Abstract
Objective
We aimed to investigate the relationship between clinical characteristics, outcomes and the severity of severe acute respiratory syndrome coronavirus 2 pneumonia.
Methods
We performed a systematic review and meta-analysis using PubMed, Embase, and Cochrane Library databases to assess the clinical characteristics and outcomes of confirmed COVID-19 cases and compared severe (ICU) and nonsevere (non-ICU) groups.
Results
We included 12 cohort studies including 2,445 patients with COVID-19. Compared with nonsevere (non-ICU) patients, severe (ICU) disease was associated with a smoking history (P = .003) and comorbidities including chronic obstructive pulmonary disease (OR = 5.08, P < .001), diabetes (OR = 3.17, P < .001), hypertension (OR = 2.40, P < .001), coronary heart disease (OR = 2.66, P < .001), cerebrovascular diseases (OR = 2.68, P = .008), and malignancy (OR=2.21, P = .040). We found significant differences between the 2 groups for fever, dyspnea, decreased lymphocyte and platelet counts, and increased leukocyte count, C-creative protein, procalcitonin, lactose dehydrogenase, aspartate aminotransferase, alanine aminotransferase, creatinine kinase, and creatinine levels (P < .05). Significant differences were also observed for multiple treatments (P < .05). Patients in the severe (ICU) group were more likely to have complications and had a much higher mortality rate and lower discharge rate than those with nonsevere (non-ICU) disease (P < .05).
Conclusions
Investigation of clinical characteristics and outcomes of severe cases of COVID-19 will contribute to early prediction, accurate diagnosis, and treatment to improve the prognosis of patients with severe illness.
Key Words: COVID-19, Clinical characteristics, Prognosis, Severe illness, ICU cases
Introduction
A series of pneumonia cases of unknown cause appeared in Wuhan, China in December, 2019. Scientists isolated the pathogen that caused the pneumonia, which was named 2019-nCoV, a novel type of beta-coronavirus.1 The World Health Organization later renamed the new virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and named the illness caused by the virus Coronavirus disease 2019 (COVID-19). As of April 27, the total number of patients with COVID-19 had risen sharply to 2,878,196 patients globally, with 198,668 deaths and a mortality rate close to 6.9% (data from the website of the WHO).2 People infected by SARS-CoV-2 have a very broad spectrum of illness, from being asymptomatic to critically ill.3 Thus, it is crucial to detect patients who are more likely to develop severe illness at diagnosis, to decrease mortality in these patients. In this study, we aimed to determine the predictors of severe disease or admission to an intensive care unit (ICU) in a meta-analysis by comparing patients with COVID-19 in severe (ICU) and nonsevere (non-ICU) groups.
Materials and methods
Search strategy
We searched the PubMed, Embase, and Cochrane Library databases for studies on COVID-19 published between December 2019 and April 14, 2020. The search terms and key words used included “novel coronavirus” or “2019-nCoV” or “2019 novel coronavirus” or “NCIP” or “novel coronavirus infected pneumonia” or “COVID-19” or “severe acute respiratory syndrome coronavirus 2” or “SARS-CoV2” and “clinical.” We also searched the medRxiv website, a free online archive and distribution service of unpublished preprints in the medical field (https://www.medrxiv.org), to identify the latest studies on the novel coronavirus. There was no language restriction. This meta-analysis followed the recommendations established by the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement.4
Inclusion and exclusion criteria
Studies included in this meta-analysis were selected based on the following inclusion and exclusion criteria. The inclusion criteria were: (1) cohort studies or case-control studies reporting the clinical characteristics of patients with SARS-CoV2infection; (2) one or more clinical features were analyzed, including epidemiology, clinical symptoms, laboratory findings, comorbidities, treatment, complications, and outcomes; and (3) patients were grouped according to the severity of disease, for example, severe and nonsevere groups or ICU and non-ICU groups. The exclusion criteria were: (1) review articles, opinions, and letters, which did not present original data; (2) studies without grouping according to the severity of illness; and (3) studies grouping patients by age or survival status.
Study selection and data extraction
All articles were managed using Endnote X 9.1 software. We first screened article titles and abstracts to exclude those that clearly did not meet the inclusion criteria. We then read the full text to select the articles to finally include in this meta-analysis. Two researchers (J. L and X. H) extracted the data from eligible studies independently, to minimize bias. If any disagreements arose, discussions were held with a third investigator (G. D) to reach consensus.
We extracted and analyzed items from eligible studies including the country, year, date of publication, the number of reported cases, sex, age, clinical symptoms and signs, comorbidities, laboratory findings, complications, and outcomes of patients with SARS-CoV2 infection who had severe (ICU) and nonsevere (non-ICU) disease.
Paper quality evaluation
To evaluate quality, we used the Newcastle-Ottawa Scale to assess cohort studies and case-control studies.5 The highest quality rating was 9 stars and the lowest was 0 stars. Assessment scores ≥7 indicated high-quality studies.
Data synthesis and statistical analysis
We used the number of events per group to calculate odds ratios (ORs) and their 95% confidence intervals (CIs) with Stata version 14.0 (StataCorp LLC, College Station, TX). The pooled results were presented as forest plots. The pooled prevalence and 95% CI in severe (ICU) cases was calculated using a single-arm meta-analysis. Measures of heterogeneity, including the Cochran's Q, I2, and chi-squared tests were estimated and reported.6 ORs were combined using the Mantel–Haenszel fixed-effects model to estimate and compare risk factors and their confidence intervals. When substantial heterogeneity was detected (I2 > 50%), the DerSimonian–Laird random-effects model was used.7 To further explore sources of heterogeneity and to examine whether the results differed by study characteristics, subgroup analyses were performed by geographic region (Wuhan area and outside Wuhan area) and by study size (≤50 cases and >50 cases). P < .05 was considered to indicate a statistically significant test result for the pooled ORs.
Results
Article selection and characteristics
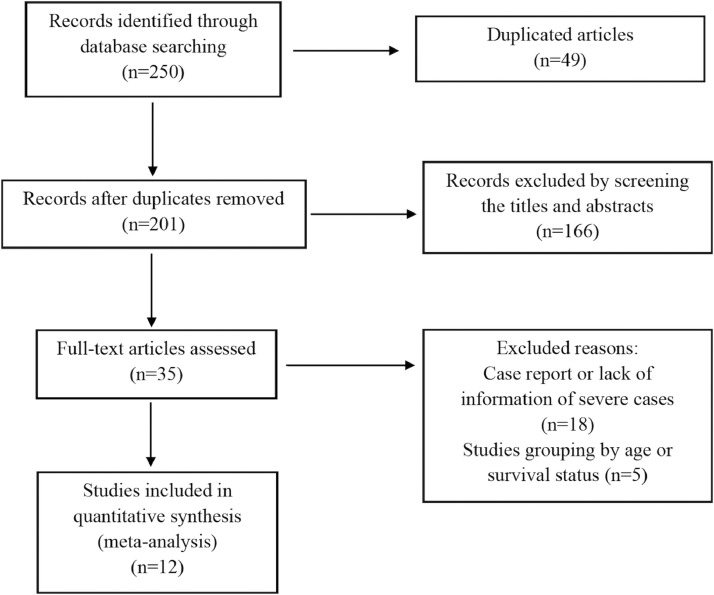
A total of 250 papers were retrieved using the search strategy, and 49 articles were excluded because of duplication. After screening the article titles and abstracts, 35 papers were selected for full-text assessment. Of these articles, 18 were excluded because of a lack of information on severe cases and 5 were excluded because study participants were grouped by age or survival status. Study selection and flow are shown in Figure 1 .
Fig 1.
Flow chart of the study selection process.
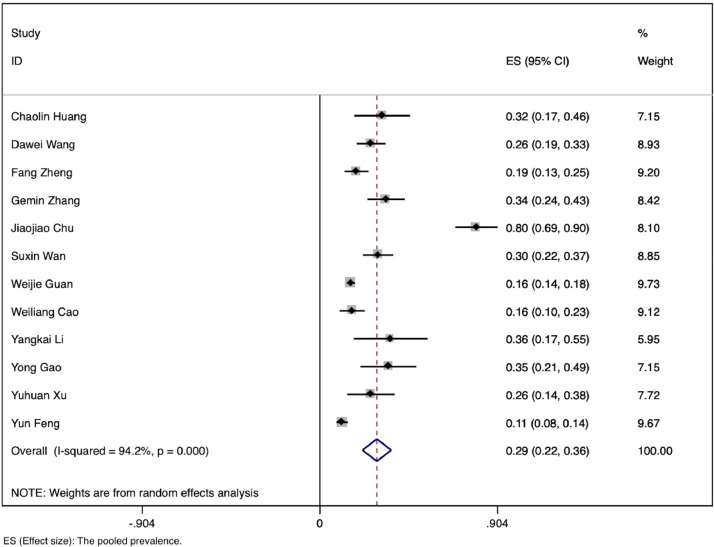
Finally, we included a total of 12 studies, conducted between January 1 and April 14, 2020 and representing 2,445 patients with confirmed SARS-CoV-2 infection; among these, 479 had severe illness or were admitted to the ICU.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 All studies were conducted in China and all articles were written in English. The characteristics of the included articles are shown in Table 1 . The pooled prevalence of severe or ICU cases was 0.33 (95% CI: 0.22-0.43) (Fig 2 ).
Table 1.
Characteristics of the literatures
| First author | Publication date | Source of cases | Sample size n (M/F) | Average age (range) | Research type | Literature quality (0-9) | Statistical index |
|---|---|---|---|---|---|---|---|
| Weijie Guan | Feb 28, 2020 | 31 provinces | 1,099 (640/459) | 47 (35-58) | Retrospective Study | 8 | Smoking history Symptoms Comorbidities Laboratory findings Complications Treatments Outcome |
| Chaolin Huang | Feb 15, 2020 | Wuhan City | 41 (30/11) | 49 (41-58) | Retrospective Study | 8 | Smoking history Symptoms Comorbidities Laboratory findings Complications Treatments Outcome |
| Dawei Wang | Feb 7, 2020 | Wuhan City | 138 (75/63) | 56 (42-68) | Retrospective Study | 8 | Symptoms Comorbidities Laboratory findings Complications Treatments |
| Weiliang Cao | Feb 23, 2020 | Xiangyang City | 128 (60/68) | NA | Retrospective Study | 6 | Symptoms |
| Yuhuan Xu | Feb 25, 2020 | Beijing City | 50 (29/21) | 43.9 (3-85) | Retrospective Study | 6 | Wuhan exposure history Symptoms Laboratory findings |
| Gemin Zhang | Mar 26, 2020 | Wuhan City | 95 (53/42) | 49 (39-58) | Retrospective Study | 7 | Symptoms Laboratory findings |
| Jiaojiao Chu | Mar 29, 2020 | Wuhan City | 54 (36/18) | 39 (26-73) | Retrospective Study | 7 | Symptoms Treatments |
| Yangkai Li | Mar 30, 2020 | Wuhan City | 25 (13/12) | 35 (22-51) | Retrospective Study | 7 | Smoking history Symptoms Comorbidities Laboratory findings |
| Yong Gao | Mar 17, 2020 | Fuyang City | 43 (26/17) | 43.47 (19-70) | Retrospective Study | 6 | Symptoms Comorbidities |
| Suxin Wan | Mar 21, 2020 | Chongqing City | 135 (72/63) | 47 (36-55) | Retrospective Study | 8 | Smoking history Symptoms Comorbidities Laboratory findings Complications Treatments Outcome |
| Fang Zheng | April 10, 2020 | Changsha City | 161 (80/81) | 45 (33.5-57) | Retrospective Study | 7 | Wuhan exposure history Symptoms Comorbidities Laboratory findings |
| Yun Feng | April 10, 2020 | Shanghai Anhui province | 476 (271/205) | 53 (40-64) | Retrospective Study | 8 | Wuhan exposure history Smoking history Comorbidities Symptoms Treatments Outcome |
Fig 2.
Pooled prevalence of severe (ICU) cases.
Demographic characteristics and comorbidities
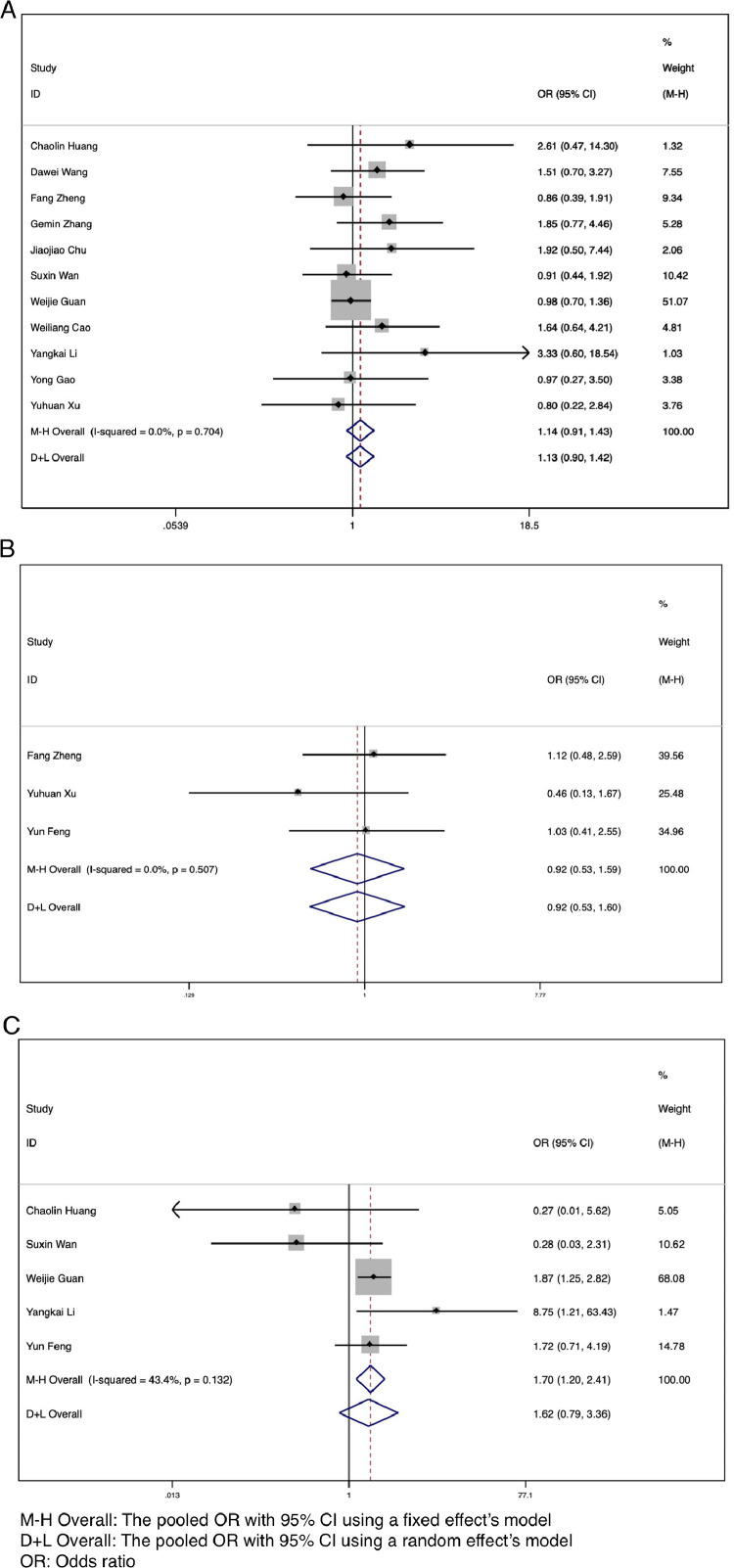
The data on demographic characteristics (sex, smoking history, Wuhan exposure history) and comorbidities (hypertension, diabetes, and so on) among patients with severe (ICU) and nonsevere (non-ICU) COVID-19 were extracted and pooled for meta-analysis. The results showed that there was no significant association between sex (OR = 1.14, 95% CI: 0.91-1.43, I2 = 0.0%, P = .267) or Wuhan exposure history (OR = 0.92, 95% CI: 0.53-1.59, I2 = 0.0%, P = .764) and increased risk of severe disease (Fig 3 A and B). However, our results showed that a smoking history was significantly associated with severe COVID-19 (OR = 1.70, 95% CI: 1.20-2.41, I2 = 43.4%, P = .003) (Fig 3C).
Fig 3.
Forest plots for the ORs for comparing demographical characteristics between severe (ICU) cases and nonsevere (non-ICU) cases in SARS-CoV-2 infected patients. (A) Sex. (B) Wuhan exposure history. (C) Smoking history.
Compared with nonsevere patients, those with severe illness or admission to the ICU were more likely to have one or more comorbidities (OR = 3.07, 95% CI: 1.56-6.05, P < .001, I2 = 79.0%), including chronic obstructive pulmonary disease (OR = 5.08, 95% CI: 2.68-9.63, P < .001, I2 = 0.0%), diabetes (OR = 3.17, 95% CI: 2.26-4.45, P < .001, I2 = 35.3%), hypertension (OR = 2.40, 95% CI: 1.47-3.90, P < .001, I2 = 51.5%), coronary heart disease (OR = 2.66, 95% CI: 1.71-4.15, P < .001, I2 = 0.0%), cerebrovascular diseases (OR = 2.68, 95% CI: 1.29-5.57, P = .008, I2 = 41.8%), and malignancy (OR = 2.21, 95% CI: 1.04-4.72, P = .040, I2 = 0.0%) (Supplementary Fig 1A-G). No significant differences were observed between the 2 groups for chronic liver disease (P = .192) and chronic renal disease (P = .404) (Supplementary Fig. 1H and I).
Clinical symptoms and laboratory findings
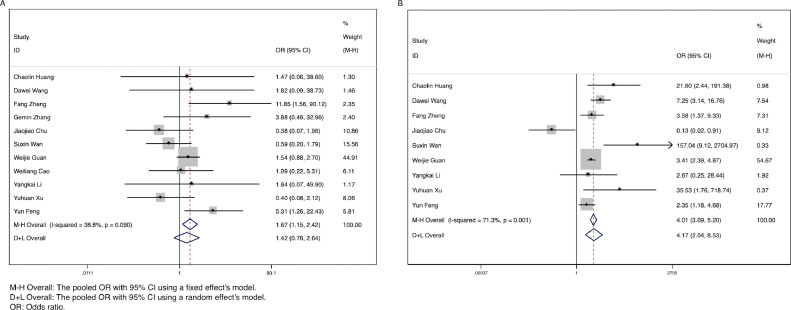
Among all clinical symptoms, fever and dyspnea were found to be significantly associated with more severe COVID-19. The combined ORs were 1.67 (95% CI: 1.15-2.42, P = .007, I2 = 38.8%) and 4.17 (95% CI: 2.04-8.53, P < .001, I2 = 71.3%), respectively (Fig 4 A and B). However, there were no marked differences for cough, nausea, headache, sore throat, expectoration, diarrhea, myalgia, and fatigue between the severe (ICU) and nonsevere (non-ICU) groups (all P > .05) (Supplementary Fig 2A-H). Among patients with fever, high fever (>39°C) was not associated with severe COVID-19 illness (P = .267) (Supplementary Fig 2I).
Fig 4.
Forest plots for the ORs for comparing fever and dyspnea between severe (ICU) cases and nonsevere (non-ICU) cases in SARS-CoV-2 infected patients. (A) Fever. (B) Dyspnea.
Regarding laboratory findings, increased leukocyte count (OR = 3.46, 95% CI: 1.06-11.28, P = .040, I2 = 75.1%), decreased lymphocyte count (OR = 4.60, 95% CI: 3.25-6.51, P < .001, I2 = 0.0%) and platelet count (OR = 2.82, 95% CI: 2.07-3.83, P < .001, I2 = 0.0%), increased levels of C-reactive protein (OR = 4.02, 95% CI: 2.80-5.79, P ≤ .001, I2 = 11.1%), procalcitonin (OR = 6.69, 95% CI: 3.99-11.20, P ≤ .001, I2 = 13.6%), lactose dehydrogenase (LDH; OR = 3.36, 95% CI: 2.46-4.58, P < .001, I2 = 48.3%), aspartate aminotransferase (AST; OR = 3.26, 95% CI: 2.40-4.42, P < .001, I2 = 5.3%), alanine aminotransferase (ALT; OR = 1.95, 95% CI: 1.35-2.80, P < .001, I2 = 39.6%), creatinine kinase (CK; OR = 2.45, 95% CI: 1.69-3.55, P < .001, I2 = 46.7%), and creatinine (OR = 2.14, 95% CI: 1.14-4.01, P = .018, I2 = 0.0%) were observed to be significantly associated with severe (ICU) cases as compared with nonsevere (non-ICU) cases (Supplementary Fig 3A-I).
Treatment, complications, and outcomes
With respect to treatment, patients in the severe (ICU) group were most likely to be treated with antibiotics therapy (OR = 3.58, 95% CI: 1.29-9.87, P = .014, I2 = 84.1%), antiviral therapy (OR = 1.79, 95% CI: 1.35-2.38, P < .001, I2 = 0.0%), systemic corticosteroids (OR = 5.46, 95% CI: 4.17-7.14, P < .001, I2 = 0.0%), mechanical ventilation including invasive and noninvasive ventilation (OR = 171.72, 95% CI: 27.38-1,077.21, P < .001, I2 = 73.2%), extracorporeal membrane oxygenation (ECMO; OR = 29.36, 95% CI: 5.36-160.68, P < .001, I2 = 0.0%), and continuous renal replacement therapy (OR = 25.45, 95% CI: 6.97-92.89, P < .001, I2 = 0.0%) (Supplementary Fig 4A-F).
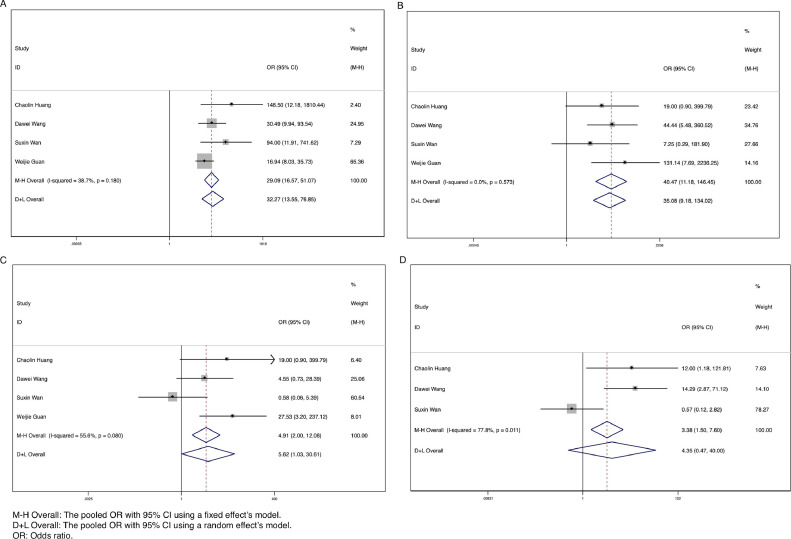
Common complications among patients with SARS-CoV-2 infection included acute respiratory distress syndrome (ARDS), shock, acute kidney injury, and acute cardiac injury. Patients who had severe illness or were admitted to the ICU were more likely to have ARDS (OR = 29.09, 95% CI: 16.57-51.07, P < .001, I2 = 38.7%), shock (OR = 40.47, 95% CI: 11.18-146.45, P < .001, I2 = 0.0%), and acute kidney injury (OR = 5.62, 95% CI: 1.03-30.61, P = .046, I2 = 55.6%) (Fig 5 A-C). No significant difference was detected for the complication of acute cardiac injury in the 2 groups (P = .194) (Fig 5D).
Fig 5.
Forest plots for the ORs for comparing the complications between severe (ICU) cases and nonsevere (non-ICU) cases in SARS-CoV-2 infected patients. (A) Acute respiratory distress syndrome (ARDS). (B) Shock. (C) Acute kidney injury. (D) Acute cardiac injury.
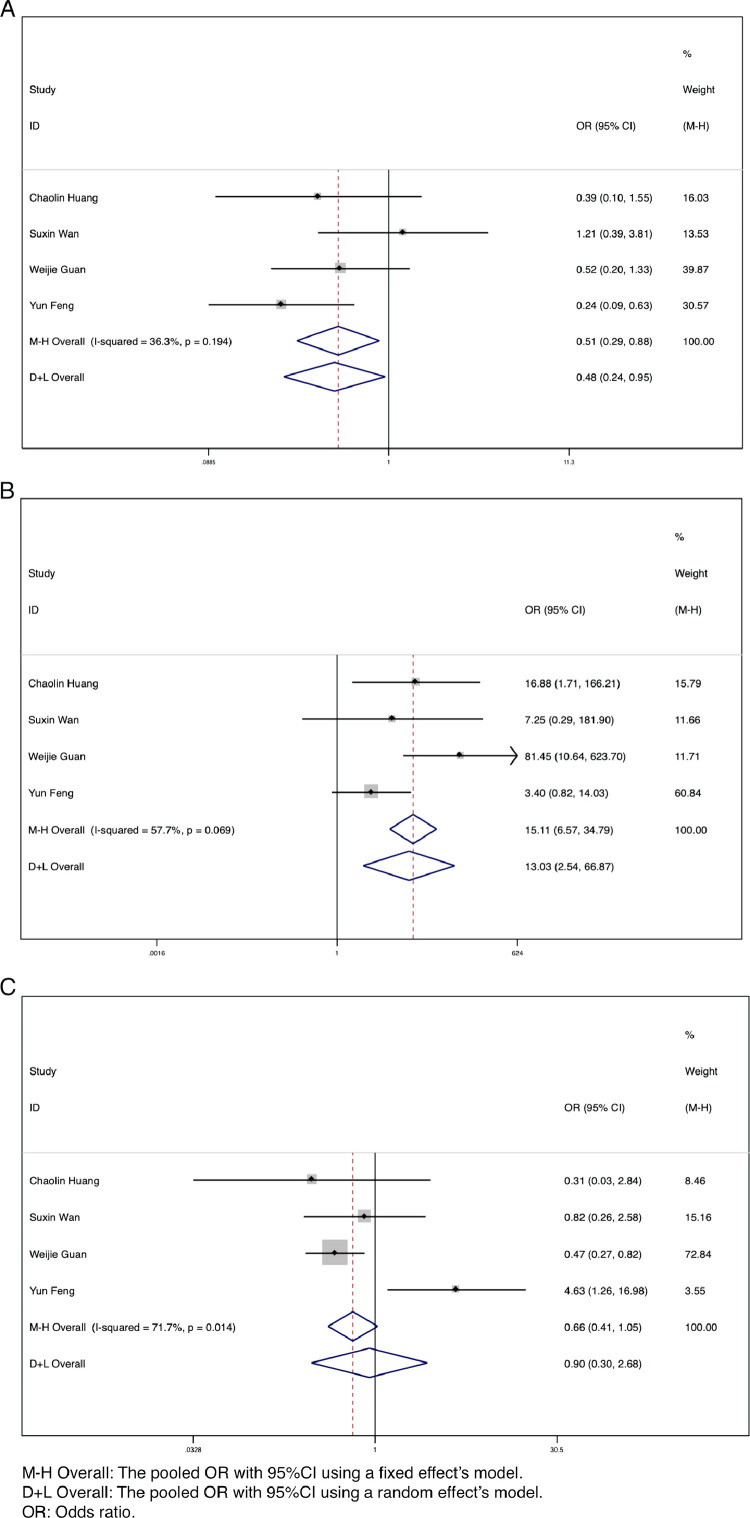
By the end of the follow-up period, the outcomes of patients with COVID-19 included hospitalization, discharge, and death. A small number of patients were lost to follow-up. There was a significant difference between the severe (ICU) group and nonsevere (non-ICU) group in the number of discharges (OR = 0.51, 95% CI: 0.29-0.88, P = .017, I2 = 36.3%) and deaths (OR = 13.03, 95% CI: 2.54-66.87, P = .002, I2 = 57.7%) (Fig 6 A and B). Patients with more severe illness have a much higher mortality rate and lower discharge rate than those with mild or moderate illness. There was no significant difference in the number of hospitalized patients (P = .848) (Fig 6C).
Fig 6.
Forest plots for the ORs for comparing the outcomes between severe (ICU) cases and nonsevere (non-ICU) cases in SARS-CoV-2 infected patients. (A) Discharge. (B) Deaths. (C) Hospitalized.
Subgroup analyses
We conducted subgroup analyses of geographic region and study size to explore potential sources of heterogeneity (I2 > 50%). No clear sources of heterogeneity were identified from these subgroup analyses (Table 2 ).
Table 2.
Subgroup analysis of pooled results of clinical characteristics with substantial heterogeneity
| Geographic region |
Sample size |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wuhan City | Outside Wuhan City | n > 50 | n ≤ 50 | |||||||||||||
| N* | OR (95%CI) | I2 | P | N* | OR (95%CI) | I2 | P | N* | OR (95%CI) | I2 | P | N* | OR (95%CI) | I2 | P | |
| Comorbidities | ||||||||||||||||
| Any comorbidities | 2 | 3.05 (1.17, 8.00) | 36.0% | 0.211 | 3 | 3.22 (1.24, 8.35) | 88.1% | < .001 | 4 | 3.41 (1.59, 7.31) | 83.8% | < .001 | 1 | 1.56 (0.39, 6.25) | / | / |
| Hypertension | 3 | 3.25 (1.24, 8.51) | 19.8% | 0.287 | 5 | 2.19 (1.24, 3.86) | 58.7% | .046 | 5 | 2.63 (1.42, 4.86) | 70.9% | .008 | 3 | 1.65 (0.60, 4.56) | 0.0% | .869 |
| Symptoms | ||||||||||||||||
| Dyspnoea | 4 | 2.79 (0.37, 21.00) | 81.5% | 0.001 | 5 | 4.36 (2.08, 9.15) | 64.9% | .022 | 6 | 3.33 (1.53, 7.26) | 77.8% | < .001 | 3 | 11.51 (2.47, 53.57) | 14.2% | .312 |
| Diarrhea | 4 | 1.58 (0.62, 3.99) | 0.0% | 0.533 | 3 | 1.91 (0.39, 9.33) | 81.1% | .005 | 5 | 2.15 (0.82, 5.67) | 62.1% | .032 | 2 | 0.46 (0.07, 3.13) | 0.0% | .773 |
| Fatigue | 4 | 0.74 (0.21, 2.58) | 70.7% | 0.017 | 3 | 2.07 (0.77, 5.54) | 76.5% | .014 | 4 | 1.19 (0.65, 2.16) | 56.7% | .074 | 3 | 1.72 (0.18, 16.42) | 82.5% | .003 |
| High fever (>39°C) | 2 | 1.66 (0.16, 17.53) | 86.2% | 0.007 | 3 | 1.55 (0.99, 2.45) | 0.0% | .450 | 3 | 2.00 (0.70, 5.70) | 70.1% | .035 | 2 | 0.95 (0.19, 4.91) | 44.8% | .178 |
| Laboratory findings | ||||||||||||||||
| Leukocyte count | 3 | 17.95 (1.41, 228.74) | 71.5% | 0.030 | 4 | 1.20 (0.74, 1.95) | 0.0% | .751 | 5 | 3.08 (0.75, 12.70) | 81.1% | < .001 | 2 | 5.46 (1.00, 29.89) | 0.0% | .317 |
| Treatment | ||||||||||||||||
| Antibiotics therapy | 1 | 0.43 (0.10, 1.85) | / | / | 3 | 5.69 (2.31, 13.99) | 79.7% | .007 | 4 | 3.58 (1.29, 9.87) | 84.1% | < .001 | 0 | / | / | / |
| Mechanical ventilation | 2 | 213.14 (6.16, 7,377.63) | 75.5% | 0.043 | 3 | 174.41 (9.87, 3,083.22) | 80.7% | .006 | 4 | 281.58 (23.01, 3,445.34) | 79.3% | .002 | 1 | 43.33 (6.28, 299.18) | / | / |
| Complications | ||||||||||||||||
| Acute kidney injury | 2 | 6.65 (1.38, 31.94) | 0.0% | 0.422 | 2 | 4.05 (0.09, 178.47) | 83.3% | .014 | 3 | 4.25 (0.54, 33.46) | 66.6% | .050 | 1 | 19.00 (0.90, 399.79) | / | / |
| Acute cardiac injury | 2 | 13.50 (3.61, 50.52) | 0.0% | 0.904 | 1 | 0.57 (0.12, 2.82) | / | / | 2 | 2.86 (0.12, 66.99) | 87.1% | .005 | 1 | 12 (1.18, 121.81) | / | / |
| Outcomes | ||||||||||||||||
| Deaths | 1 | 16.88 (1.71, 166.21) | / | / | 3 | 12.38 (1.25, 122.60) | 71.5% | .030 | 3 | 12.38 (1.25, 122.60) | 71.5% | .030 | 1 | 16.88 (1.71, 166.21) | / | / |
| Hospitalized | 1 | 0.31 (0.03, 2.84) | / | / | 3 | 1.10 (0.31, 3.93) | 80.2% | .006 | 3 | 1.10 (0.31, 3.93) | 80.2% | .006 | 1 | 0.31 (0.03, 2.84) | / | / |
Discussion
Six previously identified coronavirus types are known to cause human infection.20 Among these, SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) were responsible for serious outbreaks in Guangdong, China during 20036 and in the Middle East during 2012.21 In December 2019, an outbreak of pneumonia of unknown cause first appeared in Wuhan City of Hubei Province, the cause of which has been identified as the seventh coronavirus, named SARS-CoV-2 by the WHO. The initial outbreak led to a worldwide pandemic and the deaths of a large number of people.
Many articles have reported the historical epidemiology, clinical characteristics, and prognosis of COVID-19. However, few articles have focused on patients with severe or critical illness and those admitted to the ICU.
To date, this is the first systematic review and meta-analysis to investigate the relationship between clinical characteristics, outcomes, and severity of SARS-CoV-2pneumonia. We collected data from 12 published articles including 2,445 patients with confirmed SARS-CoV-2 infection and 479 patients with severe disease or ICU admission. The pooled prevalence of severe or ICU cases was 33%. There was no association between sex or Wuhan exposure history and severity of COVID-19. However, our results showed that smoking history might be a high-risk factor for severe illness, which was consistent with the conclusion of recently reported research.22
Among clinical symptoms, fever and dyspnea were found to be significantly associated with severe illness or ICU admission for COVID-19. However, in patients with fever, high fever (>39°C) was not a risk factor of severe illness. Based on this study, fever that is not particularly high might be associated with severe COVID-19. Thus, greater attention is needed for patients with fever or dyspnea. Patients with any comorbidities had a higher rate of severe illness; these included chronic obstructive pulmonary disease, diabetes, hypertension, coronary heart disease, cerebrovascular diseases, and malignancy. Assessment of all potential comorbidities is challenging in any study, so the most common and well-studied comorbidities assessed in the original articles were chosen for evaluation.
As for laboratory findings, increased leukocyte count, decreased lymphocyte and platelet counts, increased levels of C-creative protein, procalcitonin, LDH, AST, ALT, CK, and creatinine were related to severe illness or ICU admission with COVID-19. Different from the results of previous studies,12 , 13 , 23 our study indicated that severe illness or ICU admission for SARS-CoV-2 pneumonia had more to do with leukocytosis than leukopenia. We speculate that leukocytosis is a reflection of excessive inflammation, which is also reflected in the much higher C-reactive protein levels among patients with severe (ICU) COVID-19.Lymphopenia was more common in severe (ICU) than in nonsevere (non-ICU) patients, probably owing to translocation of lymphocytes from peripheral blood to the lungs.17 24 Our results regarding elevated levels of ALT, AST, creatinine, CK, and LDH indicated that patients with severe disease or those in the ICU had more obvious liver function, kidney function, and myocardial injury. Continuous tracking of laboratory findings is crucial to identify those patients who may progress to severe status.
Currently, there are no specific treatments for COVID-19. At present, effective measures to control the disease are early diagnosis, isolation, and supportive treatment for infected patients. As we found in our study, patients with severe illness or those in the ICU most commonly received antibiotics, antivirals, corticosteroid therapy and especially advanced life support treatments including ECMO, mechanical ventilation, and continuous renal replacement therapy. Significant differences were also observed in the complications of ARDS, shock, and acute kidney injury between the groups with different degrees of disease severity. Severely ill patients or those cared for in the ICU had a higher mortality rate and lower discharge rate than nonsevere patients or patients without ICU admission.
This study has several limitations that must be mentioned. First, the studies included in our research divided patients into severe and nonsevere groups (10 studies) or by admission to the ICU or no ICU admission (2 studies). The grouping criteria were not strictly consistent; mild and moderate cases were included in nonsevere groups. Second, there were differences in the range of normal values for laboratory indicators between different clinical laboratories. Third, all patients in the included studies were from China, and the number of cases was limited. At the time of writing, few studies have yet been published from other countries experiencing COVID-19 outbreaks such as Italy, Spain and the USA. In fact, no articles from countries other than China met the inclusion criteria for our analysis. Further investigation including a greater number of studies with a broad geographic scope and larger sample size is needed, to obtain a more comprehensive understanding of COVID-19. Finally, significant heterogeneity was detected in the statistical results for several items. To address this heterogeneity, we used random effects modeling and performed subgroup analyses to identify potential sources of heterogeneity. Unfortunately, no clear source of heterogeneity was identifiable. Clinical characteristics are related to many factors including basic physical condition, disease progression, and examination and treatment conditions. Multiple factors may have contributed to high heterogeneity; these require further research and exploration.
Conclusion
This is the first meta-analysis to investigate the differences in clinical characteristics and outcomes between patients with COVID-19 who have different degrees of disease severity. Our study results will be helpful in identifying high-risk factors in patients with severe illness, which will contribute to early prediction, accurate diagnosis, and treatment of patients with COVID-19.
Acknowledgment
We thank Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Footnotes
Conflicts of interest: None to report.
Author contributions: J. L., X. H. and G.D. participated in study design; J. L. and X. H. performed data extraction and data analysis; J. L. and Y. Y. drafted the manuscript; All authors provided critical review of the manuscript and approved the final draft for publication.
Ethical approval: Approval was not required.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.ajic.2020.06.008.
Appendix. SUPPLEMENTARY MATERIALS
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 2020. Coronavirus Disease 2019 (COVID-19) Situation Report-90. [Google Scholar]
- 3.Bai Y., Yao L., Wei T., et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 6.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 8.Cao W.L., Shi L., Chen L., Xu X.M., Wu Z.R. Clinical features and laboratory inspection of novel coronavirus pneumonia (COVID-19) in Xiangyang. Hubei. medRxiv preprint. 2020 [Google Scholar]
- 9.Chu J., Yang N., Wei Y., et al. Clinical characteristics of 54 medical staff with COVID-19: a retrospective study in a single center in Wuhan. China. J Med Virol. 2020;92:807–813. doi: 10.1002/jmv.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng Y., Ling Y., Bai T., et al. COVID-19 with different severity: a multi-center study of clinical features. Am J Respir Crit Care Med. 2020;201:1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Y., Li T., Han M., et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92:791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y.K., Peng S., Li L.Q., et al. Clinical and transmission characteristics of Covid-19 - a retrospective study of 25 cases from a single thoracic surgery department. Curr Med Sci. 2020;40:295–300. doi: 10.1007/s11596-020-2176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan S., Xiang Y., Fang W., et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y.H., Dong J.H., An W.M., et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect. 2020;80:394–400. doi: 10.1016/j.jinf.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang G., Zhang J., Wang B., Zhu X., Wang Q., Qiu S. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respir Res. 2020;21:74. doi: 10.1186/s12931-020-01338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng F., Tang W., Li H., Huang Y.X., Xie Y.L., Zhou Z.G. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur Rev Med Pharmacol Sci. 2020;24:3404–3410. doi: 10.26355/eurrev_202003_20711. [DOI] [PubMed] [Google Scholar]
- 20.Su S., Wong G., Shi W., et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Q., Meng M., Kumar R., et al. The impact of COPD and smoking history on the severity of Covid-19: a systemic review and meta-analysis [e-pub ahead of print] J Med Virol. 2020 doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.