Abstract
Aim
We previously generated transgenic (Tg) mice that expressed P123H β‐synuclein (βS), a dementia with Lewy body‐linked mutant βS. Notably, these mice recapitulated neurodegenerative features of Lewy body disease, reflected by motor dysfunction, greater protein aggregation, and memory impairment. Since recent studies suggested that non‐motor symptoms, such as depression, might be manifested in the prodromal stage of Lewy body disease, the main objective of the present study was to investigate the early expression of behavior in P123H βS Tg mice.
Methods
Nest building, locomotor activity, and depressive‐like behavior were assessed using 6‐ to 10‐month‐old male and female P123H βS Tg and wildtype mice.
Key Results
P123H βS Tg mice exhibited hyperlocomotor activity in a novel environment, a decrease in mobility time in the tail suspension test, and impairments in nest building.
Conclusions
Importantly, these non‐motor behaviors were manifested before the onset of motor dysfunction, suggesting that P123H βS Tg mice could be a valid model for investigating the early phase of Lewy body disease.
Keywords: β‐synuclein, behavior analysis, Lewy body diseases, nonmotor symptoms, transgenic mice
Transgenicmice that expressed P123H beta‐synuclein recapitulated neurodegenerative features of Lewy body disease. In the present study, we found that P123H beta‐synuclein transfenic mice exhibited non‐motor behaviors including depressive‐like behavior that were manifested before the onset of motor dysfunction. Thus, P123H beta‐synuclein transgenic mice may be a valid model for investigatig early hase of Lewy body disease.
MAIN TEXT
β‐synuclein (βS) is a presynaptic phosphoprotein that is abundantly expressed in the brain.1, 2 Although the physiological function of βS has been elusive, βS might be an inhibitor of neurodegeneration that is stimulated by its homolog α‐synuclein (αS), a central player in the pathogenesis of Lewy body disease (LBD), such as Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy.3, 4, 5 However, two missense mutations of βS, P123H and V70M, were identified in familial and sporadic cases of dementia with Lewy bodies, respectively.6 However, the accumulation of βS was not observed in a patient who carried the P123H βS mutation,6 and the pathological effect of βS was not clearly understood at that time. Therefore, to investigate the pathological effect of the βS mutant, we generated transgenic (Tg) mice that expressed P123H βS. P123H βS Tg mice exhibited several features of LBD.7 We observed the accumulation of both αS and βS P123H in the brain in Tg mice, especially in axons and axon terminals.7 Moreover, P123H βS Tg mice presented memory impairments and progressive motor dysfunction. These results suggest that P123H βS Tg mice may be a valid animal model of LBD.
Increasing attention has recently been given to nonmotor symptoms in LBD.8 These include cognitive dysfunction, neuropsychiatric manifestations, depression, autonomic complications, sleep disorders, and other nonmotor manifestations.8 The importance of the early pathogenesis of neurodegenerative diseases has been well recognized,9 and some nonmotor symptoms, such as depression, might be manifested in the prodromal stage of LBD.10 Therefore, the main objective of this study was to investigate the early expression of behavior in P123H βS Tg mice.
All of the experiments used 6‐ to 10‐month‐old male and female P123H βS Tg and wild‐type (WT) mice. Nest building (Data S3) was assessed by leaving a mouse in a cage that was covered with a Palsoft Sheet Type (ie, a sheet‐type bedding material; Oriental Yeast, Tokyo, Japan) for one night, followed by visual examination of the formation of a nest. Locomotor activity (Data S1) was assessed in a novel environment as described previously11 using a Supermex apparatus (Muromachi Kikai, Tokyo, Japan) and a sensor monitor that was mounted above the chamber. Each mouse was exposed to an illuminated chamber (30 cm × 40 cm × 25 cm). The intensity of illumination was approximately 120 lx in the center of the chamber. All locomotor activity counts were automatically summed and recorded every 15 minutes for 2 hours. Depressive‐like behavior was then assessed in the tail suspension test (Data S2) as described previously,12 with some modifications. The mice were suspended by the tail for 6 minutes, and motility was recorded from 2 to 6 minutes using an acceleration detector (Neuroscience, Inc., Tokyo, Japan). We conducted these behavioral experiments in the following order: nest building, locomotor activity, and tail suspension test. The data are presented as the mean ± SEM. For locomotor activity, the analyses were performed using two‐way analysis of variance (ANOVA). Individual post hoc comparisons were performed using Scheffe's test. The tail suspension test data were analyzed using Student's t test. Values of P < .05 were considered statistically significant. The data were analyzed using BellCurve for Excel software (Social Survey Research Information, Tokyo, Japan).
The results showed that locomotor activity in a novel environment increased in P123H βS Tg mice compared with WT mice at 60 min (F 1,136 = 5.369, P = .022) and 90 min (F 1,136 = 5.1501, P = .0248; Figure 1A), indicating that P123H βS Tg mice became hyperactive in an unfamiliar environment that was distinct from their home cage.7 This result also confirmed that locomotor activity was not impaired at this age in P123H βS Tg mice, although motor dysfunction was gradually manifested around 12 months of age in these mice.7 Furthermore, although a previous study reported “clasping” in αS/P123H βS bigenic mice but not in P123H βS single Tg mice under tail suspension conditions, based on gross observations,7 the present qualitative study unambiguously demonstrated that motility significantly decreased in P123H βS Tg mice compared with WT mice (Figure 1B). Nest building was also impaired in P123H βS Tg mice (Figure 1C,D).
Figure 1.
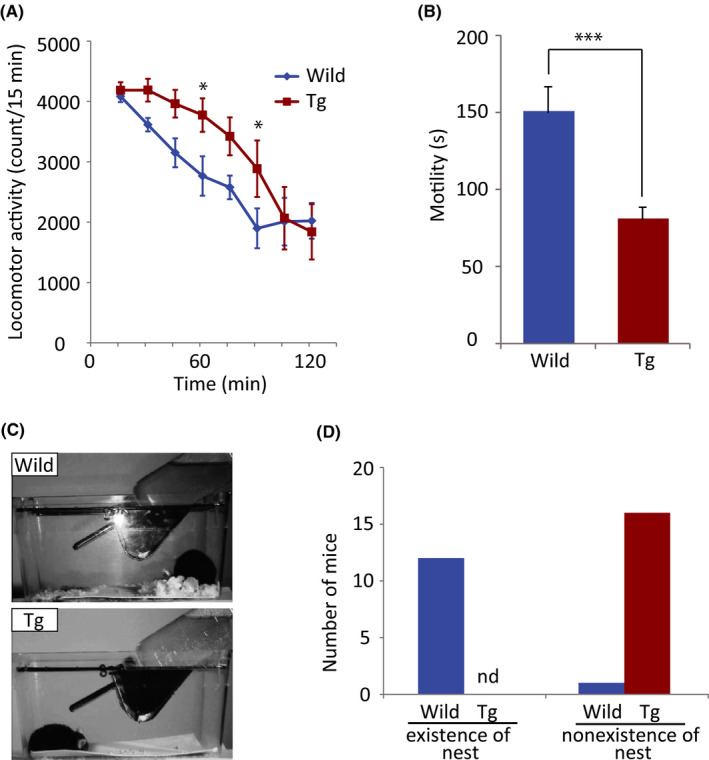
Behavioral alterations in P123H βS Tg mice. (A) Locomotor activity in a novel environment in wild‐type mice (n = 10; blue line) and P123H βS Tg mice (n = 9; red line). The data are expressed as mean ± SEM. *P < .05, compared with wild‐type mice. (B) Tail suspension test in wild‐type mice (n = 16; blue bar) and P123H βS Tg mice (n = 17; red bar). The data are expressed as mean ± SEM. ***P < .001, compared with wild‐type mice. (C) Representative images of nest building in a wild‐type mouse (wild) and P123H βS Tg mouse (Tg). (D) Twelve of the 13 wild‐type mice built a nest, whereas all of the P123H βS Tg mice (n = 16) failed to build a nest
Given that spontaneous locomotor hyperactivity has previously been observed under various neurological conditions, including manic‐depressive disorder, viral encephalopathy, attention‐deficit/hyperactivity disorder, and several other organic brain diseases,13 one may predict that the neurodegenerative status of P123H βS Tg mice might be similar to these clinical pathological conditions. A recent study found that the assessment of nest building might be a valid method for assessing depressive‐like symptoms.14 Therefore, the present results that showed a decrease in motility in the tail suspension test and impairment of nest building suggest that P123H βS Tg mice may be considered to express depressive‐like behavior.
Several mechanisms are assumed to underlie depressive‐like behavior in P123H βS Tg mice. Either a toxic gain of function or loss of function that is caused by P123H βS in the cytoplasm may result in alterations of signal transduction and transcription, followed by a decrease in synaptic plasticity, eventually leading to the manifestation of neurological phenotypes, such as depression. Indeed, similar hyperactivity and depressive‐like behavior were observed in A53T αS mice15 and a mouse model of tauopathy.16 Alternatively, recent findings suggest that neuroinflammation may be involved in depression.17 Therefore, neuroinflammation might play a key role in depressive‐like behavior in P123H βS Tg mice. Consistent with this possibility, P123H βS Tg mice presented mild features of neuroinflammation, including an increase in inflammatory cytokines (eg, interleukin‐1β and tumor necrosis factor‐α) in the hippocampus.7
In summary, P123H βS Tg mice exhibited depressive‐like behavior that was manifested before the onset of motor dysfunction. Thus, P123H βS Tg mice may be a valid model for investigating the early phase of LBD.
ACKNOWLEDGMENTS
We thank Michael Arends for assistance with editing the manuscript. This work was supported by JSPS Grants‐in‐Aid for Scientific Research 21700399 and 21300135, the Nakatomi Foundation, the Takeda Science Foundation and the Novartis Foundation for Gerontological Research.
CONFLICT OF INTEREST
Authors declare no conflict of interest for this article.
ANIMAL STUDIES
The experimental procedures and housing conditions were approved by the Institutional Animal Care and Use Committee at Tokyo Metropolitan Institute of Medical Science.
AUTHOR CONTRIBUTIONS
MF, KI, and MH conceived and designed the experiments and wrote the article. MF, YH, YuT, YS, and YoT performed the behavioral experiments and analyzed the data.
Supporting information
Fujita M, Hagino Y, Takamatsu Y, et al. Early manifestation of depressive‐like behavior in transgenic mice that express dementia with Lewy body‐linked mutant β‐synuclein. Neuropsychopharmacol Rep. 2018;38:95–97. 10.1002/npr2.12009
Contributor Information
Masayo Fujita, Email: fujita-ms@igakuken.or.jp.
Makoto Hashimoto, Email: hashimoto-mk@igakuken.or.jp.
REFERENCES
- 1. Nakajo S, Tsukada K, Omata K, Nakamura Y, Nakaya K. A new brain‐specific 14‐kDa protein is a phosphoprotein: its complete amino acid sequence and evidence for phosphorylation. Eur J Biochem. 1993;217:1057–63. [DOI] [PubMed] [Google Scholar]
- 2. Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994;345:27–32. [DOI] [PubMed] [Google Scholar]
- 3. Clayton DF, George JM. The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998;21:249–54. [DOI] [PubMed] [Google Scholar]
- 4. Hashimoto M, Rockenstein E, Mante M, Mallory M, Masliah E. β‐Synuclein inhibits α‐synuclein aggregation: a possible role as an anti‐parkinsonian factor. Neuron. 2001;32:213–23. [DOI] [PubMed] [Google Scholar]
- 5. Uversky VN, Li J, Souillac P, et al. Biophysical properties of the synucleins and their propensities to fibrillate: inhibition of α‐synuclein assembly by β‐ and γ‐synucleins. J Biol Chem. 2002;277:11970–8. [DOI] [PubMed] [Google Scholar]
- 6. Ohtake H, Limprasert P, Fan Y, et al. Beta‐synuclein gene alterations in dementia with Lewy bodies. Neurology. 2004;63:805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fujita M, Sugama S, Sekiyama K, et al. A β‐synuclein mutation linked to dementia produces neurodegeneration when expressed in mouse brain. Nat Commun. 2010;1:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schrag A, Sauerbier A, Chaudhuri KR. New clinical trials for nonmotor manifestations of Parkinson's disease. Mov Disord. 2015;30:1490–504. [DOI] [PubMed] [Google Scholar]
- 9. Leentjens AF. Parkinson disease: depression‐risk factor or early symptom in Parkinson disease? Nat Rev Neurol. 2015;11:432–3. [DOI] [PubMed] [Google Scholar]
- 10. Schapira AHV, Chaudhuri KR, Jenner P. Non‐motor features of Parkinson disease. Nat Rev Neurosci. 2017;18:509. [DOI] [PubMed] [Google Scholar]
- 11. Hagino Y, Kasai S, Fujita M, et al. Involvement of cholinergic system in hyperactivity in dopamine‐deficient mice. Neuropsychopharmacology. 2015;40:1141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamamoto H, Kamegaya E, Hagino Y, et al. Loss of GluN2D subunit results in social recognition deficit, social stress, 5‐HT2C receptor dysfunction, and anhedonia in mice. Neuropharmacology 2017;112(Pt A):188–97. [DOI] [PubMed] [Google Scholar]
- 13. Hess EJ, Jinnah HA, Kozak CA, Wilson MC. Spontaneous locomotor hyperactivity in a mouse mutant with a deletion including the Snap gene on chromosome 2. J Neurosci. 1992;12:2865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Otabi H, Goto T, Okayama T, Kohari D, Toyoda A. The acute social defeat stress and nest‐building test paradigm: a potential new method to screen drugs for depressive‐like symptoms. Behav Processes. 2017;135:71–5. [DOI] [PubMed] [Google Scholar]
- 15. Paumier KL, Sukoff Rizzo SJ, Berger Z, et al. Behavioral characterization of A53T mice reveals early and late stage deficits related to Parkinson's disease. PLoS One. 2013;8:e70274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jul P, Volbracht C, de Jong IE, Helboe L, Elvang AB, Pedersen JT. Hyperactivity with agitative‐like behavior in a mouse tauopathy model. J Alzheimers Dis. 2016;49:783–95. [DOI] [PubMed] [Google Scholar]
- 17. Yirmiya R, Rimmerman N, Reshef R. Depression as a microglial disease. Trends Neurosci. 2015;38:637–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


