Abstract
Purpose
Elevated plasma D-dimer levels were thought to be associated with decreasing survival in various cancers. The relationship between plasma D-dimer levels and clinicopathology and the optimal D-dimer cutoff as a prognostic predictor has not been determined in patients with upper tract urothelial carcinoma (UTUC). We aimed to investigate the prognostic value of preoperative plasma D-dimer levels as a predictor of patient outcomes in UTUC following radical nephroureterectomy.
Patients and Methods
We retrospectively reviewed data for 232 patients. The D-dimer cutoff value was set at 0.36 mg/L, and we used the Kaplan–Meier method and Cox’s proportional hazards regression models to analyze the association between D-dimer levels and oncological outcomes. Multivariate Cox regression was used to develop a nomogram, which we evaluated for accuracy using a receiver operating characteristic curve, calibration plot, and decision curve analysis.
Results
Plasma D-dimer levels ≥0.36 mg/L were significantly associated with advanced tumor status regarding size, location, hydronephrosis, tumor grade, lymph node involvement, grade, and stage (all p < 0.05). The Kaplan–Meier analysis showed that plasma D-dimer levels ≥0.36 mg/L predicted worse oncological outcomes vs levels <0.36 mg/L (all p < 0.001). Univariate and multivariate analyses showed that elevated preoperative plasma D-dimer level was an independent predictor of recurrence-free survival (hazard ratio (HR): 1.67, 95% confidence interval (CI): 1.07–2.63; p = 0.025), cancer-specific survival (HR: 2.34, 95% CI: 1.30–4.19; p = 0.004), and overall survival (HR: 1.98, 95% CI: 1.18–3.34; p = 0.010). We also developed a nomogram predicting 3- and 5-year overall survival probability.
Conclusion
D-dimer levels may be a useful prognostic predictor of survival and improve risk stratification and precisely individualize treatment for patients with UTUC.
Keywords: urothelial carcinoma, upper urinary tract, prognosis, predictors, D-dimer, nomogram
Introduction
Urothelial carcinoma arises from the urothelium that lines the renal pelvis, ureter, bladder, and urethra. Upper tract urothelial carcinoma (UTUC) is a relatively rare cancer with an incidence of 1.2 per 100,000 and accounts for approximately 5–10% of all urothelial malignancies.1 Radical nephroureterectomy (RNU) with ipsilateral bladder cuff excision is the surgical gold standard for the treatment of localized UTUC. For low-risk patients have the option of receiving nephron-sparing treatment. Owing to the aggressive nature of UTUC, the prognosis is generally poor, and its oncological outcomes are unsatisfactory, with an approximate 20% 5-year survival rate. Therefore, studies have been performed to identify the prognostic factors.2 The current recognized prognostic factors are derived mainly from postoperative data, such as pathological T stage, grade, lymph node involvement, and lymphovascular invasion.2–4 Preoperative factors, namely body mass index, advanced age, delayed surgery, hydronephrosis, tumor location, and neutrophil–lymphocyte ratio (NLR) are associated with poor prognosis.3
Several studies have demonstrated an association between malignancy and hemostasis biomarkers5,6 with tumors associated with high levels of coagulation markers (fibrinogen) and thrombogenesis markers (D-dimer). D-dimer is a cleavage product of fibrin, which is produced by plasmin-induced fibrinolysis,7 and this biomarker indicates the activation of hemostasis and fibrinolysis. Elevated plasma D-dimer levels may be associated with disseminated intravascular coagulation, pregnancy, infectious diseases, trauma, surgery, and venous thromboembolism.8 Although elevated circulating D-dimer levels are related to various cancers, such as lung, breast, prostate, and bladder cancer,6,9-11 the relationship between this hemostatic biomarker and UTUC survival outcomes is unclear. Therefore, the purpose of the current study was to investigate the prognostic value of preoperative plasma D-dimer levels in UTUC.
Patients and Methods
Patients
After approval by the Institutional Review Board of the Ethics Committee of Shandong Provincial Hospital (No. 2020–014), we retrospectively reviewed the electronic medical records of patients from the hospital information system, who were diagnosed with UTUC and underwent the RNU at Shandong Provincial Hospital between 1 January 2008 and 31 December 2018. The diagnosis of UTUC was confirmed by urinary cytology, ureteroscopy, retrograde pyelography, computed tomography, or magnetic resonance imaging. All patients in this study met the following criteria: 1) the first diagnosis of primary resectable UTUC and age > 18- years, 2) all needed variables in the study were complete, 3) underwent operation at our center, 4) postoperative pathological diagnosis of UTUC, and 5) adequate follow-up information. The exclusion criteria were: 1) diagnosis of another primary cancer, 2) severe cardiovascular or respiratory disease, 3) active infection, pregnancy, or autoimmune disease, 4) hematological disease, 5) history of anticoagulant or procoagulant drug use within 8 weeks before surgery, 6) incomplete blood test results, and 7) perioperative death. Patients demographics, clinical, and pathological characteristics were recorded. Preoperative plasma D-dimer levels were measured before breakfast using an automatic coagulum (Stago, Asnieres-Sur-seine, France). All patients underwent either open or laparoscopic RNU. We did not routinely perform lymphadenectomy unless patients had suspicious enlarged lymph node(s) in preoperative imaging studies or positive intraoperative findings. Tumor stage and grade were assessed according to the 2010 American Joint Committee of Cancer TNM classification and the 2004 World Health Organization/International Society of Urologic Pathology classification, respectively.
Follow-Up Regimen
We evaluated patients every 3 months postoperatively for the first year and every 6 months for the second and third years, then annually thereafter by collecting the patients’ history and performing a physical examination, routine laboratory tests, urine cytology, excretory urography, cystoscopy, and radiography of the chest, abdomen, and pelvis. Follow-up were also performed by telephone interview. According to the follow-up program, all patients were followed until the study end point of 31 December 2019, or until death. The primary endpoint was cancer-specific survival (CSS), defined as the time in months from the date of surgery to cancer-related death. Secondary endpoints were overall survival (OS) and recurrence-free survival (RFS). We defined OS as the interval between surgery and the last follow-up or death from any cause. RFS was calculated as the interval between surgery and last follow-up or recurrence.
Statistical Analysis and Nomogram Generation
We used SPSS 25.0 (IBM Corp., Armonk, NY) and GraphPad Prism 8.0 software (GraphPad Software Inc., San Diego, CA) for the statistical analysis. We analyzed continuous variables using Student’s t-test, and categorical variables using the chi-squared test. The ideal D-dimer cutoff value was calculated by using a receiver operating curve analysis to discriminate between patient survival and cancer-related outcomes. Significant prognostic predictors in univariate and multivariate analyses were determined using Cox’s proportional hazards regression model. Variable of the p-values > 0.05 were removed from further analysis. Survival outcomes were calculated using the Kaplan–Meier method and were compared between groups using the Log rank test. P < 0.05 was considered statistically significant. We developed a nomogram to predict the 3-year and 5-year OS probabilities according to the results of the multivariable regression model using R 3.3.2 (www.r-project.org/) with the Regression Modeling Strategies packages. A backward step-down selection was applied to select the predictors, and we used bootstrap validation method to estimate the bias-corrected or overfitting-corrected predictive accuracy of the model. We analyzed the predictive accuracies of the models using the area under the receiver operating characteristic curve. Calibration plots were assessed by comparing the predicted probabilities with the actual observed frequencies, and we performed a decision curve analysis to determine the clinical benefit of the prediction model.
Results
The patient selection flowchart is shown in Figure S1. We enrolled 232 consecutive patients with histologically diagnosed UTUC, and patients’ baseline characteristics are summarized in Table 1. The cohort consisted of 132 men (56.9%) and 100 women (43.1%) with a median age of 65 years (IQR (interquartile range): 58–73), of whom 81 patients (34.9%) had a history of smoking. The median follow-up duration was 39 months (IQR, 17–53). In 87 patients (37.5%), the tumor was located in the ureter; in 113 patients (48.7%), the tumor was in the renal pelvis; and in 22 patients (13.8%), tumors were present in both sites. Multifocal lesions were found in 40 patients (17.2%). Pathological T stage was pT1 in 52 patients (22.4%), pT2 in 70 patients (30.2%), pT3 in 86 patients (37.1%) and pT4 in 24 patients (10.3%). Tumor grade was low in 53 patients (22%) and high in 179 patients (78%).
Table 1.
Demographics and Clinical Characteristics of Patients with UTUC Grouped by Preoperative Plasma D-Dimer Level
| Variables | Total (n=232) | Group < 0.36 (n=128, 55.2%) | Group ≥ 0.36 (n=104 44.8%) | p |
|---|---|---|---|---|
| Sex male/female | 132/100 | 77/51 | 55/49 | 0.288 |
| Median age (IQR), years | 65 (58–73) | 65 (58–72) | 65 (57–73) | 0.926 |
| Median BMI (IQR), kg/m2 | 25.2 (23.2–27.0) | 25.4 (23.4–27.1) | 25.0 (23.0–26.7) | 0.130 |
| Smoking yes/no | 81/151 | 47/81 | 34/70 | 0.580 |
| Laterality right/left | 108/124 | 70/58 | 54/50 | 0.693 |
| Tumor size ≥ 3 cm vs <3 cm | 119/113 | 52/76 | 67/37 | 0.000 |
| ASA scores >2, n (%) | 50 (21.6) | 27 (21.1) | 23 (22.1) | 0.874 |
| Tumor location, n (%) | 0.021 | |||
| Ureter | 87 (37.5) | 47 (36.7) | 40 (38.5) | |
| Pelvicalyceal | 113 (48.7) | 70 (54.7) | 43 (41.3) | |
| Both | 32 (13.8) | 11 (8.6) | 21 (20.2) | |
| Multifocality, n (%) | 40 (17.2) | 20 (15.6) | 20 (19.2) | 0.489 |
| Hydronephrosis, n (%) | 142 (61.2) | 69 (53.9) | 73 (70.2) | 0.015 |
| Surgical approach laparoscopy vs open | 143/89 | 81/47 | 62/42 | 0.589 |
| Tumor grade, n (%) | 0.007 | |||
| Low grade | 53 (22) | 38 (29.7) | 15 (14.4) | |
| High grade | 179 (78) | 90 (70.3) | 89 (85.6) | |
| Tumor stage, n (%) | <0.001 | |||
| pT1 | 52 (22.4) | 43 (33.6) | 9 (8.7) | |
| pT2 | 70 (30.2) | 45 (35.2) | 25 (24.0) | |
| pT3 | 86 (37.1) | 35 (27.3) | 51 (49.0) | |
| pT4 | 24 (10.3) | 5 (3.9) | 19 (18.3) | |
| Concomitant CIS | 22 (9.5) | 10 (7.8) | 12 (11.5) | 0.373 |
| LN status: positive, n (%) | 38 (16.4) | 10 (7.8) | 28 (26.9) | <0.001 |
| LVI: positive, n (%) | 13 (5.6) | 5 (3.9) | 8 (7.7) | 0.257 |
| Surgical margin status: positive, n (%) | 14 (6.0) | 5 (3.9) | 9 (8.7) | 0.168 |
| Concurrent bladder cancer, n (%) | 27 (11.6) | 11 (8.6) | 16 (15.4) | 0.149 |
| NLR ≥ 2.4 vs < 2.4 | 121/111 | 60/68 | 61/43 | 0.086 |
Abbreviations: UTUC, upper tract urothelial carcinoma; IQR, interquartile; BMI, body mass index; VS, versus; ASA, American Society of Anesthesiologists; CIS, carcinoma in situ; LN, lymph node; LVI, lymphovascular invasion; NLR, neutrophil–lymphocyte ratio.
Patients were categorized into two groups, according to a D-dimer cutoff level of 0.36 mg/L to discriminate between RFS, CSS, and OS (Figure 1). The D-dimer < 0.36 mg/L group constituted 128 patients, and 104 patients (44.8%) constituted the D-dimer ≥ 0.36 mg/L group. The association between preoperative plasma D-dimer levels and patients’ clinicopathological variables is shown in Table 1. There were no differences between the groups regarding sex, age, BMI, smoking history, laterality, American Society of Anesthesiologists (ASA) scores, multifocality, surgical approach, concomitant carcinoma in situ (CIS), lymphatic vessel invasion (LVI), surgical margin status, concurrent bladder cancer and NLR (p > 0.05). However, patients with elevated D-dimer levels were significantly associated with advanced tumor status in terms of size (p < 0.001), location (p = 0.021), hydronephrosis (p = 0.015), tumor grade (p = 0.007), lymph node involvement (p < 0.001), and stage (p < 0.001).
Figure 1.
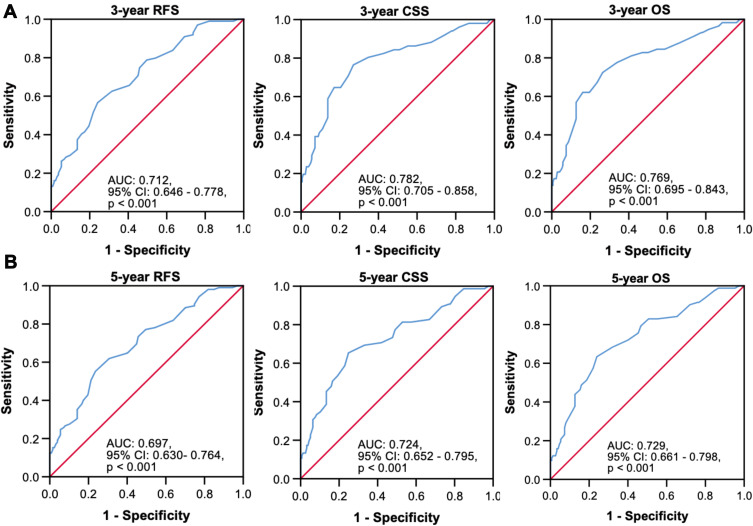
Time-dependent area under the receiver-operating characteristic curve analyses for determination of optimal cutoff values of preoperative plasma D-dimer levels to predict recurrence-free survival, cancer-specific survival and overall survival at 3 years (A) and 5 years (B).
Abbreviations: RFS, recurrence-free survival; CSS, cancer-specific survival; OS, overall survival; AUC, area under curve; CI, confidence interval.
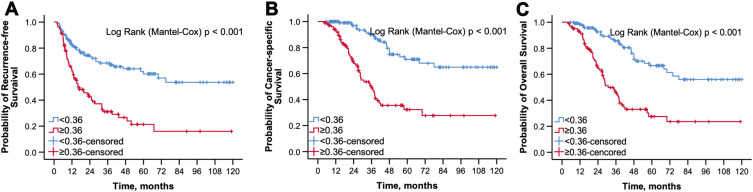
During the follow-up period, 37.9% patients (n = 88) died from all causes, 31.9% patients (n = 74) died from UTUC, and 47.0% patients (n = 109) experienced cancer recurrence. The Kaplan–Meier survival analyses revealed that patients with higher D-dimer levels had significantly worse RFS (p < 0.001), CSS (p < 0.001) and OS (p < 0.001) compared with patients with D-dimer levels below the cutoff (Figure 2). In patients with D-dimer ≥ 0.36 mg/L, the 5-year OS, CSS, and RFS were 27.5 ± 5.7%, 32.3 ± 6.1% and 21.3 ± 5.4%, respectively, and 66.7± 5.5%, 71.0 ± 5.5%, and 60.2 ± 5.3%, respectively, in their counterparts (p < 0.001).
Figure 2.
The Kaplan–Meier survival curve of RFS (A), CSS (B), and OS (C) stratified by the optimal cutoff value of preoperative plasma D-dimer.
Abbreviations: RFS, recurrence-free survival; CSS, cancer-specific survival; OS, overall survival.
In the univariate Cox analysis (Table 2), tumor size, location, multifocality, hydronephrosis tumor grade, stage, lymph node status, LVI, surgical margin status, bladder concurrence, and NLR were statistically associated with poor RFS, CSS, and OS (p < 0.05). Multivariate Cox analysis (Table 3) showed that the tumor location, lymph node status, and NLR were independent predictors of RFS, CSS and OS (p < 0.05). Univariate Cox analysis showed that increased preoperative plasma D-dimer levels (≥ 0.36 mg/L) were significantly associated with poor RFS (HR 2.85, 95% CI: 1.92–4.21, p < 0.001), CSS (HR 4.34, 95% CI: 2.64–7.15, p < 0.001) and OS (HR 3.57, 95% CI: 2.29–5.56, p < 0.001). Similarly, multivariate analysis indicated that preoperative plasma D-dimer levels ≥ 0.36 mg/L were an independent risk factor for shorter RFS (HR 1.67, 95% CI 1.07–2.63, p = 0.025), CSS (HR 2.34, 95% CI 1.30–4.19, p = 0.004) and OS (HR 1.98, 95% CI 1.18–3.34, p = 0.010).
Table 2.
Univariate Cox Regression Analyses of Relationship Between D-Dimer and Survival Outcomes in Patients with UTUC
| Variables | Recurrence-Free Survival | Cancer-Specific Survival | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Sex male/female | 1.39 | 0.95–2.02 | 0.088 | 1.27 | 0.81–2.01 | 0.300 | 1.29 | 0.85–1.97 | 0.229 |
| Age, years (≥65y vs 65y<) | 0.81 | 0.55–1.18 | 0.268 | 0.92 | 0.58–1.46 | 0.717 | 0.97 | 0.64–1.48 | 0.887 |
| BMI (≥25 vs 25<) | 0.82 | 0.56–1.20 | 0.300 | 0.68 | 0.42–1.08 | 0.100 | 0.70 | 0.46–1.07 | 0.102 |
| ASA scores >2, n (%) | 0.64 | 0.38–1.08 | 0.096 | 0.72 | 0.39–1.34 | 0.305 | 0.84 | 0.49–1.45 | 0.538 |
| Smoking yes/no | 0.75 | 0.50–1.14 | 0.174 | 0.83 | 0.51–1.37 | 0.469 | 0.92 | 0.59–1.45 | 0.727 |
| Laterality right/left | 1.01 | 0.70–1.48 | 0.949 | 0.71 | 0.44–1.12 | 0.140 | 0.83 | 0.55–1.27 | 0.389 |
| Tumor size (≥3.0cm vs < 3.0cm) | 1.97 | 1.33–2.92 | 0.001 | 2.22 | 1.36–3.64 | 0.002 | 1.83 | 1.182.84 | 0.007 |
| Tumor location | < 0.001 | < 0.001 | < 0.001 | ||||||
| Pelvicalyceal vs ureter | 0.45 | 0.28–0.71 | 0.001 | 0.22 | 0.10–0.47 | < 0.001 | 0.29 | 0.16–0.54 | < 0.001 |
| Both vs ureter | 2.97 | 1.88–4.69 | < 0.001 | 3.52 | 2.15–5.76 | < 0.001 | 2.83 | 1.78–4.51 | < 0.001 |
| Multifocality | 1.74 | 1.11–2.71 | 0.016 | 1.58 | 0.92–2.72 | 0.040 | 1.96 | 1.23–3.14 | 0.005 |
| Hydronephrosis | 1.30 | 0.88–1.91 | 0.189 | 1.80 | 1.11–2.93 | 0.018 | 2.04 | 1.29–3.23 | 0.002 |
| Surgical approach | |||||||||
| Laparoscopy vs open | 1.22 | 0.84–1.79 | 0.300 | 1.20 | 0.76–1.90 | 0.435 | 1.24 | 0.81–1.89 | 0.322 |
| Tumor grade | |||||||||
| High grade vs low grade | 1.77 | 1.08–2.90 | 0.025 | 1.92 | 1.05–3.50 | 0.033 | 1.83 | 1.06–3.15 | 0.030 |
| Tumor stage | < 0.001 | < 0.001 | < 0.001 | ||||||
| pT2 vs pT1 | 3.17 | 1.45–6.95 | 0.004 | 3.98 | 1.14–13.84 | 0.030 | 1.93 | 0.79–4.70 | 0.147 |
| pT3 vs pT1 | 5.62 | 2.64–11.95 | < 0.001 | 11.25 | 3.45–36.44 | < 0.001 | 5.70 | 2.56–12.70 | < 0.001 |
| pT4 vs pT1 | 13.36 | 5.94–30.03 | < 0.001 | 29.96 | 8.93–100.60 | < 0.001 | 12.62 | 5.36–29.74 | < 0.001 |
| Concomitant CIS | 1.01 | 0.53–1.93 | 0.980 | 0.93 | 0.40–2.14 | 0.858 | 1.23 | 0.62–2.45 | 0.562 |
| LN status | 4.59 | 3.07–6.87 | < 0.001 | 7.67 | 4.77–12.32 | < 0.001 | 5.56 | 3.57–8.65 | < 0.001 |
| LVI | 2.31 | 1.27–4.23 | 0.006 | 2.22 | 1.07–4.64 | 0.033 | 2.07 | 1.04–4.13 | 0.039 |
| Surgical margin status | 2.23 | 1.16–4.30 | 0.016 | 4.08 | 2.01–8.28 | < 0.001 | 3.61 | 1.85–7.03 | < 0.001 |
| Concurrent bladder cancer | 2.61 | 1.60–4.26 | < 0.001 | 2.33 | 1.33–4.06 | 0.003 | 2.64 | 1.61–4.32 | < 0.001 |
| NLR ≥ 2.4 vs < 2.4 | 2.11 | 1.41–3.15 | < 0.001 | 2.97 | 1.71–5.19 | < 0.001 | 2.09 | 1.32–3.33 | 0.002 |
| D-dimer (≥ 0.36 VS 0.36 <) | 2.85 | 1.92–4.21 | < 0.001 | 4.34 | 2.64–7.15 | < 0.001 | 3.57 | 2.29–5.56 | < 0.001 |
Abbreviations: UTUC, upper tract urinary carcinoma; HR, hazard ratio; CI, confidence interval; BMI, body mass index; VS, versus; ASA, American Society of Anesthesiologists; CIS, carcinoma in situ; LN, lymph node; LVI, lymphovascular invasion; NLR, neutrophil–lymphocyte ratio.
Table 3.
Multivariate Cox Regression Analyses of Relationship Between D-Dimer and Survival Outcomes in Patients with UTUC
| Variables | Recurrence-Free Survival | Cancer-Specific Survival | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Tumor size (≥3.0cm vs< 3.0cm) | 1.23 | 0.80–1.88 | 0.347 | 1.35 | 0.78–2.30 | 0.283 | 1.11 | 0.69–1.80 | 0.668 |
| Tumor location | 0.007 | < 0.001 | 0.001 | ||||||
| Pelvicalyceal vs ureter | 0.54 | 0.33–0.91 | 0.020 | 0.29 | 0.13–0.68 | 0.005 | 0.40 | 0.20–0.80 | 0.009 |
| Both vs ureter | 1.55 | 0.91–2.64 | 0.110 | 1.76 | 1.00–3.13 | 0.052 | 1.70 | 0.99–2.90 | 0.052 |
| Multifocality | 1.20 | 0.69–2.08 | 0.516 | 0.97 | 0.49–1.91 | 0.934 | 1.18 | 0.66–2.10 | 0.587 |
| Hydronephrosis | 1.09 | 0.69–1.73 | 0.717 | 1.97 | 1.03–3.75 | 0.040 | 1.89 | 1.08–3.30 | 0.026 |
| Tumor grade | |||||||||
| High grade vs low grade | 0.99 | 0.58–1.71 | 0.979 | 0.67 | 0.32–1.37 | 0.271 | 0.80 | 0.43–1.49 | 0.487 |
| Tumor stage | 0.039 | 0.038 | 0.060 | ||||||
| pT2 vs pT1 | 2.51 | 1.13–5.58 | 0.024 | 2.73 | 0.76–9.80 | 0.124 | 1.35 | 0.54–3.38 | 0.518 |
| pT3 vs pT1 | 2.95 | 1.30–6.70 | 0.010 | 4.48 | 1.28–15.68 | 0.019 | 2.53 | 1.04–6.15 | 0.041 |
| pT4 vs pT1 | 3.83 | 1.52–9.68 | 0.004 | 5.58 | 1.53–20.36 | 0.009 | 2.89 | 1.09–7.67 | 0.033 |
| LN status | 1.62 | 0.96–2.73 | 0.074 | 3.64 | 2.01–6.60 | < 0.001 | 2.57 | 1.49–4.45 | 0.001 |
| LVI | 1.42 | 0.73–2.76 | 0.305 | 1.33 | 0.58–3.08 | 0.502 | 1.17 | 0.54–2.55 | 0.691 |
| Surgical margin status | 0.98 | 0.48–2.00 | 0.962 | 1.02 | 0.47–2.20 | 0.960 | 1.17 | 0.57–2.40 | 0.673 |
| Concurrent bladder cancer | 1.36 | 0.77–2.41 | 0.294 | 1.00 | 0.51–1.95 | 0.993 | 1.16 | 0.65–2.08 | 0.607 |
| NLR ≥ 2.4 vs < 2.4 | 1.63 | 1.06–2.52 | 0.026 | 2.47 | 1.33–4.61 | 0.004 | 1.71 | 1.03–2.84 | 0.038 |
| D-dimer (≥ 0.36 vs 0.36 <) | 1.67 | 1.07–2.63 | 0.025 | 2.34 | 1.30–4.19 | 0.004 | 1.98 | 1.18–3.34 | 0.010 |
Abbreviations: UTUC, upper tract urinary carcinoma; HR, hazard ratio; CI, confidence interval; VS, versus; LN, lymph node; LVI, lymphovascular invasion; NLR, neutrophil–lymphocyte ratio.
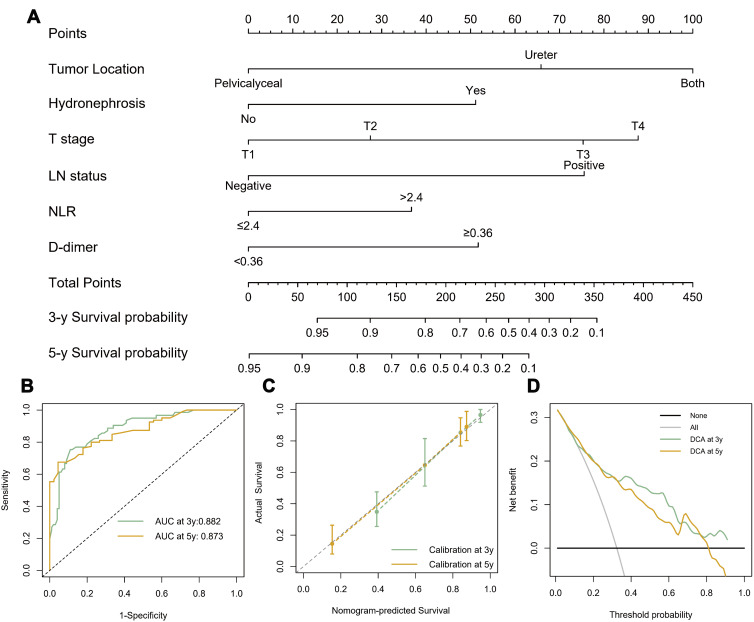
According to the predefined variables, we developed a prognostic nomogram to predict 3- and 5-year OS following RNU (Figure 3A). Receiver operating characteristic analysis was also performed to determine the specificity and sensitivity of the nomogram to predict OS (Figure 3B). Besides, the C‐index of our nomogram was 0.82, which also indicated the high predictive accuracy. The calibration curves displayed good agreements of the nomogram predicted probability with the actual probability for OS (Figure 3C), indicating that this nomogram had a high predictive value. Additionally, the decision curve (Figure 3D) showed that this model had good clinical net benefit.
Figure 3.
(A) Nomogram predicting 3- and 5-year overall survival for patients with UTUC after RNU. (B) Receiver operating characteristic curve analysis for evaluating the accuracy of the nomogram. (C) Calibration plot for predicting 3- and 5-year overall survival. (D) Decision curve analysis demonstrating the net benefit of the nomogram.
Abbreviations: UTUC, upper tract urothelial carcinoma; LN, lymph node; NLR, neutrophil–lymphocyte ratio; AUC, area under the curve.
Discussion
UTUC is a rare urinary tumor with an unfavorable prognosis. Approximately 60% of patients have invasive disease at diagnosis, and the incidence of intravesical recurrence is 22–47%. Furthermore, the 5-year CSS is < 50% for patients with stage T2/3, and < 10% for T4 patients.3 Therefore, identifying the biomarker to improve the risk stratification and personalized prediction of recurrence is a major concern in patients with UTUC. Several studies have investigated prognostic factors to stratify the risk profiles for UTUC. Ipsilateral hydronephrosis, high tumor grade, and positive urinary cytology are associated with more advanced UTUC pathology. The coagulation and fibrinolysis are often abnormally activated in malignancy. Several investigators recently reported that the elevated plasma fibrinogen levels had significance in patients with UTUC.12–14 However, to our knowledge, no studies have evaluated the effect of plasma D-dimer levels in patients with UTUC. Therefore, we validated the prognostic significance of plasma D-dimer levels in a cohort of patients with UTUC.
In the present study, we retrospectively analyzed a cohort of localized UTUC patients who underwent RNU at our center. The survival analysis showed that elevated plasma D-dimer levels predicted a higher risk of tumor recurrence and poor outcomes. Plasma D-dimer levels ≥ 0.36 mg/L were associated with higher pathological T stage, tumor grade, and lymphovascular invasion. Furthermore, univariate and multivariate analyses showed that preoperative plasma D-dimer levels ≥ 0.36 mg/L were an independent predictor of RFS (HR: 1.67, 95% CI: 1.07–2.63; p = 0.025), CSS (HR: 2.34, 95% CI: 1.30–4.19; p = 0.004) and OS (HR: 1.98, 95% CI: 1.18–3.34; p = 0.010). These findings indicate that preoperative plasma D-dimer level is a significant biomarker for predicting oncological outcomes in patients with UTUC.
Plasma D-dimer is the product of cross-linked fibrin degradation by plasmin-induced fibrinolytic activity, and this marker has been widely used as a useful indicator of hemostasis and fibrinolysis activation. Elevated preoperative plasma D-dimer levels have been reported to be an unfavorable prognostic factor in various malignancies, such as lung cancers,6,15 digestive tract cancers,16 urological cancers10,11,17 and soft tissue sarcomas.5,7
The mechanism underlying the association between elevated plasma D-dimer levels and malignancy remains speculative. One study reported that tumor cells not only activated the coagulation system directly but also broke the intactness of the vascular endothelial wall and increased platelet as well as the fibrinolytic protein activity.18 Several tumor-associated coagulation factors, including the tissue factors, fibrin, and plasmin, are dysregulated in tumor growth, metastasis, thrombosis, and angiogenesis in malignancy.19,20 The tissue factor, thrombin, and inflammatory factors from tumor cells lead to abnormal activation of the coagulation–fibrinolysis system.21 Tumor cells secret some proteins and cytokines, which disrupt the balance between the coagulation and fibrinolysis, resulting in the release of agglutinants and cytokines and causing damage to the vascular endothelium.22 The dysregulation between coagulation and fibrinolysis is reflected by the elevated levels of plasma D-dimer; therefore, the preoperative plasma D-dimer levels might be associated with prognosis of patients with UTUC.
Several studies reported that low molecular-weight heparin (LWMH) could suppress tumor growth, decrease selectin-mediated tumor cell invasion and metastasis, and inhibit angiogenesis.23–25 Several studies demonstrated that the use of LWMH enhanced patients’ response to chemotherapy, improved prognosis, reduced complication, and mortality rates. Further studies are needed to verify whether the anticoagulant therapy with prophylactic LWMH improves the prognosis of patients with UTUC with elevated D-dimer levels.
In the present study, according to the survival analysis, when the preoperative plasma D-dimer levels were higher than the cutoff, the worse survival status was seen. D-dimer levels may be a useful prognostic predictor of survival in patients with UTUC following RNU. Furthermore, patients with UTUC with elevated D-dimer levels may benefit from anticoagulant therapy. Finally, we developed a nomogram to predict individual patients’ OS. Using the nomogram, clinicians could stratify patients into different risk group with distinct prognosis according to the score obtained from the nomogram. This may help us to determine personalized treatment and follow-up strategies based on the stratified risks in patients with UTUC.
Certain limitations in the present study should be acknowledged. First, as a retrospective nature, the selection bias cannot be excluded, even though patients were enrolled consecutively, and eligibility criteria were performed to minimize the bias. More prospective studies are needed to verify the predictive value of this marker. Second, the follow-up duration was short, and the sample size was small, which decreased the statistic power to detect the significant differences between different groups. More studies with large sample sizes and longer follow-up are warranted to further validate our results. Third, this study was conducted in a Chinese cohort, and further studies with different ethnic groups are needed to validate the generalizability of the findings. Additionally, this predictive model was based on data from a single center with no external validation, which might impair its universal applicability of this model to the general patient population. Furthermore, we only analyzed the predictive value of the indicator in primary resectable UTUC without adjuvant therapy. The value of the biomarker in patients receiving adjuvant chemotherapy, and in those nonresectable UTUC requires further validating. Notwithstanding these limitations, this study provided a new point of view that preoperative D-dimer levels may predict the prognosis of patients with UTUC. Additionally, the biomarker is widely available, which can be measured rapidly and easily, do not need specific equipment. Therefore, preoperative D-dimer represents a low-cost tool that can be implemented on a large scale in clinical practice.
Conclusion
In conclusion, this study is the first to investigate the effects of preoperative plasma D-dimer levels on UTUC prognosis. We developed a nomogram for individualized prediction of survival after RNU, and our results indicated that the preoperative elevated plasma D-dimer levels were significantly associated with worse prognosis of patients with UTUC. This biomarker can improve the risk stratification and personalized prediction of recurrence and survival, which can help direct follow-up scheduling, administration of adjuvant therapies, and precise individualized treatment for patients with UTUC.
Abbreviations
UTUC, upper tract urothelial carcinomas; RNU, radical nephroureterectomy; TNM, tumor node metastasis; WHO, World Health Organization; ISUP, International Society of Urological Pathology; CSS, cancer-specific survival; OS, overall survival; RFS, recurrence-free survival; ROC, receiver operating characteristic curve; BMI, body mass index; ASA, American Society of Anesthesiologists; NLR, Neutrophil–lymphocyte Ratio; LVI, Lymphovascular invasion.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethic Approval and Informed Consent
The retrospective study was approved by the Institutional Review Board of the Ethics Committee of Shandong Provincial Hospital (No. 2020-014). The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The written informed consents from the patients were waived owing to the retrospective nature of the study and the analyses used anonymous clinical data.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Cha EK, Shariat SF, Kormaksson M, et al. Predicting clinical outcomes after radical nephroureterectomy for upper tract urothelial carcinoma. Eur Urol. 2012;61(4):818–825. doi: 10.1016/j.eururo.2012.01.021 [DOI] [PubMed] [Google Scholar]
- 3.Rouprêt M, Babjuk M, Compérat E, et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol. 2018;73(1):111–122. doi: 10.1016/j.eururo.2017.07.036 [DOI] [PubMed] [Google Scholar]
- 4.Messer JC, Terrell JD, Herman MP, et al. Multi-institutional validation of the ability of preoperative hydronephrosis to predict advanced pathologic tumor stage in upper-tract urothelial carcinoma. Urol Oncol: Semin Orig Investig. 2013;31(6):904–908. doi: 10.1016/j.urolonc.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 5.Cai H-X, Li X-Q, Wang S-F. Prognostic value of fibrinogen and D-dimer-fibrinogen ratio in resectable gastrointestinal stromal tumors. WJG. 2018;24(44):5046–5056. doi: 10.3748/wjg.v24.i44.5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou C, Jiang F, Ma H, et al. Prognostic role of preoperative platelet, fibrinogen, and D-dimer levels in patients with non-small cell lung cancer: a multicenter prospective study. Thorac Cancer. 2019;10(2):304–311. doi: 10.1111/1759-7714.12956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ay C, Dunkler D, Pirker R, et al. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica. 2012;97(8):1158–1164. doi: 10.3324/haematol.2011.054718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pabinger I, Ay C. Biomarkers and venous thromboembolism. Arterioscler Thromb Vasc Biol. 2009;29(3):332–336. doi: 10.1161/ATVBAHA.108.182188 [DOI] [PubMed] [Google Scholar]
- 9.Wen J, Yang Y, Ye F, et al. The preoperative plasma fibrinogen level is an independent prognostic factor for overall survival of breast cancer patients who underwent surgical treatment. Breast. 2015;24(6):745–750. doi: 10.1016/j.breast.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 10.Çalışkan S, Sungur M. Fibrinogen and D-dimer levels in prostate cancer: preliminary results. Prostate Int. 2017;5(3):110–112. doi: 10.1016/j.prnil.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Shu K, Zhou J, et al. Preoperative plasma fibrinogen and D-dimer as prognostic biomarkers for non–muscle-invasive bladder cancer. Clin Genitourin Cancer. 2019;18(1):11–9. doi: 10.1016/j.clgc.2019.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka N, Kikuchi E, Matsumoto K, et al. Prognostic value of plasma fibrinogen levels in patients with localized upper tract urothelial carcinoma. BJU Int. 2013;111(6):857–864. doi: 10.1111/j.1464-410X.2012.11353.x [DOI] [PubMed] [Google Scholar]
- 13.Pichler M, Dalpiaz O, Ehrlich GC, et al. Validation of the preoperative plasma fibrinogen level as a prognostic factor in a European cohort of patients with localized upper tract urothelial carcinoma. J Urol. 2014;191(4):920–925. doi: 10.1016/j.juro.2013.10.073 [DOI] [PubMed] [Google Scholar]
- 14.Liu R, Zhou X, Zou L, et al. Clinicopathological and prognostic significance of preoperative plasma fibrinogen level in patients with upper urinary tract urothelial carcinoma: a retrospective tumor marker prognostic study. Int J Surg. 2019;65:88–93. doi: 10.1016/j.ijsu.2019.03.022 [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Jia Y, Jia Y, Zhang X, Li K. Prognostic and predictive value of plasma D-dimer levels in patients with small-cell lung cancer. Int J Clin Oncol. 2018;23(6):1070–1075. doi: 10.1007/s10147-018-1320-5 [DOI] [PubMed] [Google Scholar]
- 16.Moik F, Posch F, Grilz E, et al. Haemostatic biomarkers for prognosis and prediction of therapy response in patients with metastatic colorectal cancer. Thromb Res. 2020;187:9–17. doi: 10.1016/j.thromres.2020.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He X, Huang T, Xue Y, et al. Association of preoperative plasma D-dimmer and fibrinogen and renal cell carcinoma outcome. J Cancer. 2019;10(17):4096–4105. doi: 10.7150/jca.31173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heit JA. Cancer and venous thromboembolism: scope of the problem. Cancer Control. 2005;12(Suppl 1):5–10. doi: 10.1177/1073274805012003S02 [DOI] [PubMed] [Google Scholar]
- 19.Kasthuri RS, Taubman MB, Mackman N. Role of tissue factor in cancer. J Clin Oncol. 2009;27(29):4834–4838. doi: 10.1200/JCO.2009.22.6324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakurai M, Satoh T, Matsumoto K, et al. High pretreatment plasma D-dimer levels are associated with poor prognosis in patients with ovarian cancer independently of venous thromboembolism and tumor extension. Int J Gynecol Cancer. 2015;25(4):593–598. doi: 10.1097/IGC.0000000000000415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gil-Bernabé AM, Ferjancic S, Tlalka M, et al. Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood. 2012;119(13):3164–3175. doi: 10.1182/blood-2011-08-376426 [DOI] [PubMed] [Google Scholar]
- 22.Bikdeli B, Sharif-Kashani B, Chitsaz E, et al. Dexter versus sinister deep vein thrombosis: which is the more sinister? Findings from the NRITLD DVT registry. Semin Thromb Hemost. 2011;37(3):298–304. doi: 10.1055/s-0031-1273093 [DOI] [PubMed] [Google Scholar]
- 23.Mousa SA, Mohamed S. Inhibition of endothelial cell tube formation by the low molecular weight heparin, tinzaparin, is mediated by tissue factor pathway inhibitor. Thromb Haemost. 2004;92(9):627–633. doi: 10.1160/TH04-02-0069 [DOI] [PubMed] [Google Scholar]
- 24.Norrby K. Low-molecular-weight heparins and angiogenesis. APMIS. 2006;114(2):79–102. doi: 10.1111/j.1600-0463.2006.apm_235.x [DOI] [PubMed] [Google Scholar]
- 25.Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost. 2013;11(2):223–233. doi: 10.1111/jth.12075 [DOI] [PubMed] [Google Scholar]