to severely limit or eliminate elective or nonurgent services. For many hospitals, meeting the challenges of COVID-19 has resulted in reassignment of hospital beds, repurposing of personnel, and reallocation of financial resources toward care of COVID-19 patients.
In order to manage these evolving challenges, the Heart Rhythm Society, American Heart Association, and American College of Cardiology issued a guidance document to aid electrophysiologists in defining priorities for electrophysiological procedures.1 Such collective efforts from professional societies have helped to minimize patient and health care professional exposure by postponement of elective cases and careful management of urgent or otherwise time-sensitive procedures. Even after 3 months of restrictive measures and vigilant observation, uncertainty remains in forecasting the course of this pandemic, which has seen great regional variability in surge volumes, incidence curve flattening, and outcomes.2 As stay-at-home orders are lifted and businesses reopen, concerns remain regarding the prospect of secondary peaks in disease incidence and the possibility of a continuation or expansion of existing restrictions of clinical services. It is likely that the global pandemic will continue to exert significant effects until resistance to the pathogen is developed through vaccination, herd immunity, or discovery of definitive therapy.
The degree to which patient outcomes have been adversely impacted by delaying the delivery of usual cardiac care, due to resource limitations and/or patient reluctance, is not fully understood. Early data have suggested that cardiac patients presenting with a myocardial infarction or experiencing heart failure may be suffering worse outcomes due to delayed presentations.3, 4, 5 Many chronic diseases and acute medical conditions often require a nonurgent, but time-sensitive, intervention to prevent them from becoming emergencies or having long-term sequelae. Questions remain as to how long one can delay these nonurgent medical interventions to prevent patients from developing undesirable outcomes.
Some of the immediate critical needs of the pandemic response have been met or at least partially addressed. PPE shortages have improved in many regions as a result of the efforts by industry, government, and even individuals to manufacture masks and develop methods to process N95 respirators for reuse. Effective flattening of the curve, sharing of resources across hospital systems, and increased production have eased concerns on ventilator availability. However, the pandemic is far from under control, as access to accurate testing and serology remain limited,6 potentially effective antiviral drugs are being evaluated with limited availability, and candidate vaccines are still in early stages of development and testing.
Given these remaining shortcomings and the still undetermined course of the pandemic, there is uncertainty as to how to resume effectively and deliver much-needed electrophysiology (EP) services for non–COVID-19 arrhythmia patients. COVID-19 will continue to coexist and present significant health care delivery challenges. Many patients remain fearful about exposure in health care settings.3 , 4 Creating a relatively COVID-19 safe clinical care continuum and environment is an important strategy that can regain patient confidence and enable health care institutions to start providing elective cardiovascular7 and EP procedures. “Rebooting” EP at many institutions may be more challenging than “shutting down.” Electrophysiologists may have to work with other services for limited resources and space. This may require hospital leadership understanding the urgency of EP care as it relates to other services.
Understanding regional and local COVID-19 disease status
Accurate tracking, modeling, and understanding of COVID-19 status, as well as collaboration with local, regional, state, and federal authorities, are critical to health care organizations when making informed decisions about the resumption and ramping up of services. Considerations include hospital and intensive care unit (ICU) census, ventilator and PPE availability, and staffing capability. Areas that are more severely affected, where entire hospitals were converted into COVID-19 care units, will likely require a longer time before they will have capacity to provide care for non–COVID-19, nonurgent cases. However, this may be quite different for regions that are less affected and have a significantly lower prevalence and incidence of COVID-19 cases. In general, a significant and sustained drop in local incidence should be observed before health care organizations in areas experiencing a high case level should increase elective medical interventions. The timing and rollout of this process will be dictated by governmental and health system policies. In areas fortunate enough to have avoided a high COVID-19 burden, assiduous attention to ongoing local COVID-19 incidence will be essential to managing the reboot process and the need to respond rapidly if a second wave occurs. Accordingly, resumption of nonurgent EP services should be approached in a measured and cautious manner. Contingency plans and specific criteria to limit or stop elective cases in the event of a second wave should be predefined in advance of reopening in compliance with local regulations.
Role of screening and diagnostic viral testing
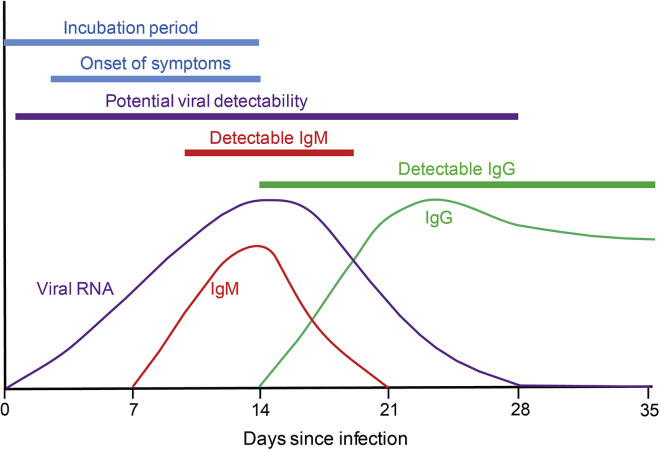
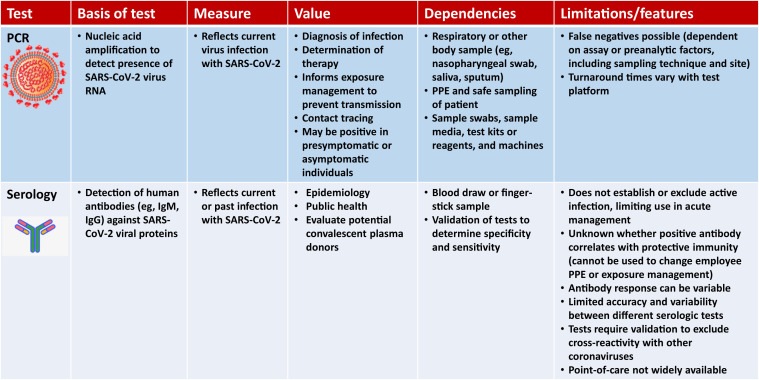
Testing for COVID-19 infection is a critical tool as we embark on safely restarting elective and semi-elective procedures. Figure 1 illustrates a model for the evolution of detectable virus and virus-specific immunoglobulin during severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Our current understanding regarding the limitations of these tests and how the timing of results over the course of exposure or infection impacts the interpretation of test results are shown in Figure 2 .
Figure 1.
Representative model illustrating the presence of viral RNA, IgM, and IgG in the human body over time after infection with SARS-CoV-2. Understanding of this is particularly important when using polymerase chain reaction (PCR) or serologic testing as tools to identify whether an individual is actively infected, in convalescence, or in a watershed time period, when test results have to be interpreted with care.
Figure 2.
The differences between polymerase chain reaction (PCR) and serologic testing as well as features and limitations that need to be understood prior to using them and incorporating them into the reboot testing and workflow. PPE = personal protective equipment.
Patient screening and diagnostic testing are important tools to limit patient and staff exposure. However, lack of widespread access to timely and accurate viral testing has been a major limitation from the onset of the pandemic,6 and there will likely be persistent variations in regional availability of testing, greatly affecting our ability to identify infected individuals, schedule cases, prevent disease transmission, and clarify policies that will minimize the risk of restarting elective procedures.
Viral testing
The test platforms now available have different advantages and limitations, including differences in turnaround time and throughput. Fortunately, false positive rates are low for established viral polymerase chain reaction (PCR) tests.8 However, sensitivity of PCR-based viral testing varies among platforms. Significantly variable false negative rates from available test kits have been reported, which may be compounded by sampling limitations and variation in presence of virus at different sites during the course of the disease.9 These considerations may prompt repeat testing6 , 10 when there is evidence of exposure, typical symptoms, or clinical presentation (although atypical symptom presentations are well described).11 Whether a positive PCR test late in the course of the disease in an asymptomatic person represents detection of noninfectious viral particles or transmissible virus remains uncertain.
Serologic antibody testing
Serologic antibody testing may supplement viral detection by PCR when available,12 , 13 but it remains controversial and susceptible to misinterpretation when used to make decisions related to individual patient management. PCR viral testing may be more accurate than IgM for assessment of early stages of infection. IgG may be more helpful in the identification of prior infection over time (Figure 1). Unfortunately, limitations in sensitivity and specificity associated with serologic testing, when compared with COVID-19 viral testing, could result in improper clinical decisions. False positive serology tests, uncertainties about whether true seropositivity confers protection from reinfection, and the potential for continued viral transmissibility could create a false sense of security. While ongoing research may clarify these issues and serologic testing will likely continue to improve, at this time, seropositivity should not be used to determine decreased standards for PPE or other containment approaches. Given the above limitations of viral and antibody testing, all patients regardless of the test results should be treated with universal precautions.
Developing testing policies for EP care
A major concern inhibiting patients from coming to hospitals is the fear of contracting COVID-19, as both patients and HCWs can be asymptomatic carriers with the potential to infect other patients and health care staff.11 The availability and implementation of universal testing policies for patients prior to procedures and for HCWs, as well as universal masking, sanitization, and hand hygiene, can favorably impact confidence.
Institutions will need to define standardized and comprehensive protocols for testing, including testing prior to planned procedures. Electrophysiologists, laboratory managers, and outpatient clinical team leaders should define workflow processes for preprocedure testing and operational plans concordant with hospital and local policies. For many institutions, testing policies will be extensive and include multiple locations, such as clinics, procedural areas (eg, cardiac catheterization laboratory, endoscopy suite, interventional radiology suite, etc), and off-site locations (including drive-through testing). Accommodations to testing will need to be based on a patient’s clinical condition, geographic location, inpatient versus outpatient status, type and urgency of intervention, test capability, and local conditions.
Ideally, viral PCR testing should be performed within 12–72 hours prior to the procedure, whenever feasible, to allow sufficient time for test results to be obtained and reviewed in the event that positive test results may change procedure planning. Mandatory preoperative isolation for the period between testing and procedure performance is important to mitigate the possibility of infection. If preoperative testing is unavailable locally in a hospital or health care system, and yet patients are to undergo nonurgent procedures, then alternative screening methods must be established in conjunction with the health care system and local public health officials. Regardless of the availability of testing, all organizations should utilize mandatory symptom screening, temperature analysis, and mandatory masking.
Postoperative or postprocedure COVID-19 testing may need to be considered in patients who develop symptoms after the procedure is performed. Atelectasis, fever, and volume overload are not uncommon in the postoperative period. Establishing operational guidelines for COVID-19 testing in these patients and management of testing results should be determined.
Testing and return to work for health care workers
Transmission of COVID-19 to exposed HCWs has been documented. Since a negative test does not preclude subsequent infection, even soon after testing, periodic viral testing for asymptomatic HCWs is not currently a standard approach, but enhanced surveillance of HCWs for even mild symptoms, fever, or a history of exposure and universal masking has generally been adopted. This may reduce patient fear of developing hospital-acquired COVID-19 infection. Quarantine of HCWs with confirmed or suspected COVID-19 and return-to-work criteria should follow Centers for Disease Control and Prevention (CDC) guidelines or local policy.14
Active viral shedding remains a possibility for asymptomatic individuals with positive IgM and/or IgG serology.15 Where available, viral testing should be performed to help determine whether the HCW is in true convalescence without active viral shedding. Some institutions offer serology testing for HCWs, which may suggest exposure to coronavirus, but whether antibodies confer immunity to recurrent infection is unproven.
Risk stratification and management strategies based on COVID-19 disease burden
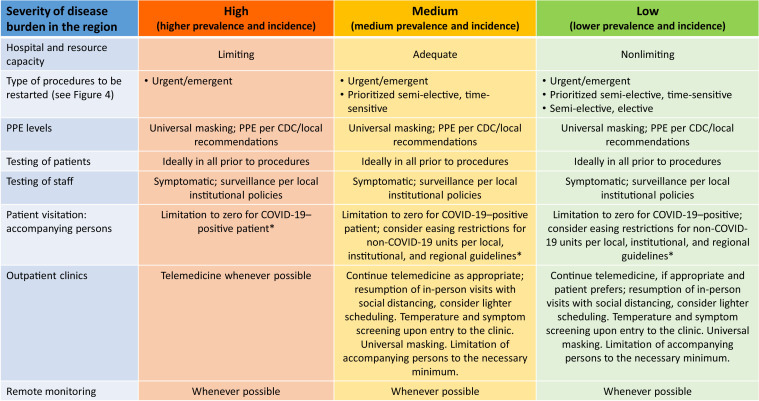
The ability to perform elective or semi-elective cases is highly dependent on the COVID-19 burden in each region. Regional risk can be categorized based on the severity of disease burden, the state of resource utilization, and projections (Figure 3 ), as follows: (1) high prevalence and incidence, (2) medium prevalence and incidence, and (3) low prevalence and incidence. These factors could impact whether the health care systems in a region have the capacity to start engaging in elective procedural or medical care.
Figure 3.
Framework for categorizing various aspects of EP reboot based on severity of regional infection. ∗With exceptions, according to Centers for Disease Control and Prevention (CDC) or local guidelines. PPE = personal protective equipment.
Institutional preparedness for resuming elective procedures
The availability of PPE and resources remains a major consideration in the timing of resumption of semi-elective and elective procedures. Adherence to PPE policies according to CDC and local policies remains critical for HCWs in the hospital and operating/procedure rooms. Supply chains for PPE needed for aerosol, airborne, droplet, and contact precautions should ideally project and maintain adequate supplies for COVID-19–related care in addition to covering the extra PPE needs for elective procedures. Comprehensive hospital-wide multispecialty operational committees and leadership that can oversee this process are valuable and include supply chain, data analytics, strategic planning, quality assessment, infection prevention, and clinical expertise. Patients and HCWs will need to continue using PPE, particularly masks, until community spread of the virus has reduced below threat level and when local and federal regulations dictate.
Important data needed to inform health care system planning include the following: bed, ICU, and ventilator capacity; procedural and recovery room availability; depth of diagnostic and laboratory services; and cleaning capacity. Housekeeping in all clinical areas along the continuum of care should be addressed (eg, clinic, preoperative, EP laboratories, recovery areas, ICUs, ventilators, transesophageal echocardiography probes, cardiac implantable electronic device [CIED] programmers, etc). Operating room and procedure schedules and staffing will need to be flexible to accommodate the influx of cases. Modifications may include limiting block time assignments to increase open scheduling time or extending hours of elective procedure scheduling. Repurposed rooms may need to be refitted with appropriate equipment to expand and return to prior capacity for specific procedures. The need to prioritize scheduling of cases according to urgency and need for hospitalization will continue. Ideally, preprocedure testing of patients will be performed outside of the preoperative assessment areas to facilitate appropriate preservation of PPE. Preferably, registration, pre- and postprocedural areas, and EP laboratories should ideally be in near proximity to minimize exposure during transport. Appropriate staffing and reorientation of redeployed staff to new and old processes is important, and case scheduling escalation increasing the caseload should be implemented gradually to allow time for assessment of impact on COVID-19 positivity and transmission. Of note, changes in use of PPE and other related precautions may increase procedure times. Adequate HCW staffing should be anticipated to accommodate a COVID-19 surge should a second wave occur.
Patient preparation and communication
Many physician practices have shifted to telehealth platforms to communicate with their patients and provide medical care. Informing patients of the organizational processes instituted for minimizing exposure to COVID-19 and the facility’s preparedness for restarting elective cases can help to allay patients’ fears on coming into hospital or clinic facilities. Honest and open communication about infection mitigation strategies, available testing options, and specific institutional plans can help the patient to decide whether to proceed with an elective procedure.
Shared decision-making concerning the risks and benefits of moving forward with procedural options versus continuing noninvasive approaches is critical. Shared decision-making should ideally be documented in the electronic medical records. Scheduling decisions are more complex, as they not only involve the provider and patient, but also public health considerations. If the procedure is being considered in a high (or medium) prevalence region (Figure 3), an elective procedure may be delayed longer even if the patient and provider (usual shared decision participants) would like to proceed.
Once a conversation is completed and the patient agrees to proceed, written instruction on preprocedural care notes, location of preanesthesia/COVID-19 testing, and details of periprocedural care can be helpful to send to the patient. Policies may continue to limit the number of family members who can accompany the patient. Accordingly, appropriate arrangements will be required for drop off and pick up of the patient, avoiding areas with known COVID-19–positive patients. Timely updates on patient condition and procedural status can be facilitated by a dedicated patient navigator or communicator who can be readily accessible for family members to call. Written instructions of routine procedural care and COVID-19 prevention strategies are helpful.
Prioritizing procedures
The suspension of elective cases due to COVID-19 has resulted in an accumulation of deferred EP procedures. The ethical values used to prioritize procedures need to balance public health societal concerns with the commitment to the individual patient. For example, the risks of postponing a procedure in an individual should be fully weighed against the risk of further COVID-19 spread. Transparency and communication regarding scheduling decisions are essential for patient and community trust. For COVID-19–positive patients, nonemergent cases should be delayed until recovery or a change in the patient’s condition warrants reconsideration.
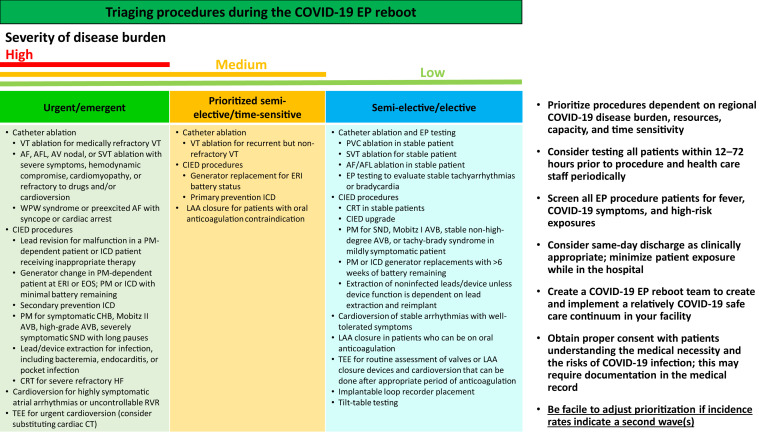
Procedure prioritization is essential and contingent upon facility capacity and the nature of deferred procedures, as well as regional or local policy and restrictions. All emergent or urgent procedures should take precedence, followed by semi-urgent or time-sensitive procedures, followed by elective procedures.1 The triaging of these procedure categories will vary with geographic and temporal variations in COVID-19 burden (Figure 4 ). The ultimate decision regarding the time sensitivity of a procedure is based on clinical judgment and individual patient factors. For many tertiary referral institutions, communication to other hospitals and referring physicians about availability will be vital to ensure that all patients are prioritized according to medical need. Prioritizing inpatient procedures may minimize the need to reschedule later visits while reducing exposure and testing. Inpatient procedures will require similar preprocedural COVID-19 testing according to local policies. Other considerations include the availability of the anesthesia team, whose personnel may have been repurposed to covering ICUs to care for sick COVID-19 patients; case type and how further delay might impact patient outcomes; how long patients have already been waiting; and procedure risk, given how this might impact bed or resource needs if complications result in prolonged hospitalization or ICU stays. One should ensure appropriate follow-up to assure there is no further deterioration of clinical status. Attention to local, state, and federal orders should also be considered, as some geographies may have ongoing restrictions limiting the scheduling of elective procedures and surgeries. In addition, some hospitals may require physician attestation about medical necessity for a time-sensitive procedure.
Figure 4.
List of EP procedures based on urgency and triaging the workflow during reboot. The ultimate decision regarding the time sensitivity of a procedure is based on clinical judgment and individual patient factors. AF = atrial fibrillation; AFL = atrial flutter; AV = atrioventricular; AVB = atrioventricular block; CHB = complete heart block; CIED = cardiac implantable electronic device; CRT = cardiac resynchronization therapy; CT = computed tomography; EOS = end of service; EP = electrophysiology; ERI = elective replacement indicator; HF = heart failure; ICD = implantable cardioverter defibrillator; LAA = left atrial appendage; PM = pacemaker; PVC = premature ventricular contractions; RVR = rapid ventricular response; SND = sinus node dysfunction; SVT = supraventricular tachycardia; TEE = transesophageal echocardiography; VT = ventricular tachycardia; WPW = Wolff-Parkinson-White.
Outpatient care pathways
COVID-19 has successfully moved many clinical practices to adopt digital telehealth platforms into care pathways to minimize patient exposure. This model, though initially cumbersome, has proved to be a useful means of providing continued care for our patients. Similarly, remote monitoring has continued to be a valuable resource for patients with CIEDs. Reestablishment of in-person visits will vary with geographic and temporal variation of viral incidence. Use of PPE for patient-facing outpatient clinic visits should continue per CDC and local authority guidelines. Clinic areas should be configured to comply with regional social distancing directives.
In regions with high COVID-19 burden, in-person clinic visits may still need to be minimized, using telehealth options. The majority of incision-site inspections following CIED implantation or catheter ablation can be managed via telehealth by inspecting the site, utilizing a video conference, or asking the patient to send a picture via secure email, often in conjunction with a few simple questions. Similarly, many of the clinic follow-ups and some new consults can be performed via telehealth, leveraging electronic medical record data and obtaining vital signs and electrocardiography (ECG) tracings using digital wearables where available. As the number of app-based technologies evolves, they will continue to be an integral part of telehealth. Examples of low-risk patients for whom in-person visits could be deferred include asymptomatic patients with satisfactory CIED battery longevity and primary prevention implantable cardioverter defibrillator (ICD) patients without symptoms suggesting worsening of heart failure or arrhythmia burden. Patients on antiarrhythmic drugs, such as dofetilide, that require QTc and laboratory monitoring may need to defer testing if prior values and their clinical condition have remained stable and if no new drugs that may prolong the QTc have been added. In addition, remote monitoring may offer a valuable supplement or perhaps short-term alternative to ECG in some situations where QTc and clinical condition have remained previously stable. Patients with borderline values may need continued access to ECG and laboratory testing. Some studies have evaluated the use of mobile ECG devices for QTc monitoring.16 , 17 As the pandemic eases, exceptions to use of less secure platforms may change; practitioners are advised to remain up to date on current reimbursement and documentation requirements.
Other urgent or semi-urgent clinical indications can be evaluated in person on an individualized basis. These might include patients with worsening heart failure associated with an uncontrolled arrhythmia; significant arrhythmia symptoms; a need for device reprogramming; ICD patients with recent shocks or syncope; or CIED patients with recent symptoms suggesting possible device malfunction (eg, syncope or heart failure exacerbation) or suspected device infection. Select patient populations, such as vulnerable infants and children with arrhythmias, may also warrant in-person evaluation. When possible, in-person visits and procedures should be coordinated on the same day to minimize multiple exposures for the patient. Patients presenting for outpatient visits and HCWs should be masked, and measures should be taken to screen for concerning symptoms (eg, fever, cough). Practitioners should be aware that COVID-19 may present with atypical symptoms, including diarrhea, anorexia, anosmia, and multisystem inflammatory disease consisting of but not limited to a rash, lymphadenopathy, swelling of hands and feet, and mucus membrane changes, which have been seen in children and adolescents.18, 19, 20, 21 If suggestive symptoms or a fever are present, patients should be redirected to an appropriate screening clinic or facility, with appropriate measures taken, including testing for COVID-19, or clinics should follow local policies (Figure 2).
In-person CIED interrogation
Depending on the regional stage of the pandemic, local, hospital, and departmental guidance may vary. In regions with continuing concern for pandemic spread, in order to minimize exposure of EP staff and device manufacturer representatives to patients with suspected or confirmed COVID-19 infection, it is prudent to consider limiting in-person CIED interrogations to the following indications:
-
•
Clinically actionable suspected CIED abnormality
-
•
Need for reprogramming
-
•
Evaluation of potential arrhythmic symptoms or alerts in patients without access to remote monitoring
Potential strategies to maintain social distancing include reconfiguring waiting areas and/or notifying patients when it is time for them to be seen. Importantly, device interrogation programmers, cables, and wands should be disinfected between all patients. Plastic sleeves to cover the cable and wand may also be considered. It may be helpful to inform patients of the disinfecting procedures being systematically performed between visits.
Remote device monitoring
A current expert consensus statement gives remote monitoring a class I recommendation for routine use in patients with CIEDs22 based on multiple studies demonstrating reduction of unnecessary ICD therapies and mortality.22, 23, 24, 25 Despite its effectiveness, prior to the pandemic, remote monitoring was significantly underutilized due to a variety of patient- and system-based issues.23 During the pandemic, use of remote monitoring is even more important and should be used in most circumstances to reduce the need for nonurgent clinic visits. When feasible, remote monitoring should be reconsidered in patients who are currently not enrolled.
Creating relatively COVID-19 safe EP care pathways
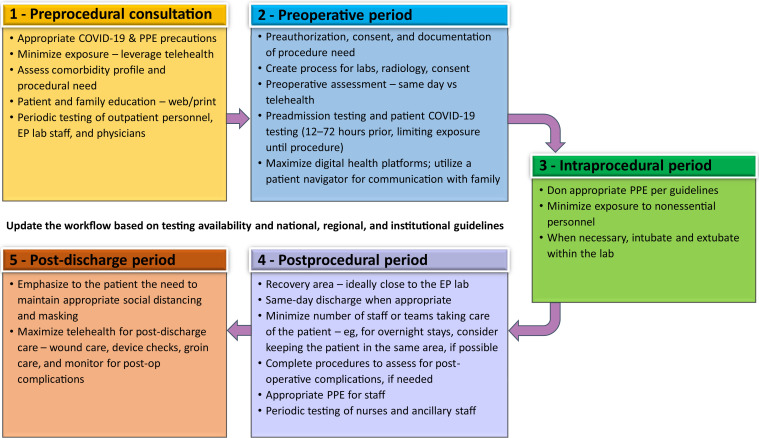
Quality improvement programs and care pathways can help to standardize and support safe, high-quality, high-value patient care. Risk-adjusted data can be used to evaluate patient care outcomes. Based on principles discussed, an example of a stepwise care pathway is summarized as follows (Figure 5 ):
Figure 5.
Stepwise approach to creating a care continuum for EP reboot. PPE = personal protective equipment.
Step 1: Initial consultation for an intervention
-
•
All appropriate COVID-19 precautions should be followed.
-
•
Unnecessary exposure of the patient to the clinic or hospital environment can be minimized by carefully prioritizing the problem and utilizing telehealth platforms wherever necessary.
-
•
The patient’s comorbidity profile should be assessed in the event that there is a potential procedural complication and the remote possibility that the patient may acquire COVID-19 infection during the periprocedural period.
-
•
Appropriate patient education should be provided, potentially through web resources, with thorough orientation to the health care environment and review of the patient’s clinical situation. Greater transparency will help the patient understand the risks, benefits, and alternatives to the planned intervention.
-
•
During outpatient clinic visits, universal masking and social distancing, which may require blocking off or rearranging waiting or exam room seating and/or limiting the number of family members accompanying the patient to a maximum of one or per local policy, should continue. Engaging other family members via telehealth video options while the patient is seeing the clinician in consultation is a way to involve them in the process.
Step 2: Preoperative period
-
•
After a decision to intervene has been made, prior authorization should be completed as necessary. In some hospitals or states, attestation to the necessity of the procedure during the COVID-19 pandemic may be necessary.
-
•
Formulation of guidance for when previously obtained laboratory testing, diagnostic imaging, history and physical, and consent can be utilized is helpful to determine whether these need to be repeated for rescheduled procedures that were previously deferred.
-
•
Avoidance of elective interventions on COVID-19–positive patients, persons under investigation (PUIs), or patients with a high comorbidity profile should be considered.
-
•
Use of telehealth or consolidation of preoperative assessment to the same day of the procedure in the preoperative area can help minimize patient exposure, if there is no significant change in patient’s clinical status.
-
•
Preoperative COVID-19 testing should be performed within 12–72 hours before the procedure, when feasible; patients should be advised to maintain isolation between the time of testing and the planned procedure. For emergent procedures when rapid testing is not feasible, patients should be treated as PUI with use of appropriate PPE. All of the preoperative testing should be consolidated as much as feasible. Determining the pathway for follow-up and reporting of results to the patient and procedure team is important, along with standardized recommendations for patients who test positive for COVID-19.
-
•
Limiting or minimizing companions in procedure facilities may still be required. Initial limitations to zero accompanying companions may be able to be relaxed to one or minimal family members or friends later in the evolution of the COVID-19 pandemic. Telehealth digital platforms can be used to communicate with and update the patient’s family. A patient navigator is an excellent resource, providing a single point of contact. Active discharge planning ahead of time can facilitate arrangements for resources needed after the procedure.
Step 3: Intraoperative period
-
•
PPE use should follow CDC or hospital guidelines, similar to rules earlier in the COVID-19 pandemic.
-
•
Each procedure patient is a PUI unless tested otherwise.
-
•
The number of personnel that are in contact with the patient should be minimized, if possible, especially for COVID-19–positive patients or PUIs.
-
•
Anesthesiology and EP care teams should take appropriate aerosol/airborne/droplet precautions. Patients can be extubated in the laboratory and then transported to the recovery area.
-
•
Smoke evacuators may be considered for procedures using electrocautery in COVID-19–positive patients.
Step 4: Postoperative period
-
•
If available, recovery in a perioperative unit that is close to the EP laboratories can minimize patient transport and recovery within a relatively COVID-19 safe environment, minimizing their exposure to other patients or hospital personnel who are not involved in their care.
-
•
When possible, same-day discharge should be considered. If patients need to be monitored overnight, they could potentially stay in the same room and be discharged the next day, if possible, to minimize contact.
-
•
Periodic viral PCR or serologic testing may become routine for HCWs in these periprocedure areas, including the housekeeping and dietary staff.
-
•
Prohibiting or minimizing family members or accompanying persons in the recovery area can reduce unnecessary exposure. Exceptions may be considered for minors or adults with special needs. Patients can be transported to a pick-up area where the discharging staff member or the patient coordinator can meet the caretaker to review the discharge instructions.
-
•
Patient needs and the potential for delayed complications should be anticipated, and appropriate standard of care testing (eg, chest X-rays, device checks, echocardiograms if needed) should be performed before discharge, especially for same-day discharges.
-
•
PPE use should be per guidelines.
Step 5: Post-discharge period
-
•
Maintaining social distancing and universal masking remain important for patients.
-
•
Patients should be educated and reminded about the importance of avoiding COVID-19 exposure and infection during the recovery phase.
-
•
There should be a single easy mechanism through which patients can get in touch with their EP care team to address any postprocedural concerns.
-
•
Incision checks, device checks, and even post-ablation follow-up in most cases can be performed using telehealth platforms.
COVID-19 EP reboot team
When possible, an EP COVID-19 working group representing the stakeholders involved in the EP care continuum pathway can coordinate with institutional or hospital-level COVID-19 leadership. The group may include an electrophysiologist, EP laboratory manager, outpatient clinic manager, EP nurse, advanced practice providers, device technician, anesthesiologist, and imaging team to provide insights into various aspects of the workflow. This team can clarify, interpret, iterate and disseminate policies, and also provide the necessary operational support to plan and successfully execute the reboot process as the efforts to contain COVID-19 continue. Effective communication with the rest of the EP team, the extended cardiology team, and other relevant clinical and hospital/health care system teams is essential. A logical and methodical approach to easing the restrictions and slowly revamping work without causing major disruptions to the work done by other care teams is extremely important. Coordination with other hospital COVID-19 teams developing similar pathways would be synergistic. Regularly scheduled appraisals of the process and adjustments should be made to fit the needs of the facility and the care teams. Decisions should be data driven. Transparency and data sharing with other teams should be encouraged so that all teams and patients benefit from the collective experiences. Establishing institutional programs to assess successes and failures so that consistent progress occurs is advantageous. Institutional teams should take a lead in understanding and implementing regulatory body policies, new information on testing, changes in PPE guidance, patient waitlists, insurance and prior authorization issues, and implementation of periodic and timely communications with the patients while keeping abreast of the ground situation of COVID-19 in the region.
Anticipating and managing the second wave
This pandemic is far from being over. As the stay-at-home orders are lifted and more people emerge from social isolation or fail to practice masking or social distancing, human-to-human spread may surge and there could be second or even recurring waves of the COVID-19 pandemic. Health care teams and hospitals must continue to be prepared and preserve adequate resources for such contingencies. Appropriate planning for dealing with a second wave should be a mandatory component of the elective reboot plan. We will have to learn to create relatively COVID-19 safe zones within the hospitals to help isolate patients from second waves and yet be able to provide regular care for non–COVID-19 patients.
Conclusions
COVID-19 has presented health care systems across the globe with novel challenges. As EP professionals, we need to determine how we can minimize the ravages of living with COVID-19 while ensuring that we provide exemplary care to our arrhythmia patients across all age-groups. In this document, we have tried to provide EP clinicians and institutional administrators with a series of guiding suggestions and principles to move forward as we start the “reboot” to provide necessary heart rhythm care to our patients, which has understandably and appropriately been delayed. Our main goal as health care professionals, whether we serve in a clinical, teaching, research, or administrative role, is to do everything we can to create a safe environment for our patients so that they receive the excellent care they deserve.
Acknowledgments
The American College of Cardiology Electrophysiology Council contributed to the development of and endorses the document.
Footnotes
For copies of this document, please contact the Elsevier Inc. Reprint Department (reprints@elsevier.com). Permissions: Multiple copies, modification, alteration, enhancement, and/or distribution of this document are not permitted without the express permission of the Heart Rhythm Society. Instructions for obtaining permission are located at https://www.elsevier.com/about/our-business/policies/copyright/permissions.
This article has been copublished in Circulation: Arrhythmia and Electrophysiology and the Journal of the American College of Cardiology: Clinical Electrophysiology.
Correspondence: Heart Rhythm Society, 1325 G Street NW, Suite 400, Washington, DC 20005. E-mail address: clinicaldocs@hrsonline.org. Dr Dhanunjaya Lakkireddy, Kansas City Heart Rhythm Institute and Research Foundation, HCA Midwest Health, 5100 W 105th Street, Suite 200, Overland Park, KS 66211. E-mail address: dhanunjaya.lakkireddy@hcahealthcare.com.
© 2020 The Heart Rhythm Society, the American Heart Association, Inc., and the American College of Cardiology Foundation.
Supplementary data associated with this article can be found in the online version at .https://doi.org/10.1016/j.hrthm.2020.06.012.
Appendix
Appendix 1.
Author disclosure table
| Writing group member | Employment | Honoraria/speaking/consulting | Speakers’ bureau | Research∗ | Fellowship support∗ | Ownership/partnership/principal/majority stockholder | Stock or stock options | Intellectual property/royalties | Other |
|---|---|---|---|---|---|---|---|---|---|
| Dhanunjaya R. Lakkireddy, MD, FHRS (Co-Chair) | Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, Kansas | 1: BIOTRONIK; 2: Abbott | 1: Abiomed; 1: Biosense Webster; 1: Boston Scientific;2: Janssen | None | None | None | None | None | None |
| Mina K. Chung, MD, FHRS (Co-Chair) | Heart, Vascular, and Thoracic Institute and Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio | 2: ABIM | None | 5: AHA; 5: NIH | None | None | None | 1: Elsevier; 1: UpToDate | 0: AHA (Chair, ECG & Arrhythmias Committee; Member, Clinical Cardiology Leadership Committee; Member, Committee on Scientific Sessions Programming); 0: Amarin (Data Monitoring Committee Member); 2: AHA (Associate Editor, Circulation: Arrhythmia and Electrophysiology) |
| Christine M. Albert, MD, MPH, FHRS | Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, California | None | None | 5: Abbott; 5: NIH; 5: Roche Diagnostics | None | None | None | None | None |
| Thomas F. Deering, MD, MBA, FHRS | Piedmont Heart Institute, Atlanta, Georgia | 1: Abbott (Adjudication Committee for IDE Trial) | None | 0: Abbott; 0:BIOTRONIK; 0: Boston Scientific; 0:CVRx, Inc.; 0: HUYA Bioscience International; 0: Medtronic; 0: Milestone | None | None | None | None | 0: EHRA (Speaker at annual Scientific Sessions); 0: ACC (Speaker at annual Scientific Sessions & other meetings); 0: HRS (Past President) |
| Laurence M. Epstein, MD | Northwell Health, Manhasset, New York | 1: Abbott; 2: Medtronic; 2: Spectranetics Corporation | None | None | None | None | None | None | 2: Boston Scientific (Clinical Events Committee) |
| Rakesh Gopinathannair, MD, MA, FHRS | Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, Kansas | 1: Abbott; 1: Boston Scientific; 1:ZOLL Medical Corporation | 1: Pfizer | None | None | None | None | None | 0: AltaThera Pharmaceuticals (Physician Advisor) |
| Clifford V. Harding, MD, PhD | Case Western Reserve University, Cleveland, Ohio | None | None | 4: NIH | None | None | None | None | None |
| Jodie L. Hurwitz, MD, FHRS | North Texas Heart Center, Dallas, Texas | 1: Medtronic | None | None | None | None | 3: Microsoft | None | 1: ABIM (CCEP Writing Committee Member) |
| Courtney C. Jeffery, MSN, APRN-C | Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, Kansas | 1: Abbott; 1: Medtronic | None | None | None | None | None | None | None |
| Andrew D. Krahn, MD, FHRS | University of British Columbia, Vancouver, British Columbia, Canada | 1: Medtronic | None | None | None | None | None | None | None |
| Fred M. Kusumoto, MD, FHRS | Mayo Clinic Jacksonville, Jacksonville, Florida | None | None | None | None | None | None | None | None |
| Rachel Lampert, MD, FHRS | Yale School of Medicine, New Haven, Connecticut | 1: Abbott; 1: Medtronic | None | 0: Amgen; 0: MediLynx;2: Abbott; 2: Medtronic | None | None | None | None | None |
| Moussa Mansour, MD, FHRS | Massachusetts General Hospital, Boston, Massachusetts | 1: Abbott; 1: Biosense Webster; 1: Boston Scientific; 1: Medtronic | None | 0: Abbott; 0: Biosense Webster | None | None | 5: NewPace Ltd; 5: EPD Solutions | None | None |
| Andrea Natale, MD, FHRS | Texas Cardiac Arrhythmia Institute, Austin, Texas | 1: Baylis Medical Company; 1: BIOTRONIK; 1: Boston Scientific; 1: Medtronic; 2: Abbott; 2: Biosense Webster | None | None | None | None | None | None | None |
| Kristen K. Patton, MD, FHRS | University of Washington, Seattle, Washington | None | None | None | None | None | None | None | 0: ACGME RC Internal Medicine; 0: AHA Clinical Cardiology Council; 0: ACC EP Council (Committee Member); 1: FDA Circulatory System Devices Panel; 1: ABIM |
| Andrea M. Russo, MD, FHRS | Cooper Medical School of Rowan University, Camden, New Jersey | None | None | 1: MediLynx; 2: Boston Scientific | None | None | None | 1: UpToDate | 0: ABIM (Member, ABIM Cardiovascular Board); 0: Apple Inc. (Steering Committee, Apple Heart Study); 0: Boston Scientific (Steering Committee, Research) |
| Amber Seiler, ANP, FHRS | Cone Health, Greensboro, North Carolina | 1: Biosense Webster; 2: Medtronic | None | None | None | 3: CV Remote Solutions | None | None | None |
| Maully J. Shah, MBBS, FHRS | Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania | 0: Medtronic | None | None | None | None | None | None | 0: SADS Foundation (Board Member); 1: JACC (Associate Editor) |
| Paul J. Wang, MD, FHRS | Stanford University, Palo Alto, California | None | None | 5: AHA; 5: Coulter Foundation | None | None | None | None | None |
Number value: 0 = $0; 1 = ≤$10,000; 2 = >$10,000 to ≤$25,000; 3 = >$25,000 to ≤$50,000; 4 = >$50,000 to ≤$100,000; 5 = >$100,000.
ABIM = American Board of Internal Medicine; ACC = American College of Cardiology; ACGME = Accreditation Council for Graduate Medical Education; AHA = American Heart Association; CCEP = Clinical Cardiac Electrophysiology; EHRA = European Heart Rhythm Association; EP = electrophysiology; FDA = Food and Drug Administration; HRS = Heart Rhythm Society; JACC = Journal of the American College of Cardiology; NIH = National Institutes of Health; RC = Review Committee; SADS = Sudden Arrhythmia Death Syndromes.
Research and fellowship support are classed as programmatic support. Sources of programmatic support are disclosed but are not regarded as a relevant relationship with industry for writing group members or reviewers.
References
- 1.Lakkireddy D.R., Chung M.K., Gopinathannair R. Heart Rhythm Guidance for cardiac electrophysiology during the coronavirus (COVID-19) pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart Rhythm. 2020;17:e233–e241. doi: 10.1016/j.hrthm.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Worldometer COVID-19 coronavirus pandemic. https://www.worldometers.info/coronavirus/ Available from.
- 3.Tam C.-C.F., Cheung K.-S., Lam S. Impact of coronavirus disease 2019 (COVID-19) outbreak on ST-segment–elevation myocardial infarction care in Hong Kong, China. Circ Cardiovas Qual Outcomes. 2020;13 doi: 10.1161/CIRCOUTCOMES.120.006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reza N., DeFilippis E.M., Jessup M. Secondary impact of the COVID-19 pandemic on patients with heart failure. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007219. [DOI] [PubMed] [Google Scholar]
- 5.Barghash M.H., Pinney S.P. Heart failure in the COVID-19 pandemic: where has all New York’s congestion gone? J Card Fail. 2020;26:477–478. doi: 10.1016/j.cardfail.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esbin M.N., Whitney O.N., Chong S., Maurer A., Darzacq X., Tjian R. Overcoming the bottleneck to widespread testing: a rapid review of nucleic acid testing approaches for COVID-19 detection. RNA. 2020;26:771–783. doi: 10.1261/rna.076232.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood D.A., Mahmud E., Thourani V.H. Safe reintroduction of cardiovascular services during the COVID-19 pandemic: From the North American Society Leadership. J Am Coll Cardiol. 2020;75:3177–3183. doi: 10.1016/j.jacc.2020.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He X., Lau E.H.Y., Wu P. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 9.Wolfel R., Corman V.M., Guggemos W. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 10.Wang X., Tan L., Wang X. Comparison of nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 detection in 353 patients received tests with both specimens simultaneously. Int J Infect Dis. 2020;94:107–109. doi: 10.1016/j.ijid.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li R., Pei S., Chen B. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallapaty S. Will antibody tests for the coronavirus really change everything? Nature. 2020;580:571–572. doi: 10.1038/d41586-020-01115-z. [DOI] [PubMed] [Google Scholar]
- 13.Xie J, Ding C, Li J, et al. Characteristics of patients with coronavirus disease (COVID-19) confirmed using an IgM-IgG antibody test [published online ahead of print April 24, 2020]. J Med Virol. https://doi.org/10.1002/jmv.25930. [DOI] [PMC free article] [PubMed]
- 14.Centers for Disease Control and Prevention Criteria for return to work for healthcare personnel with suspected or confirmed COVID-19 (interim guidance) https://www.cdc.gov/coronavirus/2019-ncov/hcp/return-to-work.html Available from.
- 15.Stowell S, Guarner J. Role of serology in the COVID-19 pandemic [published online ahead of print May 1, 2020]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa510.
- 16.Gropler M.R.F., Dalal A.S., Van Hare G.F., Silva J.N.A. Can smartphone wireless ECGs be used to accurately assess ECG intervals in pediatrics? A comparison of mobile health monitoring to standard 12-lead ECG. PLoS One. 2018;13 doi: 10.1371/journal.pone.0204403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung E.H., Guise K.D. QTC intervals can be assessed with the AliveCor heart monitor in patients on dofetilide for atrial fibrillation. J Electrocardiol. 2015;48:8–9. doi: 10.1016/j.jelectrocard.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Haldrup M., Johansen M.I., Fjaeldstad A.W. Anosmia and ageusia as primary symptoms of COVID-19 [in Danish] Ugeskr Laeger. 2020;182:V04200205. [PubMed] [Google Scholar]
- 19.Schmulson M., Davalos M.F., Berumen J. Beware: gastrointestinal symptoms can be a manifestation of COVID-19. Rev Gastroenterol Mex. 2020;85:282–287. doi: 10.1016/j.rgmx.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouaziz JD, Duong T, Jachiet M, et al. Vascular skin symptoms in COVID-19: a French observational study [published online ahead of print April 27, 2020]. J Eur Acad Dermatol Venereol. https://doi.org/10.1111/jdv.16544. [DOI] [PMC free article] [PubMed]
- 21.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slotwiner D., Varma N., Akar J.G. HRS Expert Consensus Statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm. 2015;12:e69–e100. doi: 10.1016/j.hrthm.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Akar J.G., Bao H., Jones P. Use of remote monitoring of newly implanted cardioverter-defibrillators: insights from the patient related determinants of ICD remote monitoring (PREDICT RM) study. Circulation. 2013;128:2372–2383. doi: 10.1161/CIRCULATIONAHA.113.002481. [DOI] [PubMed] [Google Scholar]
- 24.Piccini J.P., Mittal S., Snell J., Prillinger J.B., Dalal N., Varma N. Impact of remote monitoring on clinical events and associated health care utilization: a nationwide assessment. Heart Rhythm. 2016;13:2279–2286. doi: 10.1016/j.hrthm.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 25.Saxon L.A., Hayes D.L., Gilliam F.R. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation. 2010;122:2359–2367. doi: 10.1161/CIRCULATIONAHA.110.960633. [DOI] [PubMed] [Google Scholar]