Abstract
Introduction
Abiraterone acetate in combination with prednisone is now widely used for the treatment of castration‐resistant prostate cancer.
Case presentation
A 62‐year‐old patient with a bone metastatic castration‐resistant prostate cancer was started on abiraterone acetate at a dose of 1000 mg a day along with 10 mg prednisone. In spite of castrate testosterone level, the laboratory test showed a relatively high level of serum testosterone, which was 21 ng/dL. Within 6 months, the patient achieved a complete prostate‐specific antigen response. Follow‐up bone scintigraphy demonstrated no area of intense uptake. He had a history of hyperlipidemia and was started on atorvastatin at home just after starting abiraterone acetate.
Conclusion
This report is a rare case of a clinically complete response to abiraterone acetate in a patient with metastatic castration‐resistant prostate cancer.
Keywords: abiraterone acetate, bone metastases, castration resistant prostate cancer, statin
Abbreviations & Acronyms
- ADT
androgen deprivation therapy
- AR
androgen receptor
- CRPC
castration‐resistant prostate cancer
- LHRH
luteinizing hormone‐releasing hormone
- PSA
prostate‐specific antigen
Keynote message.
Abiraterone acetate is effective for CRPC patients especially with higher testosterone serum levels. This report is a rare case of a clinically complete response to abiraterone acetate in a patient with metastatic prostate cancer. The patient had a history of hyperlipidemia and was started on atorvastatin at home just after starting abiraterone acetate.
Case presentation
A 62‐year‐old man was diagnosed with a multiple bone metastatic prostate cancer. He had no symptoms and a blood test only revealed a serum PSA level of over 10 000 ng/mL. A transrectal ultrasound‐guided biopsy of his prostate revealed six cores (out of 6) of adenocarcinoma, with a Gleason score of 4 + 5. Bone scintigraphy showed diffuse increased tracer uptake with a pattern compatible with metastatic supercan (Fig. 1). Computed tomography also indicated multiple bone metastases and multiple lymphadenopathy. There were no identifiable visceral organ metastases. He received ADT. ADT included LHRH agonist and bicalutamide and his serum PSA level decreased to 0.11 ng/mL (nadir) in 10 months, but his duration of response to initial ADT (time to castration resistance) was 12 months without androgen withdrawal syndrome. His serum PSA level increased to 1.1 ng/mL. The complete blood count was normal. The level of serum lactate dehydrogenase was slightly elevated, which was 242 U/L. The level of serum testosterone was 21 ng/dL, which achieved a castrate level. All other laboratory results including serum cholesterol levels were within normal limits.
Figure 1.
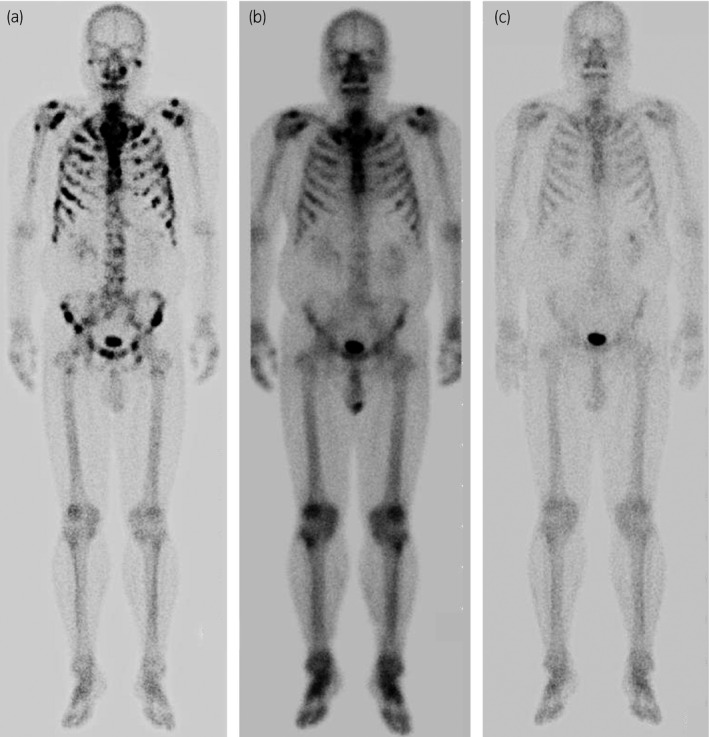
(a) Bone scintigraphy showed diffuse increased tracer uptake with a pattern compatible with metastatic supercan at the time of diagnosis of prostate cancer. (b) After ADT, diffuse bony lesions remained at the time of diagnosis of CRPC. (c) Follow‐up bone scintigraphy demonstrated no area of intense uptake, suggesting the bone metastasis had disappeared after treated with abiraterone acetate.
Abiraterone acetate at a dose of 1000 mg per day along with 10 mg prednisone was administered as a first‐line therapy for CRPC. Within 6 months, the patient achieved a complete PSA response. His serum PSA level reached a nadir (undetectable level). Follow‐up bone scintigraphy demonstrated no area of intense uptake, suggesting the bone metastasis had disappeared (Fig. 1). His serum PSA level remained at the nadir and no bone metastasis appeared for 4 years after starting abiraterone acetate (Fig. 2). We reviewed the patient's medical history and medications. The patient's medical chart showed he had a history of hyperlipidemia (his total cholesterol level was 256 mg/dL) and he was started on atorvastatin at home just after starting abiraterone acetate.
Figure 2.
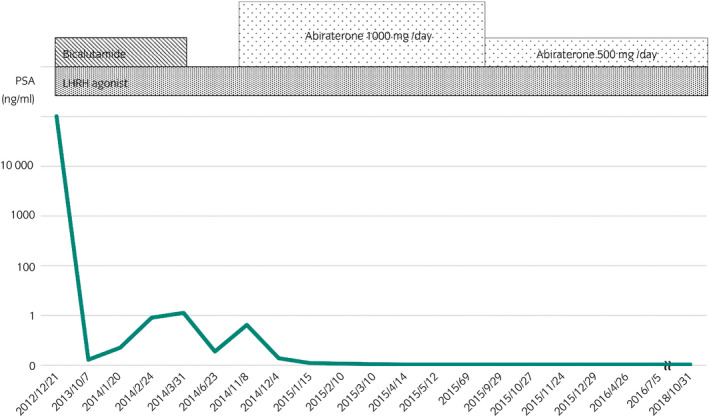
Serum PSA level remained at the nadir and no bone metastasis appeared for 4 years after starting abiraterone acetate.
Discussion
Abiraterone acetate in combination with prednisone is now widely used for the treatment of CRPC since the phase III trial COU‐AA‐302 demonstrated a significant overall survival.1 The median time to PSA progression was 11.1 months in the abiraterone‐prednisone group. Better and more complete inhibition of the transcriptional activity of the AR axis is needed to enhance the anti‐tumor efficacy of abiraterone acetate for prostate cancer cells.
In this report, abiraterone acetate was selected as a second‐line hormonal therapy against CRPC. There were two reasons. First, the patient's serum testosterone level was 21 ng/dL, which was relatively high during ADT. In a previous report, higher testosterone levels were found to predict a reduced risk of progression in CRPC patients.2 It is assumed that CRPC with a high testosterone level is associated with AR axis pathway. Abiraterone acetate specifically inhibits cytochrome P450 17α‐hydroxylase and C17,20‐lyase (CYP17A1) and thus depletes androgen biosynthesis.3 CYP17A1 inhibition provides significant benefits to CRPC patients due to suppression of androgen signaling, which stimulated prostate tumor growth. Second, the patient was a music teacher so he used his fingers a lot. Docetaxel is a highly effective treatment for metastatic CRPC, but is often associated with peripheral neuropathy.4
In Japanese patients with docetaxel‐naïve metastatic CRPC, the median time to PSA progression in the abiraterone acetate group was reported to be 9.0 months.5 Over 48 months, our patient's serum PSA continued to decrease to below 0.01 ng/mL, suggesting that abiraterone plus something may provide a good long‐term outcome in this patient. One of the biological roles of cholesterol is as the precursor for the synthesis of steroid hormones, including androgens. Statins decrease the level of cholesterol in the blood by inhibiting 3‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase in the mevalonate pathway.6 The anti‐tumor effects of statins have been described in some reports,7, 8 however, the actual mechanisms remain unclear. Further studies with larger samples will be needed in order to conclude that the addition of atorvastatin to abiraterone and prednisone contributes to the clinical response in patients with CRPC. Although further studies are needed, this report is a rare case of a clinically complete response to abiraterone acetate in a patient with metastatic CRPC.
Conflict of interest
The authors declare no conflict of interest.
References
- 1. Ryan CJ, Smith MR, de Bono JS et al Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Eng. J. Med. 2013; 368: 138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ryan CJ, Molina A, Li J et al Serum androgens as prognostic biomarkers in castration‐resistant prostate cancer: results from an analysis of a randomized phase III trial. J. Clin. Oncol. 2013; 31: 2791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jarman M, Barrie SE, Llera JM. The 16,17‐double bond is needed for irreversible inhibition of human cytochrome p450 17 alpha by abiraterone (17‐(3‐pyridyl)androsta‐5, 16‐dien‐3beta‐ol) and related steroidal inhibitors. J. Med. Chem. 1998; 41: 5375–81. [DOI] [PubMed] [Google Scholar]
- 4. Velasco R, Bruna J. Taxane‐induced peripheral neurotoxicity. Toxics 2015; 3: 152–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miyake H, Hara T, Terakawa T, Ozono S, Fujisawa M. Comparative assessment of clinical outcomes between abiraterone acetate and enzalutamide in patients with docetaxel‐naive metastatic castration‐resistant prostate cancer: experience in real‐world clinical practice in Japan. Clin. Genitourin. Cancer 2017; 15: 313–9. [DOI] [PubMed] [Google Scholar]
- 6. Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG‐CoA reductase. Science 2001; 292: 1160–4. [DOI] [PubMed] [Google Scholar]
- 7. Sassano A, Katsoulidis E, Antico G et al Suppressive effects of statins on acute promyelocytic leukemia cells. Cancer Res. 2007; 67: 4524–32. [DOI] [PubMed] [Google Scholar]
- 8. Alfaqih MA, Allott EH, Hamilton RJ, Freeman MR, Freedland SJ. The current evidence on statin use and prostate cancer prevention: are we there yet? Nat. Rev. Urol. 2017; 14: 107–19. [DOI] [PMC free article] [PubMed] [Google Scholar]


