Abstract
Background
Optimal management of patients with cancer during COVID-19 pandemic is still pending.
Methods
Our patients were advised to maintain their scheduled appointments, and planned cancer treatment was continued without unnecessary delays in an outpatient setting. Additional strict preventive infection measures were rapidly implemented at our outpatient department. When COVID-19 test became widely available, universal testing of healthcare workers and vigorous screening of all patients coming to our facility for COVID-19 infection were performed by SARS-CoV-2 real-time reverse transcription PCR on rhinopharyngeal swab.
Results
As of the data cut-off on 9 April 2020, a total of 156 oncology patients with a median age of 67 (range 26–86) years and 63 haematology patients (median age 69 years, range 23–89) were screened for COVID-19 during active cancer treatment. Prevalence (1.8%; 4/219) of COVID-19 in patients with cancer was significantly higher compared with a respective control group of asymptomatic counterparts (p=0.018). Outcomes of COVID-19 positive patients were good, with only one observed death due to progression of advanced metastatic disease.
Conclusion
Our data indicate that continuation of anticancer treatment in epidemic areas during the COVID-19 pandemic seems to be safe and feasible, if adequate and strict preventive measures are vigorously and successfully carried out.
Keywords: COVID-19, coronavirus, cancer
Key questions.
What is already known about this subject?
Patients with cancer undergoing anticancer treatment are often more susceptible to infections compared with individuals without cancer. Concerns have been addressed by both physicians and patients about the optimal management of patients with cancer during COVID-19 pandemic.
What does this study add?
We retrospectively investigated the infection rate and clinical management of patients with cancer on active outpatient cancer treatment during the COVID-19 pandemic. Prevalence of COVID-19 in patients with cancer was significantly higher compared with a respective control group of asymptomatic counterparts. Outcomes of COVID-19 positive cancer patients were good, with only one observed death due to progression of advanced metastatic disease.
How might this impact on clinical practice?
Continuation of anticancer treatment in epidemic areas during the COVID-19 pandemic seems to be safe and feasible, if adequate and strict preventive infection control measures are enforced.
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was first detected in December 2019 in the Hubei region of China, which causes a respiratory disease called COVID-19.1 This novel virus spread rapidly worldwide and has specifically high morbidity in elderly as well as in comorbid patients.2 3 On 30 January 2020, when two tourists tested positive, the first case of COVID-19 infection was recorded in Italy, which is now one of the most affected countries. As of 9 April 2020, more than 145 000 confirmed cases with about 18 000 deaths have been registered in Italy.4 Several recommendations for COVID-19 clinical management have been made by different organisations.5–9 However, data on management and outcome of patients with cancer treated during the COVID-19 pandemic in daily clinical practice is limited. This retrospective study was performed to review the management and COVID-19 infection rate of patients with cancer on active cancer treatment during the COVID-19 pandemic.
Methods
Study designs
All consecutive patients with haemato-oncological diseases treated at the haemato-oncology day unit of the “Franz Tappeiner” Hospital in Merano from 15 March 2020 to 9 April 2020 were included in this analysis. The “Franz Tappeiner” hospital is a public regional tertiary care hospital, which covers a large area of patients from South Tyrol, an autonomous province in northern Italy. Based on the national regulations, all patients have access to a unique healthcare system, which provides universal coverage to all citizens and residents. All patients were followed up until 9 April 2020. Clinical data were obtained from electronic clinical records (OncoNet, http://edp-progetti.it, Bolzano, Italy) and through review of medical records. Screening and testing for COVID-19 infections were performed according to the respective recommendations of the WHO and national standards. In the early phase of outbreak, the situation was rapidly evolving and case definitions and hospital guidelines were modified frequently.
COVID-19 infection prevention and control measures
General measures
After the start of the epidemic in Italy, the Italian government has taken a series of drastic measures to contain the spread of COVID-19. These included strict movement restrictions and self-declaration forms specifying the purpose of the movement. The first lockdowns began around 21 February 2020 ending in quarantine measures through the entire country on 9 March 2020. South Tyrol was classified as a risk area by the Robert Koch Institute (RKI) on 5 March 2020.
Specific hospital measures
From the hospital authorities, tents have been set up in front of the hospital entrance serving as triage areas. Behaviour rules including detailed instructions about hand hygiene, cough and sneeze hygiene, as well as disinfecting agents have been provided. All incoming employees and patients have been checked for symptoms of COVID-19 and temperature using hospital-issued digital auricular thermometers. Additional actions were mandatory disinfection of hands as well as denied access to visitors to the hospital. Wearing of medical face masks became mandatory for healthcare providers and for all patients on 13 March 2020.
Specific measures at the haemato-oncological outpatient department
After the first alert message from the local healthcare service, a number of additional preventive infection measures were rapidly implemented at our outpatient department on 10 March 2020. A daily list of our scheduled patients was lodged at the main entrance of the hospital to allow access. Due to significant reduction in the number of individuals within the hospital, patients could follow the usual route from the main entrance to our ward. However, a separate elevator was designated and blocked for COVID-19 positive patients. Before entering the department, access to our clinic was restricted by a second infection control by an experienced nurse who checked daily temperature and signs or symptoms of infection at a checkpoint. For early detection of potentially infectious persons, a simple questionnaire was carried out through the nurse. The questionnaire included specific questions related to signs and symptoms to COVID-19 (fever, cough, shortness of breath, loss of taste or smell, sore throat) or signs of a cold within 48 hours as well as questions about close contacts to people under quarantine or people with known or suspicion COVID-19 infection. In addition, measurement of oxygen saturation based on a pulse oximeter was performed. Patients with known contact history or individuals with symptoms suggestive of infection were isolated in a separate room and tested for the virus. Staff members attested that they have no symptoms before starting work each day to avoid also minimally symptomatic infections. Repeated disinfection of hands as well as wearing of surgical face mask to protect against droplet transmission of all individuals were mandatory before entering our outpatient department. All suspected and confirmed cases have been immediately placed under quarantine or were transferred to the hospital’s COVID-19 isolation ward. Furthermore, patients were placed to maintain a distance of at least 2 m throughout the treatment. The healthcare team was cyclic separated (shifting to the home office) to reduce the risk of infection. For patients who currently not received active therapy, routine follow-up visits were minimised or suspended, and medical care was provided by telemedicine on a case-by-case basis. In addition, patients were repeatedly educated and encouraged to apply the prescribed standards appropriately and consequently practise social and physical distancing. In the later phase of the outbreak, widely available testing kits made it possible to screen the whole healthcare team as well as many patients for COVID-19 at our clinic.
COVID-19 test
Laboratory testing for COVID-19 was performed using a routine real-time reverse transcription PCR (RT-PCR) on respiratory samples obtained from nasopharyngeal swabs. Real-time PCR for SARS-CoV-2 RNA was performed on a COBAS 6800 device (Roche) at the Pathology Laboratory Obrist and Brunhuber, Zams, Austria.
Statistical analyses
Statistical analysis was performed using the Statistical Package of Social Sciences (SPSS, V.15.0). Correlations between COVID-19 positive cancer patients and the general population were assessed with the χ2 test or Fisher’s exact test as appropriate. For all analyses, p<0.05 was considered statistically significant.
Ethics
Informed consent was obtained from all individual participants included in the study.
Results
During the observation period from 15 March to 9 April 2020, a total of 537 patients were treated at our outpatient department. Within that period of time, 1541 patient visits were carried out and 560 treatments have been intravenously applied. Visits were occasionally cyclical, with a mean number of 2.8 (range 1–5) visits per patient. Altogether, 243 (45.3%) out of 537 consecutive patients have been tested for COVID-19. Because this study predominantly focus on patients with cancer, 24 patients being treated for chronic non-malignant disorders (multiple sclerosis, n=12; inflammatory bowel disease, n=7; hypogammaglobulinaemia, n=4; alpha-1 antitrypsin deficiency, n=1; all tested negative for COVID-19) were excluded from further analyses. Demographic and clinical characteristics of patients with cancer on active outpatient treatment tested for COVID-19 are summarised in table 1.
Table 1.
Clinical characteristics of patients with cancer on active outpatient therapy tested for COVID-19
| Parameters | No. of patients (%) | Median age, years (range) |
| Total cohort | 219 (100%) | 68 (23–89) |
| Patients with oncological disease | 156 (71.2%) | 67 (26–86) |
| Patients with malignant haematological disease | 63 (28.8%) | 69 (23–89) |
| Gender | ||
| Male | 94 (42.9%) | 70 (23–87) |
| Female | 125 (57.1%) | 66 (27–89) |
| Treatment setting | Adjuvant | Palliative |
| Solid tumours (N=156) | 41 (26.3%) | 115 (73.7%) |
| Haematological malignancies (N=63) | 14 (22.2%) | 49 (77.8%) |
| COVID- | COVID+ | |
| Disease (N=219) | ||
| Solid tumours | 154 (70.3%) | 2 (0.9%) |
| Breast cancer | 49 (22.5%) | |
| Lung cancer | 23 (10.5%) | |
| Colorectal cancer | 18 (8.2%) | |
| Renal cancer | 10 (4.6%) | |
| Prostate cancer | 10 (4.6%) | |
| Ovarian cancer | 8 (3.7%) | |
| Melanoma | 5 (2.3%) | |
| Gastric cancer | 4 (1.8%) | |
| Bladder cancer | 4 (1.8%) | |
| Pancreatic cancer | 3 (1.4%) | 1 (0.4%) |
| Oesophagus caner | 3 (1.4%) | |
| Appendix cancer | 3 (1.4%) | |
| Anal carcinoma | 3 (1.4%) | |
| Glioblastoma | 3 (1.4%) | |
| Cervical cancer | 2 (0.9%) | |
| Biliary tract cancer | 1 (0.4%) | 1 (0.4%) |
| Non-seminomatous germ cell tumour | 1 (0.4%) | |
| Hypopharyngeal cancer | 1 (0.4%) | |
| Sarcoma | 1 (0.4%) | |
| Neuroendocrine tumour | 1 (0.4%) | |
| Cancer of unknown primary | 1 (0.4%) | |
| Haematological malignancies | 61 (27.9%) | 2 (0.9%) |
| Multiple myeloma | 16 (7.3%) | |
| Chronic myeloproliferative disease | 16 (7.3%) | |
| Indolent lymphoma | 10 (4.6%) | 2 (0.9%) |
| Aggressive lymphoma | 9 (4.1%) | |
| Myelodysplastic syndrome | 7 (3.2%) | |
| Hodgkin’s disease | 3 (1.4%) | |
| Ongoing treatment (N=219) | ||
| Conventional chemotherapy | 79 (36.1%) | 2 (0.9%) |
| Immunotherapy | 48 (21.9%) | |
| Chemo-immunotherapy | 36 (16.5%) | 2 (0.9%) |
| Targeted therapy | 31 (14.2%) | |
| Antihormone therapy | 13 (5.9%) | |
| Best supportive care | 7 (3.2%) | |
| Radiotherapy | 1 (0.4%) | |
A total of 156 (71.2%) patients were treated for oncological and 63 (28.8%) patients for malignant haematological diseases, respectively. The median age of oncological patients was 67 years (range 26–86) and 69 years (range 23–89) for haematological patients, respectively. Briefly, breast cancer was the most frequent diagnosis of oncological patients (49/219; 22.5%), followed by lung (23/219; 10.5%) and colorectal (18/219; 8.2%) cancer. The most common haematological diagnoses were multiple myeloma (16/219; 7.3%), chronic myeloproliferative disease (16/219; 7.6%) and indolent lymphoma (10/219; 4.6%). Of note, 118 (53.9%) of 219 patients had at least one comorbid condition (table 2). Among the total cohort, 81 (37.0%) out of 219 patients received conventional chemotherapy, whereas 38 (17.4%) out of 219 patients were treated with combination chemoimmunotherapy and 48 (21.9%) out of 219 patients were treated with single agent immunotherapy. Oral treatment was administered as targeted therapy in 31 (14.2%) out of 219 patients and antihormone treatment in 13 (5.9%) out of 219 cases, respectively.
Table 2.
Prevalence of comorbidities from patients with cancer on active outpatient therapy tested for COVID-19
| COVID- | COVID+ | |||
| Yes | No | Yes | No | |
| Comorbidity (N=219) | ||||
| Cardiovascular | 33 (15.1%) | 182 (83.1%) | 0 (0.0%) | 4 (1.8%) |
| Hypertension | 41 (18.7%) | 174 (79.5%) | 3 (1.4%) | 1 (0.4%) |
| Diabetes mellitus | 12 (5.5%) | 203 (92.7%) | 1 (0.4%) | 3 (1.4%) |
| Chronic renal failure | 8 (3.7%) | 207 (94.5%) | 1 (0.4%) | 3 (1.4%) |
| Chronic lung disease | 30 (13.7%) | 185 (84.5%) | 0 (0.0%) | 4 (1.8%) |
| Obesity (BMI >30) | 24 (10.9%) | 191 (87.3%) | 1 (0.4%) | 3 (1.4%) |
| Smoker (N=219) | 66 (30.1%) | 149 (68.1%) | 1 (0.4%) | 3 (1.4%) |
BMI, body mass index.
Until last follow-up (9 April 2020), 4 (1.8%) of 219 patients with cancer have tested positive for COVID-19 infection, which was significantly lower than the cumulative prevalence of all other diagnosed symptomatic COVID-19 cases and their contacts registered in our healthcare district (6.9%; 196/2832) over the same period (figure 1; p<0.001, χ2/Fisher’s exact test). Altogether, fever (>38°C) was detected in 6 (2.7%) of 219 patients. In one febrile patient, COVID-19 infection was confirmed by RT-PCR test, whereas five patients were diagnosed with neutropenic fever. In addition, COVID-19 testing for all healthcare professionals (9 physicians, 23 nurses, 2 technicians, 2 secretaries) from the outpatient department was performed. None was positive on repeat testing (days 0, 7 and 14; data not shown). Of note, no hospital-associated transmission was identified. All four COVID-19 positive patients were transferred to the hospital COVID-19 isolation ward, and during follow-up, no patient required admission to the intensive care unit (ICU). One out of four COVID-19 positive patients died due to progressive metastatic pancreas cancer. All other COVID-19 positive patients were discharged after a median observation period of 14 days in home quarantine. Oncological treatment was continued after resolution of symptoms and at least two consecutive negative RT-PCR tests collected >24 hours apart. A detailed description for the subgroup of COVID-19 positive patients is summarised in table 3.
Figure 1.
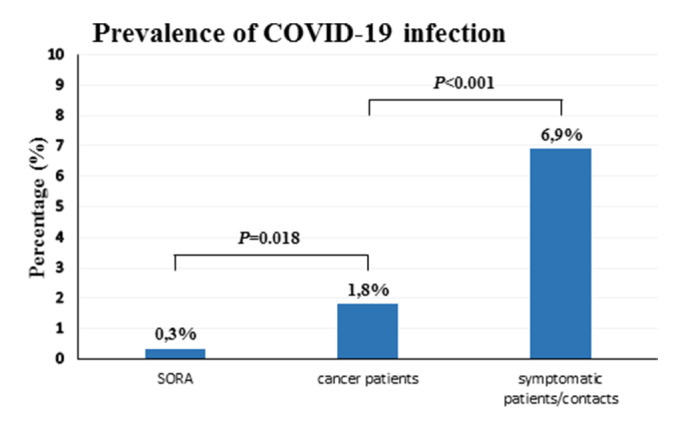
Comparison of the prevalence (in percent) of COVID-19 infection among different subgroups. SORA: prevalence of COVID-19 infection in asymptomatic individuals tested for COVID-19 within the SORA-trial; cancer patients: prevalence of COVID-19 infection in patients on active outpatient cancer treatment tested for COVID-19 from our hospital; symptomatic patients/contacts: prevalence of COVID-19 infection in symptomatic COVID-19 patients and their close contacts registered in our healthcare district. Statistical significance was determined using χ2/Fisher’s exact test.
Table 3.
Clinical characteristics of the subgroup of COVID-19 positive cancer patients on active outpatient therapy
| Subgroup of COVID+ cancer patients | 4/219 (1.8%) | Smoker | Current treatment | Comorbidities |
| Cancer type/gender | Age (years) | |||
| Solid tumours | ||||
| Pancreatic cancer/female | 71 | No | Capecitabine | Diabetes, hypertension |
| Biliary tract cancer/female | 73 | No | Cisplatin+gemcitabine | Hypertension |
| Haematological malignancies | ||||
| Mantle cell lymphoma/female | 69 | No | Bendamustine+rituximab | Hypertension, obesity |
| Chronic lymphocytic leukaemia/male | 63 | Yes | Fludarabine+cyclophosphamide+rituximab | Chronic renal failure |
Discussion
COVID-19 is characterised by rapid human-to-human transmission and currently there are no therapeutics or vaccines available.10 Consequently, strict infection control measures are of paramount importance. Because of the immunosuppressive state, patients with cancer undergoing anticancer treatment such as chemoimmunotherapy or targeted therapy are often more susceptible to infections compared with individuals without cancer.11–14 In addition, patients with cancer combine a frequent burden of comorbidities and many patients are repeatedly treated in the same area like chemotherapy rooms.
The present study describes the clinical management and examined the infection rate of COVID-19 in patients on active outpatient anticancer therapy within a tertiary care hospital in South Tyrol, an autonomous province in northern Italy. The provincial health service in South Tyrol is divided into four public healthcare districts, of these the second largest health district is Merano which provides healthcare to about 135 000 residents. As of the data cut-off on 9 April 2020, 3075 individuals have been tested for COVID-19 within the district of Merano, with registration of 200 laboratory-confirmed COVID-19 positive cases and 27 deaths. Almost 8% (243/3075) of all tests conducted were performed at our outpatient department, which was an important part of our infection control plan. As expected, COVID-19 prevalence among our screening group (1.8%; 4/219) was significantly lower than the cumulative prevalence of all other diagnosed symptomatic COVID-19 cases and their close contacts registered in our healthcare district (6.9%; 196/2832) over the same period (p<0.001). However, as per the report of the Austrian SORA-Institute for Social Research and Consulting on COVID-19 prevalence on exclusively asymptomatic individuals (0.33%; 5/1554), we found an estimated fivefold increased risk of COVID-19 in our patients with cancer (p=0.018).15
During the COVID-19 pandemic outbreak, concerns by both physicians and patients about management of patients with cancer have been recognised. In many hospitals, access to routine visits and surgical oncology may be either completely restricted or significantly reduced. Quickly, oncology societies and national authorities have published guidelines on cancer care during the pandemic. Currently, no clinical evidence supports changing or withholding chemoimmunotherapy in patients with cancer.8 16–19
We basically encouraged patients to continue treatment, provided that all infection control and prevention measures were followed. Interruption of active treatment was observed in only one patient from a nursing home, which became hotspot for COVID-19. Notably, compared with the northern Lombardy region, which is the epicentre of the country’s coronavirus outbreak, our region of South Tyrol had 7–10 days more to be prepared in advance for emergency plan. In our study, we could not identify some evidence regarding patients with a specific histology, therapy, or subpopulation of patients with cancer to be at higher risk of severe illness from COVID-19 infection.
In a study by Liang et al, a higher risk of developing severe events in COVID-19 disease in patients with cancer was reported.20 The authors suggest an intentional postponing of adjuvant chemotherapy or elective surgery for stable cancer. However, the number of COVID-19 positive cases for this subgroup was small with a large amount of clinical heterogeneity. Two out of 18 cancer patients with confirmed COVID-19 disease had unknown treatment status, and only 4 out of 16 patients had received chemotherapy or surgery within the past month. Moreover, the majority of patients (n=12) were cancer survivors in routine follow-up. Only recently, Zhang et al reported clinical outcomes from 28 COVID-19–infected cancer patients with solid tumours.21 In their retrospective study, COVID-19–infected cancer patients presented poor outcomes, with high occurrence of clinical severe event and a mortality rate of 28.6%. In addition, antitumour treatment within 14 days of COVID-19 diagnosis was an independent predictor of death or other severe events. Of note, 28.6% of the patients acquired COVID-19 infection during hospitalisation and nosocomial transmission of SARS-CoV-2 was suspected. In addition, no patients with haematological malignancies were reported and inpatient anticancer therapy may not reflect routine clinical practice in many countries. In a retrospective study on 1524 patients with cancer, Yu et al could find an infection rate of 0.79% (12/1524) compared with 0.37% within the general population.22 The authors concluded that hospital admission and recurrent hospital visits are potential risk factors for COVID-19 infection.
In total, we registered 10.5% (14/133) COVID-19 positive patients who were admitted to the ICU within all four healthcare districts of South Tyrol and having concomitant diagnosis of cancer (data not shown). Mortality rate of COVID-19 positive cancer patients in South Tyrol was 21.4% (3/14 deaths), which is comparable with that reported by Zhang et al.21 Major limitations of our study include the retrospective study design within a single institution, relative small sample size of patients and a limited observation period. However, current data to examine the effect of COVID-19 pandemic in outpatient cancer settings are still limited.
In summary, our data indicate that continuation of anticancer treatment in epidemic areas during the COVID-19 pandemic seems to be safe and feasible, if adequate and strict infection control measures are enforced. More detailed studies of COVID-19 infection in patients with cancer may help to guide management of oncology patients in future.
Footnotes
Contributors: Study design: MM and DF. Data collection: SR, CP, EH, MA, MM. Statistical analysis: MM. Drafting of the manuscript: DF. Critical revision of the manuscript for important intellectual content: DF, SR, MM.
Funding: This work was supported by the budget of the Südtiroler Sanitätsbetrieb.
Competing interests: DF declares travel, accommodation, expenses supported by Sanofi and Ipsen outside the submitted work. CP declares non-financial support from Innova outside the submitted work. EH declares travel, accommodation, expenses supported by Roche and received honoraria from Novartis, Boehringer Ingelheim and AstraZeneca outside the submitted work. MM declares travel, accommodation, expenses supported by Ipsen and Celgene and received honoraria from Janssen outside the submitted work.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the local Ethics Committee of the “Südtiroler Sanitätsbetrieb” (protocol number 35-2020) and was performed in accordance with the Declaration of Helsinki.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. The data that support the findings of this study are available from the corresponding author on reasonable request.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 2020. [DOI] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis 2020;94:91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Worldometer Coronavirus (Covid-19) mortality rate, 2020. Available: https://www.worldometers.info/coronavirus/country/italy [Accessed 9 Sep 2020].
- 5.Sorbello M, El-Boghdadly K, Di Giacinto I, et al. The Italian coronavirus disease 2019 outbreak: recommendations from clinical practice. Anaesthesia 2020;75:724–32. 10.1111/anae.15049 [DOI] [PubMed] [Google Scholar]
- 6.Nicastri E, Petrosillo N, Bartoli TA, et al. National Institute for the Infectious Diseases "L. Spallanzani", IRCCS. Recommendations for COVID-19 clinical management. Infect Dis Rep 2020;12:8543. 10.4081/idr.2020.8543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortiula F, Pettke A, Bartoletti M, et al. Managing COVID-19 in the oncology clinic and avoiding the distraction effect. Ann Oncol 2020;31:553–5. 10.1016/j.annonc.2020.03.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shankar A, Saini D, Roy S, et al. Cancer Care Delivery Challenges Amidst Coronavirus Disease - 19 (COVID-19) Outbreak: Specific Precautions for Cancer Patients and Cancer Care Providers to Prevent Spread. Asian Pac J Cancer Prev 2020;21:569–73. 10.31557/APJCP.2020.21.3.569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al‐Shamsi HO, Alhazzani W, Alhuraiji A, et al. A Practical Approach to the Management of Cancer Patients During the Novel Coronavirus Disease 2019 (COVID ‐19) Pandemic: An International Collaborative Group. Oncologist 2020. 10.1634/theoncologist.2020-0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamboj M, Sepkowitz KA. Nosocomial infections in patients with cancer. Lancet Oncol 2009;10:589–97. 10.1016/S1470-2045(09)70069-5 [DOI] [PubMed] [Google Scholar]
- 12.Cooley L, Dendle C, Wolf J, et al. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J 2014;44:1350–63. 10.1111/imj.12599 [DOI] [PubMed] [Google Scholar]
- 13.Hirsch HH, Martino R, Ward KN, et al. Fourth European conference on infections in leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis 2013;56:258–66. 10.1093/cid/cis844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai A, Sachdeva S, Parekh T, et al. COVID-19 and cancer: lessons from a pooled meta-analysis. JCO Glob Oncol 2020;6:557–9. 10.1200/GO.20.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SORA Institute for Social Research and Consulting COVID-19 prevalence. Available: https://www.sora.at/nc/news-presse/news/news-einzelansicht/news/covid-19-praevalenz-1006.html [Accessed 9 Sep 2020].
- 16.Ramirez PT, Chiva L, Eriksson AGZ, et al. COVID-19 global pandemic: options for management of gynecologic cancers. Int J Gynecol Cancer 2020. [DOI] [PubMed] [Google Scholar]
- 17.Lambertini M, Toss A, Passaro A, et al. Cancer care during the spread of coronavirus disease 2019 (COVID-19) in Italy: young oncologists' perspective. ESMO Open 2020;5:e000759. 10.1136/esmoopen-2020-000759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tagliamento M, Lambertini M, Genova C, et al. Call for ensuring cancer care continuity during COVID-19 pandemic. ESMO Open 2020;5:e000783. 10.1136/esmoopen-2020-000783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueda M, Martins R, Hendrie PC, et al. Managing cancer care during the COVID-19 pandemic: Agility and collaboration toward a common goal. J Natl Compr Canc Netw 2020:366–9. 10.6004/jnccn.2020.7560 [DOI] [PubMed] [Google Scholar]
- 20.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335–7. 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol 2020. 10.1016/j.annonc.2020.03.296. [Epub ahead of print: 26 Mar 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu J, Ouyang W, Chua MLK, et al. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol 2020. 10.1001/jamaoncol.2020.0980 [DOI] [PMC free article] [PubMed] [Google Scholar]


