Abstract
Although electroconvulsive therapy seizure duration has been shown to have limited relevance to efficacy, seizure duration remains important for clinically valid stimulus efficiency. There has been no report on seizure duration using sample entropy with Thymatron (Somatics, Inc), which is widely used in Japan. Furthermore, wavelet transform analysis is also suitable for a seizure because of the wide range of dominant frequencies. Therefore, in this study with Thymatron, the intraclass correlations of seizure duration determined by sample entropy, wavelet transform, and visual determination were investigated to determine whether these methods were applicable for clinical use. Wavelet transform, sample entropy, and the human rater had high intraclass correlations for seizure duration. The present results indicate that wavelet transform and sample entropy can be useful in the clinical electroconvulsive therapy setting, and they may also be suitable for clinical research into the mechanisms of the generalized tonic‐clonic seizures related to the efficacy of electroconvulsive therapy.
Keywords: ECT, EEG, sample entropy, seizure duration, wavelet transform
The intraclass correlations of electroconvulsive therapy‐induced seizure duration determined by sample entropy, wavelet transform, and visual determination were investigated to determine whether these methods were applicable for clinical use. sample entropy, wavelet transform, and the human rater had high intraclass correlations for the seizure durations. The present results indicate that sample entropy and wavelet transform can be useful in the clinical electroconvulsive therapy setting.

1. INTRODUCTION
Electroconvulsive therapy (ECT) is widely used to treat depression and is considered to be a rapidly acting and effective therapy.1 Although ECT‐induced EEG seizure duration (SD) has been shown to have limited relevance to efficacy, SD remains important to ensure clinically valid stimulus efficiency. Accordingly, studies on determining when a seizure terminates have been reported.2, 3, 4 Human raters showed sufficient reliability when artifact or poor postictal suppression (PS) was absent compared with the built‐in algorithm with Thymatron DGx (Somatics, Inc), though human raters had weak points when the above phenomena were present.3 However, an ECT‐induced EEG seizure was not stationary, but nonstationary5 and appropriate for nonlinear analysis. So far, fractal dimension (FD)‐ and sample entropy (SampEn)‐based algorithms, as nonlinear analyses, have been shown to have good interrater reliabilities with experienced human raters.6, 7 Gangadhar used FD to examine EEG seizures from the end of electrical stimulation6, while Yoo used SampEn with the 3‐min EEG including the prestimulus EEG with the MECTA Spectrum 5000Q device (MECTA Corp).7 There have been no reports on the EEG seizure endpoint using SampEn with Thymatron (Somatics, Inc), which is widely used in Japan. Furthermore, wavelet transform (WT) analysis is also suitable for seizures because of the wide range of dominant frequencies.8 Therefore, in this study with Thymatron, the intraclass correlations of SD by WT, SampEn, and visual determination were investigated to determine whether these methods were applicable for clinical use.
2. METHODS
Electroconvulsive therapy was conducted by Thymatron containing an inbuilt EEG system (Fp1‐A1, Fp2‐A2, International 10‐20 system). The details have been described elsewhere.9 A total of 100 continuous ECT EEGs from seven patients (4 women and 3 men; age 60.7 ± 17.7 years; age range, 40‐85 years) diagnosed with a depressive episode (depressive disorder/bipolar disorder; 6/1) treated by ECT in Nagoya City University Hospital were analyzed.10 All session EEGs were used. The EEG from the end of electrical stimulation to 2 seconds was deleted due to machine artifact contamination, and the EEG data endpoint, not the seizure endpoint, was always the point at least 15 seconds after the probable seizure endpoint. The EEG sampling frequency was 200 Hz. First, the human rater determined the SD of all sets. Second, WT and SampEn were calculated for all sets without confirming the results of the human rater.
2.1. EEG analysis
2.1.1. Human rater
The SD was determined with a precision of 1 seconds. The SD was the integer rounded down to the nearest decimal. One experienced human rater rated the SD and PS.9 The SD was defined as the point where EEG seizure activity changed to a continuously lower amplitude or flattening. The end of the seizure was divided into three categories according to the degree of PS. PS was rated by the human rater as follows: 0, cannot tell where the seizure ends; 1, seizure termination is clear, but suppression is poor (not flat); 2, good seizure suppression (very flat), but the transition to flat is gradual; and 3, good seizure suppression (very flat), and the transition is abrupt.
2.1.2. Wavelet transform
Wavelet transform analysis is a linear analysis. A seizure is characterized by dynamic localized variations in the frequency of the signal. A spectrogram is usually used to represent the spectra in the time and frequency space by means of a short‐time Fourier transform (STFT) using the window function, and a predetermined interval is used to analyze the time‐varying signals. However, a predetermined scaling method of the STFT may not be appropriate for the ictal EEG seizure because of the wide range of dominant frequencies and the nonstationary power. A method of time‐frequency localization that is scale‐independent, such as WT, should be used.11 Therefore, the ictal EEG was analyzed with a continuous wavelet transform (CWT) using the Morse wavelet. The absolute maximum magnitude of the CWT was averaged per second. The CWT analysis for ictal EEG was useful in terms of the ictal EEG gradually changing the evolution of its frequency from faster to slower according to the time elapsed.8
2.1.3. SampEn
SampEn was selected according to Yoo et al7 SampEn is a statistic measuring the complexity and regularity of clinical and experimental time series data and can be used for the analysis of relatively shorter biological data.12 A lower value of SampEn indicates more self‐similarity and regularity in the time series. SampEn (N, r, m) was calculated by means of the algorithm in PhysioNet.12, 13, 14, 15 N is the length of data, m is the length of sequences to be compared, and r is the tolerance for accepting matches.13 In the present study, N = 200 data points, m = 2, and r = 0.2 were used.
2.1.4. Exploring the seizure endpoint
One example of SD is shown in Figure 1A‐E. All raters indicated the SD of this seizure as 74 seconds. The method of determining the seizure endpoint was always calculated by the same method with both SampEn and WT analyses. After the calculations with SampEn and WT analysis, the following was applied to detect the SD for the two methods. The ictal EEG was standardized (mean = 0 and standard deviation = 1) before these calculations (Figure 1A). Figure 1B shows the SampEn analysis, and Figure 1C‐E shows the WT analysis. Because the changes in SampEn (Figure 1B) were similar to those of wavelet magnitude on WT analysis (Figure 1C), the below is explained using the WT analysis. Because the wavelet magnitude and SampEn gradually increased and decreased and then flattened (Figure 1B,C), the part of the increase was deleted to avoid duplication with the part of the decrease. Thus, SD of <15 seconds was excluded. The wavelet magnitude in Figure 1C was sorted in ascending order (Figure 1D). Thus, the index in Figure 1D means the order from the small to the large wavelet magnitude. Then, the 10 wavelet magnitudes backward from each index were averaged, according to Yoo et al7 (Figure 1C,D). Specifically, each 10 wavelet magnitudes, from index 1 (75 seconds) to 66 seconds, from index 2 (94 seconds) to 85 seconds, from index 3 (92 seconds) to 83 seconds, continuing to from index 10 (100 seconds) to 91 seconds, were averaged (Figure 1C‐E). In exploring the seizure endpoint, since the index of the seizure endpoint was the largest averaged wavelet magnitude among the above 10 averaged wavelet magnitudes, in this seizure, index 5 (74 seconds) was the seizure endpoint (Figure 1E). The ANOVA intraclass correlation coefficients (ICCs) were calculated for the SD between the human rater and the two methods.16 All calculations were performed using MATLAB R2018b.
Figure 1.
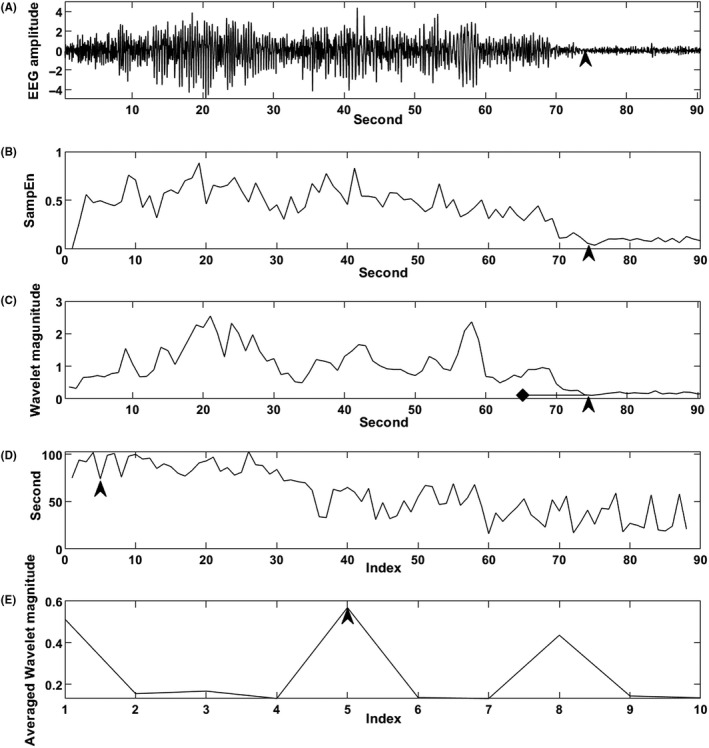
A, Electroconvulsive therapy (ECT)‐induced EEG seizure for which the seizure duration (SD) was determined to be 74 s (arrow) by all raters. B, SampEn of the seizure. The X‐axis is time (seconds), and the Y‐axis is SampEn. The arrow indicates 74 s (X‐axis). C, Wavelet magnitude per second calculated by wavelet transform (WT) analysis of the seizure. The X‐axis is time (s), and the Y‐axis is the wavelet magnitude of the continuous wavelet transform. The arrow indicates 74 s (X‐axis), which is index 5 in D (Arrow). The diamond indicates 65 s (X‐axis). Ten wavelet magnitudes from 74 to 65 s were averaged. From index 1 to index 10, the averaged wavelet magnitudes were always calculated similarly. D, The index (X‐axis) implies the ascending order of wavelet magnitude (the order from small to large wavelet magnitude). Thus, the Y‐axis is the time (s) corresponding to the wavelet magnitude in C. The smaller index shows smaller wavelet magnitude. The arrow indicates that index 5 (X‐axis) is 74 s (Y‐axis). E, The 10 wavelet magnitudes backward from each of the 10 indices were averaged. The largest averaged wavelet magnitude was index 5 (arrow) (X‐axis). The index is the same as in D
3. RESULTS
The results are shown in Table 1. One seizure with an 11‐second SD was excluded. It was confirmed that all of the data sets included no coarse artifacts. A total of 99 seizures were included. The ANOVA ICCs overall and of all pairs were high and sufficient for clinical application. The means (standard deviation) of the SD for the human rater, SampEn, and WT were 58.7 seconds (17.6 seconds), 58.7 seconds (17.5 seconds), and 59.8 seconds (17.7 seconds), respectively. The proportions with a 3‐second or greater difference between each of two methods were 6.1%, 17.2%, and 12.1% for the human rater vs SampEn, the human rater vs WT, and SampEn vs WT, respectively, while that for the human rater vs SampEn of Yoo et al7 was 20.8%. PS was rated as 1 (PS1) in 51%, 2 (PS2) in 28%, and 3 (PS3) in 21%. The ICCs [99% confidence intervals] of PS1, PS2, and PS3 were 0.99[0.98 0.99], 0.99[0.98 1.0], and 0.99[0.98 1.0], respectively. The proportions with a 3‐second or greater difference between each of two methods in PS1 were 19.6%, 23.5%, and 25.5% for the human rater vs SampEn, the human rater vs WT, and SampEn vs WT, respectively; those in PS2 were 22.2%, 33.3%, and 14.8%, respectively; and those in PS3 were 4.8%, 28.6%, and 19.0%, respectively. Thus, the present data could consist of the various types of seizure termination.
Table 1.
ANOVA ICC and 99% confidence interval (CI)
| Raters | ICC | 99% CI |
|---|---|---|
| Human vs SampEn vs WT | 0.99 | [0.98 0.99] |
| Human vs SampEn | 0.99 | [0.98 0.99] |
| SampEn vs WT | 0.99 | [0.99 1.00] |
| Human vs WT | 0.99 | [0.98 0.99] |
Abbreviations: SampEn, sample entropy; WT, wavelet transform.
4. DISCUSSION
This study demonstrated that WT, SampEn, and the human rater had high ICCs for SD. The results with SampEn were the same as Yoo et al7 (n = 24). However, Yoo et al used the 3‐minute EEG by the MECTA Spectrum 5000Q device, and the EEG consisted of the period before electrical stimulation, the ictal EEG, and the postictal EEG.7 In the present study, the ictal EEG for the calculation of the seizure endpoint was the EEG from the end of electrical stimulation to the postictal state. Furthermore, in Japan, the Thymatron is broadly used, and our method can be used as soon as the end of the seizure with the computer through the Thymatron. The present data consisted of consecutive ECT sessions (n = 99) and included difficult cases to be rated as shown in the PS ratings. So far, manually rated EEG seizure markers of ECT treatment efficacy, regularity, or PS were investigated.9, 17 Nevertheless, SD is clinically the first criterion to determine the validity of the session. The present results indicate that these methods, WT and SampEn, can assist human raters in the clinical ECT setting. These methods may also be suitable for clinical research into the mechanism of the generalized tonic‐clonic seizure related to the efficacy of ECT.
The limitation of our methods is that coarse artifact contamination between the SD and the data endpoint may cause identification of the incorrect SD. Although the data endpoint was usually 5‐10 seconds over the SD, a data endpoint that is not too long is desirable to avoid artifact contamination.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
HA designed the study and wrote the protocol. HA, HO, and ES managed data acquisition, and all authors critically reviewed the manuscript.
INFORMED CONSENT
Each patient's written, informed consent was obtained.
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEW BOARD
This research was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans and was approved by the Ethics Committee of the Nagoya City University Medical School Ethics Committee.
Supporting information
ACKNOWLEDGMENTS
The authors would like to thank all of the patients and the residents for participating in this study.
Azuma H, Ogawa H, Suzuki E, Akechi T. Intraclass correlations of seizure duration by wavelet transform, sample entropy, and visual determination in electroconvulsive therapy. Neuropsychopharmacol Rep. 2020;40:102–106. 10.1002/npr2.12095
DATA AVAILABILITY STATEMENT
Data sets are available in the Supporting Information.
REFERENCES
- 1. UK ECT Review Group . Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta‐analysis. Lancet. 2003;361(9360):799–808. [DOI] [PubMed] [Google Scholar]
- 2. Swartz CM, Abrams R, Rasmussen K, Pavel J, Zorumski CF, Srinivasaraghavan J. Computer automated versus visually determined electroencephalographic and electromyographic seizure duration. Convuls Ther. 1994;10(2):165–70. [PubMed] [Google Scholar]
- 3. Krystal AD, Weiner RD. ECT seizure duration: reliability of manual and computer‐automated determinations. Convuls Ther. 1995;11(3):158–69. [PubMed] [Google Scholar]
- 4. Rosenquist PB, McCall WV, Colenda CC, Melton BA. A comparison of visual and computer‐generated measures of “seizure quality”. J ECT. 1998;14(2):76–82. [PubMed] [Google Scholar]
- 5. Katz MJ. Fractals and the analysis of waveforms. Comput Biol Med. 1998;18(3):145–56. [DOI] [PubMed] [Google Scholar]
- 6. Gangadhar BN, Dutt DN, Janakiramaiah N, Sadasivan PK. Automation of seizure duration estimation during ECT; use of fractal dimension. Proceedings of the First Regional Conference, IEEE Engineering in Medicine and Biology Society and 14th Conference of the Biomedical Engineering Society of India; 1995.
- 7. Yoo CS, Jung DC, Ahn YM, Kim YS, Kim S‐G, Yoon H, et al. Automatic detection of seizure termination during electroconvulsive therapy using sample entropy of the electroencephalogram. Psychiatry. 2012;195(1–2):76–82. [DOI] [PubMed] [Google Scholar]
- 8. Azuma H, Akechi T. Temporal evolution of ictal physiological indices during electroconvulsive therapy. Clin Neurophysiol. 2017;128(9):e176. [Google Scholar]
- 9. Azuma H, Fujita A, Sato K, Arahata K, Otsuki K, Hori M, et al. Postictal Suppression correlates with therapeutic efficacy for depression in bilateral sine and pulse wave electroconvulsive therapy. Psychiatry Clin Neurosci. 2007;61(2):168–73. [DOI] [PubMed] [Google Scholar]
- 10. American Psychiatric Association . Diagnostic and statisitical manual of mental disorders, 5th edn Arlington, VA: American psychiatric Publishing; 2013. [Google Scholar]
- 11. Torrence C, Compo GP. A practical guide to wavelet analysis. Bull Am Meteor Soc. 1998;79(1):61–78. [Google Scholar]
- 12. Richman JS, Moorman JR. Physiological time‐series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278:H2039–H2049. [DOI] [PubMed] [Google Scholar]
- 13. Lake DK, Moorman JR,Cao H.Sample entropy estimation using sampen. Available from: http://www.physionet.org/physiotools/sampen/c/. Accessed July 01, 2019.
- 14. Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, Mark RG, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101:E215–E220. [DOI] [PubMed] [Google Scholar]
- 15. Azuma H, Nagata H, Akechi T. Linear and non‐linear EEG analyses before and after psychosis in patients with epilepsy. Epilepsy Seizure. 2019;11(1):20–9. [Google Scholar]
- 16. McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;1(1):30–46. [Google Scholar]
- 17. Perera TD, Luber B, Nobler MS, Prudic J, Anderson C, Sackeim HA. Seizure expression during electroconvulsive therapy: relationships with clinical outcome and cognitive side effects. Neuropsychopharmacol. 2004;29(4):813–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sets are available in the Supporting Information.


