Abstract
Background
AMPA receptors predominantly mediate fast excitatory synaptic transmission in the mammalian brain. Post‐translational protein S‐palmitoylation of AMPA receptor GluA subunits at their C‐termini reversibly controls the receptors trafficking to and from excitatory glutamatergic synapses. Excitatory inputs to neurons induce the expression of immediate early genes (IEGs), including Arc, with particular spatial patterns. In the hippocampal dentate gyrus, Arc is mainly expressed in the upper (dorsal) blade at the basal state. GluA1 C‐terminal palmitoylation‐deficient (GluA1C811S) mice showed enhanced seizure susceptibility and disturbed synaptic plasticity without impaired gross anatomy or basal synaptic transmission. These mutant mice also exhibited an increased expression of IEG products, c‐Fos and Arc proteins, in the hippocampus and cerebral cortex. In this report, we further analyzed excitability and Arc expression pattern in the dentate gyrus of GluA1C811S mice.
Methods and Results
Electrophysiological analysis of granule neurons to measure the evoked excitatory postsynaptic current/evoked inhibitory postsynaptic current ratio revealed that excitatory/inhibitory (E/I) balance was normal in GluA1C811S mice. In contrast, immunohistochemical staining showed an abnormal distribution of Arc‐positive cells between upper and lower (ventral) blades of the dentate gyrus in these mutant mice. These data suggest that deficiency of GluA1 palmitoylation causes perturbed neuronal inputs from the entorhinal cortex to the dentate gyrus, which potentially underlies the excessive excitability in response to seizure‐inducing stimulation.
Conclusion
Our findings conclude that an appropriate regulation of Arc expression in the dentate gyrus, ensured by AMPA receptor palmitoylation, may be critical for stabilizing hippocampal neural circuits and may suppress excess excitation.
Keywords: AMPA receptor, Arc, dentate gyrus, hippocampus, palmitoylation
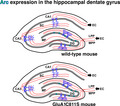
Compared with wild‐type mouse (top), AMPA receptor GluA1 Cys811Ser palmitoylation‐deficient mouse (bottom) shows abnormal expression pattern of immediate early gene Arc in the hippocampal dentate gyrus. An appropriate regulation of Arc expression in the dentate gyrus, ensured by AMPA receptor palmitoylation, may be critical for stabilizing hippocampal neural circuits and may suppress excess excitation.
1. INTRODUCTION
Activity‐induced expression of immediate early genes (IEGs) has been explored to monitor neuronal activity in the brain.1, 2, 3, 4 Excitatory inputs to neurons induce the expression of various IEGs, including Arc, with their particular temporal and spatial patterns in the brain.
In the mammalian central nervous system, glutamate is the major excitatory neurotransmitter, and the excitatory synapses throughout the brain contain α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazole propionate (AMPA)‐type glutamate receptors, which predominantly mediate fast excitatory synaptic transmission. Therefore, a quantitative control of synaptic AMPA receptor number is critical for basal synaptic transmission, mammalian synaptic plasticity, and higher brain function.5, 6, 7, 8 We have previously reported that a key modification mechanism of AMPA receptor trafficking into and from excitatory synapses is the post‐translational protein S‐palmitoylation of the receptors at their C‐termini.9, 10, 11, 12, 13, 14, 15 Among the four AMPA receptor subunits GluA1‐4 (also known as GluR1‐4, GluRA‐D, or GluRα1‐4), GluA1 mainly acts in activity‐dependent AMPA receptor trafficking into postsynapses.8, 12, 16 Recently, we reported that the GluA1 C‐terminal palmitoylation‐deficient (GluA1C811S) mice showed enhanced seizure susceptibility and disturbed synaptic plasticity without affecting gross brain structure or normal excitatory synaptic transmission in vivo.17 Significant pathophysiological spine enlargements upon strong stimulation were observed in the hippocampus of GluA1C811S mice. These mutant mice also exhibited increased expressions of IEG products, c‐Fos and Arc proteins, in the hippocampus and the cerebral cortex.
In this report, we further examined Arc expression in detail and found altered expression patterns of Arc in the hippocampal dentate gyrus of GluA1C811S mice at the basal state.
2. METHODS
2.1. Experimental animals
Mice were fed with standard laboratory chow and water in standard animal cages under a 12‐h light/dark cycle.
2.2. Antibodies
Anti‐Arc (OP1/2MBL‐2012B and OP2‐2012, H.O.) antibodies were used for the experiments.
2.3. Electrophysiology
Male mouse hippocampal slices and electrophysiological recordings were prepared as previously described.17 Each slice was perfused (approximately 3 mL/min) with artificial cerebrospinal fluid (in mM: 125 NaCl, 2.5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 4 CaCl2, 4 MgSO4, and 10 glucose) at 28‐32°C. All external solutions were equilibrated with 95% O2 and 5% CO2 (pH 7.4). For voltage‐clamp recordings, patch pipettes were filled with a Cs+‐based intracellular solution [mM: 135 CsMeSO4, 5.0 TEA‐Cl, 1 MgCl2, 0.5 EGTA, 3.0 Mg‐ATP, 0.3 Na‐GTP, 10 Na‐phosphocreatine, 2 QX314, and 10 HEPES (pH 7.2)], and the resistances were 4 to 6 MΩ. A bipolar stimulating electrode was placed in the molecular layer of the dentate gyrus to stimulate the perforant pathway, and evoked excitatory postsynaptic currents (EPSCs) of granule neurons were adjusted to approximately −200 to −400 pA at −70 mV. Subsequently, the cells were held at 0 mV and evoked inhibitory postsynaptic currents (IPSCs) were recorded. EPSC/IPSC ratios were calculated as the ratio of the peak current at −70 mV (EPSC) to peak current at 0 mV (IPSC).
2.4. Immunohistochemistry
Male mouse brains were perfused and fixed with 2% paraformaldehyde (PFA) in phosphate‐buffered saline (PBS) and transferred to an increasing gradient of 10%, 20%, and 30% sucrose in PBS solution over three days. After tissues were embedded in optimal cutting temperature (OCT) compounds, cryosections were cut at 20 μm thickness. Sagittal sections were processed for immunohistochemistry using anti‐Arc antibodies with DAPI. Confocal z‐stack images (0.3‐ to 0.8‐μm intervals, 5‐10 image sections/stack) acquired with the LSM710 using a 63× objective (N.A. 1.4, oil) were projected onto single planes by summation.
3. RESULTS
3.1. No obvious E/I imbalance in the dentate gyrus of GluA1C811S mutant mice at the basal level
Excitatory/inhibitory (E/I) imbalance occurs in very few excitatory synapses and then spreads to hippocampal circuits. Because GluA1C811S mice showed enhanced seizure susceptibility,17 we first assessed synaptic E/I balance in the dentate gyrus, the major entry gate for cortical input to the hippocampus (see also Figure 2C). Stimulation electrodes were positioned within the perforant pathway from the entorhinal cortex, and the evoked EPSC and IPSC were recorded at granule neurons in the upper (dorsal) or lower (ventral) blades of the dentate gyrus, respectively. The results showed that the EPSC/IPSC ratio recorded from GluA1C811S homozygous (CS/CS) mice was almost the same as that from wild‐type (wt) mice in both upper (Figure 1A, 0.48 ± 0.03, n = 21 cells from 3 wt mice; 0.46 ± 0.04, n = 21 cells from 3 CS/CS mice, P = 0.67; t test) and lower (Figure 1B, 0.44 ± 0.03, n = 28 cells from 4 wt mice; 0.51 ± 0.03, n = 28 cells from 4 CS/CS mice, P = 0.16; t test) blades. These data suggested no significant difference in E/I balance between wt and CS/CS mice in either the upper or lower layer under basal conditions.
Figure 1.
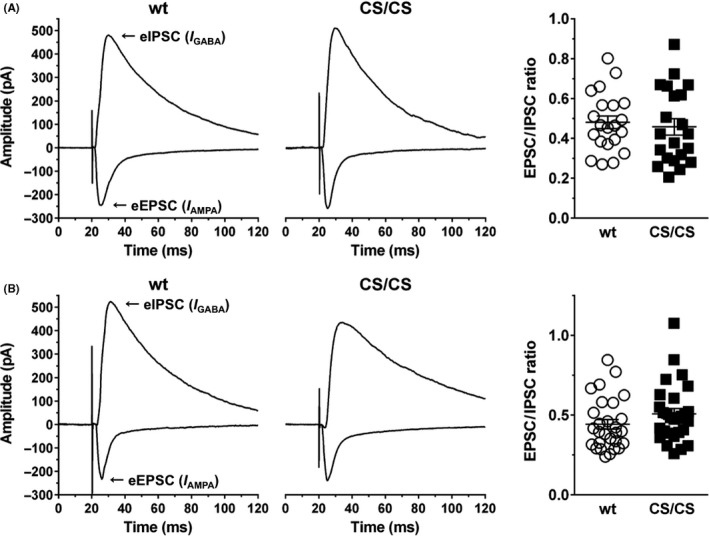
Excitatory/inhibitory balance in the hippocampal dentate gyrus was not altered in GluA1C811S mice. (A) EPSC/IPSC ratio of granule neurons in the upper blade of the dentate gyrus. Representative traces of EPSCs and IPSCs from wild‐type (wt, left) and GluA1C811S homozygous (CS/CS, middle) mice are shown. EPSC/IPSC ratios were calculated and plotted (right). wt: n = 21 cells from 3 mice; CS/CS: n = 21 slices from 3 mice. (B) EPSC/IPSC ratio of granule neurons in the lower blade of the dentate gyrus. Representative traces of EPSCs and IPSCs from wild‐type (wt, left) and GluA1C811S homozygous (CS/CS, middle) mice are shown. EPSC/IPSC ratios were calculated and plotted (right). wt: n = 28 cells from 4 mice; CS/CS: n = 28 cells from 4 mice. See also Table S1
3.2. Perturbed expression pattern of Arc in the dentate gyrus of GluA1C811S mutant mice
Previous studies have shown that Arc‐positive cells were mainly found in the upper blade, rather than the lower blade, of the dentate gyrus at the basal state as well as after spatial exploration.18, 19, 20, 21 Expression levels of Arc protein under the basal condition were comparably low in the dentate gyrus of wt and CS/CS mice.17 Notably, our immunohistochemical staining showed that Arc proteins exhibited abnormal expression patterns in CS/CS mice, compared to wt mice. Namely, Arc‐positive neurons were equally observed in both upper and lower blades of the dentate gyrus, in most but not all CS/CS mice (Figure 2A). The ratio of Arc‐positive cells to total cells in the lower blade was increased in CS/CS mice, whereas no significant difference was observed in the upper blade or in total cell numbers (Figure 2B).
Figure 2.
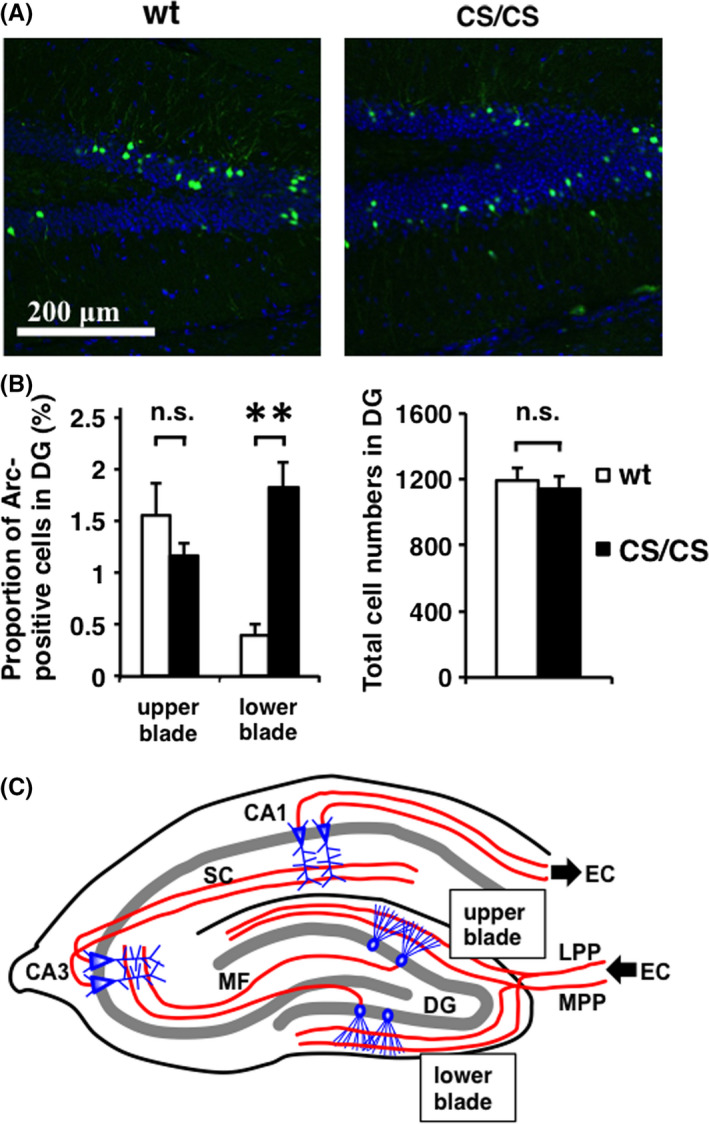
Different Arc expression in the upper and lower blades of the hippocampal dentate gyrus at basal level. (A) Arc expression (green) and DAPI staining (blue) in wild‐type (wt) and GluA1C811S homozygous (CS/CS) mice. Typical patterns are shown. (B) Proportions of Arc‐positive cells in the upper and lower blades (left) and total cell numbers (DAPI‐positive cells, right) in the dentate gyrus (DG) of wt and CS/CS mice. Wt: n = 4, CS/CS: n = 4. Error bars represent SEM **P < 0.01, t test. n.s.: not significant. (C) Schematics of major excitatory circuits in the hippocampus. The hippocampal circuits consist of a trisynaptic pathway. Granule neurons in the dentate gyrus (DG) receive excitatory input from the entorhinal cortex (EC) via the medial perforant pathway (MPP) and lateral perforant pathway (LPP). Then, the granule neuron sends the signal to CA3 pyramidal neurons via the mossy fibers (MF). CA3 pyramidal cells relay the signal to CA1 pyramidal cells via Schaffer collaterals (SC). Finally, CA1 pyramidal neurons return the signal to the EC. See also Table S2
4. DISCUSSION
Epilepsy is characterized by recurring, unprovoked seizures, resulting from the abnormally synchronous activity of excitatory neurons in a focal area of the cerebrum, and in some cases, conveyed throughout the entire brain.22, 23, 24 Disrupted E/I balance leads to perturbed neural circuit function, and repetitive seizures induce serious epilepsy.25 Recently, we demonstrated that seizures induced by pentylenetetrazol (PTZ), a GABAA receptor antagonist, were accompanied by an excessively increased expression of c‐Fos and Arc proteins in both the upper and lower blades of the dentate gyrus in adult GluA1C811S mice at 2 and 4 h after administration of PTZ.17 Our data presented in this report further indicate that the basal expression pattern of Arc is disturbed in the dentate gyrus of GluA1C811S mice, although imbalanced E/I was not obviously detected in granule neurons in the dentate gyrus as a whole (Figure 2C). These results suggest the significance of appropriate Arc expression, regulated by the palmitoylation‐dependent control of GluA1‐containing AMPA receptors in excitatory synapses. Although we have focused on analysis of excitatory synapses in GluA1C811S mice,17 there still remains a possibility that mutation in GluA1 affected the expression of cation‐chloride cotransporters, NKCC1 and KCC2, and chloride homeostasis, resulting in the alteration of GABAergic inhibitory synaptic response.26, 27, 28 In contrast to Arc, c‐fos, another IEG, is expressed in both blades of the dentate gyrus at the basal state.17 Altered Arc expression patterns potentially underlie the excessive excitability in response to seizure‐inducing stimulation. While the upper blade of the dentate gyrus mainly receives spatial information, the lower blade is supposed to receive nonspatial information from the entorhinal cortex.20, 29, 30 The abnormal Arc expression may also reflect perturbed information processing through the perforant pathway to the hippocampal dentate gyrus in GluA1C811S mice. Further analysis would precisely explain details of the causal relationship between seizure susceptibility and abnormal Arc expression. Epileptic seizures are associated with excessive cortical excitability resulting from imbalances between excitation and inhibition in some focal regions of the cerebrum. In the temporal lobe, Arc‐dependent synaptic expression of AMPA receptors under appropriate regulation in the dentate gyrus may contribute to the stability of circuits in whole hippocampus.
CONFLICT OF INTEREST
The authors declare that we have no conflicts of interest.
AUTHOR CONTRIBUTION
HO, MH, MS, and TH designed study; MI, DY, MY, MA, RN, TK, and TH performed experiments and analyzed data; MI, HO, and TH drafted the manuscript with contributions from all of the other authors; and KS, MM, and KW supervised the project. MY did Graduate Program in Department of Development and Regenerative Biology, Graduate School of Medical and Dental Science, Tokyo Medical and Dental University, Tokyo, Japan.
DATA REPOSITORY
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
n/a.
INFORMED CONSENT
n/a.
REGISTRY AND THE REGISTRATION NO. OF THE STUDY/TRIAL
n/a.
ANIMAL STUDIES
All animal care and experiments were performed in accordance with the regulations and institutional guidelines of the National Center of Neurology and Psychiatry (NCNP), Japanese Pharmacological Society, and Japan Neuroscience Society. The technical protocols for animal experiments in this study were approved by the Institutional Review Committees of the National Institute of Neuroscience, NCNP.
Supporting information
ACKNOWLEDGEMENTS
This work was supported in part by the Grants‐in‐Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT)/Japan Society for the Promotion of Science (JSPS) (Grant numbers 22680029, 23650187, 24111512, and 16K07078 to TH; 21249012, 22123008, 24249014, and 16H04676 to MM; 21300118 and 24650195 to KS; 24700321 and 26350979 to MA; 25290027 and 17K07124 to KW; 15K06730 and 17K10286 to MS; 15H04258 and 18H05127 to HO; and 15K0701 to MI), RRIME from Japan Agency for Medical Research and Development (AMED) (Grant number JP18gm5910009 to TH), the Takeda Science Foundation (TH), the Mitsubishi Foundation (TH), the Brain Science Foundation (TH), the Suzuken Memorial Foundation (TH), and the Astellas Foundation for Research on Metabolic Disorders (TH). We are grateful to our colleagues in NCNP; Dr. K. Yamamoto and Ms. M. Date for animal care; Drs. T. Owa and S. Miyashita for technical assistance; and Ms. A. Takayama, J. Sakawa, A. Tsuzuki, and A. Yanai for excellent administrative assistance.
Itoh M, Okuno H, Yamada D, et al. Perturbed expression pattern of the immediate early gene Arc in the dentate gyrus of GluA1 C‐terminal palmitoylation‐deficient mice. Neuropsychopharmacol Rep. 2019;39:61–66. 10.1002/npr2.12044
REFERENCES
- 1. Madabhushi R, Kim TK. Emerging themes in neuronal activity‐dependent gene expression. Mol Cell Neurosci. 2018;87:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Okuno H, Minatohara K, Bito H. Inverse synaptic tagging: an inactive synapse‐specific mechanism to capture activity‐induced Arc/arg3.1 and to locally regulate spatial distribution of synaptic weights. Semin Cell Dev Biol. 2018;77:43–50. [DOI] [PubMed] [Google Scholar]
- 3. Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci. 2011;14(3):279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. West AE, Greenberg ME. Neuronal activity‐regulated gene transcription in synapse development and cognitive function. Cold Spring Harb Perspect Biol. 2011;3(6):a005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5(12):952–62. [DOI] [PubMed] [Google Scholar]
- 6. Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80(3):704–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61(3):340–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–43. [DOI] [PubMed] [Google Scholar]
- 9. Hayashi T, Rumbaugh G, Huganir RL. Differential regulation of AMPA receptor subunit trafficking by palmitoylation of two distinct sites. Neuron. 2005;47(5):709–23. [DOI] [PubMed] [Google Scholar]
- 10. Lin DT, Makino Y, Sharma K, et al. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci. 2009;12(7):879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thomas GM, Hayashi T. Smarter neuronal signaling complexes from existing components: how regulatory modifications were acquired during animal evolution: evolution of palmitoylation‐dependent regulation of AMPA‐type ionotropic glutamate receptors. BioEssays. 2013;35(11):929–39. [DOI] [PubMed] [Google Scholar]
- 12. Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol. 2012;22(3):461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang J, Suppiramaniam V, Wooten MW. Posttranslational modifications and receptor‐associated proteins in AMPA receptor trafficking and synaptic plasticity. Neurosignals. 2006;15(5):266–82. [DOI] [PubMed] [Google Scholar]
- 14. Lu W, Roche KW. Posttranslational regulation of AMPA receptor trafficking and function. Curr Opin Neurobiol. 2012;22(3):470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lussier M, Sanz‐Clemente A, Roche KW. Dynamic Regulation of N‐Methyl‐D‐aspartate (NMDA) and alpha‐Amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic Acid (AMPA) Receptors by Posttranslational Modifications. J Biol Chem. 2015;290(48):28596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heine M, Thoumine O, Mondin M, Tessier B, Giannone G, Choquet D. Activity‐independent and subunit‐specific recruitment of functional AMPA receptors at neurexin/neuroligin contacts. Proc Natl Acad Sci USA. 2008;105(52):20947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Itoh M, Yamashita M, Kaneko M, et al. Deficiency of AMPA receptor‐palmitoylation aggravates seizure susceptibility. J Neurosci. 2018;38(47):10220–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Minatohara K, Akiyoshi M, Okuno H. Role of immediate‐early genes in synaptic plasticity and neuronal ensembles underlying the memory trace. Front Mol Neurosci. 2015;8:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okuno H, Akashi K, Ishii Y, et al. Inverse synaptic tagging of inactive synapses via dynamic interaction of Arc/Arg3.1 with CaMKIIbeta. Cell. 2012;149(4):886–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramirez‐Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration‐induced Arc mRNA and protein expression: evidence for selective, network‐specific reactivation. J Neurosci. 2005;25(7):1761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vazdarjanova A, Ramirez‐Amaya V, Insel N, et al. Spatial exploration induces ARC, a plasticity‐related immediate‐early gene, only in calcium/calmodulin‐dependent protein kinase II‐positive principal excitatory and inhibitory neurons of the rat forebrain. J Comp Neurol. 2006;498(3):317–29. [DOI] [PubMed] [Google Scholar]
- 22. Grone B, Baraban SC. Animal models in epilepsy research: legacies and new directions. Nat Neurosci. 2015;18(3):339–43. [DOI] [PubMed] [Google Scholar]
- 23. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314–9. [DOI] [PubMed] [Google Scholar]
- 24. Staley K. Molecular mechanisms of epilepsy. Nat Neurosci. 2015;18(3):367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paz JT, Huguenard JR. Microcircuits and their interactions in epilepsy: is the focus out of focus? Nat Neurosci. 2015;18(3):351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ben‐Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3(9):728–39. [DOI] [PubMed] [Google Scholar]
- 27. Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298(5597):1418–21. [DOI] [PubMed] [Google Scholar]
- 28. Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation‐chloride cotransporters and neuronal function. Neuron. 2009;61(6):820–38. [DOI] [PubMed] [Google Scholar]
- 29. Ramirez‐Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci 2006;26(47):12237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chawla MK, Guzowski JF, Ramirez‐Amaya V, et al. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus. 2005;15(5):579–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


