Abstract
Aim
Aging is thought to coincide with gradual and progressive changes in brain function and behavior over the lifetime. Our previous meta‐analytic study reported age‐related behavioral changes from young to middle age in male C57BL/6J mice. However, the previous study had some limitations that could affect the generalizability of the findings because of the potential influence of genetic and environmental factors on behavior, in addition to a lack of information regarding the behaviors of old‐aged mice. Here, to investigate age‐related behavioral changes from young to old age in mice, we analyzed the behaviors of male C57BL/6J mice from four different age groups (8, 47, 73, and 99 weeks of age at the beginning of the experiment) from a colony that had been maintained in a genetically controlled condition based on The Jackson Laboratory's Genetic Stability Program in an environmentally controlled animal facility.
Methods
We used a battery of behavioral tests, including the light/dark transition, open field, elevated plus maze, hot plate, social interaction, rotarod, three‐chamber social approach, prepulse inhibition, Porsolt forced swim, T‐maze, Barnes maze, tail suspension, and fear‐conditioning tests.
Results
Some behavioral changes occurred between young and middle age, and further changes in various behaviors were observed in old age. Decreased locomotor activity and increased anxiety‐like behavior were found in old‐aged mice compared to those in young and middle‐aged mice in the light/dark transition test. Similarly, an age‐dependent decrease in locomotor activity was observed in the open field test and the elevated plus maze test, while there was an age‐dependent increase in the time spent in the center area in the open field test and there were no significant differences among age groups in behavioral measures of anxiety in the elevated plus maze test. Decreases in motor performance and the auditory startle response were found in middle‐aged mice compared to those in young mice. Similar behavioral changes and increased pain sensitivity, decreased social novelty preference, reduced working and spatial memory, and impaired cued fear memory were observed in old‐aged mice compared to those in young mice. Prepulse inhibition was higher in middle‐aged mice than in young and old‐aged mice. Age‐related changes in depression‐related behavior were dependent on the type of test and the test time period.
Conclusions
This study generally confirmed our previous report regarding age‐related behavioral changes from young to middle age and expanded the previous observations by examining the behaviors of old‐aged mice. Our results show age‐related changes in a wide range of behaviors in mice from young to old age. Most behaviors showed gradual changes with advancing age, but some types of behaviors, such as vertical activity, prepulse inhibition, and depression‐related behavior, did not show unidirectional changes with age. These findings provide basic information about the behavioral characteristics of young, middle‐aged, and aged male C57BL/6J mice.
Keywords: aged C57BL/6J mice, aging, behavioral test battery, genetic stability program, middle age, old age
This study reports age‐related changes in a wide range of behaviors in male C57BL/6J mice from young to old age. The findings provide basic information about the behavioral characteristics of young, middle‐aged, and aged male C57BL/6J mice.
1. INTRODUCTION
Aging is considered to be a gradual and progressive change in brain function and behavioral performance over the lifetime. In rodents, age‐related changes in behavior have generally been demonstrated by comparisons of young and old‐aged animals. A number of studies have reported that compared to young mice, aged mice exhibit declines in behavioral performance related to sensory, motor, and cognitive functions.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 Our previous meta‐analytic study using extensive behavioral data from the public “Mouse Phenotype Database” reported that from young adulthood to middle age (age of 2‐12 months old), age‐related changes occurred in various behaviors, including locomotor activity, sensory and motor functions, anxiety‐like and depression‐related behaviors, social behavior, and learning and memory, in C57BL/6J male mice.13 However, in the previous study, the behavioral data of the different age groups were obtained from wild‐type control mice of various mutant strains with a C57BL/6J genetic background that were derived from different vendors and laboratories. In addition, our previous study provided no information about the behavioral characteristics of mice that were older than 12 months or aged mice. Therefore, some potential limitations of the previous study might affect the generalizability of the findings.
Assessment of various domains of behavior in a cohort of mice has been performed through a battery of behavioral tests covering a wide range of behaviors, such as behavioral tasks designed to measure locomotor activity, sensory reflexes, motor abilities, anxiety‐like behavior, social behavior, and learning and memory. This approach has been used to characterize behavioral phenotypes of transgenic and knockout mice, allowing us to obtain significant findings from a cohort of mice through the interpretation of behavioral outcomes that are evaluated with multiple tests.14, 15, 16 Behavioral analyses have been performed in young and old‐aged C57BL/6J mice through a battery of tests that are mainly used to assess locomotor activity, sensory and motor functions, and memory function.3, 12, 17 The majority of such studies have employed a limited number of behavioral tests to investigate the effects of age on behavior. A test battery approach measuring a wide range of behaviors will allow us to obtain detailed information on the behavioral characteristics of young, middle‐aged, and old‐aged mice.
Genetic and environmental factors can contribute to individual differences in behavior in mice.18, 19 In our previous study, as described above, some potential confounding factors, such as breeding environment and prior test experience, as well as the genetic background of the mice, might have led to the behavioral differences found between the young and middle‐aged age groups.13 These potential issues in our previous study warrant further investigations of age‐related behavioral changes in mice that have been maintained under genetically and environmentally controlled conditions. The C57BL/6J strain is an inbred strain of mice that are widely used in biomedical research and as a background strain for genetically engineered mice. There are many lines of C57BL/6J mice with genetic differences caused by possible genetic drift.20, 21 Aged C57BL/6J mice from colonies that were managed by the Jackson Laboratory's Genetic Stability Program to minimize genetic drift are now available from The Jackson Laboratory and Charles River, Inc.22, 23 In this study, to investigate age‐related changes in various domains of behavior from young to old age in the inbred mice, we used four different age cohorts of male mice from the “C57BL/6J‐Aged mice” colony (8, 47, 73, and 99 weeks old at the beginning of the behavioral analysis) that had been maintained based on the Jackson Laboratory's patented program at Charles River Japan, Inc. We analyzed the behaviors of the mice using a battery of behavioral tests, including the general health and neurological screen, light/dark transition, open field, elevated plus maze, hot plate, social interaction, rotarod, three‐chamber social approach, acoustic startle response (ASR)/prepulse inhibition (PPI), Porsolt forced swim, T‐maze spontaneous alternation, Barnes maze, tail suspension, and contextual and cued fear‐conditioning tests.
2. MATERIALS AND METHODS
2.1. Animals
Male C57BL/6J mice of different ages (7 weeks old, n = 16; 46 weeks old, n = 16; 72 weeks old, n = 16; and 98 weeks old, n = 35) were used. The C57BL/6J strain had been maintained under The Jackson Laboratory's patented Genetic Stability Program22, 23 at Charles River Laboratories Japan, Inc. The four age groups of mice were bred as “C57BL/6J‐Aged” mice and kept continuously under controlled conditions of lighting (12‐hour light/dark cycle, lights on at 6:00 am), ambient temperature (20‐25°C), and humidity (40%‐70%) at the Atsugi Breeding Center of Charles River Laboratories Japan, Inc. The mice were group‐housed in plastic cages (29.2 × 44.0 × 20.0 cm; ≤15 mice per cage) with food (Oriental Yeast Co., Tokyo, Japan) and water ad libitum. The breeding and animal care were carried out by trained professionals at Charles River Laboratories Japan, Inc., according to the protocols of The Jackson Laboratory,24 until arrival at our animal facility. All of the mice were then transported to our animal facility on the same day. After arrival, mice of the same age were housed in groups (three to four per cage) in plastic cages (22.7 × 32.3 × 12.7 cm) with sterilized PaperClean bedding (Japan SLC, Inc., Shizuoka, Japan) in a room with a 12‐hour light/dark cycle (lights on at 7:00 am). The room temperature was maintained at 23 ± 2°C. The mice were given access to food (CRF‐1, Oriental Yeast Co.) and water ad libitum throughout the study. Several days after arrival, two mice from the oldest age group died for unknown reasons.
2.2. Behavioral test battery
The animals were subjected to a series of behavioral tests (Table 1) 1 week after arrival. At least 30 minutes before the beginning of each test, the mice were transferred to sound‐attenuated rooms adjacent to the housing room and acclimated to the room set up for each test (see Refs 25, 26). The mice were tested in the following order: general health and neurological screen, light/dark transition, open field, elevated plus maze, hot plate, social interaction, rotarod, three‐chamber social approach, ASR/PPI, Porsolt forced swim, T‐maze spontaneous alternation, Barnes maze, tail suspension, contextual and cued fear‐conditioning tests, as previously described.27 Behavioral testing was performed between 9:00 am and 6:00 pm. Except for the T‐maze and Barnes maze tests, each test was completed for all the mice within 1‐4 days. The interval between tests was at least 1 day. After each test, the mice were returned to their home cages, and all apparatuses were cleaned with super hypochlorous water and/or 70% ethanol to prevent bias based on olfactory cues. The number of animals in the older age groups gradually decreased during the test battery because some mice died for unknown reasons (for the number of animals used in each test, see Table 1; one old‐aged mouse over 24 months old was excluded from the analyses for the Barnes maze, tail suspension, and fear‐conditioning tests due to health problems (the mouse would lie down on its side and was so inactive that it could not reach the target location in the Barnes maze test). All experimental procedures were approved by the Institutional Animal Care and Use Committee of Fujita Health University.
Table 1.
A behavioral test battery of young, middle‐aged, and aged C57BL/6J mice
| Order | Test | Age cohort | ||||
|---|---|---|---|---|---|---|
| Age 1 | Age 2 | Age 3 | Age 4 | Figure | ||
| 1 | General health and neurological screen | 8‐9 wk (1‐2 mo), n = 16 | 47‐48 wk (10‐11 mo), n = 16 | 73‐74 wk (16‐17 mo), n = 16 | 99‐100 wk (22 mo),n = 33 | Figure 1A‐D |
| 2 | Light/dark transition test | 9 wk (2 mo), n = 16 | 48 wk (11 mo), n = 16 | 74 wk (17 mo), n = 16 | 100 wk (23 mo),n = 33 | Figure 2E‐H |
| 3 | Open field test | 9‐10 wk (2 mo), n = 16 | 48‐49 wk (11 mo), n = 16 | 74‐75 wk (17 mo), n = 16 | 100‐101 wk (23 mo),n = 33 | Figure 2A‐D, S2A‐H |
| 4 | Elevated plus maze test | 10 wk (2 mo), n = 16 | 49 wk (11 mo), n = 16 | 75 wk (17 mo), n = 16 | 101 wk (23 mo),n = 32 | Figure 2I‐L |
| 5 | Hot plate test | 10 wk (2 mo), n = 16 | 49 wk (11 mo), n = 16 | 75 wk (17 mo), n = 16 | 101 wk (23 mo),n = 31 | Figure 1E |
| 6 | Social interaction test | 10‐11 wk (2 mo), n = 8 pairs | 49‐50 wk (11 mo), n = 8 pairs | 75‐76 wk (17 mo), n = 8 pairs | 101‐102 wk (23 mo),n = 15 pairs | Figure 3A‐E |
| 7 | Rotarod test | 11 wk (2 mo), n = 16 | 50 wk (11 mo), n = 16 | 76 wk (17 mo), n = 16 | 102 wk (23 mo),n = 31 | Figure 1F |
| 8 | Three‐chamber social approach test | 12 wk (2 mo), n = 16 | 51 wk (11 mo), n = 16 | 77 wk (17 mo), n = 16 | 103 wk (23 mo),n = 28 | Figure 3F‐I |
| 9 | Startle response/prepulse inhibition test | 13 wk (3 mo), n = 16 | 52 wk (12 mo), n = 16 | 78 wk (18 mo), n = 16 | 104 wk (23‐24 mo),n = 27 | Figure 4 |
| 10 | Porsolt forced swim test | 13 wk (3 mo), n = 16 | 52 wk (12 mo), n = 16 | 78 wk (18 mo),n = 16 | 104 wk (24 mo),n = 27 | Figure 5A |
| 11 | T‐maze spontaneous alternation test | 14‐15 wk (3 mo), n = 16 | 53‐54 wk (12 mo), n = 16 | 79‐80 wk (18 mo),n = 16 | 105‐106 wk (24 mo),n = 24 | Figure 6 |
| 12 | Barnes maze test | 16‐22 wk (3‐5 mo), n = 16 | 55‐61 wk (12‐14 mo), n = 16 | 81‐87 wk (18‐20 mo),n = 15 | 107‐113 wk (24‐26 mo),n = 15 | Figure 7A‐F |
| 13 | Tail suspension test | 22 wk (5 mo), n = 16 | 61 wk (14 mo), n = 16 | 87 wk (20 mo),n = 15 | 113 wk (26 mo),n = 14 | Figure 5B |
| 14 | Fear conditioning test | 23 wk (5 mo), n = 16 | 62 wk (14 mo), n = 16 | 88 wk (20 mo),n = 15 | 114 wk (26 mo),n = 13 | Figure 8A‐I, S3 |
n, number of animals.
2.3. General health and neurological screen
Physical characteristics, including rectal temperature and body weight, neurological reflexes (the righting reflex, whisker twitch reflex, ear twitch reflex, and visual placing reflex, which is a forepaw extension when lowered toward a visible surface) and response to key jangling were assessed. To measure neuromuscular strength in the wire hang test, each mouse was placed on a wire mesh that was then inverted, and the latency to falling from the wire was recorded with a 60‐seconds cutoff time. The forelimb grip strength was measured using a grip strength meter (O'Hara & Co., Tokyo, Japan). In the grip strength test, the mice were held by their tails and lifted so that their forepaws could grasp a wire grid of the grip strength meter. The mice were gently pulled backward by the tail until they released the grid. The peak force applied by the forelimbs was recorded in Newtons (N). Each mouse was tested three times, and the highest value was used for statistical analysis.
2.4. Light/dark transition test
The light/dark transition test, which was originally developed by Crawley and colleagues,28 was conducted to assess anxiety‐like behavior, as previously described.29 The apparatus consisted of a plastic cage with a white floor (21 × 42 × 25 cm), which was divided into two sections of equal size by a partition with a door (O'Hara & Co.). One chamber with white walls was brightly illuminated (light chamber, 390 lux), and the other chamber with black walls was dark (dark chamber, 2 lux). The mice were placed into the dark chamber and allowed to move freely between the chambers with the door open for 10 minutes. The distance traveled (cm) in each chamber, total number of transitions between chambers, latency to enter the light chamber (s), and time spent in each chamber (s) were recorded automatically using ImageLD software (see “Image analysis”).
2.5. Open field test
The open field test was carried out to evaluate locomotor activity and emotional responses.30 The apparatus was a transparent square cage (42 × 42 × 30 cm) equipped with infrared photobeam sensors (VersaMax; Accuscan Instruments, Columbus, OH, USA) with a white floor. The center of the floor was illuminated at 100 lux. Each mouse was placed in the corner of the cage. The total distance traveled (cm), vertical activity (rearing measured by counting the number of photobeam interruptions), time spent in the center area (20 × 20 cm), and stereotypic counts (number of repeated breaks of the same beam during stereotyped behaviors) were measured for 120 minutes using the VersaMax system.
2.6. Elevated plus maze test
The elevated plus maze test was conducted to assess anxiety‐like behavior, as previously described.31 The elevated plus maze consisted of two open arms (25 × 5 cm) with 3‐mm‐high ledges and two closed arms (25 × 5 cm) with 15‐cm‐high transparent walls (O'Hara & Co.). The floors of the arms and the central square (5 × 5 cm) were made of white plastic plates and elevated to a height of 55 cm above the floor. The arms of the same type were arranged on opposite sides of the maze. The center of the maze was illuminated at 100 lux. Each mouse was placed into the center of the maze facing one of the closed arms and was recorded for 10 minutes. The distance traveled (cm), number of total entries into the arms, percentage of entries into the open arms, and percentage of time spent in the open arms were measured for 10 minutes using ImageEP software (see “Image analysis”).
2.7. Hot plate test
The hot plate test was performed to evaluate sensitivity to a painful stimulus. The mice were placed on a hot plate maintained at 55.0 ± 0.2°C (Columbus Instruments, Columbus, OH, USA). The latency to the first paw response (s) was recorded with a 15‐seconds cutoff time. A paw response was defined as either a foot shake or a paw lick.
2.8. Social interaction test in a novel environment
The social interaction test was conducted to measure social behavior in a novel environment. Pairs of weight‐matched mice of the same age that had been housed in different cages were placed in a white plastic box (40 × 40 × 30 cm) and allowed to explore freely for 10 minutes. The mice were recorded by a video camera placed above the box. Images were captured at three frames per second and transferred to a computer. The distance traveled by each mouse between two successive frames was automatically calculated by ImageSI software (see “Image analysis”). The total number of contacts, total duration of contacts (s), total duration of active contacts (s), mean duration per contact (s), and total distance traveled (cm) were recorded by the software. Active contact was measured when the two mice contacted each other and one or both mice moved with a velocity of at least 10 cm/s.
2.9. Rotarod test
Mice were placed on rotating drums (3 cm diameter) of an accelerating rotarod (UGO Basile, Comerio, VA, Italy) to evaluate motor coordination and balance. The speed of the rotarod accelerated from 4 to 40 rpm over a 5‐minute period. Latency to fall off the rotating rod was recorded with a 5‐minutes cutoff time for three trials per day over 2 consecutive days.
2.10. Three‐chamber social approach test
The three‐chamber social approach test was conducted to assess sociability and social novelty preference.32 The testing apparatus consisted of a rectangular three‐chambered box and a lid with an video camera (O'Hara & Co.). The dividing walls of the chamber were made of transparent plastic, with small square openings (5 × 3 cm) allowing access to each chamber (20 × 40 × 47 cm). A small round wire cage (9 cm in diameter, 11 cm in height, with vertical bars 0.5 cm apart) was located in the corner of the left and right chambers. The test mice were first placed in the middle chamber and allowed to explore the entire test chamber for 10 minutes. Immediately after the 10‐minute period, the test mice were placed in a clean holding cage, and a male C57BL/6J mouse (stranger 1) with no prior contact with the test mice was enclosed in one of the wire cages. Next, the test mice were returned to the middle chamber and allowed to explore for 10 minutes (sociability test). After the test session, the test mice were again placed in the holding cage, and a second unfamiliar mouse (stranger 2) was enclosed in the wire cage on the opposite side. The test mice were placed in the middle chamber and had a choice between the first, already investigated unfamiliar mouse and the novel unfamiliar mouse for 10 minutes (social novelty preference test). The time spent in each chamber and the time spent around each cage were automatically measured from images using ImageCSI software (see “Image analysis”).
2.11. ASR/PPI test
A startle reflex measurement system (O'Hara & Co.) was used to measure the startle response elicited by a loud stimulus (ASR) and PPI of the startle response, as previously described.33 The mice were placed in a plastic cylinder that was mounted on a platform with a accelerometer. The mice were left undisturbed for 10 minutes and then subjected to test trials consisting of six trial types, that is, two types of startle stimulus only trials and four types of PPI trials. White noise of 110 or 120 dB (40 ms) was used as the startle stimulus for all trial types. The prepulse stimulus was presented 100 ms before the onset of the startle stimulus, and its intensity was 74 or 78 dB (20 ms). Four combinations of prepulse and startle stimuli were used (74‐110, 78‐110, 74‐120, and 78‐120 dB). Six blocks of the six trial types were presented in a pseudorandom order such that each trial type was presented once within a block. The average intertrial interval was 15 seconds (range: 10‐20 seconds). The startle response was recorded for 400 ms starting with the onset of the startle stimulus. The peak startle amplitude was used as a dependent variable. The background noise level was 70 dB during all the test sessions. Percent PPI was calculated for each mouse according to the following formula: percent PPI = 100 × [1 − (ASR amplitude in prepulse + startle trial)/(ASR amplitude in startle stimulus alone trial)].
2.12. Porsolt forced swim test
The Porsolt forced swim test, which was developed by Porsolt et al,34 was performed to assess depression‐related behavior. A Plexiglas cylinder (20 cm height × 10 cm diameter) was placed in a test chamber (49 cm height × 44 cm length × 32 cm width, inside dimensions; O'Hara & Co.). A video camera was mounted on the ceiling of the test chamber and positioned directly above the cylinder. The mice were placed into the cylinder, which was filled with water (approximately 23°C) to a height of 7.5 cm. Immobility times were recorded over a 10‐minutes test period on 2 consecutive days. Images were captured at two frames per second through the video camera and transferred to a computer. For each pair of successive frames, the area (pixels) within which the mouse moved was measured. When the area was below a certain threshold, the mouse behavior was judged as “immobile.” When the area equaled or exceeded the threshold, the mouse was considered “moving.” The optimal threshold for this judgment was determined by adjusting it to the amount of immobility measured by a trained human observer. Immobility lasting for <2 seconds was not included in the analysis. Data acquisition and analysis were performed automatically using ImagePS software (see “Image analysis”).
2.13. T‐maze spontaneous alternation test
The spontaneous alternation task was conducted to assess spatial working memory using a modified automatic T‐maze apparatus (O'Hara & Co.), as previously described.25, 35 The apparatus was constructed of white plastic runways with 25‐cm‐high walls. It was partitioned into 6 areas: the stem of the T, a straight runway, the left and right arms, and the connecting passageways from the arms to the stem of the T. The mice were subjected to a session consisting of 10 trials per day for 3 days (cutoff time, 50 minutes). Each trial consisted of a forced choice followed by a free choice (intertrial interval, 60 seconds). In the forced‐choice trial, the mice were forced to enter either the left or right arm of the T‐maze and were held in the arm for 10 seconds. After the 10‐second period, the doors of the connecting passageway from the arm to the stem of the T were opened, and the mouse could return to the starting compartment. Three seconds after the mice entered the starting compartment, a free‐choice trial started. The mice were allowed to choose one of the arms. The percentage of correct responses in which the mice entered the arm opposite to their choice in the forced‐choice trial during the free‐choice trial was calculated. Data acquisition and analysis were performed automatically using ImageTM software (see “Image analysis”).
2.14. Barnes maze test
The Barnes maze test, which was developed by Barnes,36 was conducted on a white circular surface (1.0 m in diameter, with 12 holes equally spaced around the perimeter; O'Hara & Co.). The circular open field was elevated 75 cm from the floor. The apparatus was illuminated by fluorescent lights mounted on the ceiling of the sound‐attenuating room. The illumination level was 850 lux in the center of the field. A variety of fixed extra‐maze clues surrounded the apparatus. A black Plexiglas escape box (17 × 13 × 7 cm) was located under one of the holes, the target hole, analogous to the hidden platform in the Morris water maze task. The location of the target hole was consistent for a given mouse but randomized across mice. In a training session, the mice were placed in the center of the field and allowed to explore the field freely. After entering the target hole, the mice were left undisturbed in the escape box for 30 seconds. If the mice did not enter the target hole within 300 seconds, they were gently picked up and guided to the target hole by the experimenter and were allowed to enter the target hole and remain there for 30 seconds. The training session was conducted with one trial per day for 18 consecutive days. The maze was rotated daily, with the spatial location of the target unchanged with respect to the distal visual room cues, to prevent a bias based on olfactory or proximal cues within the maze. The number of errors before first reaching the target hole, latency to reach the target hole (s), and distance traveled to reach the target hole (cm) were automatically recorded by ImageBM software (see “Image analysis”). One and 10 days after the last training session, a probe trial was conducted without the escape box for 180 seconds to assess spatial reference memory. In the probe trials, the time spent around each hole was measured. After the second probe trial, a reversal training session was performed with one trial per day for 10 days. The escape box was moved 180° from the original location in the reversal training. One day after the last reversal training session, a probe trial was conducted as described above.
2.15. Tail suspension test
The tail suspension test, which was developed by Steru et al,37 was performed to assess depression‐related behavior. Each mouse was suspended 30 cm above the floor by the tail in a white plastic chamber (44 cm height × 49 cm length × 32 cm width, inside dimensions; O'Hara & Co.) with a video camera mounted on the wall (O'Hara & Co.). The behavior was recorded for 10 minutes. Images were captured at two frames per second through the video camera. Similar to the Porsolt forced swim test, the immobility of each mouse was judged according to a certain threshold using ImagePS software.
2.16. Contextual and cued fear‐conditioning tests
Contextual and cued fear‐conditioning tests were performed as previously described.26 In the conditioning session, each mouse was placed in an acrylic chamber consisting of white (side) and transparent (front, rear, and top) walls (33 × 25 × 28 cm) with a stainless‐steel grid floor (0.2 cm diameter, spaced 0.5 cm apart; O'Hara & Co.). The mice were allowed to explore the chamber freely for 120 seconds, and 55 dB white noise then served as the conditioned stimulus (CS) for 30 seconds. During the last 2 seconds of CS presentation, a mild footshock (0.3 mA, 2 seconds) was delivered as the unconditioned stimulus (US). The mice were subjected to two more CS‐US pairings with a 2‐minutes interstimulus interval. The animals were returned to their home cages 90 seconds after the last footshock. Approximately 24 hours after conditioning, a context test was conducted for 300 seconds. In the context test, the mice were placed in the same chamber in which they had been conditioned. At least 2 hours after the context test, a cued test with altered context was performed for 360 seconds. In the cued test, the mice were placed in a triangular chamber (33 × 29 × 32 cm) made of white plastic walls and floor, which was located in a different sound‐attenuating room, and allowed to explore the triangular chamber for 180 seconds. Then, the CS was presented during the last 180 seconds of the cued test. In each session, the percentage of freezing and the distance traveled were calculated automatically using ImageFZ software (see “Image analysis”).
2.17. Image analysis
The application software used for the behavioral experiments (ImageLD/EP/SI/CSI/PS/TM/BM/FZ), based on the public domain ImageJ program (http://rsb.info.nih.gov/ij/), was developed and modified for each test by Tsuyoshi Miyakawa.
2.18. Statistical analysis
Statistical analysis was conducted using SAS University Edition (SAS Institute, Cary, NC, USA). Data were analyzed to examine the effects of age on behavior using one‐way ANOVA or two‐way repeated measures ANOVA (for the statistical results, see Table S1). We defined “study‐wide significance” as statistical significance that survived the Benjamini‐Hochberg False Discovery Rate (FDR) correction38, 39 to control for multiple testing based on the number of behavioral measures in the test battery (61 measures). “Nominal significance” was defined as achieving statistical significance without the FDR correction (uncorrected P < 0.05). Post hoc simple main effect analyses and simple interaction analyses were conducted as necessary and appropriate, and post hoc tests for pairwise comparisons were performed using Fisher's LSD with the Bonferroni correction (uncorrected p values were described, and the significance level was P < 0.05/6 = 0.0083). The values in the graphs are expressed as the mean ± SEM.
3. RESULTS
3.1. Increased body weight, reduced neuromuscular strength, increased thermal sensitivity, and decreased motor function in middle‐aged and aged C57BL/6J mice
All mice from each age group (1‐2 months old, 1‐2 mo; 10‐11 months old, 10‐11 mo; 16‐17 months old, 16‐17 mo; and 22 months old, 22 mo) appeared grossly normal at the beginning of the test battery and showed no apparent differences in neurological reflexes, including the righting reflex, whisker twitch reflex, ear twitch reflex, and visual placing reflex. Significant effects of age were found on body weight (Figure 1A: F 3,77 = 92.62, P < 0.0001), body temperature (Figure 1B: F 3,77 = 6.12, P = 0.0009), grip strength (Figure 1D: F 3,77 = 6.62, P = 0.0005), hot plate latency (Figure 1E: F 3,75 = 3.12, P = 0.0310), and rotarod latency (Figure 1F: age effect, F 3,75 = 31.15, P < 0.0001; age × trial interaction, F 15,375 = 4.80, P < 0.0001) but not on wire hang latency (Figure 1C: F 3,77 = 0.94, P = 0.4256). Middle‐aged and old‐aged mice were significantly heavier than young mice (10‐11, 16‐17, and 22 mo > 1‐2 mo, all P < 0.0001), and old‐aged mice were significantly lighter than middle‐aged mice (22 mo < 10‐11 and 16‐17 mo, all P < 0.0001). In addition, compared to young mice, 16‐ to 17‐month‐old mice and 22‐month‐old mice showed reduced body temperature (16‐17 and 22 mo < 1‐2 mo, P = 0.0048 and P = 0.0001, respectively), reduced grip strength (16‐17 and 22 mo < 1‐2 mo, P = 0.0002 and P = 0.0002, respectively), reduced hot plate latency (23 mo < 2 mo, P = 0.0032), and a reduced latency to fall off the rotating rod (11, 17, and 22 mo < 2 mo, all P < 0.0001). For all these measures except for body weight, there were no significant differences among the three age groups of middle‐aged and old‐aged mice. Similar to the results of our previous study,13 the mean rotarod latency averaged across six trials was analyzed by ANCOVA, with the body weight measured 4‐6 days before the rotarod test as a covariate because rotarod performance is negatively correlated with body weight.40, 41 ANCOVA revealed that the assumption of homogeneity of slopes was met (age × body weight interaction, F 3,71 = 1.63, P = 0.1891, Figure S1 for scatterplots of body weight with the average rotarod latency), and there were significant effects of age on rotarod latency (age effect, F 3,74 = 10.07, P < 0.0001), which confirmed that rotarod performance decreased after 11 months of age and that no significant differences in performance occurred among 11‐, 17‐, and 22‐month‐old mice.
Figure 1.
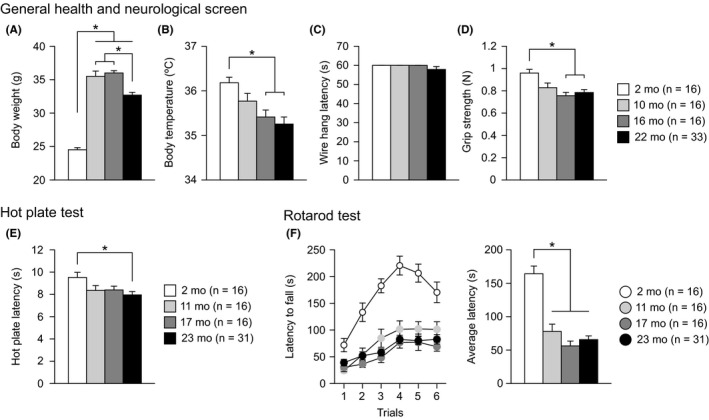
Increased body weight, decreased neuromuscular strength, increased thermal sensitivity, and decreased motor function in middle‐aged and aged C57BL/6J mice. (A) Body weight (g), (B) body temperature (°C), (C) wire hang latency (s), (D) grip strength (Newton, N), (E) latency to paw lick or foot shake (s) in the hot plate test, and (F) latency to fall off a rotating rod (s) in the rotarod test. Values are means ± SEM. Asterisks indicate significant differences between groups after Bonferroni correction for multiple comparisons (P < 0.0083)
3.2. Decreased locomotor activity and altered anxiety‐like behavior in aged C57BL/6J mice
The open field test was performed to evaluate locomotor activity and anxiety‐like behavior during the initial 5‐ and 120‐minute periods of the test in 2‐, 11‐, 17‐, and 23‐month‐old mice (Figure 2A‐D and Figure S2). A significant effect of age was found on distance traveled (Figure 2A) during the first 5‐minute period (Figure S2A: F 3,77 = 6.53, P = 0.0005) but not during the 120‐minute period (Figure S2E: F 3,77 = 0.86, P = 0.4653). During the first 5‐minute period, 23‐month‐old mice traveled shorter distances than 2‐ and 11‐month‐old mice (23 mo < 2 and 11 mo, P = 0.0002 and P = 0.0017, respectively). Similarly, there was a significant effect of age on vertical activity (Figure 2B) during the first 5‐minute period and the 120‐minute period (for 5 minute, Figure S2B, F 3,77 = 6.30, P = 0.0007; for 120 minutes, Figure S2F, F 3,77 = 6.36, P = 0.0007). Twenty‐three‐month‐old mice showed less vertical activity than 2‐, 11‐, and 17‐month‐old mice during the first 5‐minutes period (23 mo < 2, 11, and 17 mo, P = 0.0008, P < 0.0001, and P = 0.0036, respectively); during the 120‐minute period, 2‐ and 23‐month‐old mice exhibited less vertical activity than 11‐month‐old mice (2 and 23 mo < 11 mo, P = 0.0017 and P = 0.0004, respectively). A significant age effect was observed on stereotypic counts (Figure 2D) during the first 5‐minute period and the 120‐minute period (for 5 minute, Figure S2D, F 3,77 = 16.57, P < 0.0001; for 120 minutes, Figure S2H, F 3,77 = 6.07, P = 0.0009). During the first 5‐minute period, 23‐month‐old mice showed lower stereotypic counts than 11‐ and 17‐month‐old mice (P < 0.0001 and P = 0.0046, respectively), and 2‐ and 17‐month‐old mice exhibited lower stereotypic counts than 11‐month‐old mice (P = 0.0002 and P = 0.0007, respectively). Twenty‐three‐month‐old mice also displayed less stereotypic behavior than 11‐month‐old mice during the 120‐minute period (23 mo < 11 mo, P = 0.0001). Regarding the time spent in the center area (Figure 2C), there was a significant effect of age during the 120‐minute period (Figure S2G: F 3,77 = 11.25, P < 0.0001) but not during the first 5‐minute period (Figure S2C: F 3,77 = 0.81, P = 0.4924). During the 120‐minute period, 23‐, 17‐, and 11‐month‐old mice spent more time in the center area than 2‐month‐old mice (23, 17, and 11 mo > 2 mo, P < 0.0001, P = 0.0018, and P = 0.0059, respectively). These observations indicate that, in general, old‐aged mice showed decreased locomotor activity in the novel environment but showed subsequent increases in locomotor activity and time spent in the center area compared to those of younger age groups and that middle‐aged mice exhibited more vertical activity and repetitive behavior than the other age groups during the first 5‐minute period and the 120‐minute period.
Figure 2.
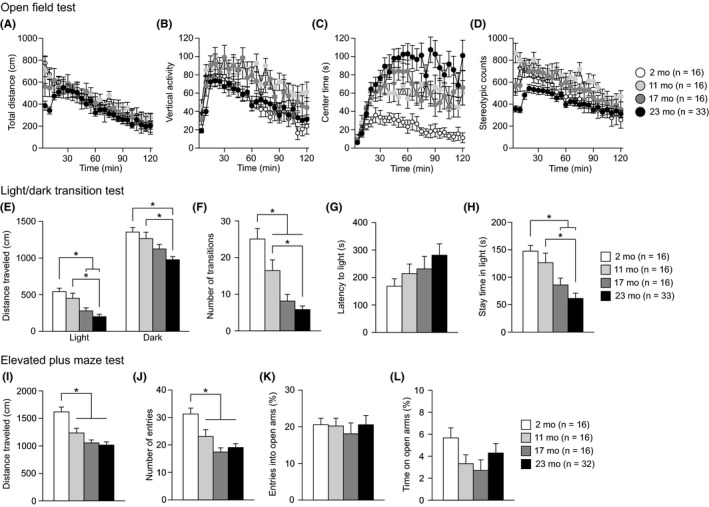
Decreased locomotor activity and altered anxiety‐like behaviors in middle‐aged and old‐aged C57BL/6J mice. (A‐D) Open field test: (A) distance traveled (cm), (B) vertical activity, (C) center time (s), and (D) stereotypic counts for each 5‐minutes block of testing. (E‐H) Light/dark transition test: (E) distance traveled (cm) in the light and dark chambers, (F) number of transitions, (G) latency to enter the light chamber (s), and (H) time spent in the light chamber (s). (I‐L) Elevated plus maze test: (I) distance traveled (cm), (J) number of arm entries, (K) entries into open arms (%), and (L) time spent in open arms (%). Values are means ± SEM. Asterisks indicate significant differences between groups after Bonferroni correction for multiple comparisons (P < 0.0083)
The light/dark transition test, which is based on the innate aversion of rodents to brightly illuminated areas, was conducted to assess anxiety‐like behavior. There were significant effects of age on distance traveled in the light chamber (Figure 2E: F 3,77 = 12.52, P < 0.0001) and dark chamber (Figure 2E: F 3,77 = 8.52, P < 0.0001), number of transitions (Figure 2F: F 3,77 = 19.70, P < 0.0001), and time spent in the light chamber (Figure 2H: F 3,77 = 10.72, P < 0.0001) but not on latency to enter the light chamber (Figure 2G: F 3,77 = 1.38, P = 0.2561). In the light chamber, 23‐month‐old mice moved a shorter distance than 2‐ and 11‐month‐old mice (23 mo < 2 and 11 mo, P < 0.0001 and P = 0.0001, respectively), and 17‐month‐old mice traveled a shorter distance than 2‐month‐old mice (17 mo < 2 mo, P = 0.0004). In the dark chamber, 23‐month‐old mice traveled a shorter distance than 2‐ and 11‐month‐old mice (23 mo < 2 and 11 mo, P < 0.0001 and P = 0.0008, respectively). Similarly, middle‐ and old‐aged mice made fewer transitions between the two chambers than 2‐month‐old mice (11, 17, and 23 mo < 2 mo, P = 0.0068, P < 0.0001, and P < 0.0001, respectively), and the number of transitions was lower in 23‐month‐old mice than in 11‐month‐old mice (23 mo < 11 mo, P = 0.0002). Furthermore, 17‐ and 23‐month‐old mice spent less time in the light chamber than 2‐ or 11‐month‐old mice (17 and 23 mo < 2 mo, P = 0.0023 and P < 0.0001, respectively; 23 mo < 11 mo, P = 0.0002). The results of the light/dark transition test show that aged mice displayed reduced exploration and marked avoidance of the brightly illuminated area, suggesting increased anxiety‐like behavior.
In the elevated plus maze test, there were significant effects of age on distance traveled (Figure 2I: F 3,76 = 14.11, P < 0.0001) and the number of entries (Figure 2J: F 3,76 = 9.87, P < 0.0001), but no significant effect of age was found on the percentage of entries into the open arms (Figure 2K: F 3,76 = 0.18, P = 0.9072) or the percentage of time in the open arms (Figure 2L: F 3,76 = 1.55, P = 0.2075). Middle‐ and old‐aged mice showed lower locomotor activity than 2‐month‐old mice, as indicated by the shorter distance traveled (11, 17, and 23 mo < 2 mo, P = 0.0010, P < 0.0001, and P < 0.0001, respectively) and the lower number of total arm entries (11, 17, and 23 mo < 2 mo, P = 0.0061, P < 0.0001, and P < 0.0001, respectively).
3.3. Normal sociability and reduced social novelty preference in aged C57BL/6J mice
In the social interaction test in a novel environment, there were significant effects of age on distance traveled (Figure 3A: F 3,35 = 6.11, P = 0.0019) and mean duration per contact (Figure 3E: F 3,35 = 5.03, P = 0.0053). Twenty‐three‐month‐old mice traveled a shorter distance than 2‐month‐old mice (23 mo < 2 mo, P = 0.0003) and showed an increased mean duration of contact compared to that of 2‐ and 11‐month‐old mice (23 mo > 2 and 11 mo, P = 0.0023 and P = 0.0051, respectively). A marginally significant effect of age was found on the total duration of contacts (Figure 3C: F 3,35 = 2.92, P = 0.0477). Post hoc analysis revealed that 17‐ and 23‐month‐old mice spent a longer time in contact with the unfamiliar conspecific of the same age than 2‐month‐old mice, although the behavioral differences among the age groups failed to reach significance after the Bonferroni correction (17 and 23 mo > 2 mo, P = 0.0365 and P = 0.0314, respectively). There was no significant effect of age on the number of contacts (Figure 3B: F 3,35 = 2.48, P = 0.0771) or the total duration of active contacts (Figure 3D: F 3,35 = 2.26, P = 0.0991). Collectively, these results indicate that old‐aged mice showed decreased locomotor activity in the novel environment, which might have resulted in increases in the total duration of contact and the mean duration of contact in old‐aged mice.
Figure 3.
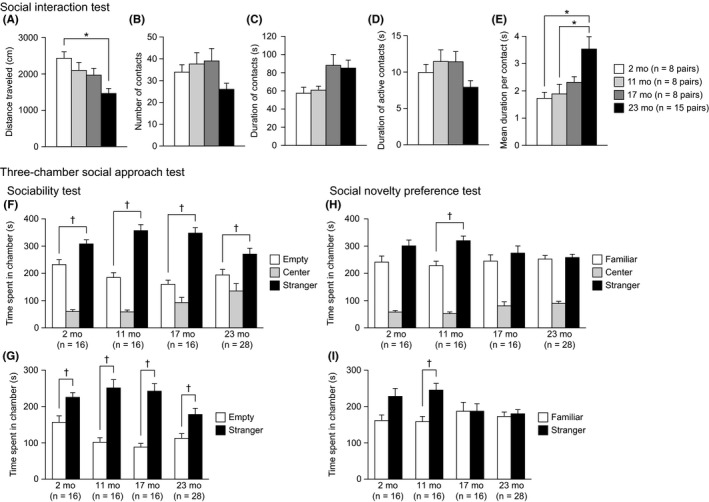
Normal sociability and reduced social novelty preference in aged C57BL/6J mice. (A‐E) Social interaction test: (A) distance traveled (cm), (B) number of contacts, (C) total duration of contacts (s), (D) total duration of active contacts (s), and (E) mean duration of contact (s). (F‐I) Three‐chamber social approach test: (F) time spent in the chamber with an empty cage, the center chamber, and the chamber with a cage containing a stranger mouse (stranger 1). (G) Time spent around the empty cage and the cage with stranger 1. (H) Time spent in the chamber with the cage containing stranger 1, the center chamber, and the chamber with a cage containing a novel unfamiliar mouse (stranger 2). (I) Time spent around the cage containing stranger 1 and the cage containing stranger 2. Values are means ± SEM. Asterisks indicate significant differences between groups after Bonferroni correction for multiple comparisons (P < 0.0083). Daggers represent statistical significance with paired t test (P < 0.05)
In the three‐chamber social approach test for assessing sociability (Figure 3F,G), each group of mice spent significantly more time in the chamber with stranger 1 than in the chamber with the empty cage (Figure 3F: 2 mo, t 15 = 2.32, P = 0.0346; 11 mo, t 15 = 4.38, P = 0.0005; 17 mo, t 15 = 6.32, P < 0.0001; 23 mo, t 27 = 2.36, P = 0.0257) and spent significantly more time around the cage with stranger 1 than around the empty cage (Figure 3G: 2 mo, t 15 = 2.43, P = 0.0279; 11 mo, t 15 = 4.54, P = 0.0004; 17 mo, t 15 = 6.31, P < 0.0001; 23 mo, t 27 = 2.75, P = 0.0106). In the social novelty preference test, 11‐month‐old mice spent significantly more time in the chamber with the unfamiliar mouse (stranger 2) than in the chamber with the now familiar mouse (stranger 1; Figure 3H: 11 mo, t 15 = 2.77, P = 0.0143) and spent significantly more time around the cage with stranger 2 than around the cage with stranger 1 (Figure 3I: 11 mo, t 15 = 2.90, P = 0.0111). Although 2‐month‐old mice exhibited no difference between the time spent in the chamber with stranger 2 and the time spent in the chamber with stranger 1 (2 mo, t 15 = 1.37, P = 0.1907), they showed a tendency to spend more time around the cage with stranger 2 than around the cage with stranger 1 (2 mo, t 15 = 1.89, P = 0.0777). In contrast, neither 17‐ nor 23‐month‐old mice showed significant differences between the time spent in each side chamber (17 mo, t 15 = 0.63, P = 0.5392; 23 mo, t 27 = 0.24, P = 0.8110) or the time spent around each cage (17 mo, t 15 = 0.01, P = 0.9941; 23 mo, t 27 = 0.39, P = 0.7027). These observations may reflect a decreased preference for social stimulus and impaired social recognition in 17‐ and 23‐month‐old mice.
3.4. Decreased ASR and decreased PPI in aged C57BL/6J mice
There were significant effects of age on startle responses to 110‐ and 120‐dB stimuli (Figure 4A: for 110‐dB, F 3,71 = 22.51, P < 0.0001; for 120‐dB, F 3,71 = 36.21, P < 0.0001). Middle‐ and old‐aged mice showed significantly smaller startle amplitudes than young mice at 110 dB (12, 18, and 23 mo < 3 mo, P = 0.0031, P < 0.0001, and P < 0.0001, respectively) and 120 dB (12, 18, and 23 mo < 3 mo, all P < 0.0001), and old‐aged mice exhibited smaller startle amplitudes than middle‐aged mice at both stimulus intensities (for 110 dB, 23 mo < 12 mo, P < 0.0001; for 120 dB, 23 mo < 12 mo, P = 0.0020). A significant effect of age was also found on PPI levels in four trial types (Figure 4B: for 74‐110 dB, F 3,71 = 5.45, P = 0.0020; for 78‐110 dB, F 3,71 = 12.11, P < 0.0001; for 74‐120 dB, F 3,71 = 4.89, P = 0.0038; for 78‐120 dB, F 3,71 = 14.64, P < 0.0001). At 74‐110 dB, 18‐ and 23‐month‐old mice showed significantly lower PPI levels than 12‐month‐old mice (P = 0.0021 and P = 0.0007, respectively). At 78‐110 dB, 18‐ and 23‐month‐old mice also exhibited significantly lower PPI levels than 3‐ and 12‐month‐old mice (18 and 23 mo < 3 mo, P = 0.0003 and P = 0.0007, respectively; 18 and 23 mo < 12 mo, all P < 0.0001). At 74‐120 dB, 18‐month‐old mice showed significantly lower PPI levels than 12‐month‐old mice (P = 0.0003). At 78‐120 dB, 3‐, 18‐, and 23‐month‐old mice exhibited significantly lower PPI levels than 12‐month‐old mice (P = 0.0042, P < 0.0001, and P < 0.0001, respectively), and 18‐month‐old mice showed lower PPI levels than 3‐month‐old mice (P = 0.0014). These results indicate that aging is associated with a gradual decrease in the ASR and with an increase and subsequent decrease in PPI levels from young to old age.
Figure 4.
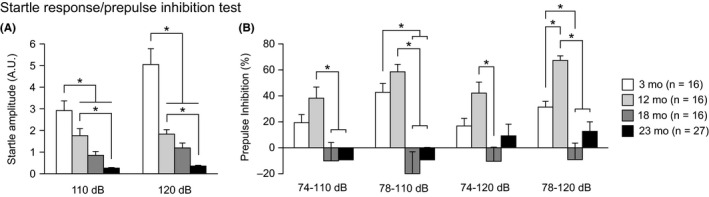
Decreased acoustic startle response and altered prepulse inhibition in middle‐aged and old‐aged C57BL/6J mice. (A) Acoustic startle response to sound stimuli (110 and 120 dB white noise) and (B) prepulse inhibition (%) of the startle response with 74 and 78 dB prepulse stimuli. Values are means ± SEM. Asterisks indicate significant differences between groups after Bonferroni correction for multiple comparisons (P < 0.0083)
3.5. Altered depression‐related behavior in aged C57BL/6J mice
Depression‐related behavior was assessed in the Porsolt forced swim test in 3‐, 12‐, 18‐, and 24‐month‐old mice (Figure 5A). There were significant main effects of age and age × time interactions on the percentages of immobility on test day 1 (age effect, F 3,71 = 14.29, P < 0.0001; age × time interaction, F 27,639 = 3.32, P < 0.0001) and test day 2 (age effect, F 3,71 = 4.50, P = 0.0060; age × time interaction, F 27,639 = 3.75, P < 0.0001). Post hoc analyses revealed that on day 1, 12‐, 18‐, or 24‐month‐old mice showed significantly less immobility than 3‐month‐old mice from the third to fifth minutes (3rd minute: 12, 18, and 24 mo < 3 mo, P = 0.0002, P < 0.0001, and P = 0.0023, respectively; 4th minute: 12, 18, and 24 mo < 3 mo, P = 0.0013, P < 0.0001, and P = 0.0017, respectively; and 5th minute: 12 and 18 mo < 3 mo, P = 0.0019 and P < 0.0001, respectively). In addition, 12‐ and 18‐month‐old mice exhibited significantly less immobility than 3‐month‐old mice from the sixth to ninth minutes (6th minute: 12 and 18 mo < 3 mo, P = 0.0409 and P = 0.0024, respectively; 7th minute: 12 mo < 3 mo, P = 0.0158; 8th minute: 12 mo < 3 mo, P = 0.0004; and 9th minute: 18 mo < 3 mo, P = 0.0017). From the fifth to tenth minutes, 24‐month‐old mice showed more immobility than 12‐ or 18‐month‐old mice (5th minute: 24 mo > 18 mo, P = 0.0004; 6th minute: 24 mo > 12 and 18 mo, P = 0.0397 and P = 0.0016, respectively; 7th minute: 24 mo > 12 and 18 mo, P < 0.0001 and P = 0.0224, respectively; 8th minute: 24 mo > 12 and 18 mo, P < 0.0001 and P = 0.0412, respectively; 9th minute: 24 mo > 18 mo, P < 0.0001; and 10th minute: 24 mo > 12 and 18 mo, P = 0.0003 and P = 0.0003, respectively), while the immobility of 24‐ and 3‐month‐old mice showed no significant differences after the fifth minute except for the tenth minute in which 24‐month‐old mice exhibited more immobility than 3‐month‐old mice (10th minute: 24 mo > 3 mo, P = 0.0027).
Figure 5.
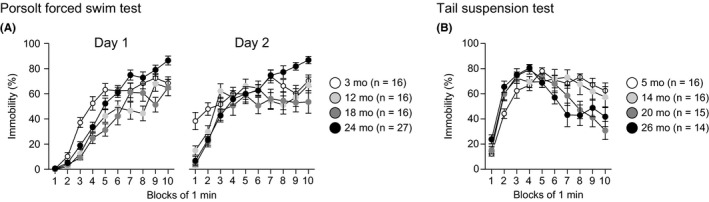
Altered depression‐related behavior in middle‐aged and old‐aged C57BL/6J mice. (A) Immobility time (%) on days 1 and 2 in the Porsolt forced swim test. (B) Immobility time (%) in the tail suspension test. Values are means ± SEM
In the Porsolt forced swim test, on day 2, 12‐, 18‐, and 24‐month‐old mice showed less immobility than 3‐month‐old mice during the first 2 minutes (1st minute: 12, 18, and 24 mo < 3 mo, P = 0.0052, P < 0.0001, and P < 0.0001, respectively; 2nd minute: 12, 18, and 24 mo < 3 mo, P = 0.0307, P = 0.0019, and P = 0.0011, respectively). In contrast, during the last 4 minutes of the session, 24‐month‐old mice generally exhibited more immobility than the other age groups (7th minute: 24 mo > 12 mo, P = 0.0063; 8th minute: 24 mo > 12 and 18 mo, P = 0.0005 and P = 0.0023, respectively; 9th minute: 24 mo > 3, 12, and 18 mo, P = 0.0037, P = 0.0009, and P = 0.0001, respectively; and 10th minute: 24 mo > 3, 12, and 18 mo, P = 0.0270, P = 0.0078, and P < 0.0001, respectively).
To further assess depression‐related behavior, the tail suspension test was performed in mice 5, 14, 20, and 26 months old (Figure 5B). There was a significant age × time interaction (age effect, F 3,57 = 2.41, P = 0.0767; age × time interaction, F 27,513 = 3.44, P < 0.0001). Post hoc analyses indicated that 26‐month‐old mice showed more immobility than 5‐month‐old mice during the second minute of the session (P = 0.0077), and 20‐ and 26‐month‐old mice exhibited less immobility than 5‐ and 14‐month‐old mice from the seventh to tenth minutes of the session (7th minute: 26 mo < 5 and 14 mo, P = 0.0018 and P = 0.0002, respectively; 8th minute: 26 mo < 5 and 14 mo, P = 0.0002 and P = 0.0021, respectively, and 20 mo < 5 and 14 mo, P = 0.0009 and P = 0.0093, respectively; 9th minute: 26 mo < 5 mo, P = 0.0218, and 20 mo < 5 and 14 mo, P = 0.0010 and P = 0.0083, respectively; and 10th minute: 26 mo < 5 mo, P = 0.0096, and 20 mo < 5 and 14 mo, P < 0.0001 and P = 0.0007, respectively).
3.6. Declines in working memory and spatial memory in aged C57BL/6J mice
Working memory was assessed in the T‐maze spontaneous alternation test at the ages of 3, 12, 18, and 24 months (Figure 6). There was a significant effect of age on the percentage of correct responses (F 3,68 = 3.89, P = 0.0126). Post hoc analysis revealed that 18‐ and 24‐month‐old mice showed a significantly lower percentage of correct responses than 3‐month‐old mice (P = 0.0070 and P = 0.0036), and 12‐month‐old mice exhibited a marginally significant decrease in the percentage of correct responses compared to that of 3‐month‐old mice (P = 0.0093). These observations indicate that aged mice show reduced working memory.
Figure 6.
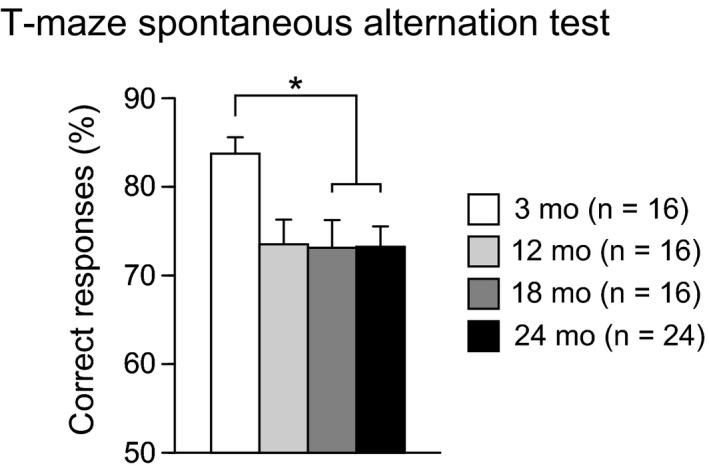
Working memory deficits in aged C57BL/6J mice. Percentage of correct responses was presented. Values are means ± SEM. Asterisk indicates significant differences between groups after Bonferroni correction for multiple comparisons (P < 0.0083)
Spatial learning and memory were assessed in the Barnes maze test (Figure 7). The training session started in the four cohorts of mice at the ages of 3, 12, 18, and 24 months, and the last training session was performed at the ages of 4, 13, 19, and 25 months in the different cohorts. In the training session, there were significant effects of age on latency to reaching the target hole (Figure 7B: age effect, F 3,58 = 9.74, P < 0.0001; age × session interaction, F 24,464 = 1.21, P = 0.2247) and distance traveled to reach the target hole (Figure 7C: age effect, F 3,58 = 4.61, P = 0.0058; age × session interaction, F 24,464 = 0.78, P = 0.7622). No significant effect of age was found in the number of errors before reaching the target hole (Figure 7A: age effect, F 3,58 = 1.39, P = 0.2546; age × session interaction, F 24,464 = 0.83, P = 0.6987). Mice aged 24‐25 months showed a longer latency to reaching the target hole than 3‐ to 4‐month‐old and 12‐ to 13‐month‐old mice (P < 0.0001 and P = 0.0006), and 18‐ to 19‐month‐old mice had a longer latency to reaching the target hole than 3‐ to 4‐month‐old mice (P = 0.0041) during the training session. The 24‐ to 25‐ and 18‐ to 19‐month‐old mice traveled longer distances to reach the target hole than the 3‐ to 4‐month‐old mice (P = 0.0066 and P = 0.0015). In the probe tests 1 and 10 days after the last training session, there were no significant effects of age on the time spent around the target hole (for the 1‐day retention test, Figure 7D, F 3,58 = 1.97, P = 0.1288; for the 10‐day retention test, Figure 7E, F 3,58 = 2.74, P = 0.0512). However, the time spent around the target hole in 25‐month‐old mice did not significantly differ from the average time spent around the adjacent holes (for the 1‐day retention test, Figure 7D, right panel, P = 0.1121; for the 10‐day retention test, Figure 7E, right panel, F 3,58 = 2.74, P = 0.1053, paired t test), whereas the mice in the other three age groups spent a longer time around the target hole than the around the adjacent holes in the 1‐day retention test (4 mo, P = 0.007; 13 mo, P = 0.0088; 19 mo, P = 0.0423, paired t test) and the 10‐day retention test (4 mo, P < 0.0001; 13 mo, P = 0.0062; 19 mo, P = 0.0301, paired t test). In the reversal learning session that started after the 10‐day retention test, a significant effect of age was found in the latency to reaching the target hole (Figure 7B: age effect, F 3,58 = 7.97, P = 0.0002; age × session interaction, F 12,232 = 0.97, P = 0.4822). A post hoc test revealed that 25‐month‐old mice showed a longer latency to reaching the target hole than 4‐ to 5‐, 13‐ to 14‐, and 19‐ to 20‐month‐old mice (P < 0.0001, P = 0.0023, and P = 0.0016, respectively). There were no significant effects of age on the number of errors (Figure 7A: age effect, F 3,58 = 0.49, P = 0.6883; age × session interaction, F 12,232 = 0.53, P = 0.8935) or the distance traveled to reach the target hole (Figure 7C: age effect, F 3,58 = 1.97, P = 0.1285; age × session interaction, F 12,232 = 0.59, P = 0.8520). The probe test 1 day after the last reversal session was conducted at the ages of 5, 14, 20, and 26 months, and there was a significant age effect on the time spent around the target hole (Figure 7F: F 3,58 = 3.42, P = 0.0230). Twenty‐six‐month‐old mice spent less time around the target hole than the mice in the other age groups (26 mo < 5, 14, and 20 mo, P = 0.0155, P = 0.0055, and P = 0.0190, respectively), although the differences between 26‐ and 5‐month‐old mice and the differences between 26‐ and 20‐month‐old mice did not reach significance after the Bonferroni correction. In this probe test, the time spent around the target hole did not significantly differ from the average time spent around the adjacent holes only in 26‐month‐old mice (for 26 mo, P = 0.2039; but for 5, 14, and 20 mo, P = 0.0055, P = 0.0012, and P = 0.0091, respectively, paired t test). These results indicate that aged mice show impaired spatial discrimination between the target hole and adjacent nontarget holes, which is suggestive of a deficit in spatial memory.
Figure 7.
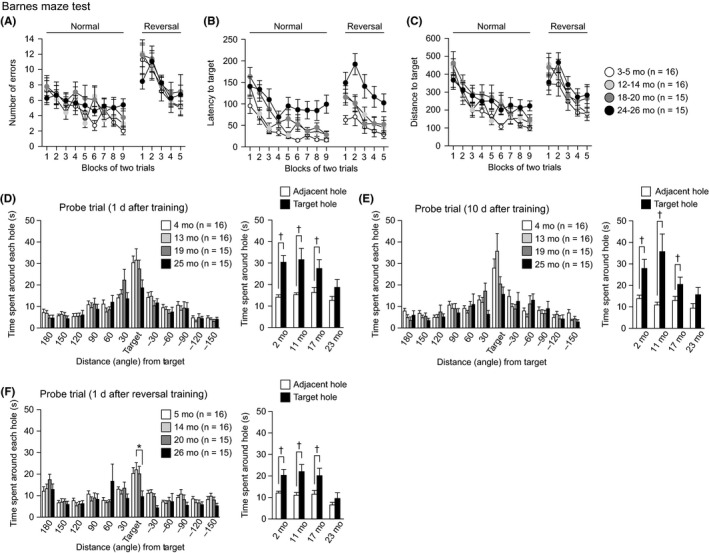
Impaired spatial memory in aged C57BL/6J mice. (A) Number of errors to first reach the target hole, (B) latency to reach the target hole, and (C) distance traveled to first reach the target hole during the training session and the reversal session of the Barnes maze test. Time spent around the target hole in the probe test 1 day (D) and 10 days (E) after the last normal training session. (F) Time spent around the target hole in the probe test 1 day after the last reversal session. In each probe trial, averaged time spent around two holes adjacent to the target one was compared to time spent around the target. Values are means ± SEM. Asterisk indicates significant differences between groups after Bonferroni correction for multiple comparisons (P < 0.0083). Daggers represent statistical significance with paired t test (P < 0.05)
3.7. Possible increase in generalized fear and reduced cued fear memory in aged C57BL/6J mice
Fear memory was assessed in the contextual and cued fear‐conditioning test in 5‐, 14‐, 20‐, and 26‐month‐old mice. In the conditioning session (Figure 8A), there was a significant main effect of age and a significant age × time interaction on freezing (age effect, F 3,56 = 27.78, P < 0.0001; age × time interaction, F 21,392 = 3.24, P < 0.0001) and distance traveled (age effect, F 3,56 = 11.02, P < 0.0001; age × time interaction, F 21,392 = 3.21, P < 0.0001). During the first minute of the conditioning session, there was no significant difference in freezing among the four age groups. During the second minute of the session, 26‐month‐old mice showed more freezing than mice in the other age groups (26 mo > 5, 14, and 20 mo, P < 0.0001, P < 0.0001, and P = 0.0020, respectively) before the CS‐US parings. Consistent with the freezing results, 26‐month‐old mice traveled significantly shorter distances than mice in the other age groups (Figure 8D: 1st minute, 26 mo < 5, 14, and 20 mo, all P < 0.0001; 2nd minute, 26 mo < 5, 14, and 20 mo, P < 0.0001, P < 0.0001, and P = 0.0032, respectively). These data indicate that old‐aged mice showed decreased basal activity levels. During the session with CS‐US parings, 20‐ and 26‐month‐old mice generally exhibited more freezing than 5‐and 14‐month‐old mice from the third to eighth minute of the session (from the 3rd to 8th minute, 26 mo > 5 and 14 mo, all P < 0.0083, except for the case of the comparison between 26 mo and 14 mo for the 7th minute (P = 0.0764); from the 4th to 6th minute and the 8th minute, 20 mo > 5 mo, all P < 0.0001; for the 4th and 6th minute, 20 mo > 14 mo, all P = 0.0083). From the sixth to eighth minutes of the session, 14‐month‐old mice showed more freezing than 5‐month‐old mice (P = 0.0053, P = 0.0161, and P = 0.0024, respectively). The distance traveled for four seconds during and after each footshock was measured to evaluate sensitivity to footshock. There were no significant effects of age and age × time interactions on the distance traveled after the first and second footshocks (Figure S3 and Table S1). For the third footshock, there was a nominally significant effect of age on the distance traveled (age effect, F 3,56 = 2.88, P = 0.0441; age × time interaction, F 42,784 = 1.76, P = 0.0025), and post hoc comparisons revealed that there were no significant differences in the total distance traveled during and after the footshock between age groups.
Figure 8.
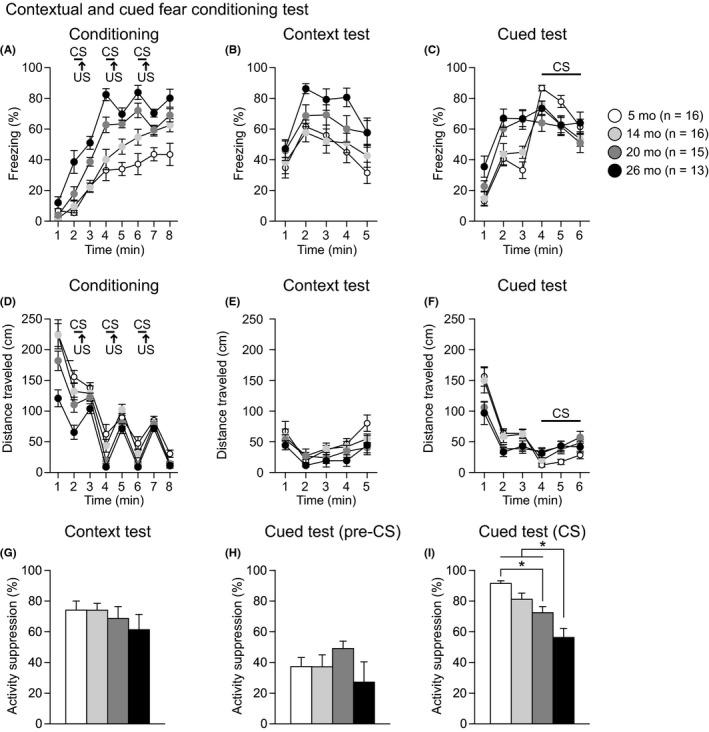
Increased generalized fear and impaired cued fear memory in aged C57BL/6J mice. Freezing (%) in the conditioning (A), context test (B), and cued test (C). Distance traveled (cm) in the conditioning (D), context test (E), and cued test (F). Percentage of activity suppression in the context test (G), pre‐CS period of the cued test (H), and CS period of the cued test (I). Values are means ± SEM. Asterisks indicate significant differences between groups after Bonferroni correction for multiple comparisons (P < 0.0083)
In the context test, approximately 24 hours after conditioning, there was a significant effect of age on freezing (Figure 8B: age effect, F 3,56 = 4.77, P = 0.0049; age × time interaction, F 12,224 = 1.01, P = 0.4417), while no significant effect of age was found on the distance traveled (Figure 8E and Table S1). Twenty‐six‐month‐old mice exhibited more freezing than 5‐ and 14‐month‐old mice (P = 0.0015 and P = 0.0041, respectively), which indicates that aged mice may show increased contextual fear memory. Considering that the age differences in freezing might be due to differences in baseline activity observed during the first 2 minutes of the conditioning session, the percentage of activity suppression (= 100 × (1 − (activity during first 2‐minute period of the test)/(baseline activity))) was calculated as a secondary fear index. There was no significant effect of age on the percentage of activity suppression (Figure 8G: F 3,56 = 0.69, P = 0.5590).
In the cued test with different contexts, significant age effects were found during the pre‐CS period on freezing (Figure 8C: age effect, F 3,56 = 9.73, P < 0.0001; age × time interaction, F 6,112 = 1.54, P = 0.1718) and on distance traveled (Figure 8F: age effect, F 3,56 = 5.13, P = 0.0033; age × time interaction, F 6,112 = 1.02, P = 0.4159). During the pre‐CS period, 20‐ and 26‐month‐old mice showed more freezing and traveled shorter distances than 5‐ and 14‐month‐old mice (for freezing, 20 mo > 5 and 14 mo, P = 0.0005 and P = 0.0090, respectively; for freezing, 26 mo > 5 and 14 mo, P < 0.0001 and P = 0.0004, respectively; for distance traveled, 20 mo > 5 and 14 mo, P = 0.0061 and P = 0.0153, respectively; for distance traveled, 26 mo > 5 and 14 mo, P = 0.0039 and P = 0.0096, respectively). The observed increases in freezing in response to the altered context and during the conditioning session imply that aged mice show enhanced generalized fear/anxiety, although the percentage of activity suppression during the first 2‐minutes of the testing period did not differ among age groups (Figure 8H: F 3,56 = 1.15, P = 0.3356). Significant age effects were also found during the CS period on freezing (age effect, F 3,56 = 4.29, P = 0.0086; age × time interaction, F 6,112 = 1.04, P = 0.4037) and on distance traveled (age effect, F 3,56 = 8.44, P = 0.0001; age × time interaction, F 6,112 = 0.51, P = 0.8009). During the CS period, 14‐, 20‐, and 26‐month‐old mice exhibited less freezing and traveled longer distance than 5‐month‐old mice (Figure 8C: for freezing, 14, 20, and 26 mo < 5 mo, P = 0.0189, P = 0.0009, and P = 0.0821, respectively; for distance traveled, 14, 20, and 26 mo > 5 mo, P = 0.0034, P < 0.0001, and P = 0.0008, respectively). In addition, 26‐month‐old mice showed a lower percentage of activity suppression than 5‐, 14‐, 20‐month‐old mice during the CS period (Figure 8I: F 3,56 = 13.20, P < 0.0001; 26 mo < 5, 14, and 20 mo, P < 0.0001, P < 0.0001, and P = 0.0080, respectively), and the percentage of activity suppression in 20‐month‐old mice was lower than that in 5‐month‐old mice (Figure 8I: P = 0.0012). Together, these data indicate that decreased freezing in response to the auditory cue in the cued test despite the increase in generalized freezing might be attributed to impaired cued fear memory in old‐aged mice.
4. DISCUSSION
This study examined various domains of behavior in four age cohorts of C57BL/6J mice from young to old age using a battery of behavioral tests, including tests to assess locomotor activity, sensory and motor functions, anxiety‐like behavior, social behavior, depression‐related behavior, and learning and memory functions. A series of behavioral tests were performed from 1 to 5 months of age in the first cohort, from 10 to 14 months of age in the second cohort, from 16 to 20 months of age in the third cohort, and from 22 to 26 months of age in the fourth cohort. One study defined a mature adult C57BL/6J mouse as 3‐6 months old, a middle‐aged mouse as 10‐14 months old, and an old mouse as 18‐24 months old.42 The present results indicate that compared to young mice, middle‐aged mice showed significant increases or decreases in various behavioral measures, including rotarod latency, center time in the open field test, number of transitions in the light/dark box test, distance traveled and total number of arm entries in the elevated plus maze test, and ASR, and spontaneous alternation in the T‐maze test and freezing in response to the conditioned cue tended to be lower in middle‐aged mice than in young mice. Increasing age from middle age to old age induces further changes in a wide range of behaviors, such as decreased locomotor activity, increased anxiety‐like behavior, reduced social behavior, decreased ASR, decreased Barnes maze performance, and increased freezing when exposed to the fear‐conditioning context and the altered context. The results of behavioral differences between young and aged male C57BL/6J mice were generally consistent with the previous reports (eg, Refs 3, 12, 17, 43, 44, 45, 46). These findings indicate that aging is associated with gradual changes in behaviors related to locomotor activity, anxiety‐like behavior, and memory functions from young to old age. In contrast, vertical activity, stereotypic counts, and PPI were increased in middle‐aged mice compared to those in young mice, while vertical activity and stereotypic counts in old‐aged mice did not significantly differ from those in young mice. In addition, decreased immobility during the forced swim test was also observed in middle‐aged mice compared to that in young mice, and old‐aged mice showed levels of immobility similar to those of young mice. These findings suggest that some behaviors do not change in a unidirectional and progressive fashion from young to old age.
Our previous study that involved a large‐scale analysis of behavioral data collected from our database for cohorts of many mutant strains of mice indicated that there are age‐related changes in behaviors from young adulthood (2‐3 months of age) to middle age (8‐12 months of age) in wild‐type control C57BL/6J mice.13 Although the present results are largely consistent with the previous findings, there appear to be some differences between the results of two studies with respect to comparisons of the behaviors of young and middle‐aged mice. For example, the present study found no statistically significant differences between young (1‐5 mo) and middle‐aged (10‐14 mo) mice in wire hang latency, distance traveled during the first 5 minutes in the open field test, distance traveled in the light box, stay time in the light box, percentage of entries into the open arms, number of social contacts, and time spent around the target hole in the probe trial of the Barnes maze test, while our previous study showed significant differences in these behavioral measures between young and middle‐aged mice. The present study also showed that vertical activity and center time in the open field test significantly increased in middle‐aged (11 mo) mice compared to those in young (2 mo) mice, whereas our previous study found no significant differences in these behaviors between young (2‐3 mo) and middle‐aged (8‐12 mo) mice, although middle‐aged mice showed a trend toward an increase in those behaviors. These inconsistent results may be due to the influences of potential confounding factors, such as the genetic background of the animals, their breeding environment, and their prior test experience, in the previous study, since the behavioral data from the previous study were obtained from many cohorts of mice derived from different vendors and laboratories and tested by different experimenters on different dates. On the other hand, all the C57BL/6J mice used in this study, which had been maintained under The Jackson Laboratory's patented Genetic Stability Program, were supplied by Charles River Laboratories Japan. The four age cohorts of mice in this study were subjected to a behavioral test battery in the same order by the same experimenters on the same day. Some cases in which behavioral differences were observed between age groups did not reach statistical significance, possibly due to the relatively small number of animals in each age group in this study. Our previous study compared behaviors between age groups using a large number of mice. A large sample size increases the statistical power to reach significance. Although the precise reasons for the differences in results between these studies are not clear, the present study generally supports our previous findings and other reports that aging from young to middle age induces increased body weight,47 reduced rotarod performance,48 decreased locomotor activity,49 reduced ASR,44 increased PPI,44 decreased immobility during the forced swim test,13 and decreased freezing during the cued test.13
In this study, PPI was higher in 12‐month‐old mice than in 3‐month‐old mice, and the PPI levels of 18‐ and 24‐month‐old mice were lower than those of 3‐ and 12‐month‐old mice. Such inverted U‐shaped changes in PPI with age were observed in our previous study and other reports in C57BL/6J mice.13, 44 Aging induces hearing loss in C57BL/6J mice,50 which might explain why the lowest ASR amplitudes were observed in old‐aged mice in this study. Mice with low ASR amplitudes were reported to show lower PPI levels than mice with intermediate and high ASR amplitudes.33 Decreased PPI levels in old‐aged mice might be partially explained by a decreased startle response. In the open field test, despite increased body weight and decreased motor performance in middle‐aged mice, increased vertical activity (possibly rearing) and repetitive stereotypic movements were observed in middle‐aged mice but not in old‐aged mice when compared to those in young mice. The behavioral tendencies in middle‐aged mice might lead to swimming and climbing against the wall of the cylinder in the Porsolt forced swim test, possibly resulting in decreased immobility.
Our previous study indicated that wire hang latency decreased after 6‐7 months of age,13 whereas this study showed no significant differences in wire hang latency among age groups, and nearly all the mice could hold on to the wire grid over the 60 seconds of the testing period. The present results imply that the middle‐ and old‐aged mice used in this study maintained physical and muscular strength similar to that of young mice. In this study, aged animals that were apparently healthy were selected and supplied by the vendor according to the company's guidelines (Tabata, K. and Goto, Y., Charles River Laboratories Japan, personal communication). This potentially biased selection of animals might have resulted in the absence of significant age‐related differences in wire hang latency. However, it must also be noted that there might be a ceiling effect regarding the results of the wire test. Reduced muscular strength might be observed in aged mice compared to that in young mice if the wire hang test was conducted for more than 60 seconds.
The open field, light/dark transition, and elevated plus maze tests are widely used to assess anxiety‐like behavior based on the natural aversion of mice to novel, bright, open spaces and based on their natural tendency to explore novel environments.51, 52, 53, 54 In the present study, a gradual decline in locomotor activity from young to old age was observed in the open field, light/dark transition, and elevated plus maze tests. In addition, aged mice exhibited a decreased number of transitions and reduced time spent in the light chamber in the light/dark transition test. The decreased locomotor activity in aged mice was unlikely to be due to muscular and motor dysfunction, since their wire hang latency and total distance traveled during the 120‐minute period in the open field test did not differ from those in young mice. These findings suggest increased anxiety‐like responses to novel environments in aged mice. Similarly, decreased locomotor activity in the social interaction test has been used to measure anxiety‐like behavior,55 which may reflect an increased anxiety state in novel environments in aged mice. In most previous studies, the open field test has been performed for 5‐30 minutes to evaluate locomotor activity and anxiety‐like behavior in response to novelty.28, 52 Our study examined open field behaviors during a 120‐minute session, during which aged mice spent a longer time in the center area than young mice. These results suggest that while the anxiety‐like behavior of old‐aged mice might increase during the initial 10‐minute period of testing in a novel environment, compared to that of young mice, the anxiety‐like behavior of aged mice might decrease after a long exposure to the same environment.
In the social interaction test, aged mice showed slightly decreased social contacts and a significant increase in the mean duration per contact, which might be due to decreased locomotor activity. Consistent with the absence of marked age differences in social behavior in aged mice, the results of the sociability test showed that middle‐ and old‐aged mice, as well as young mice, exhibited a preference for the novel mouse, which suggests that there are no clear differences in neophobic responses to a novel social stimulus, social interest, or sociability among the age groups. In the social novelty preference test conducted immediately after the sociability test, aged mice (17‐ and 23‐months old) exhibited no preference for a new stranger mouse (a decreased social novelty preference), which is consistent with a recent report.46 The decreased preference for social novelty might reflect a reduced ability to discriminate between individual mice, suggesting that a social recognition deficit is present in aged mice.
The results of a series of memory tasks indicate that old‐aged C57BL/6J mice show deficits in various types of memory functions, including working memory, spatial memory, and cued fear memory, compared to those of young mice. In the altered context of the fear‐conditioning test, compared to 5‐month‐old mice, 20‐ and 26‐month‐old mice showed increased freezing during the pre‐cue period of the test, which suggests increased generalized fear or a pattern separation deficit in old‐aged mice. In the Barnes maze test, our previous study reported that middle‐aged mice exhibited significantly decreased time spent around the target hole compared to that of young mice in the probe trial,13 whereas in this study, there were no significant differences in the behavioral measures between young and middle‐aged mice. The young cohort was 4‐5 months old when tested in the probe trials in this study. As shown by our previous report,13 the time spent around the target hole in 4‐5‐month‐old mice was markedly lower than that of 2‐3‐month‐old mice. The decreased time spent around the target hole after the age of 4‐5 months might have led to the absence of an apparent age‐related difference in the time spent around the target hole between the young and middle‐aged cohorts tested in the present study.
Decreased immobility in the forced swim test was observed in middle‐aged and old‐aged mice for the first several minutes of the session on day 2, which suggests that middle‐aged and old‐aged mice were more active than young mice in response to cold water immersion, despite their decreased locomotor activity in novel environments and their reduced motor performance in other tests. These results might reflect a stronger panic‐like response to the sudden aversive stimulus in aged animals than in young animals. During the last half of the session, old‐aged mice showed higher immobility than middle‐aged mice. This behavioral transition from an active response to a passive response might be indicative of either increased depression‐related behavior or an adaptive response that contributes to energy conservation.56 In contrast, in the tail suspension test, old‐aged mice showed less immobility than young and middle‐aged mice during the last 4 minutes of the session, which suggests decreased depression‐related behavior in old‐aged mice. From these findings from the two types of tests, it would be difficult to draw clear conclusions about age‐related changes in depression‐related behavior.
Genetic and environmental factors are recognized as critical contributors to mouse behavior. Inbred C57BL/6J mice are the most commonly used mouse strain in neuroscience and biomedical research. The present study, which used a behavioral test battery, shows the behavioral profiles of young, middle‐aged, and old‐aged male C57BL/6J mice from a colony maintained under genetically and environmentally controlled conditions. The use of the behavioral test battery has some advantages, such as reducing the number of animals used, reducing the time to prepare many animals, and increasing the interpretation of the behavioral characteristics based on the results from a variety of tests. However, the test battery approach may have one limitation, that is, increased mortality, when applied to old‐aged animals, in which the gradual decrease in the number of old‐aged mice during the test battery due to death could lead to a small sample size that could thereby have affected the statistical results. Nevertheless, our results indicate that there are age‐related changes in various domains of behavior from young to old age, such as gradual declines in motor function, locomotor activity, social behavior, startle responses, and different types of memory function, in the inbred C57BL/6J mice. The results also show that some behavioral changes that were observed from young to middle age, including increased vertical activity, increased PPI, and decreased depression‐related behavior, were reversed from middle age to old age. The findings of this study will provide fundamental information about the behavioral characteristics of different age cohorts and about age‐related behavioral changes from young to old age in male C57BL/6J mice.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
HS performed the behavioral tests and analyzed the data. HS and TM wrote the manuscript. All authors read and approved the final manuscript.
DATA REPOSITORY
Raw data of the behavioral tests are accessible on the public database “Mouse Phenotype Database” (http://www.mouse-phenotype.org/).
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
None.
INFORMED CONSENT
None.
REGISTRY AND THE REGISTRATION NO. OF THE STUDY/TRIAL
None.
ANIMAL STUDIES
All experimental procedures were approved by Institutional Animal Care and Use Committee of Fujita Health University.
Supporting information
ACKNOWLEDGMENT
We would like to thank Mr. Kazuki Tabata, Mr. Masato Sawaura, Mr. Yohei Goto, and other members at Charles River Laboratories Japan, Inc. for providing experimental animals to support this study. We also thank Tamaki Murakami, Yoko Kagami, and Harumi Mitsuya for their assistance in animal husbandry, behavioral experiment, and preparing this paper. This study was supported by Grant‐in‐Aid for Research on Innovative Areas (Platform of Advanced Animal Model Support) (16H06276) and Grant‐in‐Aid for Scientific Research (A) (16680015) from the Ministry of Education, Science, Sports and Culture (MEXT) of Japan. Behavioral analysis was carried out at the Institute for Comprehensive Medical Science, Fujita Health University, by the Joint Usage/Research Center for Genes, Brain and Behavior, which is accredited by MEXT.
Shoji H, Miyakawa T. Age‐related behavioral changes from young to old age in male mice of a C57BL/6J strain maintained under a genetic stability program. Neuropsychopharmacol Rep. 2019;39:100–118. 10.1002/npr2.12052
REFERENCES
- 1. Sprott RL, Eleftheriou BE. Open‐field behavior in aging inbred mice. Gerontology. 1974;20:155–162. [DOI] [PubMed] [Google Scholar]
- 2. Brennan MJ, Dallob A, Friedman E. Involvement of hippocampal serotonergic activity in age‐related changes in exploratory behavior. Neurobiol Aging. 1981;2:199–203. [DOI] [PubMed] [Google Scholar]
- 3. Dean RL, Scozzafava J, Goas JA, Regan B, Beer B, Bartus RT. Age‐related differences in behavior across the life span of the C57BL/6J mouse. Exp Aging Res. 1981;7:427–451. [DOI] [PubMed] [Google Scholar]
- 4. Ingram DK, London ED, Reynolds MA, Waller SB, Goodrick CL. Differential effects of age on motor performance in two mouse strains. Neurobiol Aging. 1981;2:221–227. [DOI] [PubMed] [Google Scholar]
- 5. Flood JF, Morley JE. Pharmacological enhancement of long‐term memory retention in old mice. J Gerontol. 1990;45:B101–B104. [DOI] [PubMed] [Google Scholar]
- 6. Fordyce DE, Whner JM. Effects of aging on spatial learning and hippocampal protein kinase C in mice. Neurobiol Aging. 1993;14:309–317. [DOI] [PubMed] [Google Scholar]
- 7. Ammassari‐Teule M, Fagioli S, Rossi‐Arnaud C. Radial maze performance and open‐field behaviours in aged C57BL/6 mice: further evidence for preserved cognitive abilities during senescence. Physiol Behav. 1994;55:341–345. [DOI] [PubMed] [Google Scholar]
- 8. Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age‐related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci USA. 1996;93:4765–4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frick KM, Burlingame LA, Arters JA, Berger‐Sweeney J. Reference memory, anxiety and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 1999;95:293–307. [DOI] [PubMed] [Google Scholar]
- 10. Hengemihle JM, Long JM, Betkey J, Jucker M, Ingram DK. Age‐related psychomotor and spatial learning deficits in 129/SvJ mice. Neurobiol Aging. 1999;20:9–18. [DOI] [PubMed] [Google Scholar]
- 11. Fahlström A, Yu Q, Ulfhake B. Behavioral changes in aging female C57BL/6 mice. Neurobiol Aging. 2011;32:1868–1880. [DOI] [PubMed] [Google Scholar]
- 12. Fahlström A, Zeberg H, Ulfhake B. Changes in behaviors of male C57BL/6J mice across adult life span and effects of dietary restriction. Age. 2012;34:1435–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shoji H, Takao K, Hattori S, Miyakawa T. Age‐related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol Brain. 2016;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crawley JN, Paylor R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm Behav. 1997;31:197–211. [DOI] [PubMed] [Google Scholar]
- 15. Takao K, Yamasaki N, Miyakawa T. Impact of brain‐behavior phenotypying of genetically‐engineered mice on research of neuropsychiatric disorders. Neurosci Res. 2007;58:124–132. [DOI] [PubMed] [Google Scholar]
- 16. Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57:809–818. [DOI] [PubMed] [Google Scholar]
- 17. Goodrick CL. Behavioral differences in young and aged mice: strain differences for activity measures, operant learning, sensory discrimination, and alcohol preference. Exp Aging Res. 1975;1:191–207. [DOI] [PubMed] [Google Scholar]
- 18. Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. [DOI] [PubMed] [Google Scholar]
- 19. Wahlsten D, Metten P, Phillips TJ, et al. Different data from different labs: lessons from studies of gene–environment interaction. J Neurobiol. 2003;54:283–311. [DOI] [PubMed] [Google Scholar]
- 20. Mekada K, Abe K, Murakami A, et al. Genetic differences among C57BL/6 substrains. Exp Anim. 2009;58:141–149. [DOI] [PubMed] [Google Scholar]
- 21. Zurita E, Chagoyen M, Cantero M, et al. Genetic polymorphisms among C57BL/6 mouse inbred strains. Transgenic Res. 2011;20:481–489. [DOI] [PubMed] [Google Scholar]
- 22. Flurkey K, Currer JM, eds. The Jackson laboratory handbook on genetically standardized mice, 6th edn Bar Harbor, ME: Jackson Laboratory; 2009. [Google Scholar]
- 23. Low‐Marchelli J. Strategies to minimize genetic drift and maximize experimental reproducibility in mouse research. Charles River Laboratories International, Inc., 2017. http://www.crj.co.jp/cms/cmsrs/pdf/product/Strategies_to_Minimize_Genetic_Drift_and_Maximize_Experimental_reproducibility_in_mouse_research.pdf. Accessed December 21, 2018.
- 24. Charles River Laboratories, Inc . JAXTM mice and research services provided through Charles River. https://www.crj.co.jp/cms/cmsrs/pdf/product/rm_d_JAX_EU_partnership_.pdf. Accessed January 18, 2019.
- 25. Shoji H, Hagihara H, Takao K, Hattori S, Miyakawa T. T‐maze forced alternation and left‐right discrimination tasks for assessing working and reference memory in mice. J Vis Exp. 2012;60:3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shoji H, Takao K, Hattori S, Miyakawa T. Contextual and cued fear conditioning test using a video analyzing system in mice. J Vis Exp. 2014;85:50871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shoji H, Irino Y, Yoshida M, Miyakawa T. Behavioral effects of long‐term oral administration of aluminum ammonium sulfate in male and female C57 BL/6J mice. Neuropsychopharmacol Rep. 2018;38:18–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crawley JN, Goodwin FK. Preliminary report of a simple animal behavior for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. [DOI] [PubMed] [Google Scholar]
- 29. Takao K, Miyakawa T. Light/dark transition test for mice. J Vis Exp. 2006;1:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety‐like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. [DOI] [PubMed] [Google Scholar]
- 31. Komada M, Takao K, Miyakawa T. Elevated plus maze for mice. J Vis Exp. 2008;22:1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moy SS, Nadler JJ, Perez A, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic‐like behavior in mice. Genes Brain Behav. 2004;3:287–302. [DOI] [PubMed] [Google Scholar]
- 33. Shoji H, Miyakawa T. Relationships between the acoustic startle response and prepulse inhibition in C57BL/6J mice: a large‐scale meta‐analytic study. Mol Brain. 2018;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 35. Nakao A, Miki T, Shoji H, et al. Comprehensive behavioral analysis of voltage‐gated calcium channel beta‐anchoring and‐regulatory protein knockout mice. Front Behav Neurosci. 2015;9:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74. [DOI] [PubMed] [Google Scholar]
- 37. Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. [DOI] [PubMed] [Google Scholar]
- 38. Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. [DOI] [PubMed] [Google Scholar]
- 39. Kafkafi N, Lipkind D, Benjamini Y, Mayo CL, Elmer GI, Golani I. SEE locomotor behavior test discriminates C57BL/6J and DBA/2J mouse inbred strains across laboratories and protocol conditions. Behav Neurosci. 2003;117:464. [DOI] [PubMed] [Google Scholar]
- 40. Holmes A, Yang RJ, Murphy DL, Crawley JN. Evaluation of antidepressant‐related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology. 2002;27:914–923. [DOI] [PubMed] [Google Scholar]
- 41. McFadyen MP, Kusek G, Bolivar VJ, Flaherty L. Differences among eight inbred strains of mice in motor ability and motor learning on a rotorod. Genes Brain Behav. 2003;2:214–219. [DOI] [PubMed] [Google Scholar]
- 42. Flurkey K, Currer JM, Harrison DE. Mouse models in aging research In: Fox JG, Davisson MT, Quimby FW, Barthold SW, Newcomer CE, Smith AL, editors. The mouse in biomedical research. 2nd ed Burlington, NJ: Academic Press, 2007; p. 637–672. [Google Scholar]
- 43. Gould TJ, Feiro OR. Age‐related deficits in the retention of memories for cued fear conditioning are reversed by galantamine treatment. Behav Brain Res. 2005;165:160–171. [DOI] [PubMed] [Google Scholar]
- 44. Ouagazzal AM, Reiss D, Romand R. Effects of age‐related hearing loss on startle reflex and prepulse inhibition in mice on pure and mixed C57BL and 129 genetic background. Behav Brain Res. 2006;172:307–315. [DOI] [PubMed] [Google Scholar]
- 45. Vegh MJ, Rausell A, Loos M, et al. Hippocampal extracellular matrix levels and stochasticity in synaptic protein expression increase with age and are associated with age‐dependent cognitive decline. Mol Cell Proteomics. 2014;13:2975–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Scott KA, Ida M, Peterson VL, et al. Revisiting Metchnikoff: age‐related alterations in microbiota‐gut‐brain axis in the mouse. Brain Behav Immun. 2017;65:20–32. [DOI] [PubMed] [Google Scholar]
- 47. Halloran BP, Ferguson VL, Simske SJ, Burghardt A, Venton LL, Majumdar S. Changes in bone structure and mass with advancing age in the male C57BL/6J mouse. J Bone Miner Res. 2002;17:1044–1050. [DOI] [PubMed] [Google Scholar]
- 48. Serradj N, Jamon M. Age‐related changes in the motricity of the inbred mice strains 129/sv and C57BL/6j. Behav Brain Res. 2007;177:80–89. [DOI] [PubMed] [Google Scholar]
- 49. Davis MJ, Haley T, Duvoisin RM, Raber J. Measures of anxiety, sensorimotor function, and memory in male and female mGluR4−/− mice. Behav Brain Res. 2012;229:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Keithley EM, Canto C, Zheng QY, Fischel‐Ghodsian N, Johnson KR. Age‐related hearing loss and the ahl locus in mice. Hear Res. 2004;188:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. [DOI] [PubMed] [Google Scholar]
- 52. Carola V, D'Olimpio F, Brunamonti E, Mangia F, Renzi P. Evaluation of the elevated plus‐maze and open‐field tests for the assessment of anxiety‐related behaviour in inbred mice. Behav Brain Res. 2002;134:49–57. [DOI] [PubMed] [Google Scholar]
- 53. Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety‐like behavior: the elevated plus‐maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–1205. [DOI] [PubMed] [Google Scholar]
- 54. Bourin M, Petit‐Demoulière B, Nic Dhonnchadha B, Hascöet M. Animal models of anxiety in mice. Fundam Clin Pharmacol. 2007;21:567–574. [DOI] [PubMed] [Google Scholar]
- 55. File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. [DOI] [PubMed] [Google Scholar]
- 56. De Kloet ER, Molendijk ML. Coping with the forced swim stressor: towards understanding an adaptive mechanism. Neural Plast. 2016;2016:6503162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


